Abstract
Behavioral experiments examined the effects of olivocochlear efferent lesions on performance in an intensity discrimination task. Five cats were trained with food reinforcement to signal the detection of a change in the intensity of pure tones by releasing a response lever. Intensity cues were conveyed by 1 and 8-kHz tone bursts in quiet and in the presence of continuous broadband noise. After the collection of baseline behavioral data, the olivocochlear bundle (OCB) was sectioned with bilateral knife cuts on the floor of the IVth ventricle. The completeness of OCB lesions was evaluated at the conclusion of post-lesion behavioral testing by light microscopic examination of cochlear acetylcholinesterase staining and electrophysiological measures of contralateral noise suppression of compound action potentials (CAPs). Cats with OCB lesions showed greatest performance deficits for the discrimination of 8-kHz intensity changes in continuous background noise. The subjects’ ability to discriminate 1-kHz intensity changes in noise was poor prior to OCB lesioning and did not change after the surgical procedure. Lesioning effects were not observed at either frequency when tests were conducted in quiet. These results suggest that olivocochlear feedback contributes to the auditory processing of mid-frequency acoustic signals in noisy backgrounds.
Keywords: Olivocochlear efferent lesion, intensity discrimination, noise, compound action potential, acetylcholinesterase
Rasmussen (1946, 1960) used fiber degeneration stains to perform the first anatomical description of an efferent pathway that originated from a group of neurons in the superior olivary complex and terminated within the inner ear. These projections are now known as the olivocochlear bundle. The course of olivocochlear efferents within the cochlea was later revealed when Warr (1975) traced retrograde-labeled axons of olivocochlear neurons from the site of horseradish peroxidase injections in the cochlea to cell bodies in lateral and medial regions of the superior olivary complex (SOC). In a subsequent study, Warr and Guinan (1979) noted that lateral periolivary injections of radiolabeled amino acid 35S-methionine produced autoradiographic labeling predominantly under inner hair cells in the ipsilateral cochlea; whereas, medial injections resulted in extensive labeling under outer hair cells in the contralateral cochlea. In addition to differences in the targets of their projections, olivocochlear neurons that originate in these two regions of the SOC have been shown to differ in size and shape of cell bodies, the strength of crossed vs. uncrossed projections, fiber diameter and myelination, developmental sequences, and associated neurotransmitters. Based on these differences, neurons that form the olivocochlear pathway are now characterized separately as the lateral olivocochlear (LOC) and medial olivocochlear (MOC) systems. Presumably, each efferent pathway is capable of making a unique contribution to hearing, although the functional significance of this elaborate and ubiquitous efferent network has not been convincingly demonstrated with behavioral paradigms.
Recent advances in our understanding of the anatomy and physiology of olivocochlear neurons offer new strategies for testing the functional significance of this efferent pathway. Warr’s anatomical discoveries (1975) and the intracellular labeling experiments of Liberman and Brown (1986) have described the pathway of olivocochlear projection in the brainstem and suggest surgical procedures for making more complete efferent lesions. Electrophysiological research by Liberman has described improved methods for confirming the completeness of OCB lesions using acetylcholinesterase staining procedures (1986) and suppression of the compound action potential by contralateral sound (1989). Results from single-unit recordings from olivocochlear efferent neurons propose binaural acoustic paradigms and noise effects that can achieve maximum excitation in the feedback system (Liberman 1988). The present study calls upon this wealth of new information to test the hypothesis that olivocochlear efferent feedback plays an important role in the discrimination of sound intensity.
Further evidence for the functional significance of olivocochlear feedback in the neural encoding of sound intensity was obtained in our recent electrophysiological studies of awake, behaving cats (May and Sachs 1992). The dynamic range properties of neurons in the ventral cochlear nucleus of awake cats show less of the compressive effects of background noise than ANFs in anesthetized cats, but striking similarities to those of ANFs in anesthetized cats during electrical stimulation of the OCB (Winslow and Sachs 1987). Our present behavioral study served as a test of the hypothesis that olivocochlear efferent feedback preserved the dynamic range properties of neurons in behaving cats. In support of that hypothesis, bilateral olivocochlear lesions produced deficits in the discrimination of sound intensity when behavioral performance was measured in the presence of background noise.
METHODS
All animal protocols used in our studies of intensity discrimination have been approved by the Institutional Animal Care and Use Committee of The Johns Hopkins School of Medicine.
Subjects
Five male cats were used as experimental animals. The cats were obtained at four months of age from Liberty Labs (Liberty Corners, NJ). Periodic otoscopic examinations confirmed that each cat had clean external ears and normal tympanic membranes throughout the 12 – 16 month period in which they participated in the study. Cats were housed individually with unlimited access to water. Food was available in the behavioral testing apparatus or in the home cage immediately after testing. Relative to the dry chow that was available in the home cage (Hill’s Science Diet or Purina Cat Chow), cats showed a greater appetite for the liquefied meat paste that served as rewards for correct responses in the intensity discrimination task (Hill’s Science Diet Feline Maintenance or Whiskas Mealtime). Consequently, only slight food deprivation was needed to motivate the cats to perform the task during daily 1-h testing sessions. Cats attained normal adult body weights (3 – 5 kg) and remained healthy and vigorous while on this feeding regimen.
Apparatus
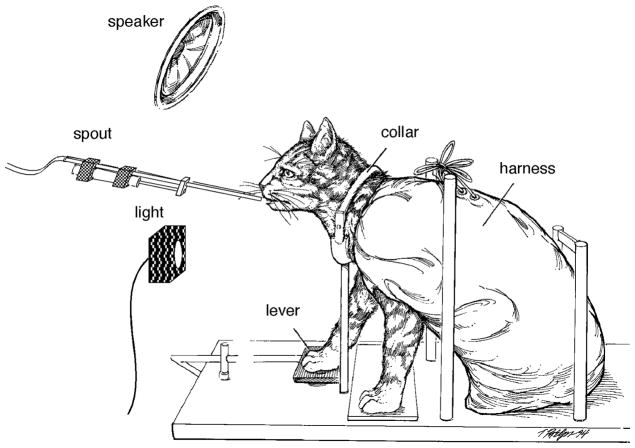
Behavioral testing was conducted inside a double-walled sound attenuating chamber (Industrial Acoustics Co.) with inner dimensions of 1.8 L × 1.8 W × 1.9 H meters. An operant testing platform was suspended at the approximate center of the chamber. All surfaces of the platform were made of hardware cloth to reduce acoustic reflections. Cats were held at a uniform location in the sound field by a canvas restraint harness and aluminum collar that attached to the framework of the operant platform (Fig. 1). The state of the behavioral task was signaled by a 4-cm cue light on the front wall of the cage. Cats made behavioral responses by pressing a lever on the floor of the cage near their right front paw. A peristaltic pump delivered liquefied food through a spout that advanced to the cat’s mouth during periods of reinforcement. Contingencies of reinforcement, data acquisition, and data analysis were controlled by digital computers (Digital Equipment PDP-11/34, Apple Macintosh).
FIGURE 1.
Operant testing platform and restraint system.
Acoustic stimuli were presented in free field from a dynamic loudspeaker (Realistic, Minimus 3.5). Prior to the start of this study, acoustic calibrations were made with the loudspeaker at several locations in the testing chamber. The loudspeaker was eventually placed slightly forward and above the operant platform because this speaker position produced a signal level at the cat’s head position that varied by less than ±5 dB for frequencies from 0.5 – 10.0 kHz. In addition, the head-related transfer function of the cat creates a relatively uniform amplitude spectrum at the eardrum when sounds arise from this direction (Musicant et al. 1990, Rice et al. 1994). Acoustic calibrations were obtained using a condenser microphone (Brüel & Kjær, model 4133) and microphone amplifier (Brüel & Kjær, model 2604).
Behavioral Procedures
Contingencies of reinforcement for the intensity discrimination task are shown in Fig. 2. Cats were trained by positive reinforcement to respond to a flashing cue light by pressing down on a response lever (May et al. 1995). The lever pressing response produced a continuous cue light and began a sequence of tone bursts. Each tone burst was presented for 250 msec and consecutive stimuli were separated by a 250-msec silent interval. These stimuli are designated the standard tones. Within a daily testing session, standard tones were of a fixed intensity (65 dB SPL) and frequency (1 or 8 kHz). The number of standard tone presentations was varied from 10 – 20 bursts on a trail by trial basis, which resulted in a hold interval of 5 – 10 sec. After the variable hold interval, standard tones began to alternate with comparison tones. Comparison tones were identical to standard tones except that these stimuli were presented at a higher sound-pressure level (SPL). Cats obtained food rewards by releasing the response lever before the termination of a 3-sec period of intensity changes (ΔI) between standard and comparison tones. Releases to comparison trials are designated hits in our data analysis.
FIGURE 2. Contingencies of reinforcement for the intensity discrimination task.
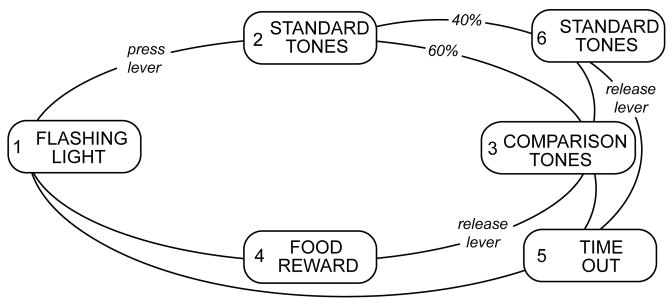
The basic behavioral paradigm cycled through a flashing cue light (1), a series of standard tones (2), intensity changes that were created by alternating standard tones with louder comparison tones (3), and a food reward that was delivered to the cat’s mouth by a pneumatic spout if the lever was released in response to the presentation of an intensity change (4).
Any release of the response lever that did not occur during comparison trials resulted in a time-out interval, as did failure to release the lever before termination of a comparison trial. Time-outs were interruptions of the testing routine in which the cue light was extinguished and contact with the response lever failed to elicit acoustic stimuli. This mildly negative reinforcement decreased the likelihood of incorrect responses or guessing. Each cat reacted in a slightly different manner to this contingency; consequently, the duration of time-out that most effectively reduced error responses ranged from 2 – 6 sec across animals.
Catch-trial intervals were presented on 40% of all trials to monitor the frequency of guessing. Catch-trials were identical to comparison trials except that intensity cues were eliminated by replacing comparison tones in the alternation sequence with standard tones. Instead of the intensity change between comparison and standard tones, the subject heard only repeating standard tones of a fixed intensity. If a subject correctly maintained the observing response for a catch-trial interval, a comparison trial followed immediately thereafter and the subject obtained a food reward by releasing the lever at that time. Alternatively, if the subject incorrectly released during a catch-trial interval, a time-out occurred. Releases to catch-trials are designated false alarms in our data analysis.
Thresholds for intensity discrimination at 1 and 8 kHz were determined for the first two cats in the study (91cv3 and 91cp2). The threshold stimulus condition was defined as the change in intensity between standard and comparison tones that produced a d′ value equal to 1.0. These measures were interpolated from psychometric functions that plotted d′ in relation to changes in the magnitude of the intensity difference between standard and comparison tones. The d′ index of sensitivity was computed for performance at each intensity change by converting response probabilities of hits and false alarms to standardized normal deviates (z-values) and combining the values according to Eq. 1.
| (1) |
d′ values were used as an estimate of discrimination sensitivity because the measure is less affected by shifts in a subject’s response criterion that may result from either surgical or stimulus conditions. For example, cats may exhibit more correct releases to comparison trials (hits) when tested in noise but also more incorrect releases to catch-trials (false alarms). Therefore, the subject is not more sensitive to intensity information; the subject is more responsive. Discrimination thresholds that are measured in terms of d′ values “correct” for such criterion shifts because the d′ statistic is derived from a subject’s rate of false alarms as well as hits.
Three cats were added to the study after extensive testing of cats 91cv3 and 91cp2. Intensity discrimination was evaluated in this second group of cats by obtaining d′ values for one fixed 3-dB intensity change. This testing strategy differed considerably from our standard psychometric procedure which randomly selected the stimulus for presentation from a fixed set of intensity changes (i.e., Method of Constant Stimuli). A 3-dB ΔI cue was selected for these tests because changes of this magnitude represented an intermediate level of task difficulty based on psychometric functions for the first two cats. Therefore, performance in the intensity discrimination task had the potential to increase or decrease after efferent lesions. Although tests with one comparison tone yielded a more limited data set than traditional threshold measures, this more efficient sampling strategy provided a rapid assessment of intensity discrimination for a variety of testing conditions. In addition, this paradigm was more sensitive to small changes in behavioral performance because discrimination of a single intensity change was extensively sampled within each testing session.
Intensity discrimination was also measured in the presence of continuous background noise. The first series of cats were trained to discriminate a fixed 3-dB ΔI cue in levels of background noise that ranged from −8 – 28 dB SPL. The intensity change was conveyed by a 65-dB standard tone and 68-dB comparison tone at frequencies of 1 and 8 kHz. The behavioral paradigm for testing in noise was identical to the one that was used for testing in quiet except that a continuous noise background changed randomly in level on a trial-wise basis. The second group of cats was tested with a 3-dB intensity change in a fixed noise spectrum level of 8 dB.
Surgical Procedures for Making OCB Lesions
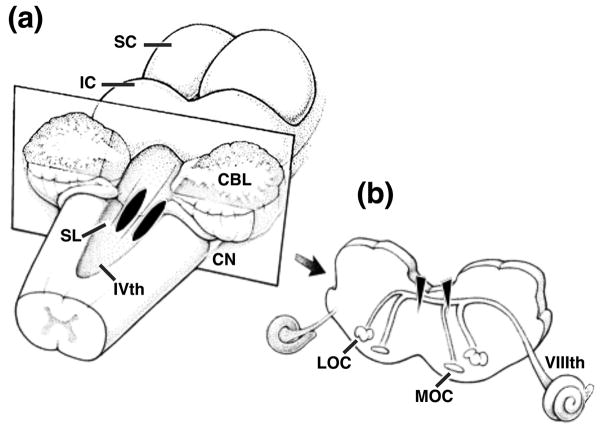
Olivocochlear lesions were performed using aseptic surgical procedures on deeply anesthetized cats. A surgical level of anesthesia was induced with xylazine (0.5 mg/kg i.m.) followed by ketamine HCl (25 mg/kg i.m.). Supplemental doses of ketamine HCl were given to maintain areflexia throughout the course of the surgery. Atropine (0.05 mg/kg i.m.) was administered to control secretions. Body temperature was maintained with a regulated heating pad. A midline incision was made over the dorsal posterior skull and rhomboideus and splenius muscles were partially dissected to expose the occipital bone. A 1-cm fenestration was made in the occipital bone just superior to the foramen magnum. Dura was excised and the posterior lobe of the cerebellum was elevated to gain access to the IVth ventricle through enlargement of the median aperture. As illustrated in Fig. 3(a), the OCB was lesioned by making bilateral knife cuts on the dorsal surface of the medulla. The knife cuts were 4 – 5 mm in length and 0.5 – 1 mm in depth and were oriented in the parasagittal plane at opposite sides of the midline. Placement of the lesions was determined visually from surface features such as the sulcus limitans. A sham lesion was performed in cat 91kj5 by exposing the floor of the IVth ventricle without making knife cuts.
FIGURE 3. Surgical schematic showing placement of OCB lesions.
(a) Surface view of the cat’s brainstem with cerebellum partially removed to reveal site of bilateral lesions (black stripes). (b) Medial and lateral olivocochlear projections to one cochlea shown in transverse view at medullar plane of section in (a). Bilateral lesions potentially transect crossed and uncrossed projections (filled triangles). IVth, fourth ventricle; VIIIth, eighth nerve; CBL, cerebellum; CN, cochlear nucleus; LOC, lateral olivocochlear system; IC, inferior colliculus; MOC medial olivocochlear system; SC, superior colliculus; SL, sulcus limitans.
The principal projections of olivocochlear neurons are schematized in Fig. 3(b). These pathways consist of two separate systems in the cat (Warr and Guinan 1975). Medial olivocochlear projections originate from large stellate cells in the medial zone of the periolivary nuclei. These thick, myelinated fibers coalesce as they climb dorsal-medially toward the facial genua on the floor of the IVth ventricle. A majority of MOC fibers (≈70%) cross the midline and project to the contralateral cochlea via the inferior vestibular branch of the eighth cranial nerve. Within the cochlea, MOC neurons follow a predominately apical course in the intraganglionic spiral bundle before entering the organ of Corti as the upper tunnel radial fibers and terminating on tonotopically organized clusters of outer hair cells. Uncrossed MOC fibers innervate OHCs in the ipsilateral cochlea. Lateral olivocochlear projections originate from small fusiform cells in the lateral zone of the periolivary nuclei. The thin, unmyelinated axons of LOC neurons approach closely to the midline as they climb toward the floor of the IVth ventricle. After nearing the dorsal medial surface of the brainstem, a majority of these fibers (≈76%) turn in a lateral direction and project to the ipsilateral cochlea where they enter the organ of Corti as the inner spiral and tunnel spiral bundles. LOC neurons terminate beneath outer hair cells on the unmyelinated peripheral processes of type I spiral ganglion cells. Crossing LOC fibers show a similar pattern of innervation within the contralateral cochlea. Our surgical technique is designed to transect all fibers that project into the vicinity of the midline, which includes all crossed OCB fibers as well as a substantial number of uncrossed fibers. Therefore, this lesioning strategy is expected to destroy a significant number of MOC projections and to spare the largely ipsilateral LOC pathway. Physiological studies suggest that the principal olivocochlear influences on auditory afferent neurons reflect activity in the medial system (Guinan and Gifford 1988).
After the lesion was completed, the cerebellum was restored to its original position. Dural flaps were approximated, and then covered with moist gel foam. The incision was dusted with antibiotic powder (Neopredef) and the scalp was closed by suturing. For the first three post-operative days, cats were placed on cephalothin sodium antibiotics (Keflin: 25 mg/kg s.c.). Some cats showed loss of balance and nystagmus immediately after the surgical procedure but all cats recovered near normal motor behavior and returned to daily behavioral testing within 1 week.
Histological Assessment of OCB Lesions
Olivocochlear lesions were evaluated by processing cochlear tissue with acetylcholinesterase stains after the collection of post-lesion behavioral data. Acetylcholine-bearing fibers and terminals of the OCB produce a dense silver precipitate when reacted with AChE stains. Since olivocochlear efferent projections are presumed to be the sole source of acetylcholine in the cochlea of the cat, AChE staining procedures reveal efferent fibers that are spared by incomplete OCB lesions. The analysis of this tissue was based on qualitative comparisons of the density of staining in lesioned cats vs normal controls. Histological processing was performed on one cochlea for cats 91cv3 and 91kj5. Both cochleae were processed successfully for cats 91cp2, 92olf1, and 92olq1.
Cats were given a lethal injection of sodium pentobarbital and perfused transcardially with 1% paraformaldehyde – 1.25% glutaraldehyde in 0.1 M phosphate buffer. Brainstem tissue at the site of the OCB lesion was also examined in four cats. This tissue was cut into 48-μm transverse sections on a freezing microtome. Sections were mounted on subbed slides, air dried and then stained with cresyl violet. Light microscopic examination of this tissue proved to be an inadequate method for identifying the site and extent of OCB lesions; consequently, acetylcholinesterase (AChE) staining techniques were applied to cochlear tissue to determine the degree of cochlear de-efferentation.
Intracochlear perfusions were performed at the time of transcardial perfusion in preparation for AChE staining. The temporal bone was dissected to expose the oval and round window of both cochleae. Window membranes were punctured and a solution of 1% paraformaldehyde – 1.25% glutaraldehyde was injected into the round window until the solution could be seen flowing out of the oval window. Cochleae were removed from the temporal bone and shaved of most of their bony covering using a drill motor with diamond burr. The cochleae were then decalcified by gentle agitation in a solution of 0.1 M disodium EDTA (Versenate) – 1% paraformaldehyde for one week and embedded in gel albumin. The tissue was cut with a vibratome into 50-μm modiolar sections and mounted before being incubated for 1 h in acetylthiocholine medium followed by 1 minute in 4% sodium sulfide and then 1 minute in 1% silver nitrate. This technique is based on the method of Osen and Roth (1969), as modified by Liberman (1990).
Electrophysiological Assessment of OCB Lesions
Compound action potentials (CAPs) were recorded in two cats to assess the completeness of OCB lesions. The objective of these recordings was to compare the magnitude of CAP suppression by contralateral noise in one cat (92olf1) whose minimal behavioral deficits suggested an incomplete lesion with that of a second cat (92olq) whose larger deficits suggested a more complete lesion. Liberman’s (1989) acute electrophysiological studies indicate that contralateral sound suppression is eliminated in cats with complete olivocochlear lesions.
CAP recordings were made using acute surgical procedures immediately before the cats were killed for histology. In preparation for electrophysiological recordings, the cats were deeply anesthetized with xylazine followed by ketamine HCL. Atropine was given to control secretions. The trachea was fitted with a cannula to keep the airway patent and the cephalic vein was catheterized. Subsequent anesthesia was maintained by intravenous injections of sodium pentobarbital. Bilateral incisions were made over the parietal skull and the temporalis muscles were dissected to reveal both bullae and ear canals. The ear canals were transected 2 – 3 mm from the tympanic membrane and the subject was placed in a stereotaxic apparatus using hollow ear bars. The ear bars were fitted with acoustic drivers and served as specula for releasing acoustic stimuli into the ear canal near the tympanic membranes (Sokolich 1977). A small fenestration was made in each bulla and a silver-wire electrode was placed just ventral to the round window niche. The bullae were sealed with cotton and covered with vaseline.
Contralateral sound suppression was demonstrated by stimulating the ipsilateral ear with a rarefaction click and the contralateral ear with broadband noise. The click was presented at a stimulus level 5 dB above the CAP threshold which produced evoked potentials with peak-to-peak amplitudes ranging from 10.5 – 18.6 μV. The noise stimulus was presented at the maximum noise level available in our acoustic system, which was 40 dB spectrum level. Contralateral noise was presented for 400 msec then turned off for 10 msec before the click to eliminate remote pickup of brainstem auditory activity (Liberman 1989). CAPs were amplified 10,000×, low-pass filtered at 5.0 kHz, and then digitized with an analog-to-digital converter using a 25 μsec sampling interval. Measures of suppression were calculated from the ratio of peak-to-peak voltages for CAPs with and without contralateral noise. The waveforms used in these calculations reflect the average of 100 stimulus presentations.
RESULTS
Subjects were trained to perform the intensity discrimination task and then tested with a variety of baseline stimulus conditions for the first 5 – 11 months of the study. Olivocochlear efferents were then lesioned by making knife cuts on the floor of the IVth ventricle. The effects of OCB lesions on intensity discrimination were evaluated during 2 – 6 months of post-lesion behavioral testing. At the conclusion of the study, OCB lesions were evaluated with histological and electrophysiological techniques. Results obtained with these two methods of lesion assessment are summarized in Table I.
TABLE I.
Summary of Histological and Electrophysiological Results
| Cat | Lesion | Density of AChE Staining |
Magnitude of CAP Suppression (%) |
||
|---|---|---|---|---|---|
|
| |||||
| Left Cochlea |
Right Cochlea |
Left Cochlea |
Right Cochlea |
||
| 91cv3 | Complete | Light | — | — | |
| 91cp2 | Complete | Light | Light | — | — |
| 92olq1 | Unilateral | Moderate | Dark | 22% | 75% |
| 92olf1 | Incomplete | Dark | Dark | 55% | 67% |
| 91kj5 | Sham | Moderate | — | — | — |
Anatomical Assessment of OCB Lesions
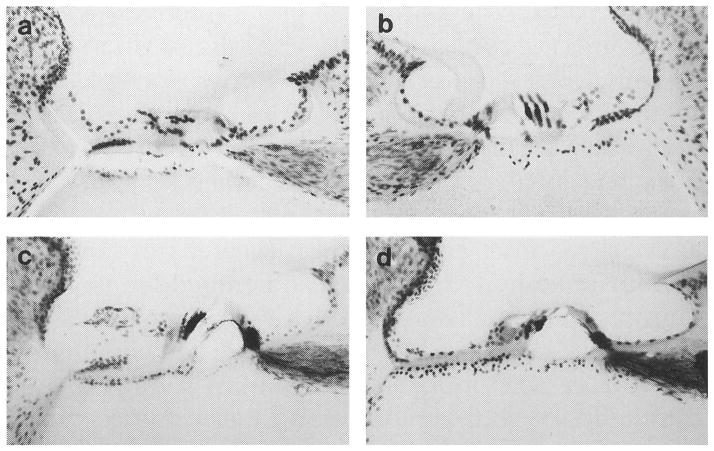
The pattern of AChE labeling for an intact non-behavioral control subject is described in Fig. 4. The tissue was lightly counter-stained with cresyl violet to enhance gross cochlear morphology for photographic purposes. In the low magnification trans-modiolar view of Fig. 4(a), a basal turn of the intact cochlea is marked by dense silver labeling for the inner and tunnel spiral fibers of LOC efferents beneath inner hair cells. This pattern of labeling is seen along the entire length of the cochlear partition. Intense staining for MOC terminals is also observed under outer hair cells in this basal turn, but less labeling is noted under the second and third rows of outer hair cells. The density of MOC labeling decreases in more apical turns. In Fig. 4(b), a higher magnification view of the basal turn relates the pattern of AChE labeling to structures in the organ of Corti and reveals labeled MOC projections as upper tunnel radial fibers (arrow). These observations are consistent with quantitative analysis of the distribution of olivocochlear projections within the cat cochlea (Liberman et al. 1990).
FIGURE 4. Photomicrographs showing the pattern of acetylcholinesterase (AChE) labeling in the cochleas of an intact control subject.
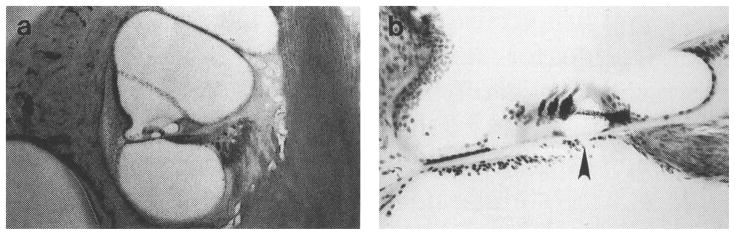
(a) Dense silver precipitate in this low magnification trans-modiolar view indicates the distribution of lateral olivocochlear terminals beneath inner hair cells in the basal turn of the left cochlea. Medial olivocochlear terminals beneath outer hair cells stain well in basal turns but AChE labeling is less intense in apical turns. Arrow points to the organ of Corti. (b) AChE labeling in this higher magnification view of a basal turn in the right cochlea of the same control animal identifies terminal zones of efferent fibers under cochlear hair cells as well as their radial course across the spiral lamina and through the tunnel of Corti. Arrow points to tunnel crossing fibers.
Figure 5 describes the pattern of AChE labeling in the basal cochlea of three behavioral cats that exhibited intensity discrimination deficits after OCB lesions. In Fig. 5(a) and (b), cochleae for cats 91cv3 and 91cp2 show light labeling relative to the intact control in Fig. 4. The depletion of acetylcholinesterase in these lesioned cats suggests that our surgical procedure created extensive damage to olivocochlear projections. A different pattern of staining is observed for cochleae of cat 92olq1. In Fig. 5(c), silver precipitate is reduced beneath the second and third rows of outer hair cells in the left cochlea but the first row of outer hair cells and inner hair cells display a high density of AChE labeling. In Fig. 5(d), the right cochlea of cat 92olq1 is also deeply stained suggesting an incomplete lesion to this ear. Staining patterns in this tissue highlights one difficulty of using AChE procedures to evaluate the completeness of OCB lesions. Remaining efferent fibers can create patterns of very dense labeling making it difficult to grade lesions that fall between the extremes of intact or completely de-efferented cochleae. OCB lesions were examined with electrophysiological measures in later experiments for this reason.
FIGURE 5. Acetylcholinesterase (AChE) labeling in cochleas of cats that showed behavioral deficits after OCB lesions.
(a, b) Light AChE labeling was observed in basal turns of cochleae from cats 91cv3 and 91cp2. Large behavioral deficits were associated with this pattern of labeling. (c, d) Cochleae of cat 92olq1 show more intense AChE labeling than lesioned cochleae in (a) and (b), but less labeling than control cochleae in Figure 4. A smaller decrease in discrimination performance were observed after the OCB was lesioned in this animal. Measures of CAP suppression are shown for cat 92olq1 in Figure 7c.
Figure 6 describes the pattern of AChE labeling for behavioral cats that did not show discrimination deficits after OCB lesions. In Fig. 6(a), the left cochlea of cat 92olf1 reveals the same general pattern of efferent distribution as that seen in the intact control animal. Silver labeling from cholinergic terminals of LOC fibers is heavily distributed beneath inner hair cells. Deeply labeled MOC fibers fill the tunnel of Corti and terminate on the base of outer hair cells enveloping the receptors with a silver precipitate. The right cochlea of this cat was also deeply stained. Such dense bilateral labeling suggests a very incomplete lesion and may account for the cat’s lack of post-lesion behavioral deficits. Gross examination of the brainstem of cat 92olf1 indicated that lesions were placed too far caudal to transect OCB projections. In Fig. 6(b), the left cochlea of cat 91kj5 also shows AChE labeling under both inner and outer hair cells. Although this subject was intentionally sham lesioned, the density of labeling was reduced relative to the intact control.
FIGURE 6. Acetylcholinesterase (AChE) labeling in basal turns of the left cochleas from (a) cat 92olf1, a behavioral subject that did not exhibit behavioral deficits after OCB lesions and (b) cat 91kj5, a behavioral subject that received a sham lesion.
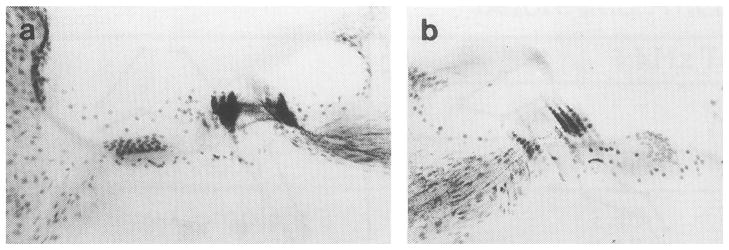
Cochleas from both cats show AChE labeling beneath inner and outer hair cell although less dense staining was noted in cat 91kj5. Gross examination of brainstem tissue suggested that lesions were made too caudal in cat 92olf1. Results of electrophysiological assessment of efferent influences in this cat are shown in Figure 7b.
Electrophysiological Assessment of OCB Lesions
Contralateral sounds suppress the magnitude of compound action potentials by activating olivocochlear neurons that project to the ipsilateral cochlea; consequently, lesioned cats will exhibit these suppression effects if olivocochlear projections are spared. CAP measures were introduced toward the end of this study and were not performed on the initial three subjects (91cv3, 91cp2, and 91kj5).
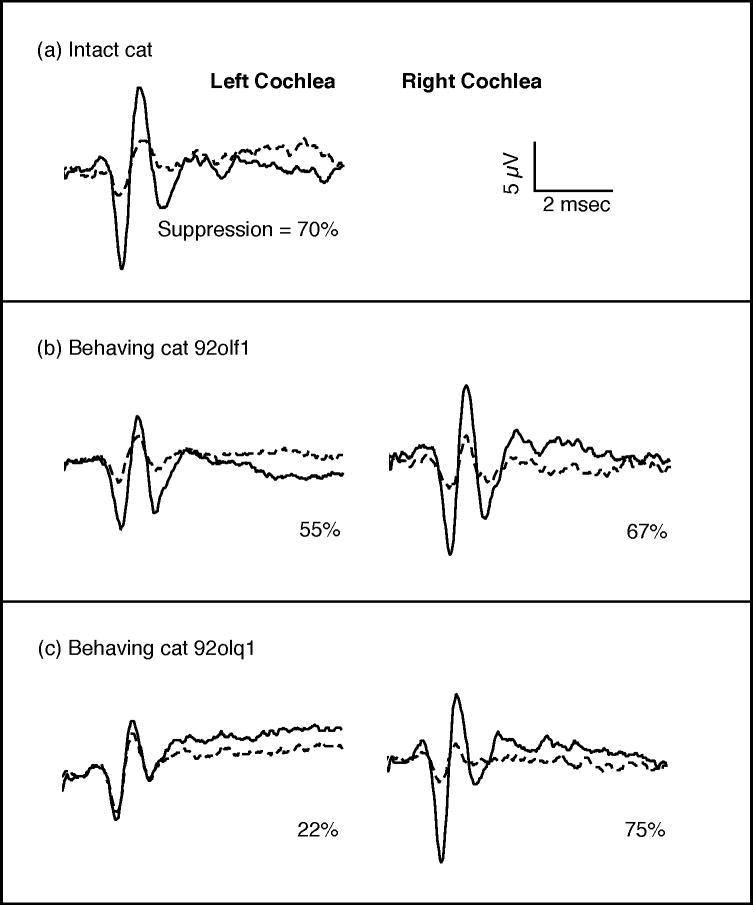
The magnitude of suppression in an intact non-behavioral control subject is described in Fig. 7(a). CAPs showed an average peak-to-peak voltage of 18.6 μV when this cat was tested with monaural rarefaction clicks 5 dB above the CAP threshold (solid line); the magnitude of the evoked potentials decreased 70% to 5.5 μV when clicks were paired with contralateral noise at 40-db spectrum level (dashed line). This pattern of strong but incomplete suppression is less than that produced by electrical OCB stimulation (Galambos 1956) but it is consistent with the effects of acoustically driven efferent activity (Liberman 1989).
FIGURE 7. Click-evoked compound action potentials (CAPs) obtained in quiet (solid lines) and in contralateral noise (dashed lines).
(a) Contralateral noise suppressed the CAP in the left cochlea of an intact control subject by 70%. (b) Both cochleae of cat 92olf1 were incompletely lesioned and like the intact cat showed large reductions of CAP in contralateral noise, (c) The left cochlea of cat 92olq1 was successfully lesioned and shows only weak efferent suppression.
The CAP suppression technique was used to assess the completeness of OCB lesions in behavioral subjects 92olf1 and 92olq1. The results of these tests are summarized for cat 92olf1 in Fig. 7(b). Evoked potentials obtained from the left cochlea of this cat decreased 55% from a peak-to-peak voltage of 12.3 μV in quiet to 5.5 μV in the presence of contralateral noise. CAPs from the right cochlea showed even greater suppression, decreasing 67% from 17.7 to 5.9 μV. These suppression effects are only slightly less than those noted for the intact cat and suggest significant olivocochlear influences remain for both cochleae after the lesioning procedure. Further evidence for incomplete lesions in cat 92olf1was provided by dense bilateral AChE labeling in Fig. 6(a) and the absence of discrimination deficits following the lesioning procedure.
CAPs for cat 92olq1 are shown in Fig. 7(c). The left cochlea of this subject showed only 22% suppression when tested with 5 dB clicks. This small suppression effect suggests that efferent feedback to the left ear was successfully reduced by the lesioning procedure. Suppression was also measured at click levels 15 dB above threshold because the 10.5 μV amplitude of the evoked potential at 5 dB was slightly less than that seen in the ears of other subjects. The CAP increased to a 40 μV peak-to-peak amplitude for the 15 dB re threshold click and showed only 13% suppression. Therefore, the reduced suppression shown by the left cochlea of cat 92olq1 was not simply an artifact of CAP magnitude. In contrast, the amplitude of evoked potentials in the right cochlea of this cat decreased 75% in the presence of contralateral noise, suggesting a relatively intact OCB projection. The apparent unilateral ablation of olivocochlear feedback in cat 92olq1 resulted in a smaller discrimination deficit than that observed in bilaterally lesioned cats.
Intensity Discrimination in Quiet
Cats were trained with large intensity changes for several months until they showed steady near-perfect performance for 1-h testing sessions. At that time, smaller intensity changes were gradually introduced until a wide range of ΔIs were tested within the same session. The final stimulus set was designed to produce a psychometric function that indicated near-perfect detection rates for large intensity changes and subthreshold levels of performance at small intensity changes. The smallest intensity change available in our testing apparatus was 1 dB. Cats performed better than chance when tested with a change of this magnitude under quiet testing conditions.
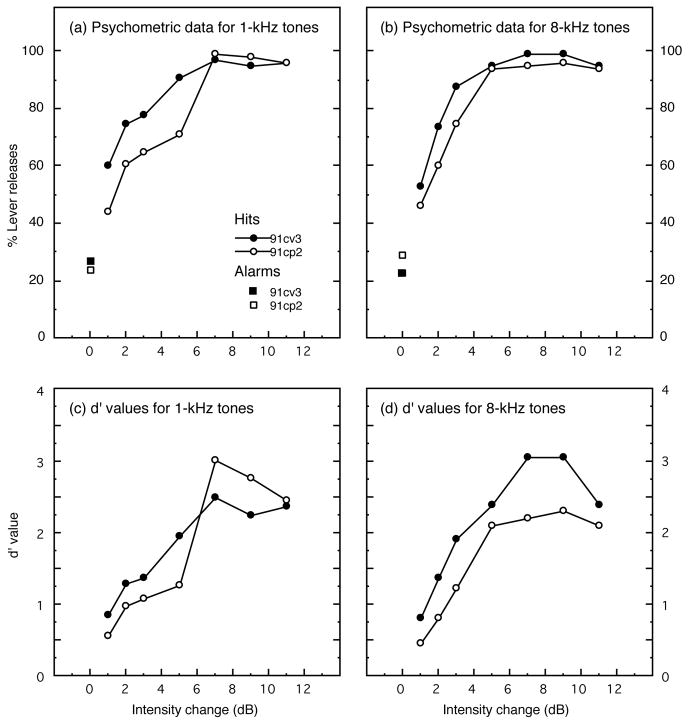
Pre-lesion discrimination of 1-kHz tones is described by psychometric functions in Fig. 8(a). These functions are based on performance over 850 trials and were collected in nine behavioral sessions for cat 91cv3 and seven sessions for 91cp2. Behavioral measures are based on a minimum of five days of testing, but more sessions were performed when difficult testing conditions decreased the number of total trials within a session, or when d′ values on a particular day varied by more than ±20% from average performance. The functions in Fig. 8 plot the percentage of lever releases (hits) at six intensity changes that ranged from 1 to 11 dB. Each intensity change was randomly selected for presentation during comparison trials within the same behavioral session. The psychometric functions asymptote at near perfect performance indicating that behavioral responses of both subjects were under good stimulus control. False-alarm rates (i.e., lever releases during catch trials) averaged 27% and 24% for cats 91cv3 and 91cp2, respectively. These data are plotted at the 0-dB intensity change on the psychometric functions. Since false alarms are needed for the calculation of the d′ index of discrimination sensitivity, contingencies of reinforcement such as the duration of the variable foreperiod and time-out intervals were arranged for each cat to achieve false-alarm rates of more than 10% and less than 35%.
FIGURE 8. Pre-lesion psychometric functions for intensity discrimination of pure tones at (a) 1 kHz and (b) 8 kHz.
Signal detection methods were used to derive d′ values from the percentage of hits at each intensity change and overall false-alarm rates, which are plotted at the 0-dB intensity change (squares). The resulting d′ values are shown in (c) for tests with 1-kHz tones and in (d) for tests with 8-kHz tones.
Psychometric functions for intensity discrimination of 8-kHz tones in quiet are shown in Fig. 8(b). These functions reflect 877 trials for cat 91cv3 and 601 trials for cat 91cp2. Cats performed more trials within a testing session and produced more stable daily thresholds for 8-kHz tones than 1-kHz tones; consequently, performance in this stimulus condition was measured in fewer sessions. The psychometric functions of both subjects rise rapidly and exceed 90% correct discriminations for intensity changes as small as 5 dB. False-alarm rates for trials with 8-kHz tones were roughly equivalent to those for trials with 1-kHz tones.
The relationship of the d′ index of sensitivity to the magnitude of 1-kHz intensity changes is plotted in Fig. 8(c). d′ values were derived from the subjects’ probability of hits for each intensity change and overall probability of false alarms. These data were taken from the psychometric functions in Fig. 8(a). Discrimination thresholds for 1-kHz tones (i.e., the intensity change predicted to yield a d′ of 1) were interpolated from the functions in Fig. 8(c) and indicated ΔI values of 1.33 dB for cat 91cv3 and 2.18 dB for cat 91cp2. A similar analysis was performed on the 8-kHz d′ functions shown in Fig. 8(d). These data produced ΔI values of 1.32 dB for cat 91cv3 and 2.45 dB for cat 91cp2. Threshold values for both subjects compared favorably with those reported in independent psychophysical studies of pure-tone intensity discrimination in cats (Igarashi et al. 1979b, Oesterreich et al. 1971).
Intensity discrimination in quiet was evaluated in three additional cats by requiring these subjects to detect a fixed 3-dB increase in the level of a 65-dB SPL standard tone. The results of tests with tone frequencies of 1 and 8 kHz are shown in Fig. 9(a) and (b); data summarizing all tests with fixed 3-dB intensity changes are presented in Table II. The d′ values that were obtained from cats 91cv3 and 91cp2 during tests with 3-dB intensity changes have been added to the figure and table.
FIGURE 9. The d′ index of discriminability for pre-lesion detection of a 3-dB intensity change at 1 kHz (a) and 8 kHz (b).
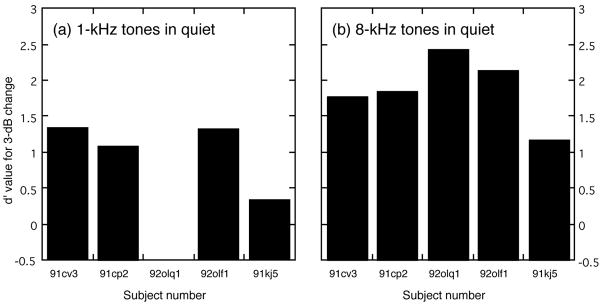
Cat 92olq1 would not perform the task when tested with 1-kHz tones.
TABLE II.
Summary of d′ Values for Discrimination of 3-dB Intensity Changes in Quiet and in 8-dB Background Noise, Before and After OCB lesions
| Cat | Lesion | 1-kHz tones | 8-kHz tones | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Quiet | Noise | Quiet | Noise | ||||||
|
| |||||||||
| Before | After | Before | After | Before | After | Before | After | ||
| 91cv3 | complete | 1.36 | 1.10 | 0.51 | 0.54 | 1.78 | 1.65 | 1.85 | 1.24 |
| 91cp2 | complete | 1.10 | 1.17 | 0.41 | 0.60 | 1.86 | 1.70 | 2.18 | 1.46 |
| 92olq1 | unilateral | — | — | — | — | 2.44 | 2.45 | 2.49 | 2.13 |
| 92olf1 | incomplete | 1.34 | 1.31 | 0.54 | 0.52 | 2.15 | 2.07 | 2.34 | 2.42 |
| 91kj5 | sham | 0.36 | 0.72 | −0.10 | 0.34 | 1.18 | 1.07 | 1.26 | 1.24 |
| All cats | |||||||||
| Mean | 1.04 | 1.08 | 0.34 | 0.50 | 1.88 | 1.79 | 2.02 | 1.70 | |
| Standard deviation | 0.47 | 0.25 | 0.30 | 0.11 | 0.47 | 0.51 | 0.49 | 0.54 | |
| Lesioned cats | |||||||||
| Mean | 1.23 | 1.14 | 0.46 | 0.57 | 2.03 | 1.93 | 2.17 | 1.61 | |
| Standard deviation | 0.18 | 0.05 | 0.07 | 0.04 | 0.36 | 0.45 | 0.32 | 0.46 | |
Every cat found discrimination of a 3-dB ΔI more difficult at 1 kHz than at 8 kHz, but the increased difficulty for tests with 1-kHz tones was especially apparent for cats 91kj5 and 92olq1 whose performance stayed near chance levels (d′ = 0) even after several months of training. Cat 92olq1 would complete only a small number of trials during a 1-kHz testing session before refusing to perform the task. This result was not predicted by earlier behavioral studies (Rosenzweig 1946, Raab and Ades 1946, Elliot and McGee 1965), which showed best intensity discrimination at low frequencies for cats. Although hearing thresholds were not obtained in our subjects (this would require lengthy training and testing in another operant task), two experimental observations suggest that poor auditory sensitivity did not create the discrimination deficit in this animal. During preliminary testing in other cats, discrimination performance was not negatively affected by decreasing the sensation level of the standard tone by 40 dB. Additionally, cat 92olq1 was able to discriminate 5-dB intensity increments at 65 dB SPL with a high degree of accuracy.
Omitting subject 92olq1 from the analysis, the mean d′ value for a 3-dB intensity change was 1.04 (±0.47 SD) at 1 kHz and 1.74 (±0.41 SD) at 8 kHz. The difference in performance that was observed for testing with 1 and 8-kHz tones was found to be statistically significant (P < 0.01, Student’s t test using paired one-tailed analysis). Cat 92olq1 showed good discrimination sensitivity for 8-kHz intensity changes; inclusion of this cat’s data increased mean performance for tests with 8-kHz tones to a d′ value of 1.88 (±0.47).
Effects of Background Noise on Intensity Discrimination
Preliminary measures of the effects of noise on intensity discrimination were obtained by requiring the first group of cats (91cv3 and 91cp2) to detect a fixed 3-dB intensity change in different levels of continuous broadband background noise. As in testing under quiet conditions, the intensity change was conveyed by pure tones of 1 and 8 kHz. Cats were first tested in low levels of background noise; then louder noise levels were introduced as cats proved able to perform the task. Final performance was evaluated in six levels of background noise ranging from −7 to 23 dB spectrum level. These noise levels were randomly selected on a trial-wise basis with equal probability of selection for all levels during the same testing session. The discrimination of 1-kHz intensity changes in background noise proved to be a challenging task for all cats, even at the lowest noise levels; consequently, the range of background noise levels did not extend to spectrum levels as high as those for tests with 8-kHz tones.
Psychometric functions for discrimination of the 3-dB ΔI cue in different levels of background noise are shown in Fig. 10(a) and (b). One indication of the difficulty of discrimination in noise was the high levels of false alarms exhibited by both cats. Cat 91cv3 averaged 35% false alarms and showed the majority of incorrect releases in high noise levels. Cat 91cp2 displayed an average false-alarm rate of 37% and exhibited most false alarms in low noise levels. The percentage of hits and false alarms were analyzed separately for each noise level to correct for these response biases.
FIGURE 10. Pre-lesion intensity discrimination in six levels of background noise and in quiet for tests with 1-kHz tones (a) and 8-kHz tones (b).
Percentages of hits (solid lines) and false alarms (dashed lines) are presented for each stimulus condition. d′ values derived from the psychometric functions are shown for 1-kHz tones (c) and for 8-kHz tones (d).
d′ values were determined for intensity discrimination in noise by applying Eq. 1 to each pair of hits and false alarms shown in Fig. 10(a) and (b). These values are presented in Fig. 10(c) for tests with 1-kHz tones and in Fig. 10(d) for tests with 8-kHz tones. The importance of signal detection analysis for these behavioral data is especially evident for the discrimination of 1-kHz tones in noise. Although both cats showed average hit rates above 50% for this testing condition, high false-alarm rates resulted in near chance d′ measures (i.e., d′ = 0). A more complex pattern of false alarm responding is apparent for tests with 8-kHz tones, but both cats showed good discrimination of the 3-dB intensity change at intermediate noise levels.
Intensity discrimination may decline in our noise paradigm not only because of the negative effects of noise on the neural representation of acoustic signals but also because any task is made more difficult by a roving background condition. This possibility was examined by presenting quiet backgrounds instead of noise on approximately 15% of trials during experiments with noise. The left-most symbols in Fig. 10(a) and (b) indicate the percentage of hits (circles) and false alarms (squares) for these tests. Intensity discrimination in quiet for cat 91cv3 was relatively unaffected when measured in conjunction with tests in background noise, but the performance of 91cp2 declined significantly under these conditions. A substantial increase in false-alarm rates contributed to this subject’s loss of discrimination sensitivity.
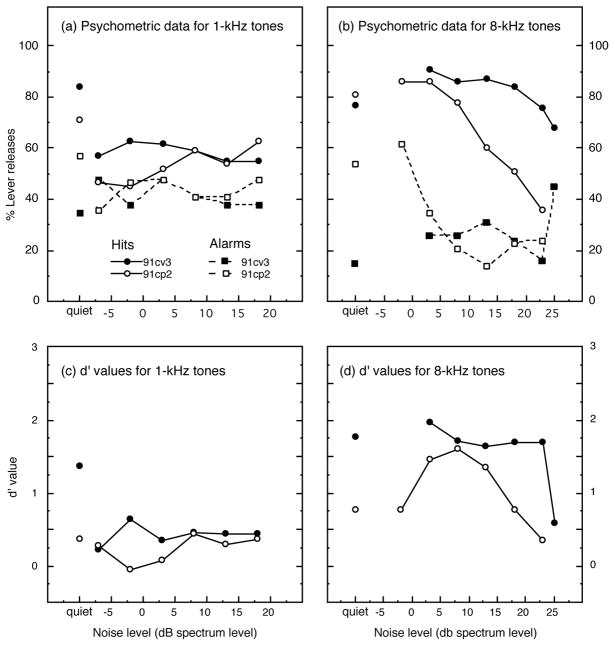
The second group of cats (92olq1, 92olf1 and 91kj5) was tested with a fixed 3-dB intensity change in a constant noise spectrum level of 8 dB to reduce the demands of the roving noise level procedure. The results of these tests are shown in Fig. 11, which includes d′ values obtained from cats 91cv3 and 91cp2 using the roving noise procedure. The strong negative effects of background noise on the discrimination of 1-kHz intensity changes are described in Fig. 11(a). Cat 92olq1 would not perform the task under these testing conditions; cat 91kj5 did not attain above-chance levels of discrimination. Omitting cat 92olq1 from the analysis, the average d′ of the four remaining subjects showed a statistically significant 67% decrease relative to performance in quiet (P < 0.005, Student’s t test using paired one-tailed analysis). In contrast, all five cats performed the discrimination well for 8-kHz intensity changes in the noise background, as shown in Fig. 11(b). The average d′ value for discrimination in noise increased by approximately 7% relative to performance in quiet. Although the difference between discrimination in quiet and noise was small for 8-kHz tones, all cats showed at least some improvement and this modest increase proved to be statistically significance (P < 0.05).
FIGURE 11. d′ values for pre-lesion detection of pure-tone intensity changes in the presence of continuous broadband noise with 8-dB spectrum level.
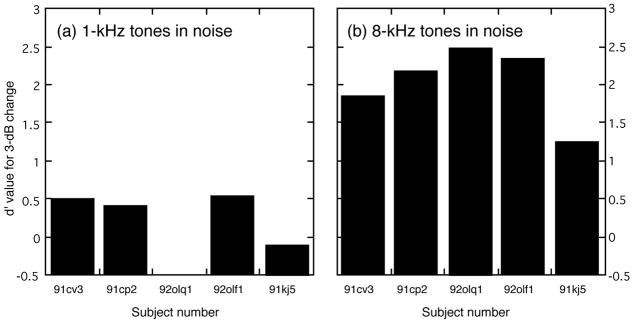
A fixed 3-dB change was conveyed by 1-kHz tones (a) and 8-kHz tones (b). All cats exhibited less sensitivity (i.e., lower d′ values) for the discrimination of low-frequency tones in noise; cat 92olq1 would not perform the task when tested under this stimulus condition.
Effects of Olivocochlear Lesions on Intensity Discrimination in Quiet
The effects of olivocochlear lesions on pure-tone intensity discrimination were measured under quiet testing conditions using a fixed 3-dB intensity change. The results of these tests are shown in Fig. 12. Subjects are ordered from left to right in this figure according to the completeness of the OCB lesion. Cats 91cv3 and 91cp2 received substantial bilateral lesions. Cat 92olq1 received an extensive unilateral lesion. The two remaining subjects maintained relatively intact olivocochlear systems because the lesions were placed too far caudal in cat 92olf1 and because of an intentional sham procedure in cat 91kj5. Each cat’s performance before the lesion is indicated by filled histograms; performance after the lesion is indicated by the stippled pattern. The effects of efferent lesions on the discrimination of 1-kHz intensity changes are shown in Fig. 12(a) and indicate a small performance deficit for cat 91cv3 and little change for cat 91cp2. Meaningful comparisons of pre and post-lesion performance are not available for the remaining three cats because cat 92olq1 failed to discriminate 1-kHz intensity changes even before the lesion, cat 92olf1 was not completely lesioned and showed not change in performance, and cat 91kj5 displayed better discrimination after an intentional sham lesion.
FIGURE 12. Effects of OCB lesions on the discrimination of 3-dB intensity changes in quiet for 1-kHz tones (a) and 8-kHz tones (b).
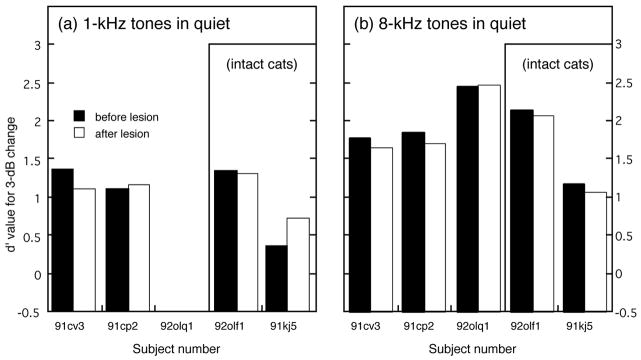
Three cats with more complete lesions (91cv3, 91cp2 and 92olq1) showed no consistent performance deficits for the discrimination of 1-kHz tones and only slight deficits for 8-kHz tones. Cat 92olf1 was relatively unaffected by an incomplete lesion, and cat 91kj5 exhibited better discrimination of low-frequency intensity changes after receiving a sham lesion.
OCB lesions resulted in small negative effects on the discrimination of 8-kHz intensity changes. As shown in Fig. 12(b), the average d′ measure decreased 5% from a pre-lesion value of 2.03 (±0.36 SD) to a post-lesion value 1.93 (±0.45 SD) for the three cats with more complete lesions. This decrease was not statistically significant, and changes of similar magnitude were noted for the incompletely lesioned cat (92olf1) and the sham-lesioned cat (91kj5). The relatively minor effects of OCB lesions on discrimination in quiet indicate that surgical procedures did not create general deficits that impaired the subjects’ ability to perform the behavioral task.
Effects of Olivocochlear Lesions on Intensity Discrimination in Noise
All cats were tested with a fixed 3-dB intensity change in 8-dB noise after bilateral olivocochlear lesions. The results of these tests are described in Fig. 13. As shown in Fig. 13(a), no significant lesioning effects were noted for the discrimination of 1-kHz tones in noise, although the sham-lesioned cat 91kj5 exhibited an improvement in performance that most likely resulted from increased experience in the task. In contrast, at 8 kHz, d′ values for the three cats with more complete lesions (91cv3, 91cp2 and 92olq1) fell 26% from an average pre-lesion value of 2.17 (±0.32 SD) to a post-lesion value of 1.61 (±0.46 SD). This decrease in performance, which is shown in Fig. 13(b), was the only statistically significant behavioral deficit that resulted from OCB lesions (P <0.05). Cats 92olf1 (incomplete lesion) and 91kj5 (sham lesion) displayed no change in their ability to discriminate 8-kHz intensity changes in noise. The stability of performance before and after the lesioning procedure for these two intact cats is another indication that general surgical impairments did not contribute to intensity discrimination deficits shown by more completely lesioned subjects.
FIGURE 13. Effects of OCB lesions on the discrimination of pure-tone intensity changes in 8-dB spectrum level background noise.
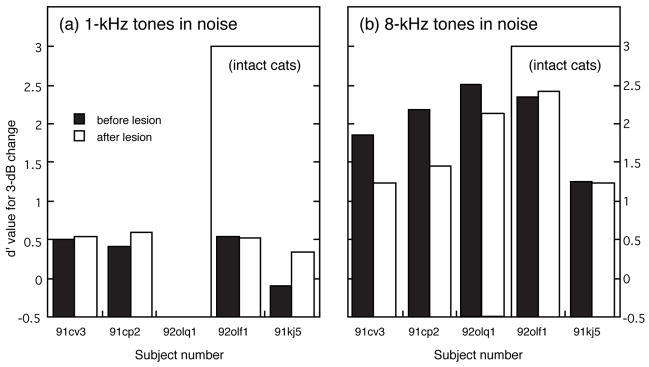
(a) All five cats showed no change or improved performance for the discrimination of a 3-dB intensity change at 1 kHz. (b) Cats with more complete lesions (91cv3, 91cp2, and 92olq1) exhibited consistent deficits in the discrimination of 8-kHz intensity changes in noise. Cats with intact olivocochlear projections (92olf1 and 91kj5) displayed no change in discrimination after the surgical procedure.
DISCUSSION
The principal finding of this study is that bilateral olivocochlear lesions induced auditory deficits that were restricted to the discrimination of intensity changes at mid frequencies (8 kHz) and were apparent only when behavioral performance was measured in continuous broadband noise. No consistent lesioning effects were observed for discrimination at low frequencies (1 kHz) in quiet or in noisy testing environments. The following discussion interprets these results in terms of previous behavioral studies, olivocochlear influences on response properties of afferent neurons, and inter-subject variability of the anatomy and physiology of the olivocochlear system.
Comparisons with Previous Behavioral Studies
The importance of olivocochlear feedback in the auditory processing of intensity information was demonstrated by measuring the accuracy of pure-tone intensity discrimination before and after bilateral lesions were placed in the olivocochlear efferent pathway. The behavioral deficits that resulted from these lesions do not contradict previous observations of Igarashi et al. (1979b) who found minimal deficits in an intensity discrimination task using an ablation paradigm that was very similar to our own. A critical difference between these two behavioral studies is that Igarashi and colleagues evaluated the effects of OCB lesions only under quiet testing conditions. Like Igarashi’s study, our investigations failed to find behavioral deficits in lesioned cats when discrimination performance was measured in quiet, but the same experimental animals showed a significant loss of the ability to discriminate intensity changes when performance was measured in background noise. Igarashi and colleagues were also unable to demonstrate OCB lesioning effects, exceeding what they termed normal biological variation, in behavioral experiments measuring absolute hearing thresholds in quiet (1972), visual discrimination in noise (1974, 1977), and frequency discrimination in quiet (1979a).
Studies of the effects of OCB lesions in primates have described large deficits in the discrimination of pure-tone frequency (Capps and Ades 1968) and human vowel sounds in noise (Dewson 1968). Some doubt must be raised about the reliability of the behavioral methods that were used by Capps and Ades because pre-lesion frequency discrimination thresholds for their subjects are a full order of magnitude higher than those consistently reported in other primate studies (Moody et al. 1986). Consequently, for many years the Dewson study has stood alone as a credible behavioral demonstration of a functional role for olivocochlear efferent feedback in hearing. Dewson’s early success may have resulted from the fortuitous decision to measure vowel discrimination in background noise.
In another early behavioral study of the olivocochlear system, Trahoitis and Elliot (1968) used OCB lesioning paradigms to evaluate the effects of efferent feedback on absolute thresholds, masked thresholds, and noise-induced threshold shifts. No significant performance deficits were observed in those experiments. Although absolute thresholds in quiet were obtained in octave steps spanning frequencies from 0.125 – 16.0 kHz, noise masking effects were only evaluated at 2 kHz and below. Our study of intensity discrimination also found no significant lesioning effect for tests with low-frequency tones. One explanation for the frequency specificity of these olivocochlear influences is offered by changes in the relationship of discharge rate to stimulus level that occur among auditory-nerve fibers (ANFs) when the OCB is stimulated with electrical current (Wiederhold 1970, Guinan and Gifford 1988). ANFs with mid-frequency tuning characteristics (8 – 10 kHz) shift their rate-level functions to higher stimulus levels and increase their maximum discharge rates during OCB stimulation. Fibers that respond best to lower or higher frequencies are less sensitive to such efferent manipulations. Anatomical studies suggest that the strength of olivocochlear influences on ANF responses may be related to the number and area of medial olivocochlear efferent terminals on outer hair cells, both of which reach a maximum at mid-frequency regions of the cat’s cochlea (Liberman et al. 1990). Sparse efferent innervation in apical turns of the cochlea may explain why background noise decreased our subjects’ ability to discriminate intensity changes at low frequencies (1 kHz) even before OCB lesions, as well as the absence of further performance deficits after lesions. It is not presently known if OCB lesions increase noise masking effects at mid frequencies although this result is predicted by physiological evidence.
An unexpected result was that cats found the discrimination of a fixed 3-dB intensity change to be more difficult when the ΔI cue was conveyed by a 1-kHz tone. The average d′ of four cats was 40% less at 1 kHz than at 8 kHz for tests in quiet and 82% less for tests in noise. The remaining cat would not perform the task at all when tested with low-frequency tones. Other psychophysical studies in cats have reported intensity discrimination thresholds to increase with stimulus frequency (Rosenzweig 1946, Raab and Ades 1946, Elliot and McGee 1965). Thresholds for cats 91cv3 and 91cp2 compared favorably with those previous results at low frequencies but were lower at mid frequencies. Our subjects may have performed the discrimination task more accurately with 8-kHz tones because the operant procedure that was used in this study restrained the subject at a fixed location in the sound field and therefore maintained good stimulus control. In contrast, the studies cited above used avoidance procedures in which cats escaped electric shocks by crossing a barrier in the testing apparatus. Movement around the testing arena results in poor stimulus control for high-frequency tones and may have contributed to increased thresholds.
An alternative explanation for our subjects’ difficulty with low-frequency intensity discrimination is that our “quiet” testing condition may have been contaminated by low levels of ambient noise. However, uncontrolled ambient noise is unlikely in these tests because our testing apparatus was situated in a double-walled sound chamber (Industrial Acoustics Company) with an inner lining of 3-inch anechoic foam (Sonex). Simultaneous free-field electrophysiological experiments obtained single-unit thresholds as low as −20 dB SPL without indication of unusual sustained noise-driven activity in the chamber (May and Sachs 1992).
Olivocochlear Influences on the Neural Representation of Pure-Tone Intensity
When tested with a range of pure-tone stimulus levels, auditory-nerve fibers undergo a rapid transition from spontaneous rates of activity at subthreshold stimulus levels to saturated rates at levels 30 – 50 dB above threshold. This transitional phase of the neuron’s rate-level function indicates the range of levels unambiguously encoded by changes in discharge rate and is designated the dynamic range of the neural response. Costalupes et al. (1984) observed that dynamic range properties of ANFs in anesthetized cats are compressed by the adaptive effects of background noise. That is, intensity information within the neuron’s dynamic range is represented by a smaller maximum rate change in the presence of noise. If stimulus level is encoded by the neuron’s discharge rate, as most neural models of intensity discrimination propose (Delgutte 1987, Winslow and Sachs 1988, Viemeister 1988), rate compression should result in a loss of discrimination accuracy. Electrical stimulation of the olivocochlear efferent pathway reduces the compressive effects of noise on ANF rate responses and therefore potentially preserves the neural representation of stimulus intensity in noise (Winslow and Sachs 1987).
Efferent action was elicited by electrical stimulation in most early studies of the olivocochlear system because traditional monaural acoustic stimulation failed to achieve physiologically relevant discharge rates (Liberman and Brown 1986). Liberman (1988) recently determined that binaural acoustic stimulation can increase the rate of responding in some efferent neurons by as much as 85%. Continuous noise in the opposite ear can add another 10 spikes/sec to the overall discharge rate. The same contralateral acoustic paradigms that effectively activate olivocochlear efferents lead to a reduced sensitivity of ANF rate responses, presumably through contributions of olivocochlear feedback mechanisms (Warren and Liberman 1989a, 1989b). These efferent influences shift the dynamic portion of the rate-level function to a higher range of stimulus levels and may help preserve the neural representation of acoustic signals in noise because less responsive ANFs are not as likely to be driven to saturation by noise alone. Free-field binaural tone bursts and continuous background noise were used as stimuli in our behavioral paradigm to promote such efferent effects.
The decrease in auditory nerve sensitivity that results from olivocochlear efferent activity is also reflected by a decrease in the magnitude of gross auditory potentials. In early studies of this phenomenon (Galambos 1956, Desmedt 1962), olivocochlear feedback was produced in anesthetized or paralyzed cats by delivering electrical shocks to the floor of the IVth ventricle, but Liberman (1989) has recently used contralateral sound suppression of the compound action potential (CAP) as a technique for rapid assessment of olivocochlear feedback. The characteristics of the contralateral stimulus that evoked most effective suppression was related to the frequency of the pure tone that elicited the CAP. Contralateral tones suppressed CAPs elicited by low-frequency stimuli (≤ 3 kHz); whereas, contralateral noise produced maximum suppression of CAPs for high-frequency stimuli (≥ 6 kHz). The strength of suppression varied substantially between cats and rarely exceeded an 8-dB change in sensitivity. Liberman also demonstrated that contralateral sound suppression of the CAP can be abolished by transecting the OCB. Our study used this electrophysiological technique to confirm the completeness of olivocochlear lesions at the conclusion of behavioral testing, but CAP monitoring can also be used intra-operatively in future behavioral studies to avoid incomplete lesions, such as those discovered in cat 92olf1 at the conclusion of behavioral testing.
Surgical lesions on the floor of the IVth ventricle may also damage the facial nerve innervation of stapedial motor neurons. Contraction of these muscles increases the stiffness of the ossicular chain and may attenuate sound conduction in the middle ear by as much as 20 dB (Pang and Peake, 1986). The middle-ear reflex can be activated by loud contralateral sound and therefore potentially contributes to contralateral sound suppression of the CAP. Several lines of evidence, however, suggest that it is unlikely that surgically induced changes of the middle-ear reflex contributed to lesioning effects shown by electrophysiological methods in Fig. 7 or behavioral data in Fig. 13. Contralateral sound suppression of auditory nerve activity (Warren and Liberman, 1989) and CAP magnitude (Liberman, 1989) is similar in cats with and without intact middle-ear muscles and occurs at sound levels well below the threshold for contralateral activation of the middle-ear reflex (Guinan and McCue, 1987). In addition, because stiffness factors mediate the transmission of low-frequency sounds through the middle ear, the stapedial reflex exerts its strongest influences at frequencies below 1–2 kHz. Our subjects showed little or no lesioning effects at these low frequencies and substantial deficits at 8 kHz.
Inter-Subject Variability
Four cats with olivocochlear lesions exhibited behavioral deficits of varying degree when discriminating 8-kHz intensity changes in continuous background noise. This inter-subject variability was directly related to the completeness of OCB lesions. d′ values for cats 91cv3 and 91cp2 fell by 33% after the most complete bilateral lesions. A smaller 14% decrease was noted for cat 92olq1 after a unilateral lesion of efferent projections to the left cochlea. Incomplete and sham lesions had little effect on the performance of cats 92olf1 and 91kj5. These behavioral results emphasize the critical importance of anatomical and electrophysiological confirmation of OCB lesions when evaluating discrimination performance.
Although the degree of lesion proved to be a good predictor for the magnitude of post-lesion performance deficits, our subjects also showed substantial variability in discrimination accuracy prior to receiving OCB lesions. This behavioral variability is not surprising given the extensive anatomical and physiological differences that previous studies of the olivocochlear efferent system have noted within and across species. Arnesen and Osen (1984) counted olivocochlear fibers in 12 vestibulocochlear anastomoses of nine cats. Within their sample, the total number of myelinated (MOC) and unmyelinated (LOC) efferent projections to one ear ranged from as few as 860 fibers to as great as 1810 fibers. Although the functional implications of such individual differences are not known, these results predict that some cats are less endowed with efferent feedback and therefore may be less able to process auditory signals in the presence of background noise. Cat 91kj5, for example, showed consistently low d′ values for intensity discrimination of 8-kHz tones in noise. Based on this poor performance, we suspected that cat 91kj5 may have entered the study with fewer efferent neurons than other subjects. The cat was sham lesioned to preserve its natural pattern of efferent innervation and cochleae were processed for AChE at the conclusion of behavioral testing. Light microscopic examination found silver labeling in the unlesioned cochleae of this cat suggesting the presence of efferent fibers, but the density of staining was less than that observed in intact controls and cat 92olq1 with the misplaced lesion. The AChE technique is not a highly quantitative measure but when combined with the behavioral performance of cat 91kj5, these anatomical results do suggest functional deficits may arise from normal inter-subject variation in efferent innervation.
Liberman and Brown (1986) observed a similar high degree of inter-subject variability when recording physiological responses of single olivocochlear fibers in the vestibulocochlear anastomosis of anesthetized cats. Less responsive animals in their study were characterized by olivocochlear neurons showing a pattern of activity which included high thresholds, depressed spontaneous rates, and low discharge rates when driven by sound stimuli; the opposite characteristics were noted in other subjects. Such differences were not apparent for cochlear afferents within the same group of animals. Although some of the physiological properties that distinguish the responsiveness of olivocochlear neurons are likely to be related to experimental factors such as depth of anesthesia or body temperature, longterm increases in efferent activity have also been observed after exposure to loud noise (Liberman 1988). Therefore, more responsive efferent systems may reflect the animal’s history of exposure to noise stimuli or even sounds arising from surgical instruments such as drill motors, vacuum pumps, or aspirators. This physiological evidence suggests that olivocochlear feedback may assume a more active role during auditory processing in noisy environments. Our results support this conclusion by demonstrating OCB-lesioning deficits that are specific to behavioral testing in continuous background noise.
Although our experiments required substantial stimulus specificity to demonstrate the effects of efferent lesions on auditory discrimination, these results should not be interpreted as evidence that olivocochlear feedback evolved only to enhance the auditory processing of 8-kHz tones in background noise. Rate responses that encode the level of simple stimuli like pure tones can be combined across populations of neurons to create neural representations of complex natural sounds like human speech. Single-unit responses in the auditory nerve of anesthetized cats suggest that such coding schemes are degraded in the presence of background noise because the rate responses of neurons with low thresholds and high spontaneous rates are easily saturated by noise-driven activity (Sachs et al. 1983). Efferent feedback in normally functioning animals may reduce neural sensitivity and thus preserve the rate representation of complex sounds in noise (May et al. 1994). This hypothesis is being tested in our current study of the effects of bilateral olivocochlear lesions on vowel discrimination in cats.
Acknowledgments
The authors thank Drs. M. C. Liberman and M. C. Brown for their guidance during the development of techniques for performing and confirming OCB lesions. The laboratory of Dr. David Ryugo provided both expertise and equipment for AChE staining procedures and photomicrographs. Cynthia Aleszczyk and Andrew Lavoie assisted in training and testing of our behavioral subjects. This research was supported by NIDCD research grant DC00954 and training grant DC00027.
References
- Arnesen AR, Osen KK. Fiber population of the vestibulocochlear anastomosis in the cat. Acta Otolaryngol. 1984;98:225–269. doi: 10.3109/00016488409107562. [DOI] [PubMed] [Google Scholar]
- Capps MJ, Ades HW. Auditory frequency discrimination after transection of the olivocochlear bundle in squirrel monkeys. Exp Neurol. 1968;21:147–158. doi: 10.1016/0014-4886(68)90133-7. [DOI] [PubMed] [Google Scholar]
- Costalupes JA, Young ED, Gibson DJ. Effects of continuous noise backgrounds on rate responses of auditory nerve fibers in cat. J Neurophysiol. 1984;51:1326–1344. doi: 10.1152/jn.1984.51.6.1326. [DOI] [PubMed] [Google Scholar]
- Delgutte B. Peripheral auditory processing of speech information: Implications from a physiological study of intensity discrimination. In: Schouton MEH, editor. Psychophysics of Speech Perception. Martinus Nijhoff; Dordrecht, The Netherlands: 1987. pp. 333–353. [Google Scholar]
- Desmedt JE. Auditory-evoked potentials from cochlea to cortex as influences by activation of the efferent olivo-cochlear bundle. J Acoust Soc Am. 1962;34:1478–1496. [Google Scholar]
- Dewson JH. Efferent olivocochlear bundle: Some relationships to stimulus discrimination in noise. J Neurophysiol. 1968;31:122–130. doi: 10.1152/jn.1968.31.1.122. [DOI] [PubMed] [Google Scholar]
- Elliot DN, McGee TM. Effect of cochlear lesions upon audiograms and intensity discrimination in cats. Ann Otol Rhinol Laryngol. 1965;74:386–408. doi: 10.1177/000348946507400209. [DOI] [PubMed] [Google Scholar]
- Galambos R. Suppression of auditory nerve activity by stimulation of efferent fibers to cochlea. J Neurophysiol. 1956;19:424–437. doi: 10.1152/jn.1956.19.5.424. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, McCue MP. Asymmetries in the acoustic reflexes of the cat stapedius muscle. Hear Res. 1987;26:1–10. doi: 10.1016/0378-5955(87)90031-1. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Gifford ML. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. I. Rate-level functions. Hear Res. 1988;33:97–114. doi: 10.1016/0378-5955(88)90023-8. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Alford BR, Nakai Y, Gordon WP. Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat. I. Pure-tone threshold and perceptual signal-to-noise ratio. Acta Otolaryng. 1972;73:455–466. doi: 10.3109/00016487209138966. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Alford BR, Gordon WP, Nakai Y. Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat. II. Conditional visual performance with intense white noise. Acta Otolaryng. 1974;77:311–317. doi: 10.3109/00016487409124630. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Cranford JL, Nakai Y, Alford BR. Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat. III. Further study of ambient light intensity discrimination under intense noise. Acta Otolaryng. 1977;83:410–416. doi: 10.3109/00016487709128865. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Cranford JL, Nakai Y, Alford BR. Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat. IV. Study on pure-tone frequency discrimination. Acta Otolaryng. 1979a;87:79–83. doi: 10.3109/00016487909126390. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Cranford JL, Allen EA, Alford BR. Behavioral auditory function after transection of crossed olivo-cochlear bundle in the cat. V. Pure-tone intensity discrimination. Acta Otolaryng. 1979b;87:429–433. doi: 10.3109/00016487909126446. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Response properties of cochlear efferent neurons: Monaural vs. binaural stimulation and the effects of noise. J Neurophysiol. 1988;60:1779–1798. doi: 10.1152/jn.1988.60.5.1779. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Rapid assessment of sound-evoked olivocochlear feedback: Suppression of compound action potentials by contralateral sound. Hear Res. 1989;38:47–56. doi: 10.1016/0378-5955(89)90127-5. [DOI] [PubMed] [Google Scholar]
- Liberman MC. Effects of chronic cochlear de-efferentation on auditory-nerve response. Hear Res. 1990;49:209–224. doi: 10.1016/0378-5955(90)90105-x. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res. 1986;24:17–36. doi: 10.1016/0378-5955(86)90003-1. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: Quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- May BJ, Sachs MB. Dynamic range of neural rate responses in the ventral cochlear nucleus of awake cats. J Neurophysiol. 1992;68:1589–1602. doi: 10.1152/jn.1992.68.5.1589. [DOI] [PubMed] [Google Scholar]
- May BJ, LePrell GS, Jain H, Sachs MB. Representation of vowel sounds in the ventral cochlear nucleus of awake cats. Abstracts of the ARO. 1994;17:16. [Google Scholar]
- May BJ, Huang AY, Aleszczyk CM, Hienz RD. Design and Conduct of Sensory Experiments for Domestic Cats. In: Dooling R, Fay R, Klump G, Stebbins W, editors. Methods in Comparative Psychoacoustics. Basil, Switzerland: Birkhäuser Verlag; 1995. pp. 95–108. [Google Scholar]
- Moody DB, May BJ, Cole DM, Stebbins WC. The role of frequency modulation in the perception of complex stimuli by primates. J Exp Biol. 1986;45:219–232. [PubMed] [Google Scholar]
- Musicant AD, Chan JCK, Hind JE. Direction-dependent spectral properties of cat external ear: New data and cross-species comparisons. J Acoust Soc Am. 1990;87:757–781. doi: 10.1121/1.399545. [DOI] [PubMed] [Google Scholar]
- Osen KK, Roth K. Histochemical localization of cholinesterases in the cochlear nuclei of the cat, with notes on the origin of acetylcholinesterase-positive afferents and the superior olive. Brain Res. 1969;16:165–185. doi: 10.1016/0006-8993(69)90092-4. [DOI] [PubMed] [Google Scholar]
- Osterreich RE, Strominger NL, Neff WD. Neural structures mediating differential sound intensity discrimination in the cat. Brain Res. 1971;27:251–270. doi: 10.1016/0006-8993(71)90252-6. [DOI] [PubMed] [Google Scholar]
- Pang XD, Peake WT. How do contractions of the stapedius muscle alter acoustic properties of the ear? In: Allen JB, Hall JL, Hubbard A, Neely ST, Tubis A, editors. Peripheral Auditory Mechanisms. Berlin: Springer; 1986. pp. 36–43. [Google Scholar]
- Raab DH, Ades HW. Cortical and midbrain mediation of a conditioned discrimination of acoustic intensities. Amer J Psychol. 1946;59:59–83. [PubMed] [Google Scholar]
- Rasmussen GL. The olivary peduncle and other fiber connections of the superior olivary complex. J Comp Neurol. 1946;84:141–219. doi: 10.1002/cne.900840204. [DOI] [PubMed] [Google Scholar]
- Rasmussen GL. Efferent fibers of the cochlear nerve and cochlear nucleus. In: Rasmussen GL, Windle WF, editors. Neural Mechanisms of the Auditory and Vestibular Systems. CC Thomas; Springfield, IL: 1960. pp. 105–115. [Google Scholar]
- Rice JJ, May BJ, Spirou GA, Young ED. Pinna-based spectral cues for sound localization in cat. Hear Res. 1994;58:132–152. doi: 10.1016/0378-5955(92)90123-5. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR. Discrimination of auditory intensities in the cat. Amer J Psychol. 1946;59:127–136. [PubMed] [Google Scholar]
- Sachs MB, Voigt HF, Young ED. Auditory nerve representation of vowels in background noise. J Neurophysiol. 1983;50:27–45. doi: 10.1152/jn.1983.50.1.27. [DOI] [PubMed] [Google Scholar]
- Sokolich WG. Improved acoustic system for auditory research. J Acoust Soc Am. 1977;62:12. [Google Scholar]
- Trahoitis C, Elliot DN. Behavioral investigation of some possible effects of sectioning the crossed olivocochlear bundle. J Acoust Soc Am. 1968;47:592–596. doi: 10.1121/1.1911934. [DOI] [PubMed] [Google Scholar]
- Viemeister NF. Auditory intensity discrimination at high frequencies in the presence of noise. Science. 1983;221:1206–1208. doi: 10.1126/science.6612337. [DOI] [PubMed] [Google Scholar]
- Viemeister NF. Psychophysical aspects of auditory intensity coding. In: Edelman GM, Gall WE, Cowan WM, editors. Auditory Function: Neurobiological Bases of Hearing. John Wiley and Sons; New York: 1988. pp. 213–241. [Google Scholar]
- Warr WB. Olivocochlear and vestibular efferent neurons of the feline brain stem: their location, morphology and number determined by retrograde axonal transport and acetylcholine histochemistry. J Comp Neurol. 1975;161:159–182. doi: 10.1002/cne.901610203. [DOI] [PubMed] [Google Scholar]
- Warr WB, Guinan JL. Efferent innervation of the organ of Corti: Two separate systems. Brain Res. 1979;173:152–155. doi: 10.1016/0006-8993(79)91104-1. [DOI] [PubMed] [Google Scholar]
- Warren EH, Liberman MC. Effects of contralateral sound on auditory-nerve responses. I. Contributions of cochlear efferents. Hear Res. 1989a;37:89–104. doi: 10.1016/0378-5955(89)90032-4. [DOI] [PubMed] [Google Scholar]
- Warren EH, Liberman MC. Effects of contralateral sound on auditory-nerve responses. II. Dependence on stimulus variables. Hear Res. 1989b;37:105–122. doi: 10.1016/0378-5955(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Wiederhold ML. Variations in the effects of electric stimulation of the crossed olivocochlear bundle on cat single auditory-nerve-fiber responses to tone bursts. J Acoust Soc Am. 1970;48:966–977. doi: 10.1121/1.1912235. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Effect of electrical stimulation of the crossed olivocochlear bundle on auditory nerve response to tones in noise. J Neurophysiol. 1987;57:1002–1021. doi: 10.1152/jn.1987.57.4.1002. [DOI] [PubMed] [Google Scholar]
- Winslow RL, Sachs MB. Single-tone intensity discrimination based on auditory-nerve rate responses in backgrounds of quiet, noise and with stimulation of the crossed olivocochlear bundle. Hear Res. 1988;35:165–190. doi: 10.1016/0378-5955(88)90116-5. [DOI] [PubMed] [Google Scholar]