Abstract
Objectives
The stationary phase of Clostridium difficile, which is primarily responsible for diarrhoeal symptoms, is refractory to antibiotic killing. We investigated whether disrupting the functions of the clostridial membrane is an approach to control C. difficile infections by promptly removing growing and non-growing cells.
Methods
The bactericidal activities of various membrane-active agents were determined against C. difficile logarithmic-phase and stationary-phase cultures and compared with known antibiotics. Their effects on the synthesis of ATP, toxins A/B and sporulation were also determined. The effect of rodent caecal contents on anti-difficile activities was examined using two reutericyclin lead compounds, clofazimine, daptomycin and other comparator antibiotics.
Results
Most membrane-active agents and partially daptomycin showed concentration-dependent killing of both logarithmic-phase and stationary-phase cultures. The exposure of cells to compounds at their MBC resulted in a rapid loss of viability with concomitant reductions in cellular ATP, toxins A/B and spore numbers. With the exception of nisin, these effects were not due to membrane pore formation. Interestingly, the activity of the proton ionophore nigericin significantly increased as the growth of C. difficile decreased, suggesting the importance of the proton gradient to the survival of non-growing cells. The activities of the lipophilic antimicrobials reutericyclins and clofazimine were reduced by caecal contents.
Conclusions
These findings indicate that C. difficile is uniquely susceptible to killing by molecules affecting its membrane function and bioenergetics, indicating that the clostridial membrane is a novel antimicrobial target for agents to alleviate the burden of C. difficile infections.
Keywords: bactericidal, drug tolerant, non-growing cells
Introduction
Clostridium difficile is a Gram-positive spore-forming anaerobe that is the leading cause of healthcare-associated diarrhoea.1,2 C. difficile infection (CDI) predominantly occurs in elderly patients, typically following the use of broad-spectrum antibiotics that perturb the host's intestinal microbiota allowing the outgrowth of and colonization with toxinogenic strains. Since 2001, there has been an alarming increase in the incidence and severity of CDI in North America and Europe.1,3 For example, annually in the USA alone there are about 500 000 cases of CDIs and more than 15 000 deaths that impose an economic burden of around $3.2 billion.3,4 This is partly a result of the emergence of epidemic strains that appear hypervirulent. The most common epidemic strain, designated as NAP1/027/BI, is responsible for several hospital outbreaks and higher rates of mortality and disease recurrence. The hypervirulence of the NAP1 clade is multifactorial, with strains showing a propensity to hyperproduce the lethal toxins TcdA and TcdB, hypersporulation5,6 and carriage of a binary toxin that may enhance mucosal adherence.7 The production of toxins and the onset of sporulation both occur in late-logarithmic-phase and stationary-phase C. difficile (i.e. quiescent cells),5 which are more refractory to antibiotic killing than rapidly dividing cells.8,9
Before the marked impact of NAP1 on healthcare, limited attention was given to the discovery and development of novel anti-difficile drugs. This is evident from metronidazole and vancomycin being the first-line treatments for more than 30 years.10–12 However, disease recurrence after treatment is common for NAP1-mediated infections, with typical relapse rates of 20%–30% or higher.13,14 The newly approved anti-difficile drug fidaxomicin does reduce the risk of relapse, but this is only for non-NAP1 infections because the rates of recrudescence in fidaxomicin-treated patients with NAP1 infections seem similar to those in patients given vancomycin.15–17 A need therefore exists for novel agents whose mode of action may improve therapeutic outcomes for NAP1 infections and reduce disease severity.
In bacteria the functions of the membrane are essential, such as maintenance of the intracellular environment, nutrient transport and the cellular ionic gradient, which are critical for cell survival and energy generation.9,18,19 To address the urgent need for novel anti-difficile agents, we hypothesize that perturbing the clostridial membrane functions, including associated bioenergetics, could be a valuable strategy to rapidly alleviate the burden of CDI by eliminating the quiescent cells responsible for toxin and spore production. Indeed, agents targeting the membrane are generally rapidly bactericidal, show a low propensity for resistance emergence and their narrow spectrum of activity for Gram-positive pathogens may make them relevant to CDI.9 In support of this hypothesis the membrane-active antimicrobials oritavancin, an analogue of vancomycin, and CB-183,315, an analogue of daptomycin, are in clinical development for CDI.20,21 Moreover, we reported that stationary-phase C. difficile is extremely sensitive to killing by membrane-active antibiotics known as reutericyclins,8 which do not kill stationary-phase Staphylococcus aureus. Therefore, in this study, we used various known membrane-active molecules to systematically investigate whether the membrane and bioenergetics are the Achilles' heel in non-growing C. difficile.
Materials and methods
Chemicals, bacterial strains and growth
The compounds 867 and 1138, which belong to the reutericyclin class of antimicrobials that are in the early stages of discovery,8 were synthesized as described by Yendapally et al.22 Thiolactomycin was synthesized as previously described.23 Daptomycin was kindly provided by Cubist Pharmaceuticals. Other compounds were obtained as follows: gatifloxacin, from Enzo Life Sciences; trimethoprim and sulfamethoxazole, from MP Biomedicals; erythromycin, from EMD Millipore; linezolid, from Selleck Chemicals; all other test compounds in Table 1 were from Sigma-Aldrich. The C. difficile strains BAA-1803 (toxinotype III, 95 NAP1) and BAA-1875 (toxinotype V, NAP7) from ATCC were primarily used. Additional test strains were C. difficile CD630, ATCC 9689, ATCC 43596 (reference strains, toxinotype 0) and CD32 (toxinotype III, NAP1 clinical isolate from Dr Scott Curry, University of Pittsburgh, USA). Unless otherwise stated all strains were routinely grown in pre-reduced TY broth [tryptone (1% w/v) and yeast extract (0.5% w/v)] or Brucella agar supplemented with vitamin K1 (1 mg/L), haemin (5 mg/L) and 5% sheep blood, at 37°C in a Whitley A35 anaerobic workstation (Don Whitley Scientific).
Table 1.
Activities of membrane-active agents and comparators against C. difficile
| Antibacterial target | Compound | Activity (mg/L) against BAA-1803a |
Activity (mg/L) against BAA-1875a |
||||
|---|---|---|---|---|---|---|---|
| MIC | MBCLOG | MBCSTA | MIC | MBCLOG | MBCSTA | ||
| DNA replication | gatifloxacinb | 32 | >64 | >64 | 32 | ≥64 | >64 |
| RNA synthesis | rifaximin | >64 | >64 | >64 | 0.0125 | 4 | >64 |
| Protein synthesis | fusidic acid | 0.078 | >64 | >64 | 0.094 | >64 | >64 |
| erythromycin | >64 | >64 | >64 | 0.75 | >64 | >64 | |
| chloramphenicol | 1 | 64 | >64 | 1 | >64 | >64 | |
| linezolid | 0.75 | >64 | >64 | 0.5 | >64 | >64 | |
| Folic acid synthesis | trimethoprim | >64 | >64 | >64 | >64 | >64 | >64 |
| sulfamethoxazole | >64 | >64 | >64 | >64 | >64 | >64 | |
| Cell wall synthesis | ramoplanin | 0.24 | 0.5 | >64 | 0.375 | 0.5 | >64 |
| vancomycinc | 0.375 | >64 | >64 | 0.375 | >64 | >64 | |
| Fatty acid synthesis | thiolactomycin | >64 | >64 | >64 | >64 | >64 | >64 |
| Membrane | CCCP | 0.75 | 3 | 1.5 | 0.125 | 4 | 4 |
| Membrane | monensin | 0.5 | 0.38 | 0.007 | 0.5 | 2 | 0.007 |
| Membrane | 867 | 0.125 | 0.25 | 0.5 | 0.094 | 0.25 | 0.25 |
| Membrane | 1138d | 0.5 | 2 | 4 | 0.5 | 1 | 2 |
| Membrane/cell wall synthesis | nisin | 0.4 | 1.25 | 0.8 | 0.4 | 1.6 | 1 |
| NDH-2/membrane | thioridazine | 32 | 64 | 48 | 24 | 64 | >64 |
| Membrane | valinomycin | 2 | 3 | 4 | 0.125 | 0.5 | 0.125 |
| Membrane | nigericin | 0.0025 | 0.005 | <0.0006 | 0.0025 | 0.005 | <0.0006 |
| Membrane/respiration | clofazimine | 0.045 | 2 | 2 | 0.25 | 2 | 8 |
| Membrane | daptomycine | 1.5 | 4 | >64 | 0.75 | 4 | 16 |
| ATP synthase | DCCD | 20 | 64 | 16 | 16 | 32 | 64 |
| NDH-1/respiration | rotenone | >64 | >64 | >64 | >64 | >64 | >64 |
| Non-specific damage by reactive species; PFOR inhibition by nitazoxanide | nitazoxanide | 0.156 | 32 | 8 | 0.25 | ≥64 | 20 |
| metronidazole | 0.5 | 0.38 | 8 | 0.094 | 0.31 | 16 | |
NDH-2, NADH dehydrogenase type 2; NDH-1, NADH dehydrogenase type 1; PFOR, pyruvate ferredoxin oxidoreductase.
aMean activity of two or more experiments.
bAgainst the wild-type strains CD630 and 9689 gatifloxacin had an MIC of 1 mg/L and only killed logarithmic-phase CD630 at 64 mg/L.
cVancomycin at 64 mg/L killed ≤2.5 logs of logarithmic-phase cells.
dData for 1138 were obtained from Hurdle et al.8 and were obtained using the same test method.
eDaptomycin MBCSTA values for 9689 and the clinical isolate CD32 were >64 mg/L and 32 mg/L, respectively.
Determination of growth inhibitory and bactericidal concentrations
The MICs of test compounds were determined in 24-well microtitre plates using bacterial inocula of ∼ 106 cfu/mL and were defined as the lowest concentrations of test compounds that inhibited visible growth after 24 h of incubation.8 MBCs for logarithmic-phase (MBCLOG) and stationary-phase (MBCSTA) cells were determined in 24-well plates8 and were defined as the lowest concentrations of test compounds causing ≥3 log reductions of the initial cell inocula (∼ 107 cfu/mL). Briefly, cells were grown to mid-logarithmic phase [optical density at 600 nm (OD600) = 0.5] or stationary phase (24 h culture), and added to 2-fold serial dilutions of compounds. After incubation for 24 h, viable counts were enumerated on Brucella agar supplemented with 2% activated charcoal to avoid the effect of carry-over test compounds. All cultures treated with daptomycin included 50 mg/L of Ca2+. All MIC and MBC measurements were performed at least twice.
Time–kill and ATP measurements
Stationary-phase cultures were either exposed to membrane-active compounds at their MBCSTA, vancomycin (10 mg/L) or PBS (controls). Samples were taken at various timepoints for determination of bacterial viability on Brucella agar or centrifuged for measurement of extracellular ATP in supernatants and intracellular ATP in cell pellets. ATP concentrations were measured using the BacTiter-Glo assay from Promega. All experiments were performed at least twice, and changes in cell survival and ATP concentration over time were expressed as a percentage of the total.
Propidium iodide (PI) uptake
To determine whether killing of C. difficile was due to the membrane pore formation and leakage of cytosolic contents, stationary-phase cells were exposed to compounds at their MBCSTA and 0.5× MBCSTA. Samples (1 mL) were obtained at 5, 30 and 120 min, and, after centrifugation, cell pellets were resuspended in PBS. PI (Invitrogen) was added to a final concentration of 3 μM. The samples were incubated anaerobically for 10 min, centrifuged and washed with PBS to remove the excess dye. PI fluorescence in cells was measured in a Biotek Synery 2 plate reader at excitation 520 nm and emission of 590 nm.
ELISA detection of toxins A and B in cultures
Stationary-phase cells grown in TY broth containing 0.5% (w/v) of Peptone 3 (Difco), to enhance toxin production,24 were first centrifuged and the pellets washed twice with pre-reduced PBS. Toxin was then removed from the supernatant by filtering through a 30K MWCO centrifugal filter device (Amicon Ultra-15, Millipore), before the pellet was resuspended in the filtered, relatively toxin-free nutrient-depleted supernatant. After resuspending the pellets, samples were removed at time 0 and compounds were then added at their MBCSTA and 0.5× MBCSTA. Additional samples were obtained at 24 h post-exposure. The entire sample culture was sonicated for 2 min using an Ultrasonic Processor to release intracellular toxins. Total toxins (i.e. intracellular and extracellular) were diluted to obtain absorbance readings within the linear detection range for the C. difficile Tox A/B II kit (TECHLAB) and toxin levels were quantified against a standard curve for toxin B (List Biological Laboratories), as described by Merrigan et al.5
Effects of compounds on sporulation
To determine whether the compounds could reduce spore production, cultures of BAA-1803 were prepared in brain heart infusion (BHI) broth containing 0.1% l-cysteine. Colonies used to start cultures were grown on BHI agar containing 1% taurocholate.25 To reduce the number of spores in the starting cultures, at day 1, logarithmic-phase cells (OD600 ∼0.5) were used as previously described.25 Compounds were added to cultures at the concentrations of their MBCLOG and 0.5× MICs and incubated anaerobically for 5 days. The number of heat-resistant cfu (i.e. spores) were determined on days 1 and 5 from samples that were heated at 60°C for 25 min and plated on BHI agar with 1% taurocholate. Dilutions of unheated cultures were also plated for total viable counts (i.e. spores and vegetative cells). The populations of spore and vegetative cells stained with Wirtz–Conklin stain26 were visualized by microscopy using Olympus BX51.
Hamster model of infection
The potential for 867, 1138 and clofazimine to treat CDI was examined in the established survival infection model in hamsters as described previously.27 Test compounds and vancomycin were formulated in polyethylene glycol:water (60 : 40) for delivery by oral gavage. All animal experiments were approved by The Institutional Animal Care and Use Committee of The University of Texas at Arlington. Briefly, on day 1 Golden Syrian hamsters (80–120 g), from Charles River Laboratories, received a single injection of clindamycin phosphate solution (50 mg/kg). Animals were maintained in sterile cages with sterile water and L-AA defined diet (Dyets Incorporated). After 20 h (day 0), hamsters were infected by oral gavage with 106 cfu of the C. difficile strain ATCC 43596 that was grown in Sporulation Medium28 and washed once in pre-reduced PBS. From days 1 to 5, hamsters (n = 8 per group) were treated twice daily with test compounds or vancomycin at 50 mg/kg per day; additional experiments with clofazimine used four hamsters. Additional controls included uninfected hamsters (n = 3 per group) that either received test compounds or vehicle only. After the treatment period, hamsters were maintained for another 30 days.
Bioavailability of compounds in hamster caecal contents
The bioavailabilities of compounds in caecal material were evaluated as follows:
-
(i) Determination of bioactivity
MICs of compounds were determined after mixing with caecal contents from drug-free Golden Syrian hamsters. The drug-free hamsters (80–120 g) were endpoint controls from an in vivo efficacy study approved by The Institutional Animal Care and Use Committee of The University of Texas at Arlington. Aliquots (1 mL) of caecal homogenates (20% w/v) were prepared in pre-reduced TY broth containing compounds at 512 mg/L. The mixtures were incubated for 1 h at room temperature with continuous shaking (400 rpm) on an orbital shaker, before collecting the supernatants (i.e. the unbound fraction of compound) by centrifugation and filtering through low protein binding 0.22 μM Acrodisc Supor® membranes (Pall Life Sciences). Controls as compounds in TY broth without caecal material were also centrifuged and filtered. Filtrates were serially diluted in 24-well plates and reduced overnight at room temperature, followed by the addition of BAA-1875 at 106 cfu/mL. MICs were recorded after 24 h of incubation.
-
(ii) Liquid chromatography–mass spectrometry (LC–MS) quantification of caecal binding
To determine the unbound quantity of compounds 867 and 1138, supernatant samples (50 μL), prepared as described in the previous experiment, were diluted with methanol (150 μL). The samples were cooled in a freezer for 1 h and then centrifuged at 10 000 rpm for 20 min to remove any protein contaminants. The purified supernatant was then quantified using a Waters AQUITY UPLC coupled with Xevo G2 QTof mass spectrometer equipped with a ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 mm × 50 mm) and using a 0.1% formic acid/H2O:0.1% formic acid/ACN 3–100% linear solvent gradient. The areas under the curve of the samples were normalized to that of an internal standard (warfarin) and the concentrations of the compounds were determined from standard curves. To determine the bound fraction, to the pelleted material (∼400 μL), prepared as described above, methanol (600 μL) was added and the mixture was sonicated for 20 min, followed by centrifugation at 15 000 rpm for 20 min. The obtained supernatant was then analysed in the same manner as described for the unbound fraction.
Results
Stationary-phase C. difficile is killed by membrane-active agents
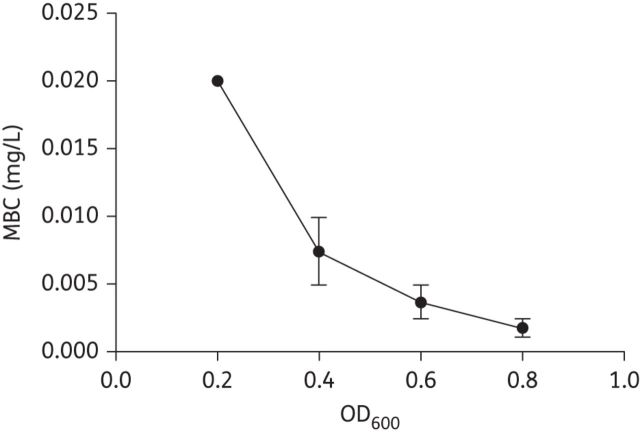
The bactericidal activities of membrane-active agents were compared with established antimicrobials to determine whether the membrane and associated bioenergetics are weak points in C. difficile (Table 1). The MICs for control compounds, including vancomycin, metronidazole, linezolid, gatifloxacin, fusidic acid and nitazoxanide, were comparable to those obtained in other studies using agar-based susceptibility testing.29–32 Many of the selected test molecules, such as nigericin, dicyclohexylcarbodiimide (DCCD), carbonyl cyanide 3-chlorophenylhydrazone (CCCP) and valinomycin have no clinical value for treating CDI, but were included in this study because they display modes of action that are common to many clinically useful agents. For example, daptomycin, lipoglycopeptides and other membrane-active agents in clinical development are known to dissipate the transmembrane potential (ΔΨ) and/or the transmembrane pH (ΔpH).9,33,34 These parameters are specifically dissipated by nigericin and valinomycin, respectively. As shown in Table 1, with the exception of rotenone, which targets NADH dehydrogenase type I, most membrane-active agents and electron transport chain uncouplers killed stationary-phase cells, in contrast to antimicrobials known to specifically inhibit macromolecular synthesis (e.g. vancomycin, fusidic acid, gatifloxacin and rifaximin). Both nigericin and valinomycin inactivated logarithmic-phase and stationary-phase cells, but the latter cell type was easier to kill with the proton ionophore nigericin (Table 1 and Figure 1), which may reflect a greater importance of ΔpH to metabolism and survival in the stationary phase. Indeed, nigericin's activity dramatically increased as cells transitioned to the stationary phase (Figure 1), as did the activity of monensin (Table 1). This information in part substantiates the recent suggestion that C. difficile like Clostridium sticklandii changes its amino acid metabolism and mechanism of energy production at different stages of growth.35 The additional test ionophore CCCP also killed logarithmic-phase and stationary-phase cells. Thioridazine and DCCD, which inhibit bacterial NADH-dehydrogenase-2 and F0F1 ATP synthase, respectively, also killed ≥99.9% of stationary-phase C. difficile at concentrations within ∼ 2-fold of their MICs (Table 1). Daptomycin displayed mean MICs of 0.75–1.5 mg/L and was bactericidal (MBCLOG = 4 mg/L) to logarithmic-phase cultures. However, for stationary-phase cultures only BAA-1875 and the NAP1 clinical isolate CD32 could be reduced by ≥3 logs by daptomycin at 16 and 32 mg/L, respectively, within four tested strains. Our observation of daptomycin's dissimilar action against growing and non-growing cells is comparable to those reported in stationary-phase S. aureus, where its activity is reduced.36,37 The nitroaromatic antibiotics nitazoxanide and metronidazole also killed stationary-phase C. difficile, but at concentrations that were collectively 16-fold to 170-fold above their MICs (Table 1). Metronidazole, and probably nitazoxanide, is activated by nitroreductases to produce reactive free radical species that affect multiple cellular processes,38 and contribute to killing of non-growing cells. Nitazoxanide's putative target is pyruvate ferredoxin oxidoreductase, but it also dissipates the ΔpH and ΔΨ in Mycobacterium tuberculosis.39
Figure 1.
Nigericin killing of C. difficile (BAA-1803) increases as cell growth decreases; a 6.8-fold difference in MBC is observed for nigericin against cultures of OD600 = 0.2 and 0.8.
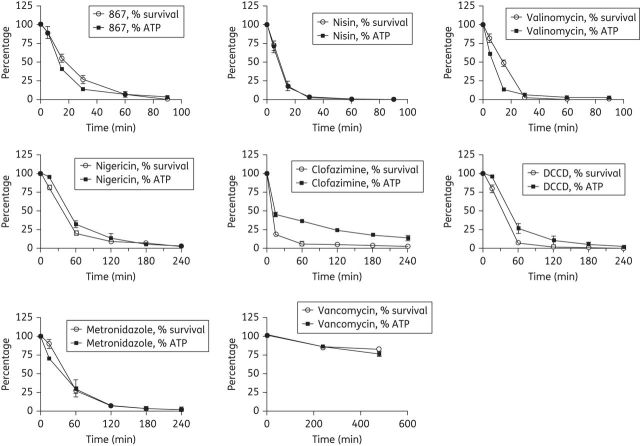
Rapid loss of cell viability corresponded with reductions in cellular ATP
Although the cellular basis for ATP reduction is multifactorial, several studies indicate that exposure of cells to membrane-active molecules results in ATP depletion, arising from uncoupling of ATP biosynthesis by the respiratory chain or by cell leakage.9,40,41 Effects on ATP levels in C. difficile were therefore assayed using a subset of membrane-active agents with representative modes of action of these compounds (Table 1) and DCCD, against stationary-phase cultures of C. difficile BAA-1803. Results showed that cell killing was rapid and correlated with reductions in intracellular ATP (Figure 2). With the exception of nisin, cell death and ATP reductions were not the result of membrane pore formation. As shown in Figure 2, for the different test compounds, the relative loss of cell viability generally correlated with reductions in intracellular ATP. The compounds nisin, reutericyclin-867 and valinomycin, killed ⩾3 logs of bacteria within 90 min, which was the most rapid rate of kill among all compounds. As expected nisin-treated cells were stained with PI after just 5 min of exposure (and leaked ATP) signifying that membrane pores had formed; with nisin at 30 min, the relative fluorescence units (RFUs) of PI were 5308.7 ± 469, compared with a value for untreated cells of 234.3 ± 19.8. Interestingly, in cells exposed to valinomycin the levels of intracellular ATP declined before cell death, because at 15 min of exposure the mean percentage of ATP was 10%, whereas the total viable cell population was ∼ 50% of starting cell numbers. This was not related to leakage of ATP from cells as no increase in the initial level of extracellular ATP was seen, at 5 and 30 min exposures. However, some pore formation was detected following 30 min of valinomycin exposure (RFU = 649.3 ± 105.6 for valinomycin versus 234.3 ± 19.8 for controls); no other test compound showed significant increases in PI fluorescence up to 120 min of exposure (data not shown). With clofazimine, the decrease in intracellular ATP was gradual and occurred after cell death, indicating that inhibition of ATP biosynthesis in C. difficile is a secondary effect of clofazimine, which parallels observations in clofazimine-treated mycobacteria.42 Corresponding decreases in ATP were also observed for metronidazole at its MBC, but vancomycin did not have a significant kill or reduction of ATP.
Figure 2.
Killing of stationary-phase C. difficile (BAA-1803) by compounds is correlated with reductions in ATP biosynthesis. The percentages of surviving cells and ATP concentrations relative to the starting point are shown. The following MBCSTA concentrations were used for compounds: nigericin, 0.0006 mg/L; DCCD, 16 mg/L; nisin, 0.8 mg/L; valinomycin, 4 mg/L; 867, 0.5 mg/L; clofazimine, 2 mg/L; metronidazole, 8 mg/L; and vancomycin, 10 mg/L (as a control). Due to differences in rates of kill for compounds, samples were recovered at various timepoints, as reflected in the graphs.
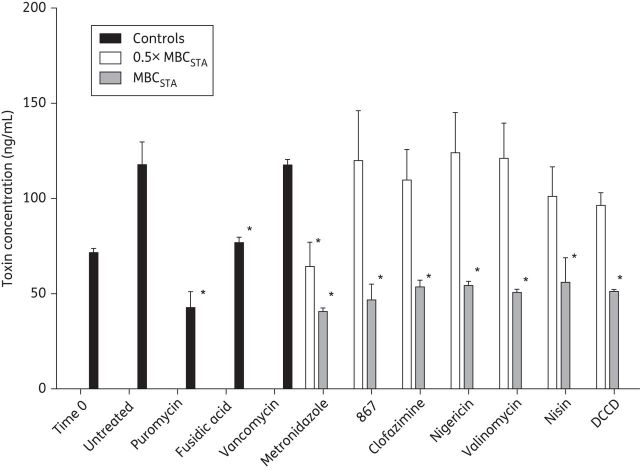
Toxin reduction results from cell death
Compounds that target the membrane often inhibit multiple cellular processes, including protein biosynthesis, as part of a pleotropic effect.9 Thus, we investigated whether the representative set of membrane-active agents, selected above, and DCCD also diminished the production of toxins A/B in hypervirulent BAA-1803. Accordingly, stationary-phase cells resuspended in toxin-free spent medium were exposed to compounds at 0.5× and 1× MBCSTA for 24 h, and total toxins (intracellular and extracellular) were measured by ELISA. At time 0, the concentration of intracellular toxin in stationary-phase cells was 71.6 ± 3.7 ng/mL. By 24 h, the total toxins in drug-free controls were 117.8 ± 20.6 ng/mL. Whilst vancomycin failed to decrease toxin production, metronidazole, DCCD and membrane-active agents at their MBCSTA prevented any increase in toxins above pre-exposure levels (Figure 3). The protein synthesis inhibitors puromycin (150 mg/L)43 and fusidic acid (10 mg/L) also reduced toxin production. However, at concentrations of 0.5× MBC, where only ≤1.5 logs of cells are killed, only metronidazole significantly decreased the production of toxins. This observation indicates that there is tight concentration dependence of membrane-active compounds, implying that a threshold number of molecules needs to be accumulated in the membrane or needs to transverse the bilayer into the cytoplasm before potent activities are displayed, to reduce toxin production. Hence, the biological basis for toxin reductions by membrane-active agents at their MBCSTA is likely to be non-specific and the result of cell killing.
Figure 3.
Effects on toxin production by membrane-active agents and comparators. At bactericidal concentrations membrane-active agents decreased the total toxin in cultures of stationary-phase C. difficile BAA-1803 within 24 h, compared with the protein synthesis inhibitors puromycin (150 mg/L) and fusidic acid (10 mg/L). Vancomycin (10 mg/L) did not alter total toxin production. The bactericidal concentrations used for membrane-active compounds are their MBCSTA: nigericin, 0.0006 mg/L; DCCD, 16 mg/L; nisin, 0.8 mg/L; valinomycin, 4 mg/L; 867, 0.5 mg/L; clofazimine, 2 mg/L; and metronidazole, 8 mg/L. Statistical significance (shown by an asterisk) was determined by one-way analysis of variance followed by Tukey's test (P < 0.05) in Graphpad prism 5 with no drug as the control.
Reduction of spore numbers is due to a decrease in viable cells
For untreated controls of BAA-1803 the number of spores at the start of the experiment was ≤102 spores/mL and by day 5 had increased to 106 spores/mL (Table 2). Compared with the untreated controls, the subset of the tested membrane-active agents, 867 (0.25 mg/L), clofazimine (2 mg/L), nigericin (0.005 mg/L) and nisin (1.25 mg/L) at their MBCLOG, significantly (P < 0.05) decreased spore numbers by 2.89, 2.18, 2.62 and 3.15 logs, respectively (Table 2), whereas decreases with vancomycin (10 mg/L) and metronidazole (0.5 mg/L) were 1.25 and 1.54 logs, respectively. With the exception of clofazimine, the spore reductions by membrane-active compounds were also significant when compared with vancomycin or metronidazole (P < 0.05). The general increased reduction in spores with tested membrane-active compounds may be attributed to their bactericidal activities, since at subinhibitory concentrations spore reductions were not observed (Table 2). Spores detected at day 5 probably arose from the population of unkilled cells. As expected, the sporulation inhibitor Acridine Orange (30 mg/L) reduced C. difficile sporulation.44
Table 2.
Effect of compounds on sporulation in C. difficile BAA-1803 after 5 days
| Compound | Concentration (mg/L)a | Log10 spores/mLb |
|---|---|---|
| Untreated | — | 6.74 ± 0.16 |
| Acridine Orange | 30 | 2.93 ± 0.037* |
| Vancomycin | 0.25 | 6.39 ± 0.08 |
| 10 | 5.49 ± 0.33 | |
| Metronidazole | 0.25 | 6.27 ± 0.09 |
| 0.5 | 5.01 ± 0.04 | |
| 867 | 0.06 | 6.23 ± 0.1 |
| 0.25 | 3.85 ± 0.27* | |
| Clofazimine | 0.03 | 6.22 ± 0.11 |
| 2 | 4.56 ± 0.18 | |
| Nigericin | 0.0012 | 6.39 ± 0.32 |
| 0.005 | 4.12 ± 0.2* | |
| Nisin | 0.2 | 6.32 ± 0.39 |
| 1.25 | 3.59 ± 0.19* |
aC. difficile cultures (OD600 ∼ 0.5) were treated with subinhibitory concentrations (0.5 × MIC) and bactericidal concentrations (MBCLOG); Acridine Orange was not bactericidal and vancomycin at 10 mg/L killed ≤2 logs of cells.
bSignificant values determined by comparison with the untreated control showed that only reductions with vancomycin (10 mg/L) and other compounds at their MBCs were significant. With metronidazole (0.5 mg/L) or vancomycin (10 mg/L) as the control comparator, reductions with membrane-active compounds at their MBCLOG were significant (indicated by an asterisk), except for clofazimine. As expected the Acridine Orange control was significant.44 Statistical comparisons were made by one-way analysis of variance (P < 0.05) followed by Tukey's test in Graphpad prism 5.
Binding to caecal material affects local bioavailability and efficacy
Since reutericyclins are potential treatments for CDI, the efficacies of current compound leads, 867 and 1138 (a related analogue),8 were examined in the established hamster infection model.27 Neither of the two compounds improved the survival of infected hamsters (n = 8 per test compound) beyond 3 days (data not shown). Hamsters that were uninfected and received only compounds survived, indicating that death was due to CDI and not toxicity. The activities of reutericyclins are reduced in the presence of serum;45 therefore, we investigated if binding to caecal material limited efficacy. As shown in Table 3, the activities of 867 and 1138 were greatly reduced by caecal contents. The mean bioactive free concentrations (i.e. MICs) in supernatants were 24 mg/L for 867 and 53.3 mg/L for 1138, which are 35.8-fold and 44.4-fold lower than their MICs in medium alone (Table 3). These results were generally confirmed by LC–MS, revealing that in supernatants and cell pellets the mean concentrations of 867 were 13 mg/L and 233 mg/L, respectively, and for 1138 these were 26 mg/L and 293 mg/L, respectively. Because 867 and 1138 are poorly water soluble and highly lipophilic (i.e. cLogPs = 6.5 and 5.42 as determined from ChemBioDraw 12.0 software),22 we speculated that they were prone to caecal binding. Therefore, bioavailabilities in caecal contents were determined for highly lipophilic clofazimine and comparators that were hydrophilic or amphipathic. Clofazimine appeared to be bound by caecal contents or may have precipitated after centrifugation, as only low bioactive concentrations were present in supernatants; this coincided with a lack of efficacy in hamsters (n = 4; data not shown). The activities of nisin and nitazoxanide were slightly reduced by 4-fold to 8-fold compared with medium alone, whereas the more water-soluble compounds metronidazole, daptomycin, vancomycin and rifaximin were unaffected by caecal contents. Although these results were obtained on a small compound set, they indicate that high hydrophobicity and/or poor solubility could hinder efficacy in the gastrointestinal tract. However, this parameter can be improved through chemical optimization, as is evident from the discovery of the investigational anti-difficile drug LFF571 from a poorly soluble lead compound.44
Table 3.
Influence of caecal material on the anti-difficile activities of compounds
| MIC for BAA-1875 (mg/L)a |
||
|---|---|---|
| Compound | without caecal contents | with caecal contents |
| Vancomycin | 0.25 | 0.25 |
| Rifaximin | 0.063 | 0.25 |
| Nisin | 0.8 | 4.65 |
| Nitazoxanide | 1 | 6 |
| Metronidazole | 1 | 0.5 |
| Daptomycin | 0.5 | 1.2 |
| Monensin | 2 | 5.3 |
| 867b | 0.67 | 24 |
| 1138b | 1.2 | 53.3 |
| Clofazimine | 0.5 | >64 |
aThe MICs of compounds in supernatant samples were determined and are shown as the mean of two or more experiments.
bFrom LC–MS analysis of the 867 and 1138 samples, the mean concentrations in supernatants were 13 mg/L and 26 mg/L, respectively, whereas in cell pellets the concentrations were 233 mg/L and 293 mg/L, respectively, indicating that compounds were bound to caecal material, explaining the poorer MICs compared with tests without caecal material.
Discussion
The pressing need for antimicrobials to treat CDI is confounded by a lack of clearly defined target sites that are essential to the survival of C. difficile. Traditionally, metronidazole and vancomycin have been the agents of choice; however, they are poor in treating NAP1 infections. Whilst the poor efficacy of metronidazole correlates with it being highly absorbed, resulting in low colonic concentrations below that associated with cell killing,8 a proposed reason for the failure of vancomycin in severe infections is its inability to clear the pathogen faster than it can produce toxins.14 Whether this reason also accounts for the failure of other agents in severe CDI is unknown. However, as demonstrated in this work quiescent cells, which are the main contributors of active disease and spore-related endogenous relapse,12 are refractory to killing by several established classes of antibiotic that inhibit specific macromolecular synthetic processes.
If hypervirulence in NAP1 strains arises from toxin hyperproduction in quiescent cells, then it is conceivable that eradication of these cell types by fast-acting antibiotics may be advantageous in yielding a prompt therapeutic response. Challenging this notion is the finding that toxin reduction is attainable with protein and RNA synthesis inhibitors such as fusidic acid, linezolid, fidaxomicin and rifamycins; but these agents are bacteriostatic or slowly bactericidal, implying that surviving cells could recrudesce the infection and act as a source for de novo resistance.29,46–48 This prompted us to explore if the clostridial membrane may be an attractive target site, given it is essential to both growing and non-growing cells, and agents affecting its structure and function are often bactericidal with a low resistance potential; they also show concentration-dependent killing that could be beneficial for drugs achieving high concentrations in the gastrointestinal tract.8,9,48 Similar arguments have been made for using bactericidal agents to treat other infections, where therapeutic outcome is challenging.49
We revealed that C. difficile is exceptionally sensitive to compounds known to dissipate the proton motive force, which in principle points to the membrane and energy metabolism as potential antimicrobial targets to control CDI. Indeed, rapid death with simultaneous depletion of ATP occurred upon exposure to most membrane-active molecules and DCCD, which irreversibly binds to ATP synthase, blocking coupled ATP synthesis. By eradicating most viable cells, toxin production was diminished along with the number of spore-forming cells, but these intriguing properties are concentration dependent and occur at bactericidal levels of compounds. From a therapeutic standpoint, compounds developed for the treatment of CDI are likely to be agents that achieve high, relatively non-absorbed concentrations in the gastrointestinal tract, which bodes well for the mode of action of concentration-dependent membrane-active agents. Therefore, such agents might prevent or even prolong the time in which endogenous relapse occurs, providing that compounds reach the colon intact; high unbound concentrations are achieved for activity; and compounds are not primarily bacteriolytic to avoid the inadvertent release of intracellular toxins. Activities against key gastrointestinal flora were not investigated in these studies, as they depend more on the individual physicochemical properties of compounds, rather than on their mode of action; though it is worth noting that most current membrane-active compounds with clinical potential, including antimicrobial peptides and mimetics, are primarily active against Gram-positive bacteria.9,50 Nevertheless, as novel anti-difficile drug candidates emerge for membrane-active molecules, it will be imperative to ensure that they are not active against major Gram-negative flora such as Bacteroides spp.51,52
Our finding for a possible crucial role of the proton motive force in C. difficile viability is unusual considering that its dissipation is not always bactericidal in many bacterial pathogens. For example, Streptococcus spp., S. aureus and Listeria monocytogenes are not killed by valinomycin or nigericin and organisms modulate their proton motive force in response to metabolic and environmental changes without significant loss of viability.19,53 Nonetheless, our observations mirror those reported in dormant M. tuberculosis, which is killed by energy uncouplers.18,54 Inhibiting respiratory metabolism is now therefore a growing paradigm for treating dormant tuberculosis. We speculate that this approach could be applicable to C. difficile, but genetic validation will be required to understand how this organism establishes its proton motive force and if the Stickland pathway is the sole mechanism involved.35,55
Although this study utilized molecules that are not suitable for C. difficile treatment, the ongoing clinical development of CB-183,315 and oritavancin somewhat validates the prospective use of membrane-active drugs to treat CDI. The action of CB-183,315 is very similar to that of daptomycin and even though it may not kill all quiescent C. difficile its efficacy is comparable to vancomycin in infected hamsters and appears to reduce and delay the onset of relapse in patients.20 Conversely, oritavancin, which rapidly kills biofilms and stationary-phase staphylococci due to its action on the membrane,33 is presumed to be effective against slow and non-growing C. difficile in an in vitro gut model system.21,29 Unlike vancomycin, oritavancin reduces both the vegetative and spore populations below detectable limits as well as toxin production.21,56 These observations have been largely corroborated in the hamster model of CDI,57 and favour a decrease in the frequency of CDI relapse caused by oritavancin and potentially other suitable membrane-active antibacterials that are bactericidal to non-growing cells.
In conclusion, targeting of the clostridial membrane and ATP biosynthesis is an emerging novel approach for discovering anti-difficile drugs to rapidly mitigate the burden of CDI and reduce relapse by removing quiescent cells that produce toxins and spores.
Funding
Funding for this research was provided by Grant 5R01AT006732 from the National Center for Complementary and Alternative Medicine at the National Institutes of Health. Additional funding was obtained from Start-up funds from the University of Texas at Arlington, and the American Lebanese Syrian Associated Charities (ALSAC), St Jude Children's Research Hospital.
Transparency declarations
None to declare.
Disclaimer
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgements
This work was presented in part at the Fifty-first Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, USA, 2011 (Poster no. C1-633).
We thank Briony L. Foster for her technical assistance.
References
- 1.Freeman J, Bauer MP, Baines SD, et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;23:529–49. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hookman P, Barkin JS. Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol. 2009;15:1554–80. doi: 10.3748/wjg.15.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol. 2009;7:526–36. doi: 10.1038/nrmicro2164. [DOI] [PubMed] [Google Scholar]
- 4.O'Brien JA, Lahue BJ, Caro JJ, et al. The emerging infectious challenge of Clostridium difficile-associated disease in Massachusetts hospitals: clinical and economic consequences. Infect Control Hosp Epidemiol. 2007;28:1219–7. doi: 10.1086/522676. [DOI] [PubMed] [Google Scholar]
- 5.Merrigan M, Venugopal A, Mallozzi M, et al. Human hypervirulent Clostridium difficile strains exhibit increased sporulation as well as robust toxin production. J Bacteriol. 2010;192:4904–11. doi: 10.1128/JB.00445-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warny M, Pepin J, Fang A, et al. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005;366:1079–84. doi: 10.1016/S0140-6736(05)67420-X. [DOI] [PubMed] [Google Scholar]
- 7.Schwan C, Stecher B, Tzivelekidis T, et al. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 2009;5:e1000626. doi: 10.1371/journal.ppat.1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurdle JG, Heathcott A, Yang L, et al. Reutericyclin and related analogues kill stationary phase Clostridium difficile at achievable colonic concentrations. J Antimicrob Chemother. 2011;66:1773–6. doi: 10.1093/jac/dkr201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurdle JG, O'Neill AJ, Chopra I, et al. Targeting bacterial membrane function: an underexploited mechanism for treating persistent infections. Nat Rev Microbiol. 2011;9:62–75. doi: 10.1038/nrmicro2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S12–8. doi: 10.1086/521863. [DOI] [PubMed] [Google Scholar]
- 11.Brazier JS. Clostridium difficile: from obscurity to superbug. Br J Biomed Sci. 2008;65:39–44. doi: 10.1080/09674845.2008.11732796. [DOI] [PubMed] [Google Scholar]
- 12.Gerding DN, Muto CA, Owens RC., Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S32–42. doi: 10.1086/521860. [DOI] [PubMed] [Google Scholar]
- 13.Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis. 2005;40:1586–90. doi: 10.1086/430311. [DOI] [PubMed] [Google Scholar]
- 14.Pepin J, Valiquette L, Gagnon S, et al. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am J Gastroenterol. 2007;102:2781–8. doi: 10.1111/j.1572-0241.2007.01539.x. [DOI] [PubMed] [Google Scholar]
- 15.Cornely OA, Crook DW, Esposito R, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis. 2012;12:281–9. doi: 10.1016/S1473-3099(11)70374-7. [DOI] [PubMed] [Google Scholar]
- 16.Louie TJ, Miller MA, Mullane KM, et al. Fidaxomicin versus vancomycin for Clostridium difficile infection. N Engl J Med. 2011;364:422–31. doi: 10.1056/NEJMoa0910812. [DOI] [PubMed] [Google Scholar]
- 17.Orenstein R. Fidaxomicin failures in recurrent Clostridium difficile infection a problem of timing. Clin Infect Dis. 2012;55:613–4. doi: 10.1093/cid/cis495. [DOI] [PubMed] [Google Scholar]
- 18.Rao SP, Alonso S, Rand L, et al. The proton motive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2008;105:11945–50. doi: 10.1073/pnas.0711697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamilton WA. Membrane-active antibacterial compounds. Biochem J. 1970;118:46P–7P. doi: 10.1042/bj1180046p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mascio CT, Mortin LI, Howland KT, et al. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for the treatment of Clostridium difficile. Antimicrob Agents Chemother. 2012;56:5023–30. doi: 10.1128/AAC.00057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baines SD, O'Connor R, Saxton K, et al. Comparison of oritavancin versus vancomycin as treatments for clindamycin-induced Clostridium difficile PCR ribotype 027 infection in a human gut model. J Antimicrob Chemother. 2008;62:1078–85. doi: 10.1093/jac/dkn358. [DOI] [PubMed] [Google Scholar]
- 22.Yendapally R, Hurdle JG, Carson EI, et al. N-substituted 3-acetyltetramic acid derivatives as antibacterial agents. J Med Chem. 2008;51:1487–91. doi: 10.1021/jm701356q. [DOI] [PubMed] [Google Scholar]
- 23.Slayden RA, Lee RE, Armour JW, et al. Antimycobacterial action of thiolactomycin: an inhibitor of fatty acid and mycolic acid synthesis. Antimicrob Agents Chemother. 1996;40:2813–9. doi: 10.1128/aac.40.12.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang A, Gerson DF, Demain AL. Production of Clostridium difficile toxin in a medium totally free of both animal and dairy proteins or digests. Proc Natl Acad Sci USA. 2009;106:13225–9. doi: 10.1073/pnas.0906425106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burns DA, Heeg D, Cartman ST, et al. Reconsidering the sporulation characteristics of hypervirulent Clostridium difficile BI/NAP1/027. PLoS One. 2011;6:e24894. doi: 10.1371/journal.pone.0024894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochsner UA, Bell SJ, O'Leary AL, et al. Inhibitory effect of REP3123 on toxin and spore formation in Clostridium difficile, and in vivo efficacy in a hamster gastrointestinal infection model. J Antimicrob Chemother. 2009;63:964–71. doi: 10.1093/jac/dkp042. [DOI] [PubMed] [Google Scholar]
- 27.Anton PM, O'Brien M, Kokkotou E, et al. Rifalazil treats and prevents relapse of Clostridium difficile-associated diarrhea in hamsters. Antimicrob Agents Chemother. 2004;48:3975–9. doi: 10.1128/AAC.48.10.3975-3979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson KH, Kennedy MJ, Fekety FR. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol. 1982;15:443–6. doi: 10.1128/jcm.15.3.443-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baines SD, Noel AR, Huscroft GS, et al. Evaluation of linezolid for the treatment of Clostridium difficile infection caused by epidemic strains using an in vitro human gut model. J Antimicrob Chemother. 2011;66:1537–46. doi: 10.1093/jac/dkr155. [DOI] [PubMed] [Google Scholar]
- 30.Fung-Tomc J, Minassian B, Kolek B, et al. In vitro antibacterial spectrum of a new broad-spectrum 8-methoxy fluoroquinolone, gatifloxacin. J Antimicrob Chemother. 2000;45:437–46. doi: 10.1093/jac/45.4.437. [DOI] [PubMed] [Google Scholar]
- 31.McVay CS, Rolfe RD. In vitro and in vivo activities of nitazoxanide against Clostridium difficile. Antimicrob Agents Chemother. 2000;44:2254–8. doi: 10.1128/aac.44.9.2254-2258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noren T, Alriksson I, Akerlund T, et al. In vitro susceptibility to 17 antimicrobials of clinical Clostridium difficile isolates collected in 1993–2007 in Sweden. Clin Microbiol Infect. 2010;16:1104–10. doi: 10.1111/j.1469-0691.2009.03048.x. [DOI] [PubMed] [Google Scholar]
- 33.Belley A, Neesham-Grenon E, McKay G, et al. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother. 2009;53:918–25. doi: 10.1128/AAC.00766-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silverman JA, Perlmutter NG, Shapiro HM. Correlation of daptomycin bactericidal activity and membrane depolarization in Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:2538–44. doi: 10.1128/AAC.47.8.2538-2544.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fonknechten N, Chaussonnerie S, Tricot S, et al. Clostridium sticklandii, a specialist in amino acid degradation: revisiting its metabolism through its genome sequence. BMC Genomics. 2010;11:555. doi: 10.1186/1471-2164-11-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ooi N, Miller K, Randall C, et al. XF-70 and XF-73, novel antibacterial agents active against slow-growing and non-dividing cultures of Staphylococcus aureus including biofilms. J Antimicrob Chemother. 2010;65:72–8. doi: 10.1093/jac/dkp409. [DOI] [PubMed] [Google Scholar]
- 37.Mascio CT, Alder JD, Silverman JA. Bactericidal action of daptomycin against stationary-phase and nondividing Staphylococcus aureus cells. Antimicrob Agents Chemother. 2007;51:4255–60. doi: 10.1128/AAC.00824-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong JY, Mukhopadhyay AK, Dailidiene D, et al. Sequential inactivation of rdxA (HP0954) and frxA (HP0642) nitroreductase genes causes moderate and high-level metronidazole resistance in Helicobacter pylori. J Bacteriol. 2000;182:5082–90. doi: 10.1128/jb.182.18.5082-5090.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Carvalho LP, Darby CM, Rhee KY, et al. Nitazoxanide disrupts membrane potential and intrabacterial pH homeostasis of Mycobacterium tuberculosis. ACS Med Chem Lett. 2011;2:849–54. doi: 10.1021/ml200157f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hobbs JK, Miller K, O'Neill AJ, et al. Consequences of daptomycin-mediated membrane damage in Staphylococcus aureus. J Antimicrob Chemother. 2008;62:1003–8. doi: 10.1093/jac/dkn321. [DOI] [PubMed] [Google Scholar]
- 41.McAuliffe O, Ryan MP, Ross RP, et al. Lacticin 3147, a broad-spectrum bacteriocin which selectively dissipates the membrane potential. Appl Environ Microbiol. 1998;64:439–45. doi: 10.1128/aem.64.2.439-445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steel HC, Matlola NM, Anderson R. Inhibition of potassium transport and growth of mycobacteria exposed to clofazimine and B669 is associated with a calcium-independent increase in microbial phospholipase A2 activity. J Antimicrob Chemother. 1999;44:209–16. doi: 10.1093/jac/44.2.209. [DOI] [PubMed] [Google Scholar]
- 43.Osgood DP, Wood NP, Sperry JF. Nutritional aspects of cytotoxin production by Clostridium difficile. Appl Environ Microbiol. 1993;59:3985–8. doi: 10.1128/aem.59.12.3985-3988.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kamiya S, Ogura H, Meng XQ, et al. Correlation between cytotoxin production and sporulation in Clostridium difficile. J Med Microbiol. 1992;37:206–10. doi: 10.1099/00222615-37-3-206. [DOI] [PubMed] [Google Scholar]
- 45.Hurdle JG, Yendapally R, Sun D, et al. Evaluation of analogs of reutericyclin as prospective candidates for treatment of staphylococcal skin infections. Antimicrob Agents Chemother. 2009;53:4028–31. doi: 10.1128/AAC.00457-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Curry SR, Marsh JW, Shutt KA, et al. High frequency of rifampin resistance identified in an epidemic Clostridium difficile clone from a large teaching hospital. Clin Infect Dis. 2009;48:425–9. doi: 10.1086/596315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noren T, Wullt M, Akerlund T, et al. Frequent emergence of resistance in Clostridium difficile during treatment of C. difficile-associated diarrhea with fusidic acid. Antimicrob Agents Chemother. 2006;50:3028–32. doi: 10.1128/AAC.00019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Babakhani F, Gomez A, Robert N, et al. Killing kinetics of fidaxomicin and its major metabolite, OP-1118, against Clostridium difficile. J Med Microbiol. 2011;60:1213–7. doi: 10.1099/jmm.0.029470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin Infect Dis. 2004;38:864–70. doi: 10.1086/381972. [DOI] [PubMed] [Google Scholar]
- 50.Van Bambeke F, Mingeot-Leclercq MP, Struelens MJ, et al. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol Sci. 2008;29:124–34. doi: 10.1016/j.tips.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Citron DM, Tyrrell KL, Merriam CV, et al. In vitro activities of CB-183,315, vancomycin, and metronidazole against 556 strains of Clostridium difficile, 445 other intestinal anaerobes, and 56 Enterobacteriaceae species. Antimicrob Agents Chemother. 2012;56:1613–5. doi: 10.1128/AAC.05655-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Louie TJ, Emery J, Krulicki W, et al. OPT-80 eliminates Clostridium difficile and is sparing of Bacteroides species during treatment of C. difficile infection. Antimicrob Agents Chemother. 2009;53:261–3. doi: 10.1128/AAC.01443-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tempelaars MH, Rodrigues S, Abee T. Comparative analysis of antimicrobial activities of valinomycin and cereulide, the Bacillus cereus emetic toxin. Appl Environ Microbiol. 2011;77:2755–62. doi: 10.1128/AEM.02671-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dhiman RK, Mahapatra S, Slayden RA, et al. Menaquinone synthesis is critical for maintaining mycobacterial viability during exponential growth and recovery from non-replicating persistence. Mol Microbiol. 2009;72:85–97. doi: 10.1111/j.1365-2958.2009.06625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson S, Calos M, Myers A, et al. Analysis of proline reduction in the nosocomial pathogen Clostridium difficile. J Bacteriol. 2006;188:8487–95. doi: 10.1128/JB.01370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chilton CH, Freeman J, Crowther GS, et al. Effectiveness of a short (4 day) course of oritavancin in the treatment of simulated Clostridium difficile infection using a human gut model. J Antimicrob Chemother. 2012;67:2434–7. doi: 10.1093/jac/dks243. [DOI] [PubMed] [Google Scholar]
- 57.Ambrose PG, Drusano GL, Craig WA. In vivo activity of oritavancin in animal infection models and rationale for a new dosing regimen in humans. Clin Infect Dis. 2012;54(Suppl 3):S220–8. doi: 10.1093/cid/cis001. [DOI] [PubMed] [Google Scholar]