Abstract
Tuberous sclerosis complex is a genetic disorder resulting in epilepsy and mental retardation. Vigabatrin has shown efficacy in the treatment of infantile spasms caused by tuberous sclerosis complex, but its effects on focal seizures caused by tuberous sclerosis complex have not been determined. We compared the efficacy of vigabatrin in patients with tuberous sclerosis complex-induced focal seizures and infantile spasms and assessed the mental outcomes in both groups.
We retrospectively evaluated 31 children with tuberous sclerosis complex and epilepsy, who were treated with vigabatrin in single tertiary center in Seoul, Korea. Vigabatrin treatment resulted in spasms cessation in 16 of 18 (88.9%) patients with infantile spasms, whereas 6 of 13 (46.2%) patients with focal seizures became seizure-free. Initial response to vigabatrin had no effect on intellectual disability. Vigabatrin was highly effective in eliminating infantile spasms caused by tuberous sclerosis complex, but was less effective in patients with focal seizures.
Keywords: Tuberous sclerosis complex, vigabatrin, infantile spasms, focal seizures
Introduction
Tuberous sclerosis complex is an autosomal dominant genetic disorder that occurs in about 1 in 5,800 individuals.1 Of these, 95% show brain involvement, and 70–80% suffer from epilepsy, which is often refractory to treatment.2 Most patients with tuberous sclerosis complex experience their first seizures during the first year of life, some experience infantile spasms, a catastrophic epileptic syndrome characterized by age-specific seizures, hypsarrhythmia on electroencephalography and profound mental retardation. During the natural history of tuberous sclerosis complex with epilepsy, focal seizures sometimes precede, coexist with or evolve from infantile spasms,3 with the latter being a major factor associated with mental retardation in patients with tuberous sclerosis complex4.
Vigabatrin is a structural analog of gamma-aminobutyric acid that greatly increases whole-brain concentrations of gamma-aminobutyric acid 5 and has shown efficacy in the treatment of patients with infantile spasms or focal seizures.6,7 Although vigabatrin is particularly effective in the treatment of infantile spasms caused by tuberous sclerosis complex,8–11 its efficacy in patients with focal seizures caused by tuberous sclerosis complex has not been determined12,13. We therefore assessed and compared the efficacy of vigabatrin in patients with infantile spasms and focal seizures caused by tuberous sclerosis complex. We also assessed mental outcomes and sought to identify the clinical risk factors associated with intellectual disability in these groups of patients.
Methods
A retrospective review of the computerized database of the Pediatric Neurology Department of the Asan Medical Center, Seoul, Korea, identified 39 patients diagnosed with tuberous sclerosis complex 14 and epilepsy and treated with vigabatrin between 1991 and 2010. The diagnosis of infantile spasms was based on age-specific spasms, a sudden flexion, extension or mixed extension-flexion of predominantly proximal and truncal muscles that is usually occur in clusters (<12 months) and/or hypsarrhythmia on electroencephalography. Seizure types and the usage of anti-epileptic drugs were identified. To avoid selection bias and to exclude the impact of drug interactions, we selected the 31 patients who had received first-line vigabatrin monotherapy (23 patients) or with second-line vigabatrin add-on therapy (8 patients) initiated within the first month after seizure onset. Vigabatrin was started from a small dosage (50mg/kg/day) and titrated to a higher dosage (maximum 150–200mg/kg/day) according to the clinical responses. If seizure stops before the usual recommended dose of 100mg/kg/day, the lowest effective dosage was maintained to minimize the dose-responsive side effects of vigabatrin. Vigabatrin efficacy in patients with focal seizures was defined as no seizures for 1 year after initiation of treatment, whereas vigabatrin efficacy in patients with infantile spasms was defined as the cessation of spasms according to the guidelines of the West Delphi Group.15 Age at onset of seizures or spasms (in 1-month intervals), brain magnetic resonance imaging findings, genetic testing, intellectual status (intellectual quotient or developmental quotient), and seizure evolution were also reviewed.
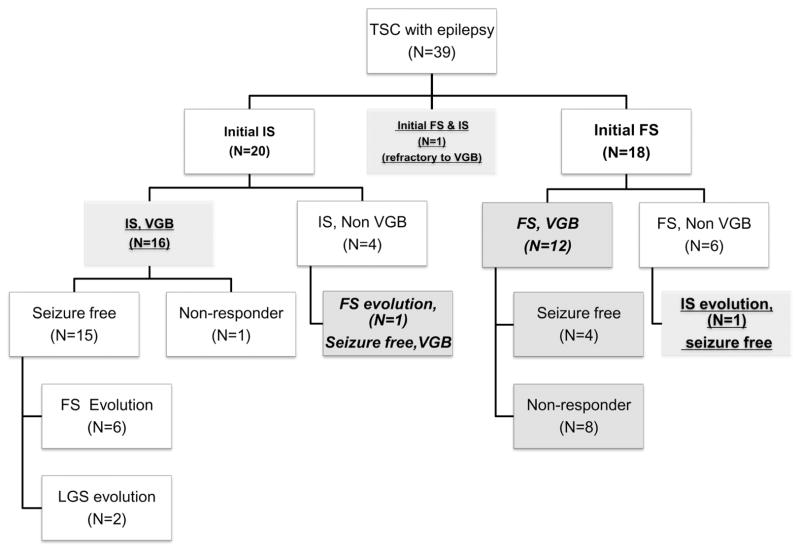
Of the 31 patients, 11 showed evolution of seizure types. To evaluate response to vigabatrin, patients were classified by seizure types at the start of vigabatrin treatment. Patients with concurrent infantile spasms and focal seizures were classified as infantile spasms. One patient developed infantile spasms after treatment of focal seizures with zonisamide and one patient developed focal seizures after adrenocorticotropic hormone-induced cessation of infantile spasms (Fig. 1).
Figure 1.
Study design. Of the 39 patients with tuberous sclerosis complex and epilepsy, 31 patients had been treated with vigabatrin as first- or second-line therapy within 1 month of seizure onset. Patients were divided into those with infantile spasms and focal seizures at initiation of vigabatrin, except for one patient with concurrent infantile spasms and focal seizures, who was regarded as having infantile spasms. Thus, 18 patients had infantile spasms (underlined) and 13 had focal seizures (italics). Of the 17 patients who initially presented with infantile spasms, 9 evolved to other types of epilepsy, whereas, of the 13 patients who initially presented with focal seizures, only one evolved to infantile spasms. Abbreviations: TSC, tuberous sclerosis complex; VGB, vigabatrin; IS, infantile spasms; focal seizures, focal seizures; LGS, Lennox-Gastaut syndrome; ACTH, adrenocorticotropic hormone; VPA, valproic acid
Intellectual outcomes were regarded as the final outcomes. Patients aged over 6 years underwent psychometric evaluation using the Korean Educational Development Institute-Wechsler Intelligence Scale for children, and patients aged less than 5 years underwent developmental evaluation using the Korean Infant and Children Developmental Tests. The developmental quotient of the Korean Infant and Children Developmental Test was defined as the mean developmental quotient of the five areas (gross motor, fine motor, social-personal, language, and cognitive-adaptive). The Korean Infant and Children Developmental Test was not administered to one patient with tuberous sclerosis complex, hemimegalencephaly and concurrent focal seizures and infantile spasms. Patients with an intellectual quotient or developmental quotient <70 were considered mentally retarded. In the three patients who underwent epilepsy surgery, we evaluated the intellectual quotient or developmental quotient before the surgery.
Demographic variables in the infantile spasms and focal seizures groups were compared using the chi-square test or Fisher’s exact test for categorical variables and the t-test for continuous variables. Multiple logistic regression analysis with backward elimination was used to determine clinical variables affecting response to vigabatrin.
Logistic regression analysis was also used to evaluate the risk factors for intellectual disability. Variables statistically significant (p<0.05) on univariate logistic regression analysis were tested in multiple logistic regression analysis with backward elimination.
Results
The clinical characteristics of the two groups of patients are shown in Table 1. The mean age of onset was earlier in the infantile spasms (5.4 months, range 1–12 months) than in the focal seizures (11 months, range 0–48 months) and the maximum dose of vigabatrin was higher in the infantile spasms (89 mg/kg/day) than in the focal seizures (81 mg/kg/day) group. After cessation of infantile spasms, seven patients developed focal seizures and two developed Lennox-Gastaut syndrome. Only one infantile spasms patient had focal seizures before the development of infantile spasms but none of the vigabatrin-treated patients with focal seizures developed infantile spasms (Fig 1). Vigabatrin treatment was significantly more effective in the infantile spasms than in the focal seizures group.
Table 1.
Clinical characteristics of patients with infantile spasms and focal seizures treated with vigabatrin.
| Infantile spasms (N=18) | Focal seizures (N=13) | P value | |
|---|---|---|---|
| Age of onset (months) | 5.4 (1–12) | 11 (0–48) | 0.046* |
| Sex (M : F) | 8 : 10 | 8 : 5 | 1.000† |
| vigabatrin as first vs second choice | 11 : 7 | 9 : 4 | 0.011† |
| Genetic testing | tuberous sclerosis complex 1: 1, tuberous sclerosis complex 2: 5, No mutation identified: 1 | tuberous sclerosis complex 2: 1 | - |
| Max. Dose (mg/kg/day) | 89 (46–145) | 81 (45–125) | 0.362* |
| Response rate** | 16/ 18 | 6/13 | 0.029† |
| Presence vs absence of intellectual disability | 13 : 5 | 5 : 8 | 0.585† |
Student’s t-test
Chi-square test
“Response” was defined as the cessation of clinical spasms in patients with infantile spasms and being seizure-free for 1 year in patients with focal seizures
For the 23 patients with vigabatrin as a first-line monotherapy, 13 (92.9%) of the 14 patients with infantile spasms became seizure free, whereas 3 (33.3%) of 9 patients with focal seizures became seizure free. Taking together with first-line monotherapy and second add-on therapy with vigabatrin, 16 (88.9%) of the 18 infantile spasms patients became seizure-free, compared with 6 of 13 (46.2%) patients with focal seizures (odds ratio, 9.3; 95% confidence interval, 1.5–58.2; p=0.017). The adjusted odds ratio for infantile spasms was 17.6 (p=0.012) by multiple logistic regression analysis with backward elimination (Table 2). We also performed a case-control analysis for intellectual disability, using the risk factors, sex, magnetic resonance imaging abnormality, history of infantile spasms, early seizure onset (<6 months), response to vigabatrin, and evolution of seizure types. The crude odds ratios and their 95% confidence intervals are shown in Table 3. Four factors were significantly associated with intellectual disability: male sex (odds ratio, 0.2; p=0.029), history of infantile spasms (odds ratio, 5.2, p=0.041), early seizure onset (<6 month) (odds ratio, 6.2, p=0.045), and evolution of seizure types (odds ratio, 13.5; p=0.023). Multiple logistic regression analysis with backward elimination showed that the adjusted odds ratios for early seizure onset (<6 month) and evolution were 9.1 (95% confidence interval 1.2–69.3; p=0.033) and 19.0 (95% confidence interval 1.7–214.9; p=0.017), respectively (Table 3).
Table 2.
Associations between clinical variables and response to vigabatrin
| Association with the response to vigabatrin | ||||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p | Adjusted | p | |
| OR | 95% CI | |||||
| Early onset (<6 months) | 0.3 | 0.1–1.8 | 0.225 | |||
| Max. dosage | 1.0 | 0.9–1.0 | 0.296 | 1.0 | 0.9–1.0 | 0.093 |
| Duration | 1.0 | 0.9–1.0 | 0.175 | |||
| Presence of infantile spasms | 9.3 | 1.5–58.2 | 0.017 | 17.6 | 1.6–164.8 | 0.012 |
Table 3.
Associations between clinical variables and intellectual outcomes.
| Association with the intellectual outcome | ||||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | P | Adjusted | P | |
| OR | 95%CI | |||||
| Male | 0.2 | 0.0–0.8 | 0.029 | |||
| MRI | 1.3 | 0.1–23.5 | 0.844 | |||
| Response to vigabatrin | 0.72 | 0.1–3.8 | 0.698 | |||
| Early onset | 6.2 | 1.0–36.8 | 0.045 | 9.1 | 1.2–69.3 | 0.033 |
| History of infantile spasms | 5.2 | 1.1–25.3 | 0.041 | |||
| Evolution | 13.5 | 1.4–128.3 | 0.023 | 19 | 1.7–214.9 | 0.017 |
Abbreviations: MRI, magnetic resonance imaging
Of the 12 patients with focal seizures only, 4 (33%) had intellectual disability during follow-up. Of the 10 patients with seizure evolution, 9 (90%) had intellectual disability.
Discussion
Vigabatrin has been consistently shown effective in the treatment of focal seizures and infantile spasms,12,16 as well as more effective than adrenocorticotropic hormone in the treatment of infantile spasms caused by tuberous sclerosis complex.17,18 We also found that vigabatrin was highly effective for patients with infantile spasms caused by tuberous sclerosis, with 16 of 18 patients becoming seizure-free after treatment. Vigabatrin, however, was less effective in patients with focal seizures caused by tuberous sclerosis complex, with only 6 of 13 (46.2%) becoming seizure free, making the adjusted odds ratio for infantile spasms relative to focal seizures 17.6. Previously, Chu-Shore et al.19 reported that most patients with epilepsy and tuberous sclerosis developed multiple seizure types with typical onset in the first year of life. Although most patients with infantile spasms later evolved to other seizure types, either focal seizures or Lennox-Gastaut syndrome, no patient with focal seizures developed infantile spasms during vigabatrin treatment irrespective of their response to vigabatrin. This suggests the specific efficacy of the vigabatrin for infantile spasms caused by tuberous sclerosis.
At present, the mechanisms of development of epilepsy in tuberous sclerosis complex patients and the pathophysiologic mechanisms of infantile spasms are still unknown. Tubers are widely believed to serve as epileptogenic foci, and tuber resection is often performed to treat intractable epilepsy in patients with tuberous sclerosis complex.20,21 Nevertheless, the relative importance of abnormal glioneuronal development of the brain, tubers and normal neighboring neurons irritated by dysgenetic areas of the brain has not yet been determined.22 The mechanisms by which infantile spasms and focal seizures develop may differ, resulting in different response to vigabatrin.
Vigabatrin is a selective and irreversible inhibitor of gamma-aminobutyric acid-transaminase that greatly increases whole-brain concentrations of gamma-aminobutyric acid.5 Vigabatrin has been shown to increase gamma-aminobutyric acid concentrations in human cerebrospinal fluid,23 and vigabatrin-induced increases in gamma-aminobutyric acid concentrations in the brain have been found to correlate with seizure control.24 During the development of infantile spasms, dysfunctional gamma-aminobutyric acid may be involved in generalization of the epileptogenic activity of tuberous sclerosis complex, and vigabatrin may act during this step to ameliorate the infantile spasms. Namely, the vigabatrin-induced increase in gamma-aminobutyric acid concentration may attenuate the development of infantile spasms by disturbing the generalization of epileptiform activity due to its dysfunction in cortical-subcortical circuits,25 but not the focal seizures caused by the region itself. Peri-lesional and lesional molecular analyses in patients with tuberous sclerosis complex have shown decreased expression of gamma-aminobutyric acidA receptors and gamma-aminobutyric acidergic neurons at the circuit level,26–28 a finding that may explain, at least in part, why vigabatrin was less effective in focal seizures than in infantile spasms associated with tuberous sclerosis complex.
There was only one case with initial focal seizures followed by infantile spasms in present study and none of the vigabatrin-treated patients with focal seizures developed infantile spasms irrespective of their response to vigabatrin. It is plausible that some subtle focal seizures preceding infantile spasms can be underdetected and vigabatrin could be effective in stopping focal seizures at the very early onset and preventing the evolution toward an epileptic encephalopathy. If so, early intervention with vigabatrin in infants is recommended in patients with tuberous sclerosis complex to prevent this evolution of epileptic encephalopathy.
In agreement with previous results, we found that evolution of seizure types and early seizure onset were the main risk factors for intellectual disability.19,29 We also found that response to vigabatrin did not affect the mental outcomes of our patients with tuberous sclerosis complex and epilepsy, suggesting that vigabatrin does not affect the natural history of epilepsy in tuberous sclerosis complex patients. However, of the seven patients with infantile spasms who responded to vigabatrin and did not experience further seizures, four (57%) had normal intellectual quotient or developmental quotient, in agreement with results showing that early control of seizures improved long-term outcomes in children with tuberous sclerosis.4,13,30 Additional studies with longer term follow-up are required to assess the ability of vigabatrin treatment to induce the cessation of infantile spasms in patients with tuberous sclerosis complex.
It is currently unclear whether cognitive deficits in tuberous sclerosis complex patients arise from the effects of early seizures or by a distinct mechanism, such as an association with infantile spasms.29 We found that the risks and degree of intellectual impairment were associated with the evolution of seizures and early seizure onset, indicating that the seizures themselves have some role in the severity of subsequent neurologic deficits.31,32 Seizure evolution or early onset may reflect the strong epileptogenic tendency, consistent with neuronal disorganization and hyperexcitability, and may in turn, cause severe intellectual disability.
There are several limitations in this study. The retrospective nature of this study and relatively small number of patients are major weaknesses. And we could not perform the prolonged ambulatory electroencephalography recordings for the patients with infantile spasms. Subclinical focal seizures proved to be very frequent in tuberous sclerosis patients with infantile spasms before, so that pure clinical approach may be not enough to define the patients as purely infantile spasms. 33 Thus, the patients classified as infantile spasms in this study might have simultaneous focal seizures at the time of vigabatrin trial and we considered the patients with concurrent infantile spasms and focal seizures as infantile spasms group.
But this is the first Korean study in this field and we have shown that vigabatrin has rather specific effects in patients with infantile spasms, but not focal seizures, caused by tuberous sclerosis complex as shown previously. 6,34 This distinct efficacy of vigabatrin, coupled with the possibility of vigabatrin-induced visual field defects suggest that vigabatrin can be carefully used as a first choice for treatment of infantile spasms but not for focal seizures in patients with tuberous sclerosis complex. Additionally, initial response to vigabatrin was not associated with the mental outcome or epilepsy outcome of tuberous sclerosis complex patients in this study, suggesting the need for further research of epilepsy and intellectual outcome in tuberous sclerosis complex and more discreet use of vigabatrin in tuberous sclerosis complex patients.
Acknowledgments
The authors would like to thank Seon-Ok Kim for the statistical analysis. No benefits in any form have been received from a commercial party related to this manuscript. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Financial Disclosure/Funding
We have nothing to declare about financial support related to this study.
This study was presented in American Epilepsy Society annual meeting 2010.
Author contribution
Mi-Sun Yum, MD, PhD; First author, who proposed the hypothesis, performed data analysis and wrote this whole manuscript
Eun Hye Lee, MD; Second author who contributed to data collection and correction of the manuscript
Tae-Sung Ko, MD, PhD: Corresponding author who directed this study, gave guidance of the paperwork, and corrected this manuscript
Conflict of Interest
None of the authors has any conflict of interest to disclose.
Ethical Approval
We confirm that this retrospective study was approved by the Asan Medical Center institutional review board.
References
- 1.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 2.Thiele EA. Managing and understanding epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51 (Suppl 1):90–91. doi: 10.1111/j.1528-1167.2009.02458.x. [DOI] [PubMed] [Google Scholar]
- 3.Curatolo P, Bombardieri R, Jozwiak S. Tuberous sclerosis. Lancet. 2008;372:657–668. doi: 10.1016/S0140-6736(08)61279-9. [DOI] [PubMed] [Google Scholar]
- 4.Jambaque I, Chiron C, Dumas C, et al. Mental and behavioural outcome of infantile epilepsy treated by vigabatrin in tuberous sclerosis patients. Epilepsy Res. 2000;38:151–160. doi: 10.1016/s0920-1211(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 5.Angehagen M, Ben-Menachem E, Ronnback L, et al. Novel mechanisms of action of three antiepileptic drugs, vigabatrin, tiagabine, and topiramate. Neurochem Res. 2003;28:333–340. doi: 10.1023/a:1022393604014. [DOI] [PubMed] [Google Scholar]
- 6.Elterman RD, Shields WD, Mansfield KA, et al. Randomized trial of vigabatrin in patients with infantile spasms. Neurology. 2001;57:1416–1421. doi: 10.1212/wnl.57.8.1416. [DOI] [PubMed] [Google Scholar]
- 7.Vigevano F, Cilio MR. Vigabatrin versus ACTH as first-line treatment for infantile spasms: a randomized, prospective study. Epilepsia. 1997;38:1270–1274. doi: 10.1111/j.1528-1157.1997.tb00063.x. [DOI] [PubMed] [Google Scholar]
- 8.Hancock E, Osborne JP. Vigabatrin in the treatment of infantile spasms in tuberous sclerosis: literature review. J Child Neurol. 1999;14:71–74. doi: 10.1177/088307389901400201. [DOI] [PubMed] [Google Scholar]
- 9.Parisi P, Bombardieri R, Curatolo P. Current role of vigabatrin in infantile spasms. Eur J Paediatr Neurol. 2007;11:331–336. doi: 10.1016/j.ejpn.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Curatolo P, Seri S, Verdecchia M, et al. Infantile spasms in tuberous sclerosis complex. Brain Dev. 2001;23:502–507. doi: 10.1016/s0387-7604(01)00300-x. [DOI] [PubMed] [Google Scholar]
- 11.Chiron C, Dumas C, Jambaque I, et al. Randomized trial comparing vigabatrin and hydrocortisone in infantile spasms due to tuberous sclerosis. Epilepsy Res. 1997;26:389–395. doi: 10.1016/s0920-1211(96)01006-6. [DOI] [PubMed] [Google Scholar]
- 12.Nabbout RC, Chiron C, Mumford J, et al. Vigabatrin in partial seizures in children. J Child Neurol. 1997;12:172–177. doi: 10.1177/088307389701200304. [DOI] [PubMed] [Google Scholar]
- 13.Cusmai R, Moavero R, Bombardieri R, et al. Long-term neurological outcome in children with early-onset epilepsy associated with tuberous sclerosis. Epilepsy Behav. 2011;22:735–739. doi: 10.1016/j.yebeh.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Roach ES, Smith M, Huttenlocher P, et al. Diagnostic criteria: tuberous sclerosis complex. Report of the Diagnostic Criteria Committee of the National Tuberous Sclerosis Association. J Child Neurol. 1992;7:221–224. doi: 10.1177/088307389200700219. [DOI] [PubMed] [Google Scholar]
- 15.Lux AL, Osborne JP. A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: consensus statement of the West Delphi group. Epilepsia. 2004;45:1416–1428. doi: 10.1111/j.0013-9580.2004.02404.x. [DOI] [PubMed] [Google Scholar]
- 16.Willmore LJ, Abelson MB, Ben-Menachem E, et al. Vigabatrin: 2008 update. Epilepsia. 2009;50:163–173. doi: 10.1111/j.1528-1167.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- 17.Riikonen RS. Favourable prognostic factors with infantile spasms. Eur J Paediatr Neurol. 2010;14:13–18. doi: 10.1016/j.ejpn.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Hancock E, Osborne J, Milner P. Treatment of infantile spasms. Cochrane Database Syst Rev. 2003:CD001770. doi: 10.1002/14651858.CD001770. [DOI] [PubMed] [Google Scholar]
- 19.Chu-Shore CJ, Major P, Camposano S, et al. The natural history of epilepsy in tuberous sclerosis complex. Epilepsia. 2010;51:1236–1241. doi: 10.1111/j.1528-1167.2009.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh S, Jayakar P, Dunoyer C, et al. Epilepsy surgery in children with tuberous sclerosis complex: presurgical evaluation and outcome. Epilepsia. 2000;41:1206–1213. doi: 10.1111/j.1528-1157.2000.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 21.Weiner HL. Tuberous sclerosis and multiple tubers: localizing the epileptogenic zone. Epilepsia. 2004;45 (Suppl 4):41–42. doi: 10.1111/j.0013-9580.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- 22.Chu-Shore CJ, Major P, Montenegro M, et al. Cyst-like tubers are associated with TSC2 and epilepsy in tuberous sclerosis complex. Neurology. 2009;72:1165–1169. doi: 10.1212/01.wnl.0000345365.92821.86. [DOI] [PubMed] [Google Scholar]
- 23.Grove J, Schechter PJ, Tell G, et al. Increased gamma-aminobutyric acid (GABA), homocarnosine and beta-alanine in cerebrospinal fluid of patients treated with gamma-vinyl GABA (4-amino-hex–5-enoic acid) Life Sci. 1981;28:2431–2439. doi: 10.1016/0024-3205(81)90511-7. [DOI] [PubMed] [Google Scholar]
- 24.Petroff OA, Behar KL, Mattson RH, et al. Human brain gamma-aminobutyric acid levels and seizure control following initiation of vigabatrin therapy. J Neurochem. 1996;67:2399–2404. doi: 10.1046/j.1471-4159.1996.67062399.x. [DOI] [PubMed] [Google Scholar]
- 25.Chugani HT, Shewmon DA, Sankar R, et al. Infantile spasms: II. Lenticular nuclei and brain stem activation on positron emission tomography. Ann Neurol. 1992;31:212–219. doi: 10.1002/ana.410310212. [DOI] [PubMed] [Google Scholar]
- 26.Yeung RS. Tuberous sclerosis as an underlying basis for infantile spasm. Int Rev Neurobiol. 2002;49:315–332. doi: 10.1016/s0074-7742(02)49019-8. [DOI] [PubMed] [Google Scholar]
- 27.Crino PB. Gene expression analysis as a strategy to understand the molecular pathogenesis of infantile spasms. Int Rev Neurobiol. 2002;49:367–389. doi: 10.1016/s0074-7742(02)49022-8. [DOI] [PubMed] [Google Scholar]
- 28.White R, Hua Y, Scheithauer B, et al. Selective alterations in glutamate and GABA receptor subunit mRNA expression in dysplastic neurons and giant cells of cortical tubers. Ann Neurol. 2001;49:67–78. doi: 10.1002/1531-8249(200101)49:1<67::aid-ana10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 29.Goh S, Kwiatkowski DJ, Dorer DJ, et al. Infantile spasms and intellectual outcomes in children with tuberous sclerosis complex. Neurology. 2005;65:235–238. doi: 10.1212/01.wnl.0000168908.78118.99. [DOI] [PubMed] [Google Scholar]
- 30.Bombardieri R, Pinci M, Moavero R, et al. Early control of seizures improves long-term outcome in children with tuberous sclerosis complex. Eur J Paediatr Neurol. 2010;14:146–149. doi: 10.1016/j.ejpn.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Joinson C, O’Callaghan FJ, Osborne JP, et al. Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychol Med. 2003;33:335–344. doi: 10.1017/s0033291702007092. [DOI] [PubMed] [Google Scholar]
- 32.Jansen FE, Vincken KL, Algra A, et al. Cognitive impairment in tuberous sclerosis complex is a multifactorial condition. Neurology. 2008;70:916–923. doi: 10.1212/01.wnl.0000280579.04974.c0. [DOI] [PubMed] [Google Scholar]
- 33.Plouin P, Dulac O, Jalin C, et al. Twenty-four-hour ambulatory EEG monitoring in infantile spasms. Epilepsia. 1993;34:686–691. doi: 10.1111/j.1528-1157.1993.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 34.Greiner HM, Lynch ER, Fordyce S, et al. Vigabatrin for childhood partial-onset epilepsies. Pediatr Neurol. 2012;46:83–88. doi: 10.1016/j.pediatrneurol.2011.11.020. [DOI] [PubMed] [Google Scholar]