Abstract
Developmental Coordination Disorder (DCD) affects a relatively large proportion (5–6%) of the childhood population. Severity of the disorder varies but there is a great need for therapeutic intervention. We propose a method for the training of manual actions in children with DCD. Our solution is achieved by applying haptic virtual reality technology to attack the difficulties that children with DCD evidence. Our results show that children with DCD are able to learn complex motor skills if proper training methods are employed. These findings conflict with reports of impaired motor learning in DCD because of under-activation of cerebellar and parietal networks.
Keywords: Developmental coordination disorder, sensori-motor training, manual actions
Developmental Coordination Disorder (DCD) is understood to be, first and foremost, a motor disorder1,2,3,4,5,6,7,8,9 although it is often co-morbid with Autism Spectrum Disorder and Attention Deficit Hyperactive Disorders (ADHD), among other general perceptual and cognitive disorders10. Children with DCD can exhibit poor gross motor control, poor fine motor control, or both3. Moreover, this motor disorder can lead to emotional and academic problems10,11,12. For instance, children with DCD commonly have problems with spelling and reading13 in addition to difficulties with tasks like writing.
DCD is also thought to be a learning disability as children with DCD often have persistent trouble learning or acquiring motor skills6,14,15,16,17. Given the difficulties that children with DCD have learning or acquiring motor skills and given the cerebellum’s known role in motor learning processes, it has been hypothesized that cerebellar dysfunction is a possible source of motor disruptions observed in individuals with DCD18,19,20 and there is some evidence supporting these claims. Specifically, Zwicker and collaborators17 found that children with DCD demonstrated under-activation in cerebellar–parietal and cerebellar–prefrontal networks. However, there is also some evidence suggesting that dysfunction of the parietal brain regions (left posterior parietal cortex and left postcentral gyrus) may underpin impaired motor skill performance in children with DCD21. It is not clear whether neural differences are cause or correlation raising the question of whether children with DCD are able to exhibit effective perceptuo-motor learning. In the present study, we find that they can learn effectively with appropriate support.
Researchers have investigated a number of possible etiologies of DCD including deficits in attention22,23 or in kinesthesia24,25,26. There has also been the suggestion that children with DCD are reliant on visual information and show kinesthetic deficits26,27,28. It has been further suggested that the root of the problem for children with DCD lies in deficits in the mappings from sensory to motor systems4,5,6,8,9. This notion is consistent with deficits in both parietal and cerebellar areas of the brain22,25. The result is poor performance in a variety of sensori-motor tasks such as targeted reaching, manual manipulation and coordination tasks6,8,9,29,30,31.
Research on the control and coordination of limb movements has shown that the sensori-motor control of limb stiffness and compliance is a key element in the organization of motor systems32,33,34,35,36,37,38,39,40. Logically, the best training for children with DCD would focus on the sensori-motor organization intrinsic to the control and coordination of the limbs i.e. limb stiffness and compliance. The therapeutic goal must be to allow the children to improve the perceptual abilities intrinsic to the concurrent generation and experience of their own movements and the use of that information to guide their movements.
This sort of training (“sensori-motor training”) is difficult to implement with traditional therapeutic tools and methods. However, robot-assisted therapies such as those involving the MIT-MANUS41,42, the ARM Guide43 (Assisted Rehabilitation and Measurement guide), and the MIME44,45 (Mirror-Image Motion Enabler) are being developed and should assist in the development of new therapeutic tools. One of the motivations behind the development of such robotic systems is the relative disparity between the number of therapists and the number of patients, coupled with the amount of “therapy time” required for functional improvements. Robot-assisted therapies allow for training to occur independently of a therapist; that is, without the direct supervision of a trained therapist. In addition, robots can apply various constraints to the required movement patterns and, thus, the complexity and/or difficulty of a motor task can be controlled very precisely46. Ben-Pazi and collaborators47, for example, showed how robots could be used with children to improve the generation of handwriting movements. In this experiment, Ben-Pazi et al. determined that the mechanical properties (inertia and viscosity) of a robot pen (Phantom 1.5) affected handwriting quality of 8–14 year old children. Specifically, Ben-Pazi et al. found that increased inertia and viscosity of the pen reduced high frequency components in handwriting movements and improved handwriting quality. The improvements in handwriting legibility were found for both teacher ratings and layperson ratings of handwriting quality.
The results from Ben-Pazi et al.47 are very promising regarding the utility of robot-assisted therapies for children. However, the nature of the support provided by the robot needs to be examined. For example, Bingham and collaborators (personal communication) found that passive training of the sort provided by some of these robot-assisted therapies failed to enable good sensori-motor learning of new movement tasks (i.e. the training failed to transfer beyond the very specific movements that were practiced). Instead, active sensori-motor generation and control of movement trajectories was required for learning that generalized to task related movements other than those specifically practiced. This result suggests that children with DCD face a difficult ‘catch-22’ problem.
Motor learning was described by Newell48 as having two stages. First, the learner acquires a qualitative approximation to the movements to be learned. Once this is achieved, the learner can quantitatively improve the performance through practice. The apparent problem for children with DCD is they cannot achieve a sufficiently good qualitative approximation to be able to then make good quantitative improvements through practice. This is the ‘catch-22.’ Robot-assisted therapies could, in principle, help them achieve the essential movement form, but it is likely that this must be done under active sensori-motor control to be effective. Most of the existing robot-assisted therapies do not allow such active control. The problem is to find a way to support and guide the movements while requiring them to be actively generated and controlled. The best method of support would allow children with DCD to perform with support as well as age-match typically developing (TD) children. This would keep the motivation and, potentially, self-esteem of the learners high. If the approach to learning is effective, then the level of support can be gradually (that is, parametrically) reduced while maintaining the high level of performance until finally the learners are able to perform without support as well as TD children.
So, the initial question is how to support performance of movements while requiring that they be actively generated and controlled?
Initial study: Finding appropriate control variables
Much of the research on children with DCD focuses on identifying differences between children with DCD and typically developing children – children with DCD are typically found to be slower and less accurate on most tasks when compared to their typically developing peers. de Oliveira and Wann49 indicated that these differences were important for diagnostic purposes but suggested that it is essential to find conditions where children with DCD are relatively unimpaired to better understand the underlying causes of DCD. The purpose of this study was to test robot-generated properties to identify a parametric variable that would allow children with DCD to perform similarly to typically-developing (TD) children or even adults when interacting with a robotic haptic device. Ben-Pazi et al.47 showed that viscosity and inertial properties may provide appropriate support, but these do not allow provision of a template for the movement form. Furthermore, there was no evidence that the method did or even could generalize to performance without the support provided by the method. We adopted an alternative approach. We provided a movement template as a wire path that was to be followed or traced by the tip of a stylus held, like a pencil or pen, by the child. This is difficult for any performer, but it is nearly impossible for a child with DCD, because that child inevitably comes off the path and has to regain it over and over again. How might such a child be supported to avoid the extreme frustration this would provoke? The method would need to be open to parametric variation. The potential solution that we investigated was to make the wire path ‘magnetically attractive’ to the stylus tip. With strong attractive force, the child can concentrate on moving the stylus along the 3D path in space. The most compliant motions become the most successful and this is exactly what a child with DCD needs to learn. (A common observation is that these children press their pencil or pen into a writing surface to try to gain some measure of control through highly non-compliant movement.) The question is whether this parameter allows children with DCD to perform as well as age-matched TD children. Here, we test the effect of both ‘magnetic attraction’ and ‘friction’ along the path.
Method
Participants
Three boys with DCD aged 12:2 (years: months), 9:9, and 12:4 were tested along with two age-matched TD boys (12:9, 10:4) and three normal adults, aged 27, 30 and 53. The children with DCD were recruited at a local Children’s Physical & Occupational Therapy clinic and were identified as having motor problems that significantly interfered with their activities at school and at home. One of these children had a Bruininks-Oseretsky upper-limb speed and dexterity z-score of −1.66 and a bilateral coordination z-score of −1.46 (both of which indicate “poor performance”). Another child had Beery VMI scores of: 0.8th percentile (visual perception skills), 19th percentile (motor coordination skills) and 23rd percentile (overall visual motor integration skills). The 3rd child had Beery VMI scores of: 19th percentile (visual perception skills) and 13th percentile (overall visual motor integration skills). The TD children were not formally evaluated, but had normal vision and no history of motor or neurological impairments. This study was approved by the Indiana University Institutional Review Board; the children participated with informed assent with consent from their parents/guardians.
Procedure
All participants performed the same basic 3D tracing task. The task was to use a virtual stylus (controlled in a similar manner to a computer mouse) to push a bead along a 3D path visible in a computer graphic display (see Figure 1) from a starting location (the plain square) to a finishing point (the checkered square). The participant grasped a stylus that was attached to a desktop force feedback haptic virtual reality device, a Phantom Omni from Sensable Technologies, and used the stylus to control the virtual stylus to feel the path and push the bead. The path attracted the stylus to hold it on the path (as if a magnetic force were present). The ‘magnetic’ strength was parametrically varied to alter task difficulty. Path ‘friction’ was a second parameter. Participants performed three random order blocks of nine trials (27 total trials) in which the bead was pushed around the wire path seen in Figure 1. In a block, a trial was a combination of level of friction (low, medium, or high) and level of magnetic attraction (low, medium, or high).
Figure 1.
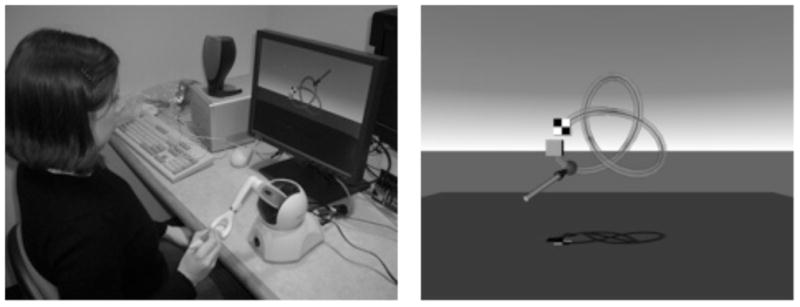
A) Example of display and target path. B) The Phantom together with the display.
Data analysis
The three dimensional Cartesian coordinates of the virtual stylus tip and red bead were recorded at 50 Hz. These data were filtered using a dual-pass, second order Butterworth filter with a 5 Hz cut-off frequency. Using these data with the known coordinates of the target trajectory (the wire), the trial duration and path length were computed to evaluate performance. Path length was then normalized so that ideal performance was equal to 1. We averaged trial duration and normalized path length, for each participant, over the trials performed in a given condition.
Results
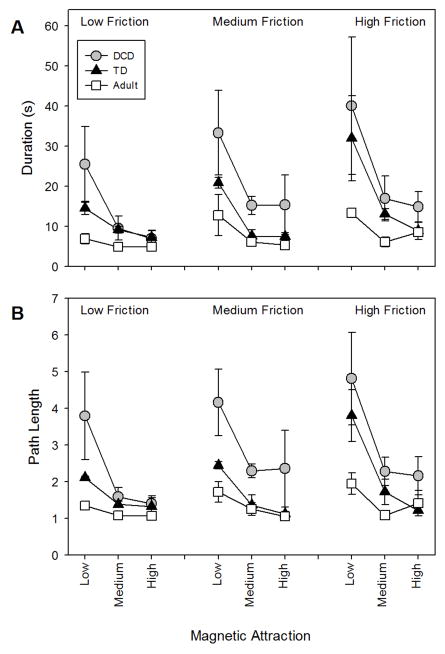
Trial durations for all participants under all conditions are reported in Figure 2a while path length is reported in Figure 2b. Overall, adult performance was best, followed by that of TD children and then, children with DCD. Importantly, the performance of children with DCD was comparable to that of TD children when the magnetic attraction was strong and the friction was low. Performance was strongly affected by magnetic attraction. When magnetic attraction was low, everyone tended to come off the wire and had to spend time getting back onto it, but children with DCD did this more often than did TD children and adults. The differences in performance were obvious at the time of testing. The task was successful in two respects: it differentiated children with DCD from TD children while, at the same time, allowing children with DCD to perform like TD children with appropriate variation in the magnetic parameter, yielding those children good efficacy. Again, adjustment of the task parameters could make the child with DCD appear normal in terms of his or her comparative level of performance.
Figure 2.
A) Trial durations and B) Normalized path length for each condition (friction level and level of magnetic attraction) by groups of participants: adults (white squares); TD children (filled triangles); children with DCD (filled circles).
Main Study: Do Children with DCD Improve?
The purpose of this experiment was to determine whether the quality of movements generated by children with DCD can be improved with progressively less support from the robot. Given the results from the first study, we elected to remove friction as a parameter and only to vary level of magnetic attraction.
Participants
Eight 7- and 8-year old children with DCD participated in this study; four were recruited from a local physical and occupational therapy clinic and four were recruited from a local elementary school. The children recruited from the local clinic were evaluated (given a standardized test of the clinician’s choice) by a trained therapist and referred to us for enrollment. These children all scored lower than the 10th percentile on a relevant standardized test (Beery VMI, Developmental Test of Visual Perception – 2: eye-hand coordination subtest, Wide Range Assessment of Visual Motor Abilities: pegboard/fine motor subtest). The children recruited from the local elementary school were evaluated by a trained clinical psychology student using the Beery VMI; the parents/guardians also evaluated their child using the DCD questionnaire50 (DCD-Q ’07). These children scored lower than the 10th percentile on the overall visual motor integration test or the coordination subtest of the Beery VMI and were identified by their parents/guardians as having “suspected DCD” were considered to have DCD. The average coordination subtest score for these children is 4.0%.
Eight 7- and 8-year old typically developing children were recruited from a local elementary school. Twenty-eight children, seven children from four different classrooms, were initially screened using the Beery VMI and the DCD-Q. In order to be included in the analyses, the typically-developing children had to closely match the children with DCD with respect to age, gender and handedness, had to be free from any known medical or neurological conditions, and were not suspected of having DCD as indicated by the DCD-Q and also scored > 16% on the Beery VMI. The average coordination subtest score for these children was 34.6%. A t-test revealed that coordination scores for TD children were significantly higher than those of children with DCD (t = −4.3714, p < 0.01).
This study was approved by the Indiana University Institutional Review Board; the children participated with informed assent with consent from their parents/guardians.
Procedure
All participants performed the same basic 3D tracing task before and after training. The task was to push a brightly colored fish along a visible path on a computer screen from the starting location (the plain square) to the finish point (the checkered square) while racing a competitor fish. The purpose of the competitor fish was to give the children a clear temporal goal. As in Experiment 1, the participants grasped a stylus that was attached to a desktop force feedback haptic virtual reality device, a Phantom Omni from Sensable Technologies, and used the stylus to feel the path and push the fish. The path magnetically attracted the stylus to hold it on the path. The magnetic strength was parametrically varied to alter task difficulty. Participants attempted two trials at each of eight levels of magnetic attraction on the path pictured in Figure 1b while racing a competitor fish that took 20s to travel the path from start to finish. From pilot testing, it was clear that most children would spend many minutes to complete a path and would become very frustrated with the lack of progress so each trial was terminated if a child could not complete more than one half of the path within 60s.
All participants were then given up to five, 20 minute, training sessions that were separated by one week (sometimes two in the case of illness). During the training, there were three different paths that varied in length, curvature, and torsion (see Figure 3). There were also two different competitors against whom the participants were racing; one that completed the path in 30s, one that completed the path in 10s. On a few occasions, we used a third competitor whose speed was in between the other competitors (20s) but this was only if a participant was struggling with the fastest competitor.
Figure 3.
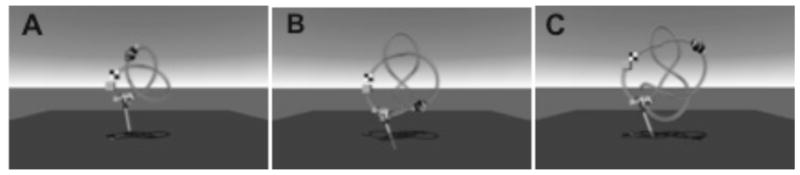
A) Shortest path. B) Middle length path. C) Longest path.
The training started with the highest level of magnetic attraction, slowest competitor, and shortest path. The goal of the training was to allow the children to progress at their own pace through the different combinations of levels of attraction, paths, and competitors so we used a “two-wins-in-a-row” rule in order to determine when the children progressed. (On a few occasions, we allowed a participant to progress without “winning” two times-in-a-row; these instances happened only after a participant tried the type of trial a few times and expressed a great deal of frustration about not winning.) After the participant “beat” the slowest competitor two times-in-a-row they progressed to the faster competitor. Once the participant beat both competitors they then moved to the next longest path (with slowest competitor). After all paths and competitors were “beaten”, then the level of magnetic attraction was decreased and the participant restarted with the shortest path and slowest competitor.
Data analysis
The three dimensional Cartesian coordinates of the virtual stylus tip and fish were recorded at 50 Hz. These data were filtered using a dual-pass, second order Butterworth filter with a 5 Hz cut-off frequency. Using these data with the known coordinates of the target trajectory (the wire), the trial duration and normalized path length were computed to evaluate performance. We then averaged trial duration, for each participant, over the trials performed in a given condition (path, competitor, level of magnetic attraction). For the (baseline) trials where children were unable to complete the path, a value of 60s was given for the trial duration; the average path length of the last level completed was given for all subsequent levels. Average trial duration and normalized path length, before and after training, were then analyzed using three-way mixed design analysis of variance (ANOVA) with the following conditions and levels: group (DCD, TD), level of magnetic attraction (1–8), and session (baseline, post-training). Group was between subjects while level and session were within subjects.
Finally, we derived learning curves from the training data. The training method was designed to preserve high self-efficacy by allowing the children with DCD to continue to perform well with support. They started training with high levels of support and as they improved the level of support was gradually decreased. This meant that the mean durations during training remained fairly constant and that level of support effectively represented time over the course of training. We derived learning curve data by scaling durations at each successive level of support over training by the mean duration for that level of support obtained in baseline trials before training (for the shortest path only). We then performed linear regression on the resulting data to reveal the respective rates of change for the two groups. Similar analysis was performed with duration and path length measures.
Results
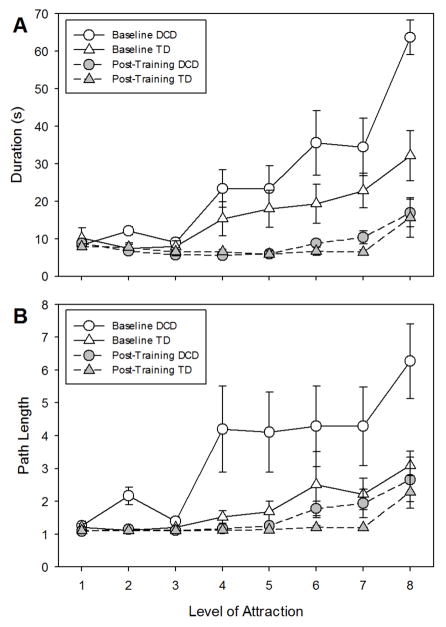
Figure 4A shows trial duration, before and after training, for TD children and children with DCD across the different levels of magnetic attraction (1 = highest level, 8 = lowest level). Before training, performance by children with DCD was significantly worse than performance by TD children. Trial durations for children with DCD were much longer without support (although by design performance was comparable with high levels of support). After training, both groups improved significantly with the important result that performance levels for both groups were the same both with and without support.
Figure 4.
A) Trial durations and B) Normalized path length across different levels of magnetic attraction for TD children (triangles) and children with DCD (circles) before (open symbols) and after (filled symbols) training.
The ANOVA yielded a group by level by session interaction (F(7,98) = 3.35, p < 0.01). There were also significant interactions of group by level (F(7,98) = 3.52, p < 0.01), group by session (F(1,14) = 5.59, p < 0.05), and level by session (F(7,98) = 15.75, p < 0.01) as well as main effects of level (F(7,98) = 28.71, p < 0.01) and session (F(1,14) = 49.54, p < 0.01). The three-way interaction indicates that the groups’ performance across the levels of support changed differently, with respect to each other, from baseline to post-training. Further testing revealed that there was a significant interaction of group and level (F(7,98) = 4.05, p < 0.01) as well as a main effect of level (F(7,98) = 26.01, p < 0.01) during baseline but not at post-test. There was only an effect of level at post-test (F(7,98) = 8.07, p < 0.01).
Figure 4B shows normalized path length, before and after training, for TD children and children with DCD across the different levels of magnetic attraction (1 = highest level, 8 = lowest level). The pattern of results was essentially the same as for the duration measure. The ANOVA yielded several significant two-way interactions (group by session: F(1,14) = 5.78, p < 0.05, and level by session: F(7,98) = 4.02, p < 0.01) as well as main effects (group: F(1,14) = 4.81, p < 0.05, level: F(7,98) = 11.30, p < 0.01, and session: F(1,14) = 17.83, p < 0.01) but no three-way interaction. The group by week interaction indicates that the groups’ performances changed differently, with respect to each other, from baseline to post-training. Further testing revealed that there were significant main effects of group (F(1,14) = 5.34, p < 0.05) and level (F(7,98) = 8.07, p < 0.01) during baseline but not after training; there was only an effect of level at post-test (F(7,98) = 9.00, p < 0.01).
These combined results show that both groups of children improved as a result of training but that children with DCD made more substantial improvements that enabled them to catch-up with their TD peers.
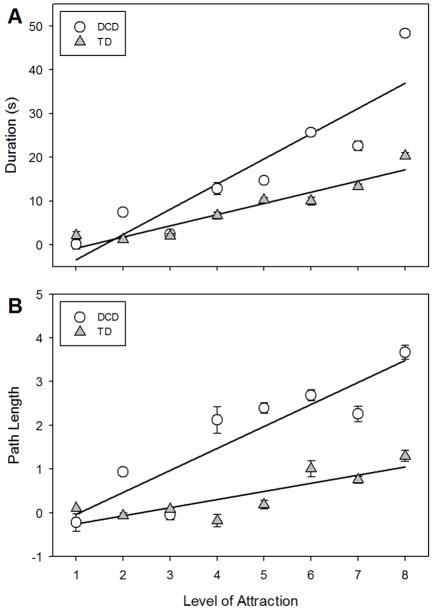
Figure 5 shows the improvement that both TD children and children with DCD exhibited over the course of training in both duration (Figure 5a) and path length (Figure 5b), where level of magnetic attraction effectively represented time during training. For both groups and both measures, the resulting regressions indicated that improvement is related to level of magnetic attraction. DCD: duration improvement = 6.51 × Level − 11.84 (r2 = 0.74); path improvement = 0.50 × Level − 0.51 (r2 = 0.47). TD: duration improvement = 2.73 × Level − 4.00 (r2 = 0.64); path improvement = 0.21 × Level − 0.58 (r2 = 0.28). Using multiple regression to test these apparent differences in slopes (and intercepts) revealed that group (DCD vs. TD) had a significant effect on the relationship between level and duration improvement (slope: t = −13.77, p < 0.01; intercept: t = 5.01, p < 0.01; overall: F(3,568) = 634.2, p < 0.01, r2 = 0.77), and path length improvement (slope: t = −7.36, p < 0.01; intercept: −0.33, p > 0.05; overall: F(3,568) = 251.7, p < 0.01, r2 = 0.57); that is, the slopes for children with DCD, for both measures, were higher than those of the TD children. These results indicate that improvement was accelerated during training for children with DCD relative to their TD peers.
Figure 5.
Improvement in A) trial duration and B) path length across the different levels of magnetic attraction during training for TD children (triangles) and children with DCD (circles).
Finally, we found that the children performing the task in this study clearly exhibited enjoyment of and enthusiasm for the task and expressed disappointment when we told them that the study was completed. We had made every effort to maintain high self-efficacy in the children and their evident positive response indicated that we had succeeded.
Discussion
The purpose of these experiments was to investigate the effectiveness of a novel sensori-motor paradigm for the training of manual actions performed by children with DCD. The children with DCD initially produced less successful actions resulting in high trial durations with longer path lengths. With training, however, these children were able to catch up with their typically-developing peers. These findings are particularly significant because they are among a small set of motor learning data which show that children with DCD are able to learn even complex motor skills when given an appropriate learning environment.
Action theory suggests that learners need to generate movements actively to be successful51. Children with DCD, however, largely cannot improve their motor performance because reliable approximations of a target action are needed to do so and these children are unable to achieve these approximations on their own. Here, we have demonstrated a method that enables children with DCD to overcome this ‘catch-22’ to be able to improve manual performance progressively to match typically developing children and to do so while performing (with implicit support) at a level comparable to that exhibited by age-matched, TD children. This motivated children with DCD to work to develop good motor skills.
Our solution was achieved by applying haptic and visual virtual reality technology developed for visualization of knots by topologists to attack the difficulties that children with DCD experience. The technology provided adjustable but essential support in a way that required active sensori-motor generation of movements and kept the task challenging so the children learned. The method provided support for development of good compliance control of the arm and hand in a tracing task. This meant that the children were able to produce the requisite initial ballpark movements that could be practiced to yield quantitative improvements in sensori-motor sensitivity and control. This adjustability is an important feature because while nearly all children with DCD have persistent trouble learning or acquiring motor skills6,14,15,16,17 no single neurological condition gives rise to DCD. Some children with DCD will demonstrate under-activation in cerebellar–prefrontal networks17. However, others will demonstrate dysfunction of the parietal brain regions21. So, it is likely that different remediation strategies may be required depending on the specific nature of the deficit exhibited by children with DCD and we are able to achieve this flexibility through the use of virtual reality technology.
The results from this method of training allow us to revisit and re-assess the relationships between brain and behavior. A number of studies, both behavioral and imaging, have demonstrated that cerebellar dysfunction and/or parietal dysfunction are plausible sources of motor disruptions observed in children with DCD21,22,25. At present, however, there is only one study that has examined whether children with DCD recruit a different set of brain regions than typically developing children during a motor learning task. Zwicker and collaborators17 mapped brain activity, using fMRI, that was associated with the learning of a trail-tracing task in children with DCD and typically developing children. They examined the reduction in tracing error from early practice to retention and found that children with DCD demonstrated poorer tracing accuracy than TD children at retention (when testing effect size). They also found that children with DCD showed less blood-oxygen-level-dependent signal as compared to TD children in cerebellar–parietal and cerebellar–prefrontal networks and in other brain regions which Zwicker et al. associated with visual-spatial learning. Zwicker et al. suggested that their data support a neurobiological correlation with impaired learning of motor skills in children with DCD; that is, under-activation of cerebellar and parietal networks is related to, and perhaps causes, poor motor learning outcomes for children with DCD. Our data, however, suggest that under-activation of cerebellar or parietal networks observed in children with DCD might reflect the absence of recruitment of a neural circuit underpinning a skill but the DCD population are able to recruit brain networks that support perceptuo-motor learning nevertheless, and develop the requisite neural circuits for a particular skill, when they are provided appropriate support in the context of a training regime designed to maintain good self-efficacy.
In conclusion, our findings support the view that children with DCD perform manual actions differently than typically developing children but that they are able to learn to control the movement of their limbs when given training that includes appropriate parametrically controlled support, enabling maintenance of high self-efficacy during practice. The successful learning was particularly evident when the initial poor performance of children with DCD was compared to their performance after training as well as to the performance of their typically-developing peers. In addition, we have identified a rate of learning that might be used to assess the progress that children with DCD exhibit during the course of treatment. This learning rate measure also showed good perceptuo-motor learning by these children with DCD.
Acknowledgments
This work was performed at the Bloomington Hospital Children’s Therapy Clinic in Bloomington, IN and South Elementary School in Martinsville, IN. Part of the results were presented at the DCD-9 International Conference in Lausanne, Switzerland.
Funding
This work was partially supported by NIH NIDCD Training Grant T32DC00012 and NIH NICHD R01HD070832.
Footnotes
Conflict of Interest
We have no commercial, financial, or other outside interests that could pose a conflict of interest in connection with this article.
Author Contributions
All authors made substantial contributions to the conception and design and interpretation of the data and approved the final article version. The first author drafted the article; the second and third authors revised the article for important intellectual content.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 2.Polatajko HJ, Fox AM, Missiuna C. An international consensus on children with developmental coordination disorder. Can J of Occup Ther. 1995;62:3–6. [Google Scholar]
- 3.Sugden DA, editor. Leeds Consensus Statement: Developmental Coordination Disorder as a Specific Learning Difficulty. Leeds: DCD-UK/Discovery Centre; 2006. [Google Scholar]
- 4.Grove CR, Lazarus J-AC. Paired re-weighting of sensory feedback for maintanence of postural control in children with developmental coordination disorder. Hum Movement Sci. 2007;26:457–76. doi: 10.1016/j.humov.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 5.Inder JM, Sullivan SJ. Motor and postural response profiles of four children with developmental coordination disorder. Ped Phys Ther. 2005;17:18–29. doi: 10.1097/01.pep.0000154184.06378.f0. [DOI] [PubMed] [Google Scholar]
- 6.Mon-Williams M, Wann JP, Pascal E. Visual-proprioceptive mapping in children with developmental coordination disorder. Dev Med Child Neurol. 1999;41:247–54. doi: 10.1017/s0012162299000523. [DOI] [PubMed] [Google Scholar]
- 7.Smits-Engelsman BCM, Niemeijer AS, Galen GP. Fine motor deficiencies in children diagnosed as DCD based on poor grapho-motor ability. Hum Movement Sci. 2001;20:161–82. doi: 10.1016/s0167-9457(01)00033-1. [DOI] [PubMed] [Google Scholar]
- 8.Smyth M, Mason UC. Direction of response in aiming to visual and proprioceptive targets in children with and without Developmental Coordination Disorder. Hum Movement Sci. 1998;17:515–39. [Google Scholar]
- 9.Volman MJM, Geuze RH. Relative stability of bimanual and visuomotor rhythmic coordination patterns in children with a Developmental Coordination Disorder. Hum Movement Sci. 1998;17:541–72. [Google Scholar]
- 10.Kamps PH. The Source for Developmental Coordination Disorder: A Childhood Disorder Characterized by Poor Coordination and Clumsiness. East Moline, IL: LinguiSystems, Inc; 2005. [Google Scholar]
- 11.Gibbs J, Appleton J, Appleton R. Dyspraxia or developmental coordination disorder? Arch Dis Child. 2007;92:534–39. doi: 10.1136/adc.2005.088054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geuze RH, Jongmans M, Schoemaker M, Smits-Engelsman B. Developmental coordination disorder. Hum Movement Sci. 2001;20:1–5. doi: 10.1016/s0167-9457(01)00026-4. [DOI] [PubMed] [Google Scholar]
- 13.Alloway TP, Temple KL. A comparison of working memory skills and learning in children with developmental coordination disorder and moderate learning difficulties. Appl Cognitive Psych. 2007;21:473–87. [Google Scholar]
- 14.Biancotto M, Skabar A, Bulgheroni M, et al. Neuromotor deficits in developmental coordination disorder: Evidence from a reach-to-grasp task. Res Dev Disabil. 2011;32:1293–1300. doi: 10.1016/j.ridd.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Geuze RH. Postural control in children with developmental coordination disorder. Neural Plast. 2005;12:183–96. doi: 10.1155/NP.2005.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasmussen P, Gillberg C. Natural outcome of ADHD with DCD at age 22 years. J Am Acad Child Psy. 2000;39:1424–31. doi: 10.1097/00004583-200011000-00017. [DOI] [PubMed] [Google Scholar]
- 17.Zwicker JG, Missiuna C, Harris SR, Boyd LA. Brain activation associated with motor skill practice in children with developmental coordination disorder: An fMRI study. Int J Dev Neurosci. 2011;29:145–52. doi: 10.1016/j.ijdevneu.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Cantin N, Polatajko HJ, Thach WT, Jaglal S. Developmental coordination disorder: exploration of a cerebellar hypothesis. Hum Movement Sci. 2007;26:491–509. doi: 10.1016/j.humov.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 19.Ivry RB. Cerebellar involvement in clumsiness and other developmental disorders. Neural Plast. 2003;10:141–53. doi: 10.1155/NP.2003.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwicker JG, Missiuna C, Boyd LA. Neural correlates of developmental coordination disorder: A review of hypotheses. J Child Neurol. 2009;24:1273–81. doi: 10.1177/0883073809333537. [DOI] [PubMed] [Google Scholar]
- 21.Kashiwagi M, Iwaki S, Narumi Y, et al. Parietal dysfunction in developmental coordination disorder: A functional MRI study. Neuroreport. 2009;20:1319–24. doi: 10.1097/WNR.0b013e32832f4d87. [DOI] [PubMed] [Google Scholar]
- 22.Castelnau P, Albaret JM, Chaix Y, Zanone P-G. Developmental coordination disorder pertains to a deficit in perceptuo-motor synchronization independent of attentional capacities. Hum Movement Sci. 2007;26:477–90. doi: 10.1016/j.humov.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Miyahara M, Piek J, Barrett N. Accuracy of drawing in a dual-task and resistance-to-distraction study: Motor or attention deficit? Hum Movement Sci. 2006;25:100–109. doi: 10.1016/j.humov.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Coleman R, Piek JP, Livesey DJ. A longitudinal study of motor ability and kinaesthetic acuity in young children at risk of developmental coordination disorder. Hum Movement Sci. 2001;20:95–110. doi: 10.1016/s0167-9457(01)00030-6. [DOI] [PubMed] [Google Scholar]
- 25.Mon-Williams M, Tresilian JR, Wann JP. Perceiving limb position in normal and abnormal control: An equilibrium point perspective. Hum Movement Sci. 1999;18:397–419. [Google Scholar]
- 26.Schoemaker MM, Wees M, Blapper B, et al. Perceptual skills of children with developmental coordination disorder. Hum Movement Sci. 2001;20:111–33. doi: 10.1016/s0167-9457(01)00031-8. [DOI] [PubMed] [Google Scholar]
- 27.Przysucha EP, Taylor MJ. Control of balance and developmental coordination disorder: The role of visual information. Adapt Phys Act Q. 2004;21:19–33. [Google Scholar]
- 28.Wann JP, Mon-Williams M, Rushton K. Postural control and co-ordination disorders: The swinging room revisited. Hum Movement Sci. 1998;17:491–513. [Google Scholar]
- 29.Plumb MS, Wilson AD, Mulroue A, et al. Online corrections in children with and without DCD. Hum Movement Sci. 2008;27:695–704. doi: 10.1016/j.humov.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Wilmut K, Wann JP, Brown JH. Problems in the coupling of eye and hand in the sequential movements of children with developmental coordination disorder. Child Care Hlth Dev. 2006;32:665–78. doi: 10.1111/j.1365-2214.2006.00678.x. [DOI] [PubMed] [Google Scholar]
- 31.Zoia S, Castiello U, Biason L, Scabar A. Reaching in children with and without developmental coordination disorder under normal and perturbed vision. Dev Neuropsychol. 2005;27:257–73. doi: 10.1207/s15326942dn2702_4. [DOI] [PubMed] [Google Scholar]
- 32.Feldman AG. Functional tuning of the nervous system with control of movement or maintenance of a steady posture. II Controllable parameters of the muscle. Biophysics. 1966;11:565–78. [Google Scholar]
- 33.Feldman AG. Once more on the equilibrium-point hypothesis (lambda model) for motor control. J Motor Behav. 1986;18:17–54. doi: 10.1080/00222895.1986.10735369. [DOI] [PubMed] [Google Scholar]
- 34.Feldman AG. Spatial frames of reference for motor control. In: Latash ML, editor. Progress in Motor Control: Bernstein’s Tradition in Movement Studies (V1) Champaign, IL: Human Kinetics; 1996. pp. 289–313. [Google Scholar]
- 35.Feldman AG, Adamovich SV, Ostry DJ, Flanagan JR. The origin of electromyograms: Explanation based on the equilibrium point hypothesis. In: Winters JM, Woo SLY, editors. Multiple muscle systems: Biomechanics and movement organization. New York: Springer-Verlag; 1990. pp. 195–213. [Google Scholar]
- 36.Hogan N. The mechanics of multi-joint posture and movement. Biol Cybern. 1985;52:315–31. doi: 10.1007/BF00355754. [DOI] [PubMed] [Google Scholar]
- 37.Hogan N. Mechanical impedance of single- and multi-articular systems. In: Winters JM, Woo SLY, editors. Multiple Muscle Systems: Biomechanics and Movement Organization. New York: Springer-Verlag; 1990. [Google Scholar]
- 38.Hogan N, Bizzi E, Mussa-Ivaldi S, Flash T. Controlling multijoint motor behavior. Exerc Sport Sci Rev. 1987;15:153–89. [PubMed] [Google Scholar]
- 39.Hogan N, Winters JM. Principles underlying movement organization: Upper limb. In: Winters JM, Woo SLY, editors. Multiple Muscle Systems: Biomechanics and Movement Organization. New York: Springer-Verlag; 1990. [Google Scholar]
- 40.Mussa-Ivaldi FA, Hogan N, Bizzi E. Neural and geometric factors subserving arm posture. J Neurosci. 1985;5:2732–43. doi: 10.1523/JNEUROSCI.05-10-02732.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot-aided neurorehabilitation. IEEE T Rehabil Eng. 1998;6:75–87. doi: 10.1109/86.662623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krebs HI, Volpe BT, Aisen ML, Hogan N. Increasing productivity and quality of care: robotic-aided neurorehabilitation. J Rehabil Res Dev. 2000;37:639–52. [PubMed] [Google Scholar]
- 43.Reinkensmeyer DJ, Kahn LE, Averbuch M, et al. Understanding and treating arm movement impairment after chronic brain injury: progress with the ARM Guide. J Rehabil Res Dev. 2000;37:653–62. [PubMed] [Google Scholar]
- 44.Burgar CG, Lum PS, Shor PC, Van der Loos HFM. Development of robots for rehabilitation therapy: The Palo Alto VA/Stanford experience. J Rehabil Res Dev. 2000;37:663–73. [PubMed] [Google Scholar]
- 45.Lum PS, Burgar CG, Shor PC. Evidence for improved muscle activation patterns after retraining of reaching movements with the MIME robotic system in subjects with post-stroke hemiparesis. IEEE T Neural Syst Rehabil Eng. 2004;12:186–94. doi: 10.1109/TNSRE.2004.827225. [DOI] [PubMed] [Google Scholar]
- 46.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: A systematic review. Neurorehab Neural Re. 2008;22:111–21. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ben-Pazi H, Ishihara A, Kukke S, Sanger TD. Increasing viscosity and inertia using a robotically controlled pen improves handwriting in children. J Child Neurol. 2010;25:674–80. doi: 10.1177/0883073809342592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Newell KM. Annual Review of Psychology. Englewood Cliffs, NJ: Prentice Hall; 1991. Motor skill acquisition. [DOI] [PubMed] [Google Scholar]
- 49.de Oliveira RF, Wann JP. Integration of dynamic information for visuomotor control in young adults with developmental coordination disorder. Exp Brain Res. 2010;205:387–94. doi: 10.1007/s00221-010-2373-5. [DOI] [PubMed] [Google Scholar]
- 50.Wilson BN, Crawford SG, Green D, et al. Psychometric Properties of the Revised Developmental Coordination Disorder Questionnaire. Phys Occup Ther Ped. 2009;29:182–202. doi: 10.1080/01942630902784761. [DOI] [PubMed] [Google Scholar]
- 51.Bingham GP. Task-specific devices and the perceptual bottleneck. Hum Movement Sci. 1988;7:225–64. [Google Scholar]