Abstract
We have previously demonstrated that PKC-potentiated inhibitory protein of protein phosphatase-1 (CPI-17) is expressed in lung endothelium. CPI-17, a specific inhibitor of myosin light chain phosphatase (MLCP), is involved in the endothelial cytoskeletal and barrier regulation. In this paper, we report identification of fourteen putative CPI-17 interacting proteins in the lung using BacterioMatch Two-Hybrid System. Five of them: plectin 1 isoform 1, alpha II spectrin, OK/SW-CL.16, gelsolin isoform a, and junction plakoglobin are involved in actin cytoskeleton organization and cell adhesion, suggesting possible significance of these binding partners in CPI-17-mediated cytoskeletal reorganization of endothelial cells. Furthermore, we confirmed the specific interaction between plakoglobin and CPI-17, which is affected by phosphorylation status of CPI-17 in human lung microvascular endothelial cells.
Keywords: BacterioMatch Two-Hybrid System, cytoskeleton, partner proteins, CPI-17, plakoglobin
Introduction
Pulmonary vascular endothelium serves as a semi-selective barrier between circulating blood vessels and surrounding tissues. Endothelial cell (EC) barrier integrity, therefore, is critical for tissue and organ homeostasis. Disruption of the endothelial barrier is considered as a major factor causing potentially lethal physiological dysfunctions, such as alveolar flooding, hypoxemia, and pulmonary edema (reviewed in (Dudek and Garcia, 2001)). Reorganization of the cytoskeleton plays a crucial role in EC barrier compromise. Actomyosin-driven contraction, in turn, is a key event in the regulation of endothelial barrier function. EC contraction is initiated by Ser/Thr phosphorylation of the 20-kDa regulatory myosin light chain (MLC), which is tightly linked to F-actin filament reorganization (Goeckeler and Wysolmerski, 1995; Verin et al., 1995). Edemagenic agonists, like thrombin and histamine, induce rapid and dramatic increase in MLC phosphorylation, actomyosin interaction, stress fiber formation, and accompanying increase of the endothelial permeability (van Nieuw Amerongen et al., 1998). Studies from our laboratory (Kolosova et al., 2004) and others (Diwan et al., 1997) demonstrated the involvement of MLCP, protein phosphatase type 1 possessing catalytic subunit (CS)1 β (formerly δ), a targeting subunit termed myosin phosphatase targeting subunit 1 (MYPT1), and a smaller subunit, M20, of unknown function (Alessi et al., 1992; Csortos et al., 2007; Hartshorne et al., 1998), in the regulation of EC gap formation and barrier dysfunction in pulmonary endothelium (Verin et al., 2000). Despite the importance of MLCP in the regulation of endothelial integrity, molecular mechanisms underlying its activity are not clearly understood. Besides MLCP activity can be regulated by several other targeting subunits and endogenous regulatory proteins such as MYPT3 (Skinner and Saltiel, 2001), TGF-β-inhibited membrane-associated protein (TIMAP), which is highly expressed in EC (Cao et al., 2002), and a specific MLCP inhibitor, CPI-17 (Eto et al., 1995). However, the exact roles of these regulatory proteins in endothelial cytoskeletal and barrier regulation are yet to be found. It has been proposed that Rho kinase (ROCK) and protein kinase C (PKC) mediate inhibition of smooth muscle MLCP activity via direct phosphorylation of MYPT1, leading to increased MLC phosphorylation in response to various agonists (reviewed in (Ito et al., 2004; Somlyo and Somlyo, 2000)). Apart from direct phosphorylation of the MYPT1 subunit, the activity of MLCP is also regulated by a specific inhibitor protein, CPI-17, that is predominantly expressed in smooth muscles and neurons (Eto et al., 2002; Eto et al., 1997; Woodsome et al., 2001). CPI-17 is composed of 147 amino acids with a molecular mass of 17 kDa (Yamawaki et al., 2001). The amino acid sequences of CPI-17 are well conserved in mammalians (more than 80%), especially in the N-terminal half (residues 1–67) encoded by exon 1 (Yamawaki et al., 2001). In the humans, the presence of a splicing variant of CPI-17, which lacks 27 residues (sequence 68–94) encoded by exon 2, in the aorta is also reported (Yamawaki et al., 2001). Phosphorylation of CPI-17 at Thr38 by PKC, which enhances its inhibitory potency around 1,000-fold, is necessary to convert the protein into a potent inhibitor of MLCP (Eto et al., 1995; Kitazawa et al., 2000; Pang et al., 2005). In our previous study, we found the expression of endogenous CPI-17 in human lung microvascular endothelial cells (HLMVEC) and its involvement in the regulation of EC cytoskeleton and permeability (Kolosova et al., 2004). However, little is known about the mechanistic linkage between CPI-17 and EC cytoskeleton. As of yet, protein partners that bind to the CPI-17 (except MLCP) have not been identified. In this study, we employed a two-hybrid system using Escherichia coli as a host for screening and identifying putative CPI-17-binding proteins in the human lung cDNA library. Several candidates of the CPI-17-interacting proteins were identified by the bacterial two-hybrid system screening. Among them, the interaction between CPI-17 and plakoglobin was reconfirmed in HLMVEC by co-immunoprecipitation. Furthermore, we demonstrated a decreased association between these two proteins by PMA-induced CPI-17 phosphorylation, suggesting physiological significance of the interaction in response to barrier-disrupting conditions.
Materials and methods
Bacterial strains, plasmids and culture conditions
E. coli strain TOP10 (Invitrogen, Carlsbad, CA) was used as a host for DNA manipulation, and 100 μg/ml of ampicillin (Sigma-Aldrich, St. Louis, MO) as used for selection of transformants. pCR 4-TOPO vector (Invitrogen) was used for general DNA manipulation. E. coli XL1-Blue MRF’ Kan and BacterioMatch Two-Hybrid System reporter strains including pBT bait vector and premade human lung cDNA library constructed in the pTRG target plasmid were used for two-hybrid analysis (Stratagene, West Cedar Creek, TX). Reagents for bacterial culture were purchased from Fisher Scientific (Hanover Park, IL). All E. coli strains were grown in Luria-Bertani (LB) medium. All selection and screening plates were prepared according to the Stratagene instruction manual. LB-CTCK (carbenicillin, chloramphenicol, tetracycline, and kanamycin) screening plates were prepared with 250 μg/ml carbenicillin, 15 μg/ml tetracycline, 34 μg/ml chloramphenicol and 50 μg/ml kanamycin. X-gal indicator plates for the verification of the specific protein-protein interaction were supplemented with 15 μg/ml of tetracycline, 34 μg/ml of chloramphenicol, 50 μg/ml of kanamycin, 80 μg/ml of X-gal, and 0.2 mM of β-galactosidase inhibitor (X-gal LB-TCK indicator plates). Restriction and modifying enzymes were purchased from New England Biolabs (Ipswich, MA). All other materials were from Sigma unless otherwise noted.
Construction of two-hybrid library plasmids
cDNA cloning of CPI-17 coding region was performed as we have previously described in detail (Kolosova et al., 2004). cDNA of human CPI-17 cloned in pcDNA3.1/myc-His (Invitrogen) was amplified using PCR and inserted in frame into EcoRI and BamHI sites of pBT bait plasmid downstream of the λcI. Forward and reverse primers for the PCR amplification were synthesized at DNA Analysis Facility (Johns Hopkins University, Baltimore MD): 5′-ATGCGAATTCCATGGCAGCTCAG-3′ (forward) and 5′-TTATCGGATCCGGGGTGAGCAGT-3′ (reverse). For amplification of the CPI-17, GeneAmp PCR System 9600 (Perkin Elmer) was used, and DNA sequences of the PCR product were confirmed. Glycerol stock of the premade human lung cDNA library (7.15 × 108 cfu/ml) constructed in the pTRG vector was amplified and then purified using QIAGEN plasmid maxi kit (Valencia, CA).
E. coli two-hybrid system screen
To determine putative protein partners for CPI-17, a BacterioMatch Two-Hybrid System (Stratagene) was employed according to the instructions provided by the manufacturer. Bacterial two-hybrid system developed by Dove, Joung, and Hochschild with a similar concept to the classical yeast two-hybrid system, is based on the transcriptional activation (Dove et al., 1997). In the bacterial two-hybrid system, a protein of interest (bait) is fused to the bacteriophage λcI protein containing the DNA-binding domain, which binds to the λ operator sequence followed by weak reporter promoter. The other protein of interest (target) is fused to the N-terminal domain of RNA polymerase α subunit. If bait and target protein interact with each other, RNA polymerase recruited to the promoter causes transcriptional activation of two reporter genes; Ampr and β-galactosidase. The resulting activations of these two reporter genes can then be detected by the formation of blue colonies on appropriate plates containing ampicillin.
For the determination of the co-transformation efficiency of both bait and target recombinant plasmids per 100 μl of BacterioMatch Two-Hybrid System reporter strain competent cells (cfu yield), experiment was performed using 10 ng of the pTRG plasmid containing human lung cDNA library and 10 ng of the recombinant pBT plasmid containing bait, CPI-17. The cfu yield of co-transformation for 100 colonies on the plate was calculated using following formula:
Based on the total number of colonies required to represent an entire library and the calculated cfu yield for the co-transformation of the reporter strain, numbers of the co-transformations for screening of the entire library were determined. To ensure the representation of entire cDNA library, at least 1×106 colonies were required for screening. The number of co-transformations required for the library screening was estimated as described below:
For the analysis of protein-protein interactions, a co-transformation experiment was performed by using 50 ng of each recombinant pBT-CPI-17 (chloramphenicol-resistant bait plasmid) and pTRG-cDNA libraries (tetracycline-resistant plasmid). The co-transformation of pBT-LGF2 encoding the dimerization domain of Gal4 transcriptional activator protein and pTRG-Gal11p encoding a domain of the mutant form of Gal11 known as interacting proteins, was used as a positive control to confirm the efficiency of the co-transformation. Co-transformation of pBT-LGF2 and empty pTRG vector served as a negative control. After co-transformation, bacterial cells were spread on LB-CTCK plates and incubated at 30°C for 24 hours. After incubation, transformants were counted and putative positive colonies were used for further validation analysis. The specificity of the interaction between CPI-17 and each putative positive target protein was examined in two ways: growths of carbenicillin-resistant colonies and following detection of X-gal activity of re-co-transformed E. coli with the isolated target plasmid from the carbenicillin-resistant colonies with either the bait plasmid containing CPI-17 or empty vector into the reporter strain. Carbenicillin-resistant colonies validated with light blue to blue color were used for further verification. After purification of the target plasmid from a positive colony on the X-gal LB-TCK plates, the plasmid was re-co-transformed to the reporter strain with either pBT-CPI-17 bait plasmid or empty pBT vector. Isolated target plasmids that form colonies on CTCK agar plates in the presence of the bait plasmid encoding CPI-17 cDNA but not with the empty vector was selected as a real positive target protein and sequenced for its identification. Sequence data was queried onto the NCBI public database, and its corresponding protein was identified using BLAST search program.
Cell culture and transfection
Human lung microvascular EC (HLMVEC) were obtained from Lonza Group Ltd. (Walkersville, MD). Cells were cultured in EGM-2-MV (Lonza) supplemented with 5% (v/v) fetal bovine serum and maintained at 37°C in humidified atmosphere of 5% CO2-95% air. Cells were plated on glass coverslips or 10 cm dish. When the cells reached ~70% confluence, human CPI-17 with myc tag was introduced into the EC using X-tremeGENE HP transfection reagent (Roche, Indianapolis, IN) according to manufacturer’s protocol. Empty pcDNA3.1-myc vector was used as a negative control. 48 hours after the transfection, cells were harvested for further experiments. Phorbol 12-myristate 13-acetate (PMA, EMD Chemicals, NJ) was used for the treatment as we have previously reported (Kolosova et al., 2004).
Immunoprecipitation
Transfected cells were washed three times with ice-cold PBS and lysed with lysis buffer (20mM Tris-HCl, pH 7.6, 0.5% NP-40, 150 mM NaCl, 3 mM EDTA, 3mM EGTA, and protease inhibitor cocktail). The cell lysate was centrifuged at 15,000 g for 15 min at 4°C. The supernatant was pre-cleared with protein G Sepharose (GE Healthcare, Little Chalfont, UK) at 4°C for 3 hours with gentle rotation to prevent nonspecific binding. The pre-cleared supernatant was incubated with appropriate volume of anti-myc antibody (SantaCruz, Santa Cruz, CA) for 1 hour and then fresh protein G Sepharose or EZ-view Red Anti c-myc Affinity gel was added (Sigma-Aldrich, St. Louis, MO). After overnight incubation at 4°C with gentle rotation, supernatant was removed, and the remained beads were washed three times with IP buffer, resuspended with 1× SDS sample buffer, and then boiled for 5 min. This supernatant was further analyzed by Western blotting.
Western Blotting
Western blotting was performed according to protocol as we have previously described (Kim et al., 2012; Kolosova et al., 2004). Supernatants were separated on SDS-PAGE, transferred to nitrocellulose membranes, incubated with primary antibody of interest diluted 1:500, and then with HRP-conjugated secondary antibody. Immunoreactive proteins were detected on X-ray film with Lumiglo reagents (Cell Signaling, Danvers, MA) according to manufacturer’s directions.
Immunofluorescence
Cultured HLMVE cells grown on glass coverslips were transfected with CPI-17-containing plasmid. After 48 hours of incubation, cells were treated with 0.1μM PMA or vehicle for 30 min, then washed with PBS, fixed in 3.7% formaldehyde solution in PBS for 10 minutes at 4°C and washed three times with PBS. The cells were permeabilized with 0.2% Triton X-100 in TBS supplemented with 0.1% Tween 20 (TBST) for 5 min, washed three times with PBS and blocked with 2% BSA in TBST for 1 hr. Incubation with specific antibodies diluted with blocking solution was performed for 1 hour at room temperature. Anti-c-myc was used for immunfluorescent detection of myc-tagged CPI-17. Specific antibody was used to detect plakoglobin. After three washes with PBS, the cells were incubated with appropriate secondary antibody (1:300) conjugated with fluorescent dye Alexa 488 (green) and Alexa 594 (red) for 1 hr at room temperature. After the immunostaining, the cells were analyzed using a Nikon Eclipse TE2000 microscope.
Results
Identification of CPI-17 binding proteins using E.Coli two-hybrid system
To identify putative binding partners of the larger isoform of CPI-17 in lung endothelium, we used two-hybrid library screening system. Because E. coli grows faster than yeast with higher transformation efficiency, allowing screening larger numbers of interactions, we selected a bacterial version of two-hybrid system. In addition, use of E. coli eliminates extra transformation steps and makes the system faster than yeast two-hybrid systems. The human pulmonary EC cDNA library for the bacterial two-hybrid screening is not currently available; therefore, we employed a human lung cDNA library in the pTRG target plasmid, provided by Stratagene’s E. coli BacterioMatch Two-Hybrid System, in order to identify potential CPI-17 interacting proteins in the lung. To test self-activation induced by the co-transformation of recombinant pBT or pTRG in the BacterioMatch reporter strain, preliminary co-transformations were performed using either recombinant pBT containing CPI-17 and the empty pTRG vector or empty pBT vector and recombinant pTRG containing human lung cDNA library. No significant growth of colonies on the LB-CTCK selection plates was observed in both cases. All positive and negative controls provided expected results. The data indicates that recombinant pBT-CPI-17 plasmid and human lung cDNA library are suitable for detecting protein-protein interactions in the two-hybrid system. Two rounds of selection were used for the detection and its validation of putative positive interaction: the growths of carbenicillin-resistant colonies and following detection of X-gal activity of re-co-transformed E. coli with the isolated target plasmid from the carbenicillin-resistant colonies with either the bait plasmid containing CPI-17 or empty vector. Twenty two colonies were identified as positive candidates on the LC-CTCK plates from around 1.3 × 106 of co-transformants. Eight cDNA clones were identified as false-positives because they generated blue colonies in the presence of empty pBT vector, instead of pBT-CPI-17 plasmid. Finally, fourteen candidates were matched with known proteins in the protein sequence database (Table 1). Five of them: plectin 1 isoform 1, alpha II spectrin, OK/SW-CL.16, gelsolin, and junction plakoglobin are reported to be involved in actin cytoskeleton organization and cell adhesion (Aberle et al., 1994; Bennett and Baines, 2001; Hartwig, 1994; Knudsen and Wheelock, 1992; Pytela and Wiche, 1980; Silacci et al., 2004; Togashi et al., 2007; Van Troys et al., 1999).
Table 1.
Putative CPI-17 binding partner proteins
| Protein | GenBank accession # | Position of binding fragment, aa | Protein length, aa | Cellular process in which the protein is involved | Ref. |
|---|---|---|---|---|---|
| Plectin 1 isoform 1 | NP_000436 | 4457–4574 | 4574 | Cytoskeleton-membrane attachment in the different tissues. Intermediate filament- associated protein that interlinks all three major protein cytoskeletal systems. | 44 |
| Alpha II spectrin | NP_003118 | 2163–2472 | 2472 | Cytoskeletal membrane protein. Links membrane proteins to the actin and microtubule filamentous skeleton. | 39 |
| OK/SW-CL.16 | BAB93516 | 1–134 35–134 |
134 | Might act as a linker between α-actinin-4 and some SH3 domain-containing signaling proteins. | 40 |
| Gelsolin isoform a | NP_000168 | 55–462 | 782 | Control actin assembly, severing and capping of actin filaments. Plays role in cell motility and control of apoptosis. | 41 |
| Junction plakoglobin | AAH00441 | 458–745 | 745 | Plays a role in two intercellular junctions, the adherens junction and the desmosomes. | 43, 62 |
| NSP1 | NP_005481 | 275–356 | 576 | May function as adaptor or scaffolding protein to integrate growth factor receptor and integrin signals. | 64 |
| Microfibrillar- associated protein 4 (MFAP4) | NP_002395 | 146–256 | 256 | Extracellular matrix protein which is involved in cell adhesion or intercellular interactions. | 72 |
| FGF receptor activating protein 1 (FRAG1) | NP_055304 | 158–201 | 315 | Processing and expression of GPI-anchored proteins in lipid rafts. | 73 |
| Synaptogyrin 2 | NP_004701 | 163–224 | 224 | Integral membrane protein that may play a role in regulating membrane traffic in non- neuronal cells. | 76 |
| Interferon-induced transmembrane protein 3 (IFITM3) | NP_066362 | 7–133 | 133 | Interferon inducible transmembrane protein. Mediates homotypic adhesion and transduction of anti-proliferative signals. | 78 |
| Granulin precursor (GEP) | P28799 | 168–407 | 593 | Tumorigenesis, cell proliferation, normal development, inflammation and wound healing. | 82, 83 |
| Catepsin D | NP_001900 | 222–267 | 412 | Progression of cancer, wound healing and tissue remodeling. | 87 |
| eIEF associated protein (HSPC021) | NP_057175 | 366–564 | 564 | Protein-protein interaction | 88 |
| Rab36 | NP_004905 | 302–333 | 333 | May function as regulator of intracellular vesicular transport. | 89 |
Immunoprecipitation of putative binding proteins of CPI-17 in human endothelial cells
To confirm these newly found protein interactions in human endothelial cells, we transfected human CPI-17 with myc tag into the HLMVEC and conducted immunoprecipitation (IP) experiments with anti-myc antibody to pull down the overexpressed CPI-17 and its binding proteins. In the following western blotting, anti-myc antibody was used to confirm the success of pull-down, and antibodies against plectin, spectrin, gelsolin, and plakoglobin were used to detect each protein which was consider to be a putative binding partner from the previous bacterial two-hybrid screening. The ten remaining candidates were excluded from this study due to their lesser relevance to the cytoskeletal regulation. As shown in Figure 1A, we confirmed the interaction between overexpressed CPI-17 and plakoglobin. The interactions between CPI-17 and other putative binding partners, plectin, spectrin, and gelsolin were not detected in HLMVEC. The effect of PMA-induced phosphorylation of CPI-17 on the binding affinity of plakoglobin was also examined. As shown in Figure 1B, the binding affinity between CPI-17 and plakoglobin was decreased in the cells that were pretreated with PMA (0.1 μM, 30 minutes). A phospho-CPI-17 specific antibody was also employed to demonstrate that PMA induced the phosphorylation of CPI-17 (Fig 1B). The specificity of the plakoglobin-CPI-17 interaction was confirmed by further IP experiments. These myc IP samples were probed not only for plakoglobin, but also for β-catenin, VE-cadherin and p120, however, only binding of plakoglobin was detected, further confirming the specificity of CPI-17-plakoglobin binding (Fig 1C). Although the MLCP subunit, MYPT1, was not identified in our screening, it was co-immunoprecipitated with the overexpressed CPI-17 as it was expected (Fig 1C). Further, immunofluorescent staining of HLMVEC revealed co-localization of recombinant CPI-17 and endogenous plakoglobin (Fig 2A–C) at the attachment sites neighboring cells. Interestingly, PMA treatment increased plakoglobin staining, while total CPI-17 staining is rather become scarce in the cell membrane (Fig 2D–F).
Figure 1. Protein-protein interactions between CPI-17 and putative partner proteins in the EC.
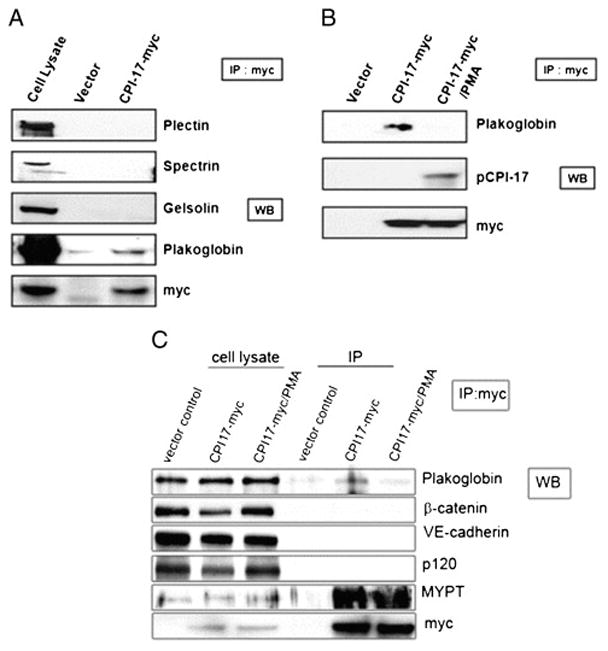
(A) CPI-17 containing plasmid with myc tag was transiently transfected in HLMVEC. After 48 hrs, the harvested lysates were immunoprecipitated (IP) with anti-myc antibody, and then subjected to Western blotting with antibodies against plectin, spectrin, gelsolin, plakoglobin, and myc. CPI-17-transfected cell lysates were used for Western blotting as a control of IP efficiency. (B) The alteration of the binding pattern between plakoglobin and CPI-17 by PMA-induced CPI-17 phosphorylation was examined. In the same condition with (A), cells were pretreated with 0.1 μM of PMA for 30 minutes. After washing with PBS, IP and following Western blotting were conducted using anti-plakoglobin, -phospho-CPI17, and -myc antibodies. (C) To clarify the CPI-17-plakoglobin interaction specificity, CPI-17-transfected cell lysates (control and PMA-treated) and empty c-myc vector-transfected cell lysates were IP with anti-c-myc Affinity gel, then subjected to Western blotting with anti-plakoglobin, β-catenin, VE-cadherin, p-120, MYPT1, and c-myc. Aliquots of cell lysates of vector control, CPI-17- myc, and CPI-17-myc/PMA were also loaded as controls. Representative blot of three independent experiments is shown.
Figure 2. Co-localization of plakoglobin and CPI-17 in HLMVEC.
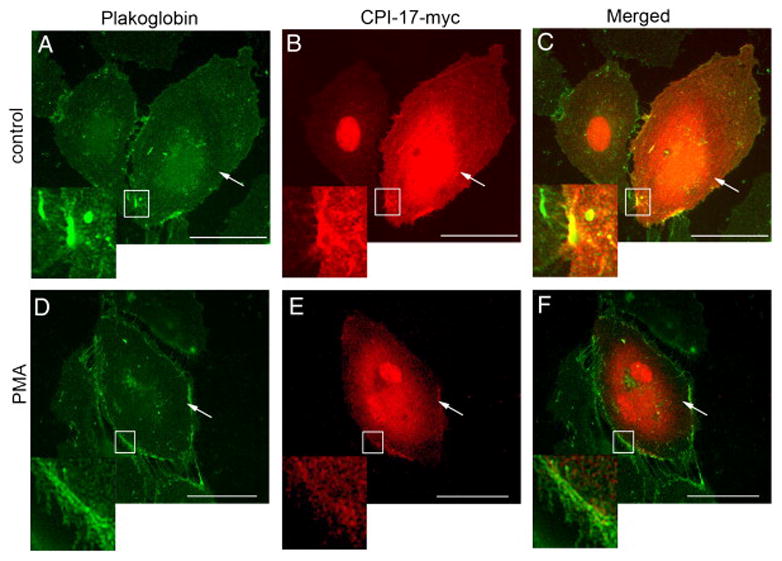
CPI-17-containing plasmid with c-myc tag was transiently transfected to HLMVEC. 48 hours later, the cells were treated either with vehicle (0.1% DMSO) or with PMA (0.1 μM for 30 min) then immunofluorescence was performed. Cells were double stained to visualize CPI-17(B, E) and plakoglobin (A, D). Panel C shows a merged image of images from panels A and B, and panel F is a merged image of D and E. Double stained images are shown in parallel. Transfected cells are indicated with arrows. Co-localization of CPI-17 and plakoglobin on panel C is shown enlarged. The similar area (lack of co-localization) is shown enlarged fragment of panel F. Scale bars: 200 μm. Same experiments were repeated three times.
Discussion
CPI-17, one of known regulators of MLCP activity in smooth muscle cells (Eto et al., 2001; Kitazawa et al., 2000; Li et al., 1998), was also identified in platelets (Watanabe et al., 2001) and brain (Dubois et al., 2003). Using cDNA purified from human pulmonary artery EC (HPAEC) as a template, we successfully cloned (Kolosova et al., 2004) the larger isoform of CPI-17 found originally in human aorta (Yamawaki et al., 2001). Since we did not obtain any PCR product corresponding to the smaller CPI-17 isoform (120 amino acids) characterized previously (Yamawaki et al., 2001), in this study we used only the coding DNA sequence of the larger isoform of CPI-17 in search for its interacting protein partners in human endothelium. As a result, fourteen potential binding partners of CPI-17 were identified using bacterial two-hybrid system (Table 1). Among them, plectin 1 isoform 1, alpha II spectrin, OK/SW-CL.16, gelsolin, and junction plakoglobin are known to be involved in actin cytoskeleton organization and cell adhesion. These findings are consistent with our published data demonstrating the involvement of CPI-17 in endothelial cytoskeletal and barrier regulation (Kolosova et al., 2004). On the other hand, we failed to identify binding of any MLCP subunits to CPI-17 in this system. This can be explained by the methodology employed. We used the bacterial two-hybrid system, which has the advantages of high efficiency and speed, but in contrast to the yeast system it lacks the ability to perform posttranslational modifications of proteins including phosphorylation. Additionally, these putative partner proteins were inferred from fragments identified in the bacterial two-hybrid system and the tertiary structures of these fragments are not necessarily the same as for the corresponding native proteins. Therefore, real interaction between CPI-17 and a putative partner protein can be affected by the three-dimensional protein structure of each protein and by posttranslational modification(s) of CPI-17. Also, the tissue-specific environment is an important determinant in protein-protein interactions. Therefore, the interaction between CPI-17 and its putative partners were re-examined in HLMVEC. As shown in Figure 1A, among the four retested putative partners only plakoglobin demonstrated significant binding with CPI-17. Further, the possible changes of binding affinity due to posttranslational modifications were examined using direct PKC activator, PMA. We have previously reported PKC-dependent CPI-17 phosphorylation induced by PMA or pro-inflammatory agonist, histamine in human pulmonary EC (Kolosova et al., 2004). Based on these data, we examined the effect of PMA-induced phosphorylation of CPI-17 on its binding affinity toward plakoglobin. As shown in Figure 1B, the binding between CPI-17 and plakoglobin was significantly decreased in PMA-treated cells.
Plakoglobin (γ-catenin) was originally discovered as a cytoplasmic component of two distinct intercellular junctions: adherens junctions and desmosomes (Cowin et al., 1986). Plakoglobin is highly homologous to β-catenin, which found in adherens junctions but not in desmosomes. Plakoglobin plays an important role in cadherin/catenin complex (cadherin, α, β, γ-catenins) assembly, as a linker between this complex and F-actin cytoskeleton. Plakoglobin binds to the cytoplasmic domains of classical and desmosomal cadherins in adherens junctions and desmosomes, respectively (Aberle et al., 1994; Knudsen and Wheelock, 1992; Mathur et al., 1994). At the same time, plakoglobin binds to α-catenin which can bind to several actin-binding proteins, such as α-actinin, and zonula occludens-1 (ZO-1) (Weis and Nelson, 2006). Through this serial connection with several proteins, the cadherin complex on the plasma membrane is connected and communicates with actin cytoskeleton. Our results indicate (Figure 1B,C and 2) that the interaction of CPI-17 with plakoglobin could be decreased upon PKC-dependent CPI-17 phosphorylation and activation. We speculate that it may affect myosin phosphatase activity. In addition, these data suggest a novel role for CPI-17 as an important modulator that regulates the interaction between cadherin/catenin complex on the plasma membrane and actin cytoskeleton machinery.
Collectively, our data suggests that CPI-17, through its binding partner, plakoglobin, can interact with F-actin cytoskeleton, scaffolding proteins, and signaling molecules. The significances of these interactions on regulation of EC cytoskeletal organization and barrier function should be determined by further experiments.
Highlights.
Using Two-Hybrid System, several binding partners of CPI-17 were identified.
Plakoglobin was identified as a real binding partner of CPI-17 in human cells.
The phosphorylation of CPI-17 interrupted its interaction with Plakoglobin.
Acknowledgments
This work was supported by NIH grants HL 58064, HL 67307, HL101902 (for ADV), ALA Research Grant for Maryland (for IK), GHSU CVDI and AHA 11SDG7670035 grants (for EAZ), and UD Faculty of Medicine Research Fund (Bridging Fund 2012) (for CsCs). The authors wish to thank Aigerim Adysheva for the technical assistance in preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature
- Aberle H, et al. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107(Pt 12):3655–63. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- Alessi D, et al. The control of protein phosphatase-1 by targetting subunits. The major myosin phosphatase in avian smooth muscle is a novel form of protein phosphatase-1. Eur J Biochem. 1992;210:1023–35. doi: 10.1111/j.1432-1033.1992.tb17508.x. [DOI] [PubMed] [Google Scholar]
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–92. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- Cao W, et al. TIMAP, a novel CAAX box protein regulated by TGF-beta1 and expressed in endothelial cells. Am J Physiol Cell Physiol. 2002;283:C327–37. doi: 10.1152/ajpcell.00442.2001. [DOI] [PubMed] [Google Scholar]
- Cowin P, et al. Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell. 1986;46:1063–73. doi: 10.1016/0092-8674(86)90706-3. [DOI] [PubMed] [Google Scholar]
- Csortos C, et al. Regulation of vascular endothelial cell barrier function and cytoskeleton structure by protein phosphatases of the PPP family. Am J Physiol Lung Cell Mol Physiol. 2007;293:L843–54. doi: 10.1152/ajplung.00120.2007. [DOI] [PubMed] [Google Scholar]
- Diwan AH, et al. Inhibition of serine-threonine protein phosphatases decreases barrier function of rat pulmonary microvascular endothelial cells. J Cell Physiol. 1997;171:259–70. doi: 10.1002/(SICI)1097-4652(199706)171:3<259::AID-JCP4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Dove SL, et al. Activation of prokaryotic transcription through arbitrary protein-protein contacts. Nature. 1997;386:627–30. doi: 10.1038/386627a0. [DOI] [PubMed] [Google Scholar]
- Dubois T, et al. Novel in vitro and in vivo phosphorylation sites on protein phosphatase 1 inhibitor CPI-17. Biochem Biophys Res Commun. 2003;302:186–92. doi: 10.1016/s0006-291x(03)00130-x. [DOI] [PubMed] [Google Scholar]
- Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- Eto M, et al. Cerebellar long-term synaptic depression requires PKC-mediated activation of CPI-17, a myosin/moesin phosphatase inhibitor. Neuron. 2002;36:1145–58. doi: 10.1016/s0896-6273(02)01107-8. [DOI] [PubMed] [Google Scholar]
- Eto M, et al. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C alpha and delta isoforms. J Biol Chem. 2001;276:29072–8. doi: 10.1074/jbc.M103206200. [DOI] [PubMed] [Google Scholar]
- Eto M, et al. A novel protein phosphatase-1 inhibitory protein potentiated by protein kinase C. Isolation from porcine aorta media and characterization. J Biochem. 1995;118:1104–7. doi: 10.1093/oxfordjournals.jbchem.a124993. [DOI] [PubMed] [Google Scholar]
- Eto M, et al. Molecular cloning of a novel phosphorylation-dependent inhibitory protein of protein phosphatase-1 (CPI17) in smooth muscle: its specific localization in smooth muscle. FEBS Lett. 1997;410:356–60. doi: 10.1016/s0014-5793(97)00657-1. [DOI] [PubMed] [Google Scholar]
- Goeckeler ZM, Wysolmerski RB. Myosin light chain kinase-regulated endothelial cell contraction: the relationship between isometric tension, actin polymerization, and myosin phosphorylation. J Cell Biol. 1995;130:613–27. doi: 10.1083/jcb.130.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorne DJ, et al. Myosin light chain phosphatase: subunit composition, interactions and regulation. J Muscle Res Cell Motil. 1998;19:325–41. doi: 10.1023/a:1005385302064. [DOI] [PubMed] [Google Scholar]
- Hartwig JH. Actin-binding proteins 1: spectrin superfamily. Protein Profile. 1994;1:706–78. [PubMed] [Google Scholar]
- Ito M, et al. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- Kim KM, et al. Molecular characterization of myosin phosphatase in endothelium. J Cell Physiol. 2012;227:1701–8. doi: 10.1002/jcp.22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa T, et al. Agonists trigger G protein-mediated activation of the CPI-17 inhibitor phosphoprotein of myosin light chain phosphatase to enhance vascular smooth muscle contractility. J Biol Chem. 2000;275:9897–900. doi: 10.1074/jbc.275.14.9897. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Wheelock MJ. Plakoglobin, or an 83-kD homologue distinct from beta-catenin, interacts with E-cadherin and N-cadherin. J Cell Biol. 1992;118:671–9. doi: 10.1083/jcb.118.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosova IA, et al. Role of CPI-17 in the regulation of endothelial cytoskeleton. Am J Physiol Lung Cell Mol Physiol. 2004;287:L970–80. doi: 10.1152/ajplung.00398.2003. [DOI] [PubMed] [Google Scholar]
- Li L, et al. Possible involvement of the novel CPI-17 protein in protein kinase C signal transduction of rabbit arterial smooth muscle. J Physiol. 1998;508(Pt 3):871–81. doi: 10.1111/j.1469-7793.1998.871bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur M, et al. Interactions of the cytoplasmic domain of the desmosomal cadherin Dsg1 with plakoglobin. J Biol Chem. 1994;269:14075–80. [PubMed] [Google Scholar]
- Pang H, et al. RhoA-Rho kinase pathway mediates thrombin- and U-46619-induced phosphorylation of a myosin phosphatase inhibitor, CPI-17, in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2005;289:C352–60. doi: 10.1152/ajpcell.00111.2005. [DOI] [PubMed] [Google Scholar]
- Pytela R, Wiche G. High molecular weight polypeptides (270,000–340,000) from cultured cells are related to hog brain microtubule-associated proteins but copurify with intermediate filaments. Proc Natl Acad Sci U S A. 1980;77:4808–12. doi: 10.1073/pnas.77.8.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silacci P, et al. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004;61:2614–23. doi: 10.1007/s00018-004-4225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner JA, Saltiel AR. Cloning and identification of MYPT3: a prenylatable myosin targetting subunit of protein phosphatase 1. Biochem J. 2001;356:257–67. doi: 10.1042/0264-6021:3560257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–85. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi M, et al. Interaction of alpha-actinin-4 with class I PxxP motif-containing OK/SW-CL.16 protein. Nephron Exp Nephrol. 2007;107:e65–72. doi: 10.1159/000108644. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, et al. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circ Res. 1998;83:1115–23. doi: 10.1161/01.res.83.11.1115. [DOI] [PubMed] [Google Scholar]
- Van Troys M, et al. Structural modules in actin-binding proteins: towards a new classification. Biochim Biophys Acta. 1999;1448:323–48. doi: 10.1016/s0167-4889(98)00152-9. [DOI] [PubMed] [Google Scholar]
- Verin AD, et al. Regulation of endothelial cell gap formation and barrier function by myosin-associated phosphatase activities. Am J Physiol. 1995;269:L99–108. doi: 10.1152/ajplung.1995.269.1.L99. [DOI] [PubMed] [Google Scholar]
- Verin AD, et al. Immunochemical characterization of myosin-specific phos–phatase 1 regulatory subunits in bovine endothelium. J Cell Biochem. 2000;76:489–98. [PubMed] [Google Scholar]
- Watanabe Y, et al. Protein kinase C-catalyzed phosphorylation of an inhibitory phosphoprotein of myosin phosphatase is involved in human platelet secretion. Blood. 2001;97:3798–805. doi: 10.1182/blood.v97.12.3798. [DOI] [PubMed] [Google Scholar]
- Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281:35593–7. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodsome TP, et al. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol. 2001;535:553–64. doi: 10.1111/j.1469-7793.2001.t01-1-00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamawaki K, et al. Identification of human CPI-17, an inhibitory phosphoprotein for myosin phosphatase. Biochem Biophys Res Commun. 2001;285:1040–5. doi: 10.1006/bbrc.2001.5290. [DOI] [PubMed] [Google Scholar]


