Summary
Fatty acids (FA) and FA-derived metabolites have long been implicated in the development of insulin resistance and type 2 diabetes. Surprisingly, application of metabolomics technologies has revealed that branched-chain amino acids (BCAA) and related metabolites are more strongly associated with insulin resistance than many common lipid species. Moreover, the BCAA-related signature is predictive of incident diabetes and intervention outcomes, and uniquely responsive to therapeutic interventions. Nevertheless, in animal feeding studies, BCAA supplementation requires the background of a high-fat diet to promote insulin resistance. This article develops a model to explain how lipids and BCAA may synergize to promote metabolic diseases.
Introduction
The pandemic of obesity that burdens the world population is well documented. In the United States, more than 65% of adults are characterized as overweight or obese (Flegal, et al, 2002). This epidemic of obesity is linked to increasing incidence of chronic diseases such as type 2 diabetes, cardiovascular disease and cancer. A common perception is that this constellation of maladies is driven principally by increased consumption of fat in the diet, and accordingly, other articles in this compendium are focused on the impact of excess lipids on various cellular functions, including mitochondrial metabolism, endoplasmic stress responses, inflammation, and generation of reactive oxygen species. However, numerous recent reports have described a particularly strong association of branched-chain and aromatic amino acids with metabolic disease. Therefore, this article reviews these recent findings, and then focuses on the potential role of these metabolites in disease pathogenesis, including a discussion of interactions between amino acids and lipids in development of metabolic disorders.
A strong relationship between glucose and lipid metabolism has been recognized for decades, with key features of this regulatory dynamic unveiled by the pioneering studies of Sir Phillip Randle and J. Denis McGarry (Randle, 1998; McGarry, 2002). Randle showed that the surge in lipolysis and resultant increase in circulating free fatty acids in the fasted state contributes to increased reliance of tissues on fatty acid oxidation to provide energy. Glucose oxidation is reduced under these conditions in part via the generation of byproducts of fatty acid oxidation that suppress key steps of glucose metabolism. Conversely, McGarry demonstrated that increased availability of glucose and insulin in the fed state leads to production of malonyl CoA, a potent allosteric inhibitor of fatty acid oxidation. Overnutrition results in perturbation of these elegant reciprocal control mechanisms, leading to a condition termed “metabolic inflexibility” (Kelley and Mandarino, 2000). A subject debated in other articles in this compendium is whether the metabolic inflexibility and insulin resistance of obese states is due to intrinsic mitochondrial deficiency and consequent accumulation of bioactive lipid species that interfere with insulin signaling (Savage, et al., 2007), or is due instead to “overloading” of normally active mitochondria with lipid substrates, leading to mitochondrial dysfunction and activation of alternative pathways for impairment of insulin action (Koves, et al., 2008). The latter model seems particularly compatible with the emergent role of certain amino acids in development of insulin resistance and type 2 diabetes, and will therefore be a focus here.
Comprehensive metabolic profiling, also known as “metabolomics”, has recently provided unique insights into mechanisms underlying development of insulin resistance (Bain, et al., 2009). For example, metabolic profiling of muscle samples from normal rats fed on a high fat compared to a standard chow diet, or from obese and insulin resistant Zucker diabetic fatty (fa/fa) compared to lean Zucker rats, reveals accumulation of a broad array of acylcarnitine species, which report on the pool of mitochondrial acyl CoA metabolites and the β-oxidative pathway (Koves, et al., 2008; Koves, et al., 2005). Several studies demonstrate that feeding of a high fat (HF) diet to humans or rodents, or culture of muscle cells in the presence of high levels of fatty acids cause clear induction of genes of β-oxidation, but with either no effect or a decrease in expression of enzymes involved in the tricarboxylic acid (TCA) cycle or oxidative phosphorylation (Koves, et al., 2005; Sparks, et al., 2005). Fatty acid culture or feeding of a high fat (HF) diet also causes a decrease in expression of peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) and an increase in acylcarnitine levels in muscle cells. Two weeks of exercise intervention in HF-fed mice results in increased PGC-1α expression and lowering of acylcarnitines in muscle, and normalization of insulin sensitivity (Koves, et al., 2005). Finally, overexpression of PGC-1α in muscle cells also causes increased expression of TCA cycle enzymes. Taken together, these findings have led Muoio and associates to suggest that HF feeding leads to the appropriate adaptive response of an increase in enzymes of fatty acid disposal (oxidation) in muscle, a response that is not matched in sedentary animals or humans by a parallel increase in enzymes of the TCA cycle. This disconnect results in accumulation of incompletely oxidized lipid species in mitochondria (represented by the acylcarnitines) and a decrease in multiple TCA cycle intermediates. The model further holds that accumulation of incompletely oxidized substrates causes mitochondrial stress, leading to impaired insulin action (Koves, et al., 2005; Koves, et al., 2008; Muoio and Newgard, 2008).
Emergence of novel associations of branched-chain amino acids with the incidence, progression, and therapy of human metabolic diseases
Metabolomics has also been applied to human disease studies. Using targeted gas chromatography/mass spectrometry and tandem mass spectrometry coupled with biochemical methods, > 100 analytes were measured in plasma samples from obese (BMI 37) and insulin-resistant versus lean (BMI 23) and insulin-sensitive subjects, and data were analyzed by principal components analysis (Newgard, et al., 2009). Five principal components were found to describe most of the variance in the data, including several lipid-related components (e.g. long chain fatty acids and ketone metabolites, medium-chain acylcarnitines), but surprisingly, the component most strongly associated with insulin sensitivity (HOMA score) was not lipid-related, but rather comprised of the branched-chain amino acids (BCAA; Val, Leu/Ile), the aromatic amino acids (Phe, Tyr), C3 and C5 acylcarnitines, as well as Glx (mostly Glu with some Gln) and Ala. The preferential association of this BCAA-related metabolite cluster with insulin resistance was confirmed in a cross-sectional study of sedentary, metabolic syndrome subjects, using the frequently sampled glucose tolerance test to measure insulin sensitivity (Huffman, et al., 2009), and in cohorts of Chinese and Asian-Indian men in Singapore in which BMI was matched at around 24 (Tai, et al., 2010). These studies demonstrate the strong and preferential association of the BCAA-related metabolite cluster and insulin resistance in studies of different design (case-control or cross-sectional), and across multiple ethnic groups and geographical locales. A subsequent study also demonstrated a strong association of a similar BCAA-related metabolite cluster with coronary artery disease in both a reference and validation cohort, with this association persisting even after correction for type 2 diabetes and other clinical variables (Shah, et al., 2010).
In addition to the strong correlation of BCAA and related metabolites with metabolic disease, recent studies have demonstrated that these analytes can be predictive of disease progression and intervention outcomes. For example, we recently studied 500 obese subjects from the weight loss maintenance (WLM) trial (Svetkey, et al., 2008), in which blood samples were taken at baseline, followed by a six-month behavioral/dietary (DASH diet) intervention. The amount of weight lost over the six months of intervention was very poorly correlated with improvement in homeostatic model assessment (HOMA) score. In contrast, targeted metabolic profiling of plasma samples collected at baseline revealed that the BCAA-related principal component factor score was a strong predictor of improvement in insulin sensitivity with intervention, whereas lipid-related factors had no predictive association (Shah, et al., 2011). Metabolomics has also been applied to baseline plasma samples from 189 subjects in the Framingham longitudinal cohort that developed type 2 diabetes over as much as 12 years of follow-up, compared to 189 control subjects that did not develop diabetes despite being matched for weight, lipid profile and other clinical variables (Wang, et al., 2011). The five metabolites with the strongest association with incident diabetes were Leu, Ile, Val, Phe, and Tyr, remarkably similar to the composition of the metabolite principal component found to associate with metabolic diseases and conditions in our studies. The ability of these metabolites to predict incident diabetes was confirmed in a second group of subjects available from the Malmo cohort (Wang, et al., 2011).
Finally, there is evidence that changes in BCAA levels may correlate with the efficacy of interventions for affecting improvement in metabolic control. Thus, our group has shown that obese subjects undergoing gastric bypass (GBP) surgery have a much more dramatic decline in circulating BCAA, C3 and C5 acylcarnitines, Phe, and Tyr than found in response to dietary intervention, despite equal weight loss in the two study groups (Laferrère, et al., 2011). This is significant because GBP causes greater improvement in glucose homeostasis than dietary intervention (Laferrère, et al., 2011; Clifton, 2010). Moreover, the efficacy of a set of thiazolidinedione (TZD) drugs for controlling glucose homeostasis in Zucker-obese rats has been correlated with their unique ability to enhance expression of the BCAA catabolic pathway in adipose tissue (Hsiao, et al., 2011), as discussed further below.
It is important to note that associations of BCAA and other amino acids with insulin resistance and type 2 diabetes have been noted over more than 30 years by Cahill, Felig, Marliss, and others (Felig, et al., 1969; Felig, et al., 1974; Gougeon, et al., 2008). However, by taking a more broad-based metabolomics approach, the newer findings provide several insights that move the field beyond these important historical observations. First, principal components analysis demonstrates that BCAA and aromatic amino acids form an independent clustered variable that also includes byproducts of BCAA catabolism such as Glu, Ala, and C3 and C5 acylcarnitines, suggesting that the meaningful association is not with BCAA per se but rather with altered flux through the BCAA catabolic pathway. Second, the global analysis conducted by several groups demonstrates a stronger association of BCAA and related metabolites with insulin resistance and type 2 diabetes than with other metabolite clusters, including lipid-related clusters. Third, the newer studies demonstrate the potential prognostic power of this group of analytes. Taken together, the combined historical and newer studies show that BCAA and related metabolites are associated with insulin resistance, diabetes, and CAD, predictive of diabetes development, predictive of intervention outcomes, and highly and uniquely responsive to therapeutic interventions.
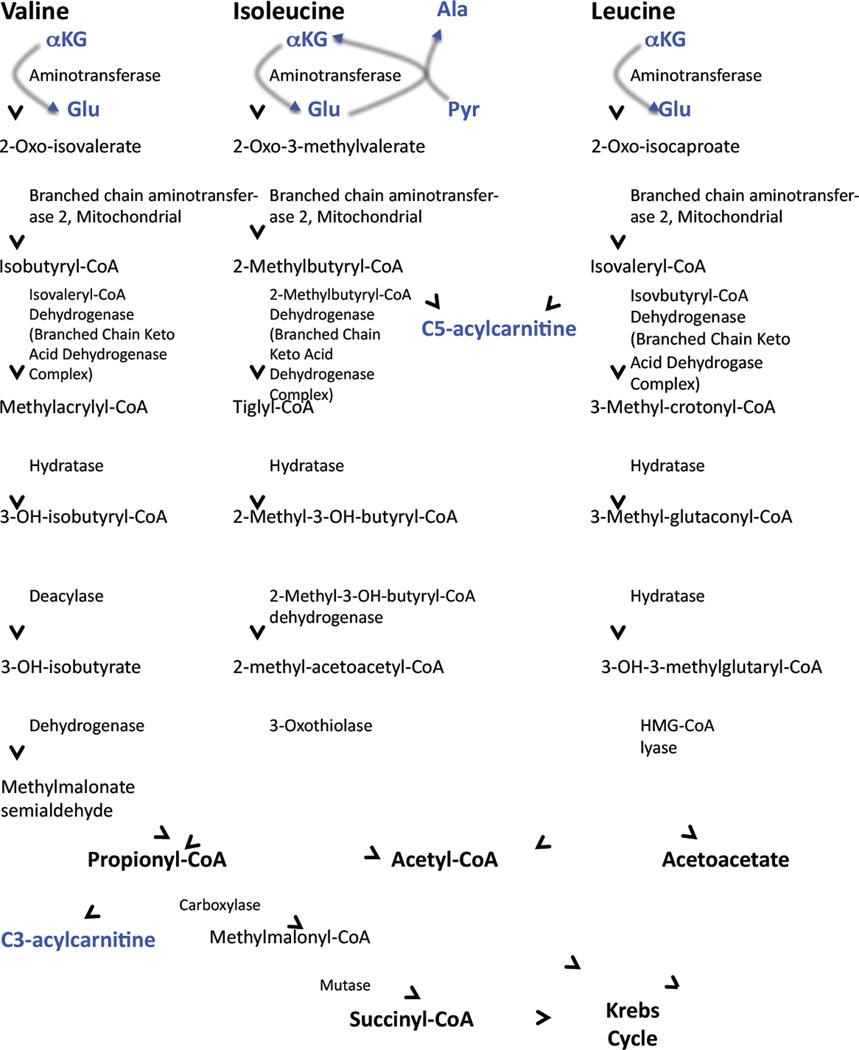
The metabolites embodied in the BCAA-related principal components that so consistently associate with insulin resistance and metabolic disease have a biochemical as well as statistical connection (Figure 1). Thus, glutamate is produced in the first step of BCAA catabolism, transamination by the mitochondrial form of branched-chain amino acid transaminase (BCATm). C5 acylcarnitines are comprised of α-methylbutyryl and isovalerylcarnitine species, intermediates in mitochondrial Ile and Leu catabolism, respectively. C3-acylcarnitine reflects the propionyl CoA pool, which is a byproduct of both Ile and Val catabolism. BCAA are not the only metabolic fuels that can generate C3 and C5 acylcarnitines (for example, methionine is also degraded to propionyl CoA), but a direct link of BCAA to C3 and C5 acylcarnitines is demonstrated by a rise in circulating and tissue levels of these metabolites in response to BCAA supplementation (Newgard, et al., 2009). Accumulation of Glu may increase transamination of pyruvate to Ala. Finally, the elevation in the aromatic amino acids Phe and Tyr may be explained by the fact that Trp, Phe, Tyr, Leu, Ile, Val compete for transport into mammalian cells by the large neutral amino acid transporter (LAT1) (Fernstrom, 2005).
Figure 1. Pathways of branched-chain amino acid catabolism.
Shown in blue are the reactions that produce metabolites found in the BCAA-related principal component that associates with insulin resistance and other metabolic diseases.
Potential cause-effect relationships between BCAA and metabolic diseases
Recent studies demonstrating strong associations of BCAA and related metabolites with disease, disease progression, and intervention outcomes suggest a possible cause/effect relationship between these metabolites and disease development. Moreover, the large body of extant literature implicating fatty acids and other lipids in development of tissue dysfunction and metabolic disease raises the possibility that these abnormalities might be driven by combined effects of lipids and BCAA. To begin to test these ideas, we fed Wistar rats on HF, HF + BCAA, or standard chow (SC) diets (Newgard, et al., 2009). Interestingly, animals fed on the HF + BCAA diet consumed less food than the HF group, consistent with reports of reduced food intake in response to intracerebroventricular injection of Leu in mice (Cota, et al., 2006). Despite a lower rate of food intake and body weight gain equivalent to the SC group, HF + BCAA rats were equally insulin resistant as HF rats, as demonstrated by glucose and insulin tolerance tests, and by impaired insulin signaling in liver and muscle (Newgard, et al., 2009). Pair-feeding of HF diet to match the food intake of HF + BCAA animals, or feeding of SC + BCAA did not cause insulin resistance. Insulin resistance induced by HF + BCAA was accompanied by chronic activation of mammalian target of rapamycin (mTOR), P70-S6 kinase 1 (S6K-1), c-Jun N-terminal kinase (JNK), and phosphorylation of insulin receptor substrate-1 (IRS1(ser307)), and was reversed by the mTOR inhibitor rapamycin. These data suggest an interaction between excess fat and BCAA in development of insulin resistance, and demonstrate a contribution of BCAA to impaired glucose homeostasis that can occur independent of body weight. Although our studies with rapamycin suggest a role for mTOR and its downstream targets in BCAA-mediated insulin resistance, full consideration of the extant literature presents a more confusing picture (reviewed in Um, et al., 2006; Avruch, et al., 2009). For example, one study shows that depletion of leucine in the diet results in reduced mTOR activity in concert with an improvement in hepatic insulin sensitivity (Xiao, et al., 2011), whereas another shows that leucine supplementation activates mTOR without impairing insulin action (Macotela, et al., 2011). Given the need for further investigation in this area, the remainder of this article focuses on a metabolic model for explaining the interaction of fatty acids and BCAA in promoting insulin resistance.
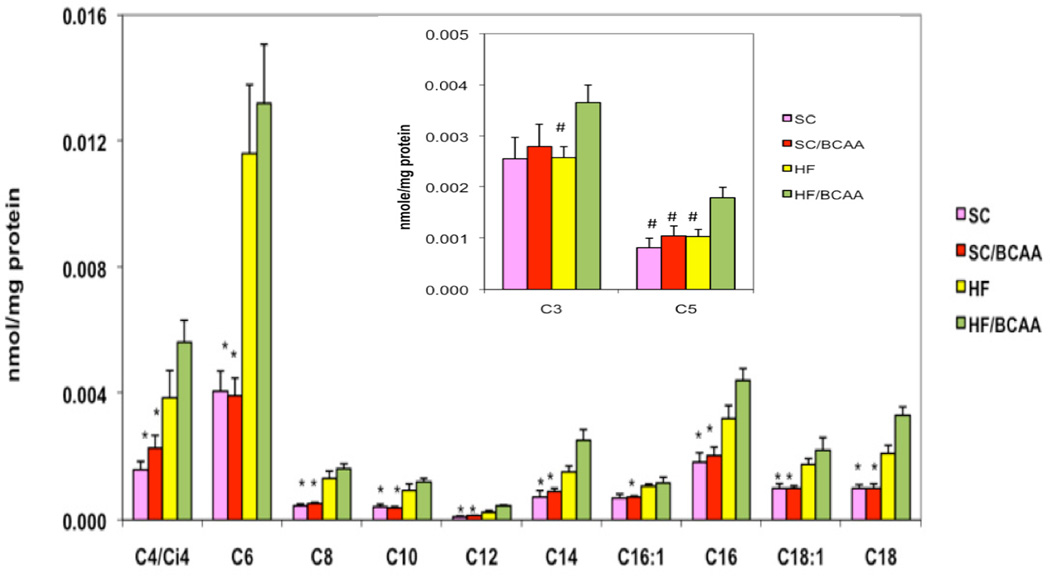
Despite the lower rate of food intake of HF + BCAA-fed animals, they accumulate acylcarnitine species in muscle to the same extent as HF-fed rats (Figure 2). As described earlier, accumulation of acylcarnitines has been interpreted by Muoio and associates as an index of incomplete fatty acid oxidation in mitochondria (Koves, et al., 2005; Koves, et al., 2008; Muoio and Newgard, 2008). We suggest that when BCAA accumulate in plasma as a consequence of dietary supplementation or via other potential mechanisms as discussed below, flux of these amino acids into skeletal muscle and through the BCAA catabolic pathway is increased, as also implied by the increase in C3 and C5 acylcarnitines in blood of obese and insulin resistant subjects. Metabolism of BCAA and/or their cognate α-ketoacids may also increase in liver. Increased BCAA catabolism in muscle and liver would result in increased production of propionyl CoA and succinyl CoA. The mechanism by which accumulation of these intermediates could contribute to incomplete oxidation of fatty acids (as evidenced by accumulation of long, even-chained acylcarnitines in HF + BCAA-fed animals (Figure 2)) remains to be defined. One possibility is that in the background of a high-fat diet, these substrates act to “fill” the TCA cycle (a process known as anaplerosis), contributing to the accumulation of incompletely oxidized intermediates of fatty acid and BCAA oxidation. Under these conditions, glucose is rendered superfluous as an energy substrate, resulting in decreased glucose utilization and glucose intolerance. It should be noted that such a model is not consistent with findings of reduced levels of TCA cycle intermediates in muscle of ZDF compared to lean rats (Koves, et al., 2008) as described earlier, but direct measures of TCA cycle intermediates in muscle of rats fed on HF or HF + BCAA diets have yet to be reported. Alternatively, propionyl CoA and succinyl CoA are allosteric inhibitors of citrate synthase (Lee, S., et al, 1997), and recent studies have also indicated that mitochondrial proteins can be reversibly post-translationally modified and regulated by succinylation and malonylation in addition to the more familiar mechanism of acetylation (Peng, et al., 2011; Hirschey, 2011), providing other possible mechanisms for cross-talk between BCAA, fatty acid, and glucose oxidative pathways. Also, in liver, excess carbon from BCAA catabolism may contribute to elevated rates of lipogenesis and gluconeogenesis. Overall, the data shown in Figure 2 suggest that BCAA can “clog” the β-oxidative machinery in a manner analogous to the effect of excess fat, even when less HF + BCAA food is ingested compared to the HF group. Importantly, this effect seems to require the presence of high fat in the diet, as supplementation of standard chow diet with BCAA does not induce insulin resistance or cause acylcarnitine accumulation in skeletal muscle (Figure 2 and Newgard, et al., 2009).
Figure 2. Acylcarnitines in skeletal muscle in rats fed on various diets for 12 weeks.
SC, standard chow, SC/BCAA, standard chow supplemented with branched-chain amino acids (Val, Leu, Ile); HF, high fat diet (35% calories from fat); HF/BCAA, HF diet supplemented with branched-chain amino acids. Inset. C3 and C5 acylcarnitines in rats fed on various diets. Data adapted from Newgard, et al., 2009.
The foregoing model seems counter to traditional thinking about pathways of BCAA catabolism, in that BCAA catabolic flux is expected to be low in muscle, consistent with that tissue’s abundant expression of BCATm, but much lower expression of the branched chain keto-acid dehydrogenase complex (BCKDH), with an opposite ratio of expression of these enzymes in liver (Shimomura, et al., 2006). This gives rise to the commonly held view that a large fraction of the ketoacid pool produced from transamination of BCAA in muscle is metabolized in liver. It is important to emphasize that this does not mean that the ketoacid skeletons of BCAA cannot be oxidized in muscle, or that increased rates of their catabolism in muscle tissue cannot contribute to mitochondrial overload. Our finding of increased levels of C3 and C5 acylcarnitine species (byproducts of BCAA, but not fatty acid catabolism) in muscle and plasma of HF + BCAA fed rats (Figure 2, inset) and in plasma of insulin resistant humans (Newgard, et al., 2009; Huffman, et al., 2009; Tai, et al., 2010; Laferrère, et al., 2011) is consistent with this view.
How do the levels of BCAA and related metabolites rise in obese and insulin resistant humans? The answers are not clearly known, but several possibilities exist. One route is through increased protein consumption, since both the BCAA and aromatic amino acids are essential (not synthesized de novo in mammalian tissues). However, in both the studies of Asian-Indian and Chinese subjects in Singapore (Tai, et al., 2010) and in the longitudinal study of the Framingham cohort (Wang, et al., 2011), associations of BCAA and aromatic amino acids with insulin resistance and risk for diabetes were not influenced by protein consumption, as estimated by feeding questionnaires, suggesting that other factors are likely to contribute. Genetic variation in expression of genes encoding key BCAA catabolic enzymes or proteins that control protein synthesis and turnover may be another contributor. A third possibility is the gut microbiome, since many bacterial species are capable of de novo synthesis of BCAA (Park, et al., 2010), and could contribute to alterations in circulating BCAA in the host, although such an effect of the microbiome has yet to be demonstrated directly.
Yet a fourth possibility is suggested from several recent studies of the interplay between adipose tissue, BCAA metabolism, and glucose homeostasis. An important finding is that subtle alterations in expression of the genes in the BCAA catabolic pathway in adipose tissue can have a very significant impact on circulating BCAA levels. This was uncovered in part via studies of mice with adipose-specific overexpression of GLUT-4, which resulted in modest but concerted decreases in expression of multiple BCAA catabolic enzymes in fat tissue, coupled with a significant increase in circulating BCAA (Herman, et al., 2010). The activity of key BCAA catabolic enzymes in adipose tissue is also strongly influenced by obesity. For example, BCKDH complex enzymatic activity is decreased and phosphorylation of the E1 component of the complex is increased in adipose tissue of Zucker-obese rats and ob/ob mice relative to lean controls. BCATm and BCKDH gene expression is also decreased, accompanied by increased levels of circulating BCAA in these models (She, et al., 2007). Interestingly, no deficits in expression or activity of these enzymes were reported in skeletal muscle in the same animals. In addition, the large decrease in BCAA in response to bariatric surgery in humans that has been described by several groups (Laferrère, et al., 2011; She, et al., 2007), is accompanied in one study by increased expression of BCATm and BCKDH in omental and subcutaneous fat post-surgery (She, et al., 2007), suggesting a possible contribution of the adipose depot to re-establishment of BCAA homeostasis with weight loss. A similar mechanism may also be at work with application of pharmaceutical therapies, as suggested in a recent study of insulin resistant Zucker obese rats treated with four different thiazolidinedione drugs with varying efficacy for control of glucose homeostasis. Microarray analysis of multiple tissues in the treated rats revealed a striking and unique correlation between the efficacy of the drugs for glycemic control and their ability to upregulate multiple enzymes of the BCAA catabolic pathway in adipose tissue (Hsiao, et al., 2011). Microarray studies in humans also revealed a strong correlation between expression of BCAA catabolic genes in adipose tissue and insulin sensitivity (Sears, et al., 2009). These data suggest that decreased catabolism of BCAA in adipose tissue may contribute to increases in BCAA levels in insulin resistant states, although such an interpretation fails to explain how C3 and C5 acylcarnitines are consistently increased in our multiple cohorts of insulin resistant humans (Newgard, et al., 2009; Huffman, et al., 2009; Tai, et al., 2010; Laferrère, et al., 2011). We suggest that flux through BCAA catabolic pathways is not universally restricted in obesity, and may in fact be enhanced in a subset of tissues, including skeletal muscle. Flux through protein biosynthetic pathways may also be reduced in obesity and type 2 diabetes (Pereira, et al., 2008), although it remains to be determined if such changes are primary drivers of elevations of circulating BCAA, or are a secondary consequence of impaired insulin action on protein metabolism.
Working model of the interplay of lipids and BCAA in development of insulin resistance and metabolic disease
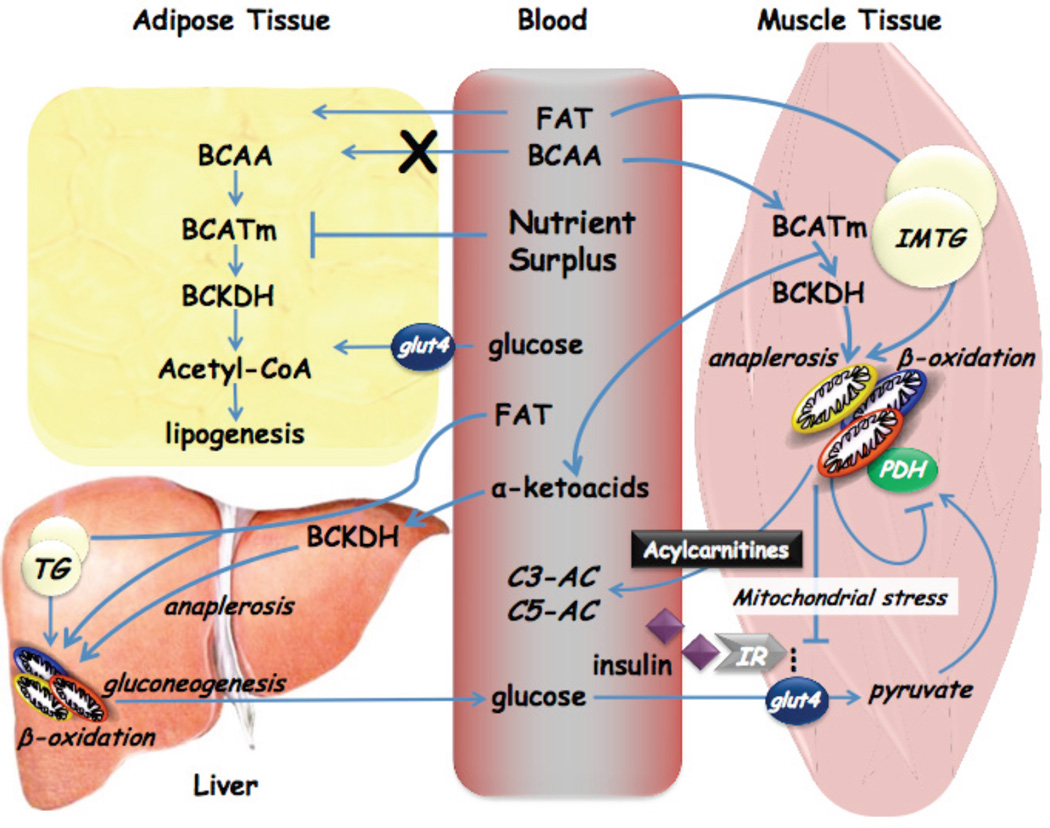
Based on our own data and that of others in the field, we propose that BCAA synergize with hyperlipidemia to make a contribution to the development of insulin resistance via the model shown in Figure 3. In this model, the rise in circulating BCAA is driven in part by an obesity-related decline in their catabolism in adipose tissue. In obesity and overnutrition, readily usable glucose and lipid substrates may obviate the need for amino acid catabolism in adipose tissue, but the mechanism by which increased supply of these substrates causes down-regulation of the BCAA catabolic enzymes remains to be explored. The fact that TZD drugs can restore expression of the BCAA catabolic genes to normal (Hsiao, et al., 2011) may suggest a role of suppressed peroxisome proliferator-activated receptor-γ (PPAR-γ) signaling in this metabolic adaptation. The resultant increase in circulating BCAA, possibly supplemented by contributions from the diet, intrinsic genetic differences in BCAA or protein turnover, and/or the gut microbiome, leads to an expanded pool of BCAA and related metabolites in obese and insulin resistant subjects. The model further holds that this expanded pool of BCAA “spills” into catabolic pathways in skeletal muscle and liver, explaining our highly consistent observation of an increase in circulating C3 and C5 acylcarnitines in humans with insulin resistance (Newgard, et al., 2009; Huffman, et al., 2009; Tai, et al., 2010; Laferrère, et al., 2011). As explained earlier, the further consequence of this enhanced flux and the generation of the catabolic intermediates propionyl CoA and succinyl CoA is to reduce the efficiency of oxidation of fatty acids and glucose, leading to accumulation of incompletely oxidized substrates, mitochondrial stress, impaired insulin action, and ultimately to perturbation of glucose homeostasis.
Figure 3. Schematic working model of potential cross-talk between lipids and BCAA in development of obesity-related insulin resistance.
See text for details. “Anaplerosis” refers to repletion or filling up of TCA cycle intermediates via entry points other than acetyl CoA. TG, triglyceride; IMTG, intramyocellular triglyceride; IR, insulin receptor.
It is important to note that while there are a number of studies consistent with our findings that BCAA contribute to development of insulin resistance, there are also some that seem to refute this conclusion. Findings consistent with ours include the induction of insulin resistance in response to infusion of amino acids in humans or animals, accompanied by chronic activation of mTOR, S6K1, and serine phosphorylation of IRS-1 (Krebs, et al., 2002; Tremblay, et al., 2005). Moreover, feeding of ob/ob mice with Leu-depleted diets increases insulin sensitivity (Xiao, et al., 2011). Finally, addition of BCAA or aromatic amino acids to cultured muscle cells results in activation of mTOR, impairment in insulin-stimulated phosphorylation of Akt/protein kinase B, and reduced insulin-stimulated glucose uptake (Tremblay and Marette, 2001; Saha, et al., 2010).
In contrast, other studies in mice report either improvement in insulin sensitivity (Macotela, et al., 2011; Zhang, et al., 2007), or no effect (Nairizi A, et al., 2009) in response to Leu supplementation. Also, global knock-out of BCATm in mice results in severe elevations of BCAA (14–37-fold), coupled with resistance to diet-induced obesity and improved glucose tolerance (She, et al., 2007). We feel that these studies are less faithful models of human obesity and related conditions, for the following reasons. First, Leu supplementation causes Leu to rise in the circulation, but causes a decline in the other BCAA (Nairizi, et al., 2009), thereby failing to mimic conditions in human insulin resistance and type 2 diabetes, where all three BCAA and several other amino acids are elevated (Newgard, et al., 2009; Huffman, et al., 2009; Tai, et al., 2010; Wang, et al., 2011; Laferrère, et al., 2011). Second, not all of the studies on Leu supplementation were conducted in the context of high fat feeding, which we have shown is necessary to unveil effects of BCAA on insulin sensitivity in rats. Third, mice with BCATm knockout have high rates of protein futile cycling and increased energy expenditure (She, et al., 2007); HF + BCAA-fed rats or obese humans do not exhibit these changes (Newgard, et al., 2009). Finally, if BCAA were protective against metabolic disease, one would not expect them to be elevated early in disease progression, as in Asian subjects with insulin resistance but relatively normal body weight (Tai, et al., 2010), or in pre-diabetic subjects from the Framingham and Malmo cohorts (Wang, et al., 2011). Nevertheless, further studies on the mechanisms by which excess BCAA may interfere (or not) with insulin action are warranted, as are studies focused on deeper understanding of the cross-talk between lipids and amino acids in development of metabolic dysfunction.
Lipids, BCAA, and the β-cell
There may also be interactions of excess BCAA and lipids in the development of β-cell dysfunction, which drives the transition from the obese, insulin-resistant state to type 2 diabetes. The metabolic basis for the gradual dysregulation of glucose-stimulated insulin secretion (GSIS) in type 2 diabetes is not completely understood, in part because fatty acids and amino acids have complex effects on the β-cell. Fatty acids can serve as secretagogues that potentiate GSIS, apparently via a combination of messengers produced during metabolism and via activation of cell-surface G-protein coupled receptors (Stein, et al., 1997; Latour, et al., 2007). Islets must also maintain a minimal supply of cellular lipids to allow stimulus/secretion coupling. Thus, total depletion of islet triglyceride stores in rats by experimental hyperleptinemia (Koyama, et al., 1997) or by treatment with the anti-lipolytic agent nicotinic acid (Stein, et al., 1996) results in loss of insulin secretion in response to a range of secretagogues; this loss of function can be rescued by re-provision of fatty acids. Conversely, the dysfunctional islets in animal models of obesity and type 2 diabetes exhibit accumulation of stored lipids (triglyceride) and other potentially harmful lipid-derived products (ceramides) preceding the onset of β-cell dysfunction (Lee, Y., et al., 1997; Shimabukuro, et al., 1998). Finally, chronic exposure of islets to elevated concentrations of fatty acids causes impairment of GSIS (Segall, et al., 1999; Boucher, et al., 2004).
Amino acids have similarly complex effects on β-cells. Two of the analytes found in the principal component associated with insulin resistance, Glu and Leu, are potent insulin secretagogues, and the two analytes interact in regulating insulin secretion. Glutamate-stimulated insulin secretion is enhanced by allosteric activation of glutamate dehydrogenase by Leu, as well as metabolism of Leu to create anaplerotic substrates (Li, et al., 2008), and activating mutations in GDH cause a form of familial hyperinsulinism (Stanley, 2009). Obese, insulin-resistant but non-diabetic humans have elevated levels of BCAA and Glu, and also exhibit an increase in the acute insulin secretion response to glucose (over 10 minutes) during a glucose tolerance test (Newgard, 2009). Moreover, these analytes as well as others in the BCAA-related principal component discussed earlier are strongly associated with fasting insulin levels, HOMA, and C-peptide secretion during a glucose challenge following bariatric surgery (Laferrère, et al., 2011). Taken together, these findings suggest that chronic elevations in BCAA and related metabolites may synergize with a similar slow rise in circulating fatty acids to drive a state of chronic hyperinsulinemia. This constant secretory pressure on the β-cell may ultimately contribute to β-cell dysfunction by causing endoplasmic reticulum stress (see Muoio and Newgard, 2008 for review). The same amino acids may contribute to the rapid improvement in glycemic control in response to bariatric surgery, in part by tempering the chronic hyperinsulinemia.
Concluding Remarks
In summary, recent studies from several laboratories have rekindled interest in the potential role of protein and amino acid metabolism in development of metabolic disease. In particular, the multiple new examples of strong associations between BCAA and related metabolites with incidence, progression, and remission of insulin resistance, type 2 diabetes, and cardiovascular disease provide the impetus for gaining better understanding of the potential synergies between this group of metabolites and lipids in development of metabolic dysfunction in multiple tissues. It is hoped that the working models advanced here will help in the design of experiments to further pursue the mechanistic underpinnings of such cross-talk in the future.
Acknowledgements
The author wishes to thank the outstanding collaborators that have contributed to work described in this review emanating from the Sarah W. Stedman Nutrition and Metabolism Center at Duke. Work from our group cited herein was supported by NIH grant PO1-DK58398 and sponsored research agreements with Glaxo Smith-Kline and Pfizer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am. J. Physiol. 2009;296:E592–E602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain JR, Stevens RD, Wenner BR, Ilkayeva O, Muoio DM, Newgard CB. Metabolomics applied to diabetes research: Moving from information to knowledge. Diabetes. 2009;58:2429–2443. doi: 10.2337/db09-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher A, Lu D, Burgess S, Telemaque-Potts S, Jensen M, Mulder H, Wang M-Y, Unger RH, Sherry AD, Newgard CB. Mechanism of lipid-induced impairment of glucose-stimulated insulin secretion and its reversal by an analogue of malate. J. Biol. Chem. 2004;279:27263–27271. doi: 10.1074/jbc.M401167200. [DOI] [PubMed] [Google Scholar]
- Clifton P. Diabetes: treatment of type 2 diabetes mellitus with bariatric surgery. Nature Rev. Endocrinol. 2010;6:191–193. doi: 10.1038/nrendo.2010.23. [DOI] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. New Engl. J. Med. 1969;281:811–816. doi: 10.1056/NEJM196910092811503. [DOI] [PubMed] [Google Scholar]
- Felig P, Wahren J, Hendler R, Brundin T. Splanchnic glucose and amino acid metabolism in obesity. Journal of Clinical Investigation. 1974;53:582–590. doi: 10.1172/JCI107593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom JD. Branched-chain amino acids and brain function. J. Nutr. 2005;135:1539S–1546S. doi: 10.1093/jn/135.6.1539S. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults,1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Gougeon R, Morais JA, Chevalier S, Pereira S, Lamarche M, Marliss EB. Determinants of whole-body protein metabolism in subjects with and without type 2 diabetes. Diabetes Care. 2008;231:128–133. doi: 10.2337/dc07-1268. [DOI] [PubMed] [Google Scholar]
- Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched cahin amino acid (BCAA) metabolism modulates circulating BCAA levels. J. Biol. Chem. 2010;285:11348–11351. doi: 10.1074/jbc.M109.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD. Old enzymes, new tricks: sirtuins are NAD(+)-dependent de-acylases. Cell Metabolism. 2011 doi: 10.1016/j.cmet.2011.10.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao G, Chapman J, Ofrecio JM, Wilkes J, Resnik JL, Thapar D, Subramaniam S, Sears DD. Multi-tissue, selective PPARg modulation of insulin sensitivity and metabolic pathways in obese rats. Am. J. Physiol. 2011;300:E164–E174. doi: 10.1152/ajpendo.00219.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman KM, Shah SH, Stevens RD, Bain JR, Muehlbauer M, Slentz CA, Tanner CJ, Kuchibhatla M, Houmard JA, Newgard CB, Kraus WE. Relationships between circulating metabolic intermediates and insulin action in middle-aged overweight to obese inactive men and women. Diabetes Care. 2009;32:1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- Koves TR, Ussher JR, Slentz DS, Mosedale M, Ikayeva OJ, Stevens R, Dyck J, Newgard CB, Lopaschuk G, Muoio DM. Mitochondrial Overload and Incomplete Fatty Acid Oxidation Contribute to Skeletal Muscle Insulin Resistance. Cell Metabolism. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Koves TR, Li P, An J, Slentz D, Ilkayeva O, Akimoto T, Dohm GL, Yan Z, Newgard CB, Muoio DM. PGC1a-mediated metabolic remodeling of skeletal muscle mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- Koyama K, Chen G, Wang MY, Lee Y, Shimabukuro M, Newgard CB, Unger RH. Beta-cell function in normal rats made chronically hyperleptinemic by adenovirus-leptin gene therapy. Diabetes. 1997;46:1276–1280. doi: 10.2337/diab.46.8.1276. [DOI] [PubMed] [Google Scholar]
- Krebs M, Krssak M, Bernroider E, Anderwald C, Brehm A, Meyerspeer M, Nowotny P, Roth E, Waldhausl W, Roden M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes. 2002;51:599–605. doi: 10.2337/diabetes.51.3.599. [DOI] [PubMed] [Google Scholar]
- Laferrère B, Arias S, Swerdlow N, Gorroochurn P, Bose M, Bawa B, Tiexeira J, Stevens RD, Wenner BR, Bain JR, Muehlbauer MJ, Haqq A, Lien L, Shah S, Svetkey LS, Newgard CB. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Science Transl. Med. 2011;3:80re2. doi: 10.1126/scitranslmed.3002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latour MG, Alquier T, Oseid E, Tremblay C, Jetton TL, Luo J, Lin DC, Poitout V. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes. 2007;56:1087–1094. doi: 10.2337/db06-1532. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Park C, Yim J. Characterization of citrate synthase purified from Drosphila melanogaster. Mol. Cells. 1997;7:599–604. [PubMed] [Google Scholar]
- Lee Y, Hirose H, Zhou YT, Esser V, McGarry JD, Unger RH. Increased lipogenic capacity of the islets of obese rats: a role in the pathogenesis of NIDDM. Diabetes. 1997;46:408–413. doi: 10.2337/diab.46.3.408. [DOI] [PubMed] [Google Scholar]
- Li C, Najafi H, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Matschinsky FM, Stanley CA. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J. Biol. Chem. 2008;278:2853–2858. doi: 10.1074/jbc.M210577200. [DOI] [PubMed] [Google Scholar]
- Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR. Dietary leucine -- an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- Muoio DM, Newgard CB. Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nature Rev. Mol. Cell. Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- Nairizi A, She P, Vary TC, Lynch CJ. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J. Nutr. 2009;139:715–719. doi: 10.3945/jn.108.100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arolotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WE, Eisenson H, Musante G, Surwit R, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contibutes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee SY. Fermentative production of branched chain amino acids: a focus on metabolic engineering. Appl. Microbiol. Biotechnol. 2010;85:491–506. doi: 10.1007/s00253-009-2307-y. [DOI] [PubMed] [Google Scholar]
- Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, Tan M, Luo H, Zhang Y, He W, Yang K, Zwaans BM, Tishkoff D, Ho L, Lombard D, He TC, Dai J, Verdin E, Ye Y, Zhao Y. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell Proteomics. 2011 doi: 10.1074/mcp.M111.012658. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira S, Marliss EB, Morais JA, Chevalier S, Gougeon R. Insulin resistance of protein metabolism in type 2 diabetes. Diabetes. 2008;57:56–63. doi: 10.2337/db07-0887. [DOI] [PubMed] [Google Scholar]
- Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 1998;14:263–283. doi: 10.1002/(sici)1099-0895(199812)14:4<263::aid-dmr233>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Saha AK, Xu XJ, Lawson E, Deoliverira R, Brandon AE, Kraegen EW, Ruderman NB. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59:2426–2434. doi: 10.2337/db09-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–520. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears DD, Hsiao G, Hsiao A, Yu JG, Courtney CH, Ofrecio JM, Chapman J, Subramaniam S. Mechanisms of human insulin resistance and thiazolidinedione. 2009 doi: 10.1073/pnas.0903032106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall L, Lameloise N, Assimacopoulos-Jeannet F, Roche E, Corkey P, Thumelin S, Corkey BE, Prentki M. Lipid rather than glucose metabolism is implicated in altered insulin secretion caused by oleate in INS-1 cells. Am. J. Physiol. 1999;277:E521–E528. doi: 10.1152/ajpendo.1999.277.3.E521. [DOI] [PubMed] [Google Scholar]
- Shah SH, Bain J, Crosslin DR, Muehlbauer MJ, Stevens R, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, Newgard CB, Kraus WE. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet. 2010;3:207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- Shah SH, Crosslin DR, Haynes C, Nelson S, Boling CL, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrere B, Gorroochurn P, Teixeira J, Brantley PJ, Stevens V, Hollis JF, Appel LJ, Lien L, Batch B, Newgard CB, Svetkey LP. Branched chain amino acids levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2011 doi: 10.1007/s00125-011-2356-5. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. 2007;293:E1552–E1563. doi: 10.1152/ajpendo.00134.2007. -mediated insulin sensitization. Proc. Natl. Acad. Sci USA 106, 18745–18750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, Hutson SM. Disruption of BCATm in mice leads to increased energy expenditure associated with activation of a futile protein turnover cycle. Cell Metab. 2007;6:181–194. doi: 10.1016/j.cmet.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl. Acad. Sci. USA. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura Y, Honda T, Shiraki M, Murakami T, Sato J, Kobayashi H, Mawatari K, Obayashi M, Harris RA. Branched-chain amino acid catabolism in exercise and disease. J. Nutr. 2006;136:250S–253S. doi: 10.1093/jn/136.1.250S. [DOI] [PubMed] [Google Scholar]
- Sparks LM, Xie H, Koza RA, Mynatt R, Hulver MW, Bray GA, Smith SR. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- Stanley CA. Regulation of glutamate metabolism and insulin secretion by glutamate dehydrogenase in hypoglycemic children. Am. J. Clin Nutr. 2009;90:862S–866S. doi: 10.3945/ajcn.2009.27462AA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DT, Stevenson BE, Chester MW, Basit M, Daniels MB, Turley SD, McGarry JD. The insulinotropic potency of fatty acids is influenced profoundly by their chain length and degree of saturation. J. Clin. Invest. 1997;100:398–403. doi: 10.1172/JCI119546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, Chen S, McGarry JD. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J. Clin. Invest. 1996;97:2728–2735. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, Vollmer WM, Gullion CM, Funk K, Smith P, Samuel-Hodge C, Myers V, Lien LF, Laferriere D, Kennedy B, Jerome GJ, Heinith F, Harsha DW, Evans P, Erlinger TP, Dalcin AT, Coughlin J, Charleston J, Champagne CM, Bauck A, Ard JD, Aicher K Weight Loss Maintenance Collaborative Research Group. Comparison of strategies for sustaining weight loss: the weight loss maintenance randomized controlled trial. Journal of the American Medical Association. 2008;299:1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- Tai E-S, Tan MLS, Stevens RD, Low Y-L, Muehlbauer M, Goh DLM, Ilkayeva O, Wenner B, Bain JR, Lee JJM, Lim S-C, Shah SH, Newgard CB. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia. 2010;53:757–767. doi: 10.1007/s00125-009-1637-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhausl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–2684. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Marette A. Amino acid and insulin signaling via the mTOR/p70 S6 kinase pathway. J. Biol. Chem. 2001;41:38052–38060. doi: 10.1074/jbc.M106703200. [DOI] [PubMed] [Google Scholar]
- Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance and ribosomal protein S6 kinase 1, D6K1. Cell Metab. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolic profiles and the risk of developing diabetes. Nature Medicine. 2011;121:1402–1411. doi: 10.1038/nm.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, Liu Y, Guo F. Leucine deprivation increases hepatic insulin sensitivity via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60:746–756. doi: 10.2337/db10-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu Y-H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56:1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]