Abstract
Pten (phosphatase and tensin homolog deleted on chromosome 10), a kind of tumor suppressor gene, plays important roles in female reproductive system. But its expression and roles in the formation of polycystic ovaries are yet to be known. In this study, we constructed a rat model of PCOS using norethindrone and HCG injections and found the expressions of pten mRNA and PTEN protein increased significantly in the polycystic ovary tissue by immunohistochemistry, RT-PCR, and western blot. Furthermore, the results showed that in vivo ovaries could be effectively transfected by lentiviral vectors through the ovarian microinjection method and indicated that pten shRNA may inhibit the formation of polycystic ovaries by pten down-regulation. Our study provides new information regarding the role of PTEN in female reproductive disorders, such as polycystic ovary syndrome.
Keywords: Polycystic ovary, pten, RNA interference, Follicular development
Introduction
Polycystic ovary syndrome (PCOS), an endocrine disorder most common in women during their reproductive years, is characterized by at least two of the following: hyperandrogenism, oligo-anovulation, hirsutism, and polycystic ovaries [1–3]. In addition, affected women are often obese and insulin-resistant [4, 5] PCOS is known to be a major cause of female infertility.
The importance of PTEN (phosphatase and tensin homolog deleted on chromosome ten) is illustrated by its frequent mutation in cancers. By suppressing the phosphoinositide 3-kinase (PI3K)-AKT-mammalian target of the rapamycin (mTOR) pathway through its lipid phosphatase activity, PTEN governs a plethora of cellular processes, including survival, proliferation, energy metabolism, and cellular architecture [6]. Furthermore, it has been shown that a lack of PTEN in oocytes can cause premature ovarian failure in mice and that controlling the initiation of the oocyte growth PTEN-PI3K pathway can regulate the follicle activation [7]. PTEN has recently been characterized as a regulator of the survival and life span of granulosa/luteal cells, facilitated ovulation and the persistence of non-steroidogenic luteal structures [8]. PTEN expression may be a key to the regulation of function in human granulose cells as well as the pathogenesis of PCOS [9]. However, further studies are needed to explore whether pten expression is related to abnormal follicular development (e.g., early excessive follicular growth and impeded dominant follicular formation) in PCOS.
For this paper, we used an animal model of PCOS to study the expression of PTEN in polycystic ovaries and to investigate its role in the formation of polycystic ovaries. Our research will provide a better understanding of the pathogenic mechanisms involved in PCOS and of the molecular mechanisms necessary for the diagnosis of related female reproductive disorders.
Materials and methods
Animals and experimental protocols
The experimental design and animal care and use conformed to the National Institute of Health guidelines on the ethical use of animals. Twenty-four-day-old Sprague–Dawley (SD) female rats were purchased from the Medical Experimental Animal Center of Nanchang University. The rats were divided into the model group (n = 36) and the control group (n = 12) according to their age, size, and vitality. Rats in both groups received a subcutaneous implant of norethindrone. After three days, rats in the model and in the control groups were injected with HCG (1.5 IU/0.2 mL) and normal saline (0.2 mL), respectively, twice a day for the next 9 days [10].
The rats were killed by decapitation, and blood was collected to measure serum hormone levels. The serum was separated from the red blood cells by centrifugation and stored at 4 °C for subsequent assay. 1 week after killing the rats, the levels of sex hormones, including FSH, LH, T (testosterone), E (estradiol), and INS (insulin), were measured by electrochemiluminescence immunoassays. The ovaries were first removed and weighed and then fixed in 4 % formaldehyde and stained with HE (hematoxylin and eosin) for light microscopy. Immunohistochemical methods were used to compare the level of PTEN expression in the polycystic ovarian tissues of each group.
RNA extraction and RT-PCR
Total RNA was extracted from the isolated ovary tissues of each rat with Trizol Reagent (Invitrogen). The integrity of the extracted total RNA was detected by 1 % agarose gel electrophoresis, and the RNA concentration was determined under ultraviolet (UV) light at 260 nm. RT-PCR was performed with a one-step RT-PCR kit (Qiangen) according to the manufacturer’s protocol, using 32 cycles on 2 ng total RNA. Primers for pten and β-actin (control) were as follows:
pten forward: 5′-ACCATAACCCACCACAGC-3′;
pten reverse: 5′-CTGCCTGACCACATTACTAA-3′;
β-actin forward: 5′-TCAGGTCATCACTATCGGCAAT-3′;
β-actin reverse: 5′-AAAGAAAGGGTGTAAAACGCA-3′;
The PCR products were loaded onto 2.5 % agarose gel and then visualized and quantified by the GeneGenius Gel Imaging System and Image Quanta Analysis Software (Syngene, American). The optical density ratio (OD pten/OD β-actin) was used to indicate the expression levels of pten mRNA.
Western blot
Total protein was extracted from ovary tissues. Western blotting was performed according to the method proposed by Jiang et al. [11].
Vectors for pten shRNA
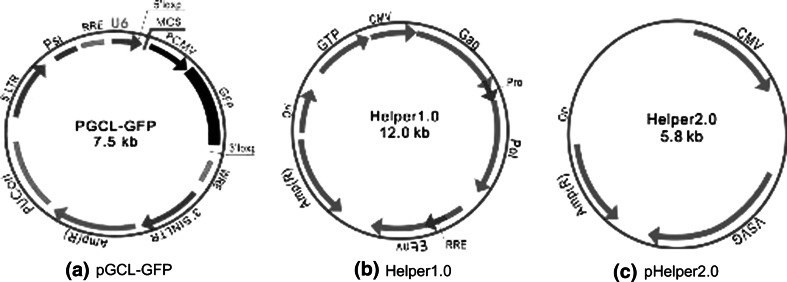
The pten shRNA target sequences were designed by GeneChem Company (Table 1). The lentiviral vector system included three plasmids, pGCL-GFP, pHelper 1.0, and pHelper 2.0 (Fig. 1), which was extracted with the Qiagen plasmid extraction kit. The 293T cells were transfected by cationic liposomes (Lipofectamine 2000) to harvest the active virosomes, which was stored at −70 °C.
Table 1.
pten shRNA target sequences
| Forward 5′ → 3′ | Reverse 3′ → 5′ | |
|---|---|---|
| pten shRNA1 | GGCGCUAUGUAUAUUAUUATT | UAAUAAUAUACAUAGCGCCTT |
| pten shRNA2 | GUAUAGAGCGUGCGGAUAATT | UUAUCCGCACGCUCUAUACTT |
| pten shRNA3 | GAUCUUGACCAAUGGCUAATT | UUAGCCAUUGGUCAAGAUCTT |
Fig. 1.
Lentiviral vectors for pten shRNA
Transfection of shRNA lentivirus
We rapidly engineered transgenic rats by directly injecting the shRNA lentivirus into their ovaries [12]. 2 weeks later, the rats were killed to harvest their ovaries, which were directly frozen and then sliced with a thermostatic frozen slicer. The thickness of the slices was approximately 5 μm. The GFP expression of the slices was observed under fluorescence microscopy.
Statistical analysis
All data are presented as the mean ± SE (). Analysis of variance and an LSD test between the groups were performed using SPSS (Version 10.0 Software, Tsinghua University, China). A value of P < 0.05 was considered statistically significant.
Results
The establishment of PCOS rat mode
Morphology and weight of ovaries of PCOS model rat
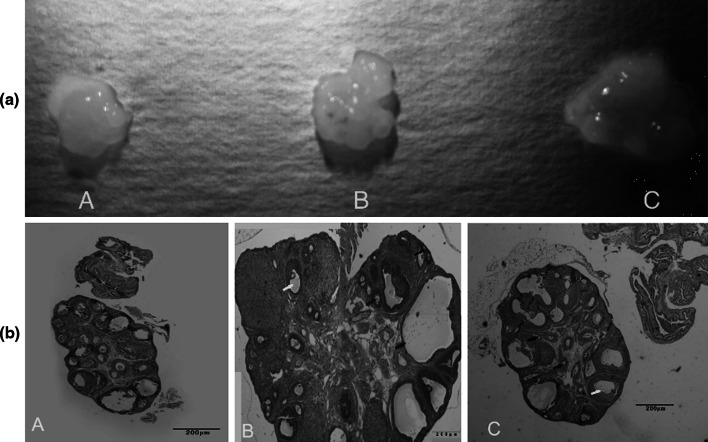
Figure 2a, b shows that the volume of the ovaries and the number of cystic dilatation ovarian follicles have both increased. Table 2 indicates that the average weight of the ovaries in the model group treated with norethindrone and HCG was significantly higher than those in the control group.
Fig. 2.
Appearances of ovaries in the control groups and the model group. a, b Indicate external ovarian appearance and tissue morphological appearances (HE ×40), respectively. A day 0 control, B day 12 control, C model. Black arrow primordial follicle, red arrow preantral follicle, yellow arrow antral follicle, blue arrow cystic follicle
Table 2.
Weight of ovaries in control and model groups
| Group | Sample number | Unilateral ovary weight (mg) |
|---|---|---|
| 0 day control | 12 | 11.42 ± 1.46 |
| 12 day control | 12 | 20.82 ± 4.04* |
| Model | 36 | 48.44 ± 8.04##,△△ |
* Showed significant difference between day 0 control and day 12 control at 0.05 level; ## and △△ showed significant difference between the model group and the control group at day 0 and day 12 (P < 0.01), respectively
Serum hormone levels
From Table 3, we found that the levels of all hormones except hormone E (estradiol) were significantly higher in the model group than in the control group at both day 0 and day 12 (P < 0.01). There was no significant difference in the hormone levels of the control group at day 0 and day 12.
Table 3.
Serum hormone analysis of the control group and the model group
| Group | FSH (IU/L) | LH (IU/L) | T (ng/dL) | E (pg/mL) | INS (μIU/mL) | LH/FSH |
|---|---|---|---|---|---|---|
| 0 day control | 1.64 ± 0.78 | 0.94 ± 0.22 | 6.84 ± 0.34 | 25.78 ± 2.28 | 12.84 ± 2.65 | 0.66 ± 0.18 |
| 12 day control | 1.21 ± 0.09 | 1.01 ± 0.17 | 5.49 ± 0.80 | 24.11 ± 1.11 | 13.44 ± 2.95 | 0.83 ± 0.08 |
| Model | 4.80 ± 0.74##,△△ | 10.93 ± 1.25##,△△ | 15.83 ± 10.44##,△△ | 28.3 ± 7.98 | 17.35 ± 3.51##,△△ | 2.29 ± 0.09##,△△ |
* Showed significant difference between day 0 control group and day 12 control group at 0.05 level; ## and △△ showed significant difference between the model group and the control group at day 0 and day 12 (P < 0.01), respectively
Expression pattern of pten in polycystic ovary tissue
Immunohistochemical results showed that pten protein was expressed in each follicular stage for both the control and the model groups (Fig. 3). However, the location of the expression seemed to be based on the developmental stage of the follicle. Pten protein was expressed mainly in the oocyte cytoplasm for the primordial follicles, while expressed in granulosa cells for the preantral and antral follicles, as well as the dilation and atretic follicles.
Fig. 3.
Immunohistochemical results of PTEN protein expression in the ovaries of the control and model groups (×40). A–C Indicate primordial follicles, preantral follicles, and antral follicles, respectively
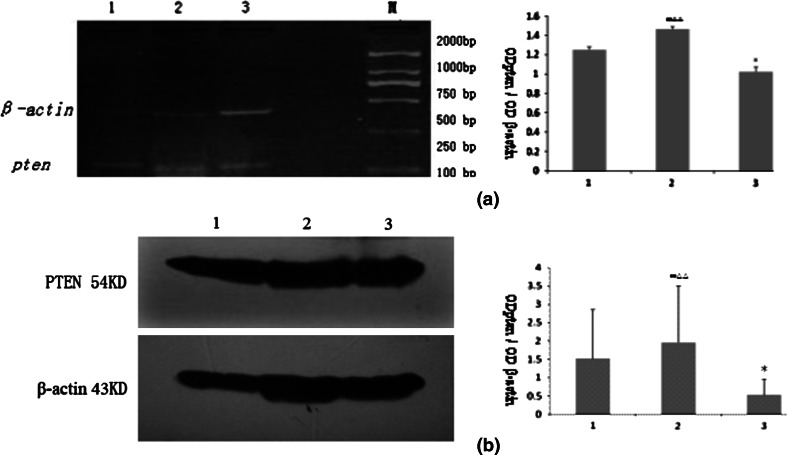
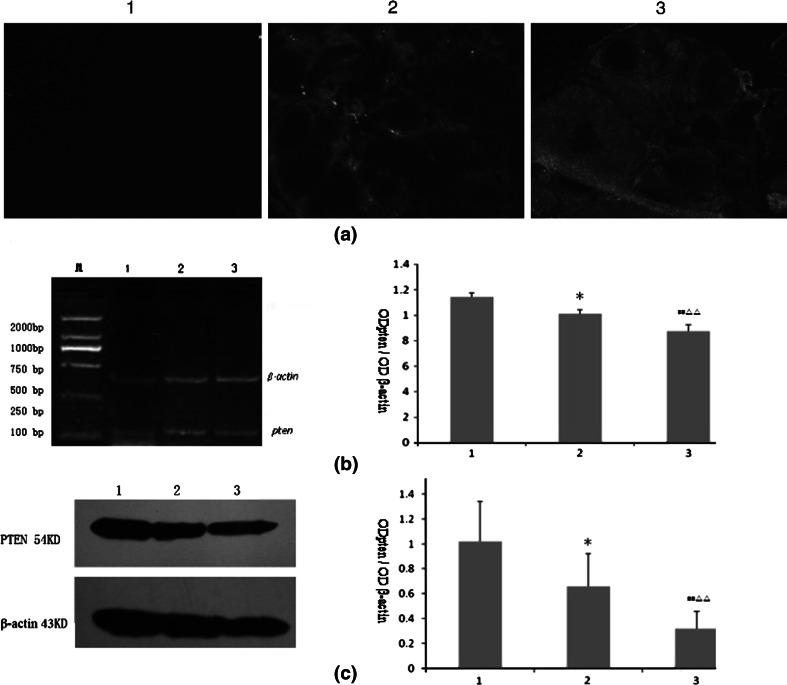
Semi-quantitative analyses of RT-PCR and Western blot revealed that the expression of pten mRNA and PTEN protein in the day 0 control was significantly higher than in the day 12 control (P < 0.05), while the expression of pten in the model was significantly higher than those in both the day 0 control and day 12 control (P < 0.01) (see Fig. 4a, b).
Fig. 4.
Expressions of PTEN in the ovaries of the control and model groups. a, b Indicate the pten mRNA expression revealed by RT-PCR ( ± s, n = 3) and Expressions of PTEN protein by western-blot, respectively. 1, 2, and 3 represent day 0 control, model, and day 12 control, respectively
Formation of the polycystic ovary by the transfection with shRNA lentivirus
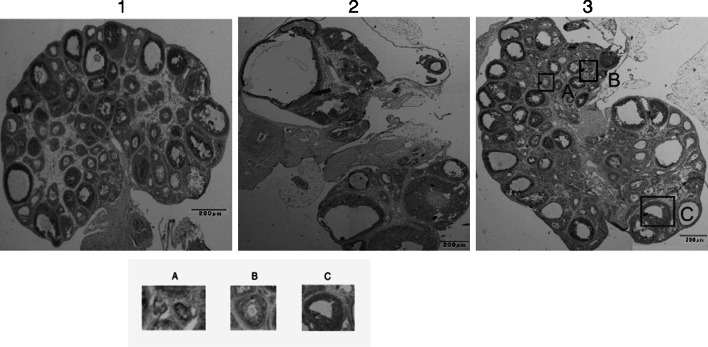
After the transfection of the lentiviral vectors carrying pten shRNA into the in vivo ovaries of each group (Fig. 5), ovarian size and weight in the lentivirus pten shRNA + model group decreased markedly (Table 4; Fig. 6). GFP was expressed in the pten shRNA + model group and in the blank vector + model group, when we viewed the frozen ovarian sections under a fluorescent microscope 12 days after transfection. Semi-quantitative analysis of RT-PCR and Western blot showed that the expression of pten mRNA and PTEN protein in the pten shRNA + model group was significantly lower than the expression of the model and blank vector + model groups (P < 0.01) (Fig. 7a–c). Serum hormone analysis demonstrated that only the T hormone of the pten shRNA + model was significantly higher than those of the model control and the empty vector + model at the 0.05 and 0.01 levels, respectively (Table 4). In addition, HE staining results revealed that cystic dilatation of the follicles and atretic follicles was obviously reduced (Fig. 8).
Fig. 5.
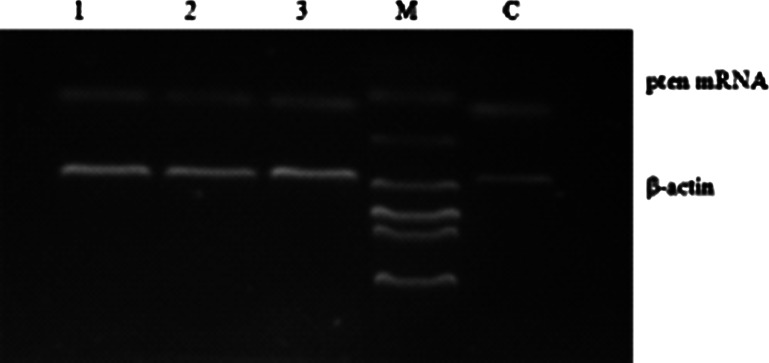
Changes of pten mRNA levels in ovaries after pten-shRNA1, pten-shRNA2, and pten-shRNA3 interference by RT-PCR. M marker, C control, 1 pten-shRNA1, 2 pten-shRNA2, 3 pten-shRNA3
Table 4.
Changes of polycystic ovarian weight and serum hormone analysis after pten shRNA lentivirus transfection
| Group | One ovary weight (mg) | FSH (IU/L) | LH (IU/L) | T (ng/dL) | INS (μIU/mL) |
|---|---|---|---|---|---|
| Model control | 50.02 ± 2.5 | 4.60 ± 0.58 | 9.55 ± 1.50 | 14.95 ± 5.54 | 17.06 ± 2.55 |
| The empty vector + model | 41.42 ± 1.24* | 4.72 ± 1.09 | 8.87 ± 2.07 | 12.33 ± 3.73 | 16.44 ± 3.07 |
| pten shRNA + model group | 30.26 ± 1.04##,△△ | 4.42 ± 0.60 | 9.03 ± 2.25 | 5.18 ± 2.69##,△△ | 16.35 ± 3.61 |
* Showed significant difference between 0 day control group and 12 day control group at 0.05 level; ## and △△ showed significant difference of model group with control group at 0 and 12 day (P < 0.01), respectively
Fig. 6.
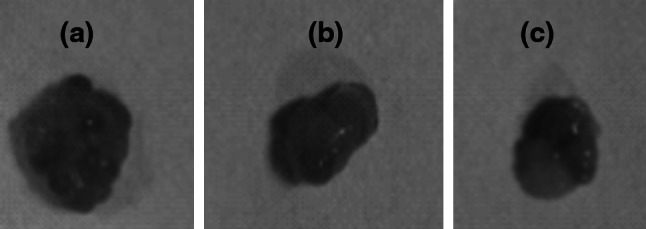
Changes of polycystic ovarian morphology after pten shRNA lentivirus transfection. A–C showed the model control group, the empty vector + model group and the pten shRNA + model group, respectively
Fig. 7.
Expressions of PTEN after transfecting the pten shRNA lentivirus into the ovaries of the control and model groups. 1, 2, and 3 represent day 0 control, model, and day 12 control, respectively. a Expression of GFP in ovaries after pten shRNA lentivirus transfection (×40). b Expressions of pten mRNA revealed by RT-PCR. c PTEN protein expression revealed by western-blot ( ± s, n = 3)
Fig. 8.
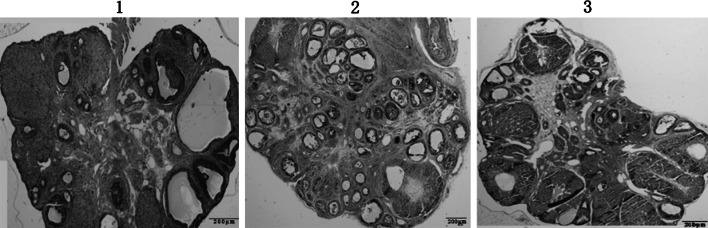
Morphological observations of ovarian tissue after pten shRNA lentivirus transfection (HE ×40). Note: 1, 2 and 3 referred to the model control group, the empty vector + model group and the pten shRNA + model group, respectively
Discussion
Importance of constructing PCOS rat models
Due to the difficulties of acquirement of clinical patient samples, the construction of PCOS animal models seemed to be very important to the study of the pathogenic mechanisms of PCOS. In 1989, Bogoviol et al. [10] established the norethindrone + HCG modeling method. In accordance with this method, we constructed the PCOS rat model used in this study, and we found that size and weight for ovarian of the model group was significantly higher than those of the control groups (P < 0.01). An increased cystic dilatation of follicles was observed on the surface of the ovaries in the model group. HE staining results showed that the number of the primordial follicles was significantly lower in the model group than in the day 12 control group (P <0.01), while the number of preantral follicles was significantly higher (P < 0.01). The level of antral follicles in the model group was significantly reduced (P < 0.01) from the day 12 control group, and in the model group, the cystic dilatation of follicles and atretic follicles was increased significantly (P < 0.01). In addition, in the model group, fewer granular layers were identified, and the granulosa cells were arranged loosely, or even lost. In addition, the results of serum hormone detection showed that levels of LH, T, INS, and LH/FSH increased significantly (P < 0.01) in the model group. These results further demonstrate the importance of constructing PCOS animal models and show that norethindrone + HCG modeling is a satisfactory technique. Furthermore, steps of constructing this PCOS model is simple, and experimental period is short. Maybe, the norethindrone + HCG model is the good model for PCOS research in rat.
There are many methods of building PCOS rat models, such as using dehydroepiandrosteron subcutaneous injection [13], letrozole gavage [14] and by exposing rats to continuous light [15], etc. But these models imitate one or a few aspects of the pathological characteristic of PCOS. Perhaps, there is no model that can be fully reflected the characteristics of PCOS. Therefore, when considering the choice of a model, according to the research purpose, choosing the Suitable rat model is the best solution.
Expression of pten in polycystic ovarian follicles
Different patterns of pten expression in polycystic ovaries have been reported in different animals at different growth stages. For example, Ding et al. [16] reported that PTEN protein was found only in the cytoplasm of the pig oocyte. Goto et al. [17] observed that the expression of PTEN protein in the granular cell increased as the human ovarian follicle grew into its later stages.
In the present study, our immunohistochemical results showed that pten could be expressed in all stages of the follicles (Fig. 3); however, pten expression was mainly found in the oocyte cytoplasm of primordial follicles and in the granulosa cells of preantral follicles and antral follicles, as well as dilation and atretic follicles. These results indicate that the spatio-temporal expression of pten varies according to the development of the rat ovarian follicles. Furthermore, semi-quantitative analysis of RT-PCR and Western blot revealed that the expression of pten mRNA and PTEN protein decreased significantly as the ovary grew in the control group but increased significantly (Fig. 4a, b) in the model group, suggesting that PTEN protein might play an important role in the pathogenesis of PCOS [18]. Others, in Fig. 7a–c the expression of pten mRNA and PTEN protein in the blank vector + model group is significantly lower than the expression of the model group (P < 0.01). Perhaps, just because some normal saline was injected in the blank vector + model group, while normal saline was not injected in the model group, and the normal saline may dilute the concentration of testosterone, thereby reducing related to PCOS symptoms in the blank vector + model group.
Roles of pten in the development of polycystic ovarian follicles
The pten gene plays a key role in the initiation, development, apoptosis, and atresia of the primordial follicle. For example, by knocking down and silencing pten, Li et al. [19] found that a previously activated ovarian follicle could develop to demonstrate the capacity for fertilization after being cultured outside the body. Moreover, Fan et al. [8] reported that rats lacking pten expression in granular cells (conditional knockdowns) demonstrated a higher frequency of ovulation and greater number of offspring. These results suggest that in the early growth stages of the ovarian follicle, PTEN protein can insure that the primordial follicle remains quiescent, while in later growth stages of the ovarian follicle, PTEN protein can regulate not only the proliferation and apoptosis of granular cells but also the development and atresia of the sinusoid ovarian follicle.
In the present research, we have found that the expression of pten mRNA and PTEN protein increased significantly in the PCOS model rat group. Pten shRNA effectively reduced the severity of PCOS, resulting in a lower ovarian volume and weight, a smaller quantity of preantral follicles, cystic dilatation, and atretic follicles. Moreover, we found that the content of T hormone was also obviously reduced, which could also interfere the pathogenesis of PCOS. Therefore, we suggest that: (1) PTEN plays an important role in the pathogenesis of PCOS; (2) a high level expression of PTEN in the PCOS can induce apoptosis of the preantral follicle and sinusoid follicle; (3) the final result may be the arrested development of the ovarian follicle and anovulia.
Moreover, PTEN has also been reported to be an important signal molecule in the pathway of PTEN-PI3K-AKT, which is closely correlated with the pathogenesis of PCOS. In addition, previous research suggested that transfecting pten into an ovarian cancer cell might be a new interventional method for curing ovarian cancer. The results of the present study also indicate that pten might be a new target gene to treat PCOS; however, the specific functional pathway relating pten and the apoptosis of polycystic ovarian cells remains to be explored.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81260098,81160081), the Natural Science Foundation of Jiangxi Province (No.2010GQY0214, 20114BAB205009) and the Science and Technology Research Projects of the Department of Education of Jiangxi Province (GJJ11313).
Footnotes
Jie-Xiu Ouyang, Tao Luo, Li-Ping Zheng contributed equally to this work.
Contributor Information
Yue-Hui Zheng, Email: yuehuizheng@163.com.
Li-Ping Zheng, Email: stone91021@163.com.
References
- 1.Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362:1017–1021. doi: 10.1016/S0140-6736(03)14410-8. [DOI] [PubMed] [Google Scholar]
- 2.Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, Erickson GF. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89:5321–5327. doi: 10.1210/jc.2004-0643. [DOI] [PubMed] [Google Scholar]
- 3.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 4.Houten EV, Kramer P, Karels B, McLuskey A, Themmen A, Visser J (2012) Dihydrotestosterone treatment in mice induces a persistent polycystic ovary syndrome phenotype. Endocr Abstr 29, OC6.5
- 5.Zhang X, Fu L, Zhang Q, Yan L, Ma Y, Tu B, Liu N, Qiao J. Association of TRB3 Q84R polymorphism with polycystic ovary syndrome in Chinese women. Reprod Biol Endocrinol. 2011;9:46–55. doi: 10.1186/1477-7827-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 7.Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, et al. Oocyte-specific deletion of PTEN causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- 8.Fan HY, Liu Z, Cahill N, Richards JS. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol. 2008;22:2128–2140. doi: 10.1210/me.2008-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwase A, Goto M, Harata T, Takigawa S, Nakahara T, Suzuki K, et al. Insulin attenuates the insulin-like growth factor-I(IGF-I)-Akt pathway, not IGF-I-extracellularly regulated kinase pathway, in luteinized granulosa cells with an increase in PTEN. J Clin Endocrinol Metab. 2009;94:2184–2191. doi: 10.1210/jc.2008-1948. [DOI] [PubMed] [Google Scholar]
- 10.Bogovich K. Induction of ovarian cysts in progesterone—synchronized immature rats: evidence that suppression of follicular aromatase activity is not a prerequisite for the induction of cystic follicles. Endocrinology. 1989;124:1646–1653. doi: 10.1210/endo-124-4-1646. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, Hou XX, Geng Z. Interpretation criteria for standardized western blot for the predominant species of Borrelia burgdorferi sensu lato in China. Biomed Environ Sci. 2010;23:341–349. doi: 10.1016/S0895-3988(10)60074-8. [DOI] [PubMed] [Google Scholar]
- 12.Yang SY, Wang JG, Cui HX, Sun SG, Li Q, Gu L, Hong Y, et al. Efficient generation of transgeic mice by direct intraovarian injection of plasmid DNA. Biochem Biophy Res Commun. 2007;358:266–271. doi: 10.1016/j.bbrc.2007.04.112. [DOI] [PubMed] [Google Scholar]
- 13.Anderson E, Lee GY, O’Brien K. Polycystic ovarian condition in the dehydroepiandrosterone-treated rat model: hyperandrogenism and the resumption of meiosis are major initial events associated with cystogenesis of antral follicles. Anat Rec. 1997;249:44–53. doi: 10.1002/(SICI)1097-0185(199709)249:1<44::AID-AR6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Mannerås L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148(8):3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- 15.Baldissera SF, Motta LD, Almeida MC, Antunes-Rodrigues J. Proposal of an experimental model for the study of polycystic ovaries. Braz J Med Biol Res. 1991;24:747–751. [PubMed] [Google Scholar]
- 16.Ding W, Wang W, Zhou B, Zhang W, Huang P, Shi F, Taya K. Formation of primordial follicles and immunolocalization of PTEN, PKB and FOXO3A protein in the ovaries of fetal and neonatal pigs. J Reprod Dev. 2010;56:162–168. doi: 10.1262/jrd.09-094H. [DOI] [PubMed] [Google Scholar]
- 17.Goto M, Iwase A, Ando H, Kurotsuchi S, Harata T, Kikkawa F. PTEN and Akt expression during growth of human ovarian follicles. J Assist Reprod Genet. 2007;24:541–546. doi: 10.1007/s10815-007-9156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei JJ, Josette W, Serdar B. Endometriosis and ovarian cancer: a review of clinical, pathologic, and molecular aspects. Int J Gynecol Pathol. 2011;30:553–568. doi: 10.1097/PGP.0b013e31821f4b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, et al. Activation of dormant ovarian follicles to generate mature eggs. Proc Natl Acad Sci USA. 2010;107:10280–10284. doi: 10.1073/pnas.1001198107. [DOI] [PMC free article] [PubMed] [Google Scholar]