Retinopathy of prematurity is a vision-threatening disease associated with abnormal retinal vascular development that occurs only in premature infants.1 Low birth weight and prematurity are strongly associated with an increased risk of the disease.2 In the Early Treatment for Retinopathy of Prematurity study, the disorder developed in 68% of premature infants born in the United States and weighing less than 1251 g; among infants with the disorder, severe retinopathy of prematurity developed in almost 37%.1 The incidence of premature births is increasing throughout the world, and with it, retinopathy of prematurity is now appearing in countries with the technology to save preterm infants. Thus, retinopathy of prematurity has become a leading cause of childhood blindness worldwide.
The management of retinopathy of prematurity is evolving. Screening and treatment interventions include frequent retinal examinations of at-risk preterm infants, laser treatment of the peripheral avascular retina in eyes with severe retinopathy of prematurity, and visual rehabilitation (Table 1 and Fig. 1).1,3 In this article, we review our changing understanding of retinopathy of prematurity, particularly its relation to oxygen use4-7 (Table 28-12), and describe current, new, and potential therapies based on mechanistic studies in models relevant to oxygen stresses in preterm infants.
Table 1.
Current Management of Retinopathy of Prematurity.
| Criteria for screening |
| United States: infants with a gestational age of ≤30 weeks or birth weight of <1500 g (and selected infants with a gesta- tional age of >30 weeks and an unstable clinical course3 |
| United Kingdom: infants with a gestational age of ≤31 weeks or birth weight of ≤1500 g |
| Canada: infants with a gestational age of ≤30 weeks, 6 days, or birth weight of ≤1250 g |
| Timing of screening and examinations |
| First examination at chronologic age of 4–6 weeks or post-gestational age of 31 weeks |
| Repeated examinations recommended by examining ophthalmologist on the basis of retinal findings and suggested schedule3 |
| Type of examination |
| Dilated binocular indirect ophthalmoscopy |
| Ongoing studies of validation and reliability of retinal imaging as a potential telemedicine alternative for screening by indirect ophthalmoscopy |
| Classification of retinopathy of prematurity determined in examinations |
| Zone (area of retinal vascularization) |
| I: vascularization within a circle centered on the optic nerve, the radius of which is twice the distance from the optic nerve to the macula |
| II: vascularization extending beyond zone I, within a circle the radius of which is the distance from the optic nerve to the nasal ora serrata |
| III: vascularization extending beyond zones I and II |
| Stage (disease severity) |
| 1: line |
| 2: ridge (with volume) |
| 3: intravitreal angiogenesis |
| 4: partial retinal detachment |
| 5: total retinal detachment |
| Plus disease: dilatation and tortuosity of retinal vessels |
| Treatment |
| Application of laser to peripheral avascular retina for type 1 retinopathy of prematurity |
| Zone I: stage 3, or stage 1 or 2 with plus disease |
| Zone II: stage 2 or 3 with plus disease |
| Under consideration, anti-VEGF agents for stage 3 and plus disease in zone I; additional study needed to determine dose, safety, and type of anti-VEGF therapy* |
| Visual rehabilitation |
| Correction often needed for associated refractive errors (ametropia and anisometropia); ongoing screening and treat ment recommended for commonly associated amblyopia or strabismus; protective eyewear and low-vision aids may be indicated |
VEGF denotes vascular endothelial growth factor.
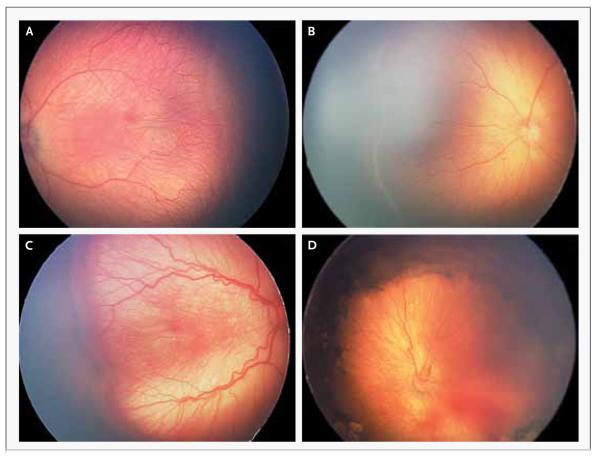
Figure 1. Stages of Retinopathy of Prematurity in Zone II in Preterm Infants.
The images were obtained with a neonatal retinal imaging system (RetCam, Clarity Medical Systems). Panel A shows a line between the vascularized and avascularized retina (stage 1). Panel 2 shows a ridge between the vascularized and avascularized retina (stage 2). Panel 3 shows a thickened ridge with aberrant intravitreal angiogenesis (stage 3). Panel 4 shows partial retinal detachment (stage 4), which is seen best at the right side of the image where the underlying choroidal vascular detail is out of focus.
Table 2.
Major Clinical Trials of Retinopathy of Prematurity.*
| Trial | Enrollment Criteria | Intervention | Outcome† |
|---|---|---|---|
| Oxygen-regulation trials | |||
| STOP-ROP8 (February 1994–March 1999) | Prethreshold retinopathy of pre- maturity in one eye |
96–99% SaO2 vs. 89–94% SaO2 | No significant difference in retinopathy of prematurity between the two groups; adverse pulmonary effects with 96–99% SaO2 |
| SUPPORT9 (February 2005–February 2009) | Gestational age 24 wk–26 wk 6 days at birth |
85–89% SaO2 vs. 91–95% SaO2 | Increased mortality with 85–89% SaO2; among survivors, lower rate of retinopathy of prematurity with 85–89% SaO2 |
| BOOST II10 (2006–2011) | Gestational age <28 wk at birth | 85–89% SaO2 vs. 91–95% SaO2 | Higher survival rate with 91–95% SaO2 |
| Conclusions from these trials: avoid high SaO2 (recommendations vary; generally agreed to keep SaO2 at <95% for the first few weeks of life), until more is known about morbidity and mortality with low SaO2 (85–89%)10 |
|||
| Treatment trials | |||
| CRYO-ROP11(January 1986–November 1987) | Birth weight <1251 g | Cryotherapy to peripheral avascu- lar retina in threshold (severe) retinopathy of prematurity |
Fewer infants with visual acuity of 20/200 or worse with cryo- therapy (44.7%) than with observation (64.3%) and lower rate of unfavorable structural outcomes at 15 yr (30% vs. 52%)‡ |
| ETROP1 (October 1999–September 2002) | Birth weight <1251 g | Laser therapy to peripheral avascular retina in type 1 retinopathy of prematurity |
Fewer infants with visual acuity of 20/200 or worse with early treatment- for severe retinopathy of prematurity than with conven- tional treatment, significant reduction in unfavorable structur- al outcomes at 6 yr§ |
| BEAT-ROP12 (March 2008–August 2010) | Birth weight <1500 g, gestational age <30 wk, stage 3 retinopa- thy of prematurity in zone I or II¶ |
0.625 mg bevacizumab in 0.025- ml solution injected into vit- reous vs. laser therapy |
Fewer cases of recurrence of stage 3 retinopathy of prematurity with bevacizumab (4%) than with laser therapy (22%); no significant difference between groups in recurrence of zone II disease; no visual-acuity outcomes (too early to assess) |
| Conclusions from these trials: treat type 1 reti- nopathy of prematurity with laser therapy; consider bevacizumab when laser therapy is not possible for zone I, stage 3 retinopathy of prematurity with plus disease |
BEAT-ROP denotes Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity, BOOST Benefits of Oxygen Saturation Targeting, CRYO-ROP Cryotherapy for Retinopathy of Prematurity, ETROP Early Treatment for Retinopathy of Prematurity, SaO2 oxygen saturation, STOP-ROP Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity, and SUPPORT Surfactant, Positive Pressure, and Pulse Oximetry Randomized Trial.
Differences are significant unless otherwise noted.
Unfavorable structural outcomes are partial or complete retinal detachment (stage 4B or 5), media opacity precluding view of macula or of posterior pole, and enucleation for any reason.
For risk of visual acuity of 20/200 or worse, there was a significant difference between groups at 9 months but not at 6 years.
The severity of retinopathy of prematurity was greater in the BEAT-ROP study than in the ETROP study.
PATHOGENESIS
During the 70 years since retinopathy of prematurity was initially described by Terry, who used the term “retrolental fibroplasia,”4 our perspective on the condition has changed. We now think that the initial 1942 description may have represented stage 5 retinopathy of prematurity, the most advanced stage of the disease, characterized by total retinal detachment. In addition, the early studies by Michaelson,5Ashton et al.,6 and Patz et al.,7 which examined the effects of high oxygen levels in newborn animal models in which the retinas normally vascularize postnatally (unlike human infants, in whom the retina is vascularized at term but not in preterm births), must now be reconsidered in light of advancements in neonatal care. Although these early investigators exposed animals to a high-oxygen milieu similar to that used in the treatment of preterm infants at the time, they did not consider the fact that the newborn animals they studied had healthy lung function. In addition, the oxygen levels used then differ considerably from those currently used in preterm infants. Ashton and colleagues reported that 70% to 80% inspired oxygen delivered continuously for at least 4 days in healthy kittens caused “vaso-obliteration” of the newly formed capillaries6; when the animals were returned to ambient air, a “vasoproliferative” effect was observed. Thus, a two-phase hypothesis of retinopathy of prematurity was developed.6
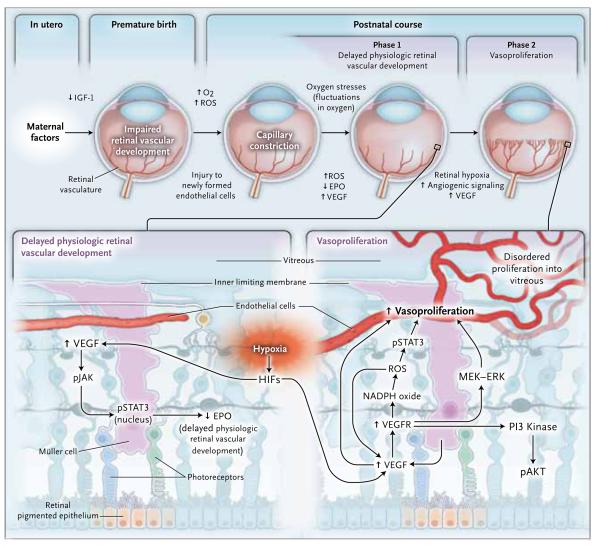
Now, with an improved understanding of the disorder from clinical examination and through the use of relevant animal models, the hypothesis has been refined: phase 1 involves delayed physiologic retinal vascular development, and phase 2 involves vasoproliferation (Fig. 2). Note that the two-phase hypothesis was proposed more than 30 years before the classification of human retinopathy of prematurity according to zone and stage (Table 1 and Fig. 1 and 2).
Figure 2. Revised Two-Phase Hypothesis of Retinopathy of Prematurity.
In retinopathy of prematurity in the United States today, there is initially delayed physiologic retinal vascular development, resulting in a peripheral avascular area of the retina (phase 1). Later, vasoproliferation in the form of intravitreal angiogenesis can occur at the junction of avascularized and vascularized retina (phase 2). EPO denotes erythropoietin, ERK extracellular signal-regulated kinase, HIF hypoxia-inducible factor, IGF-1 insulin-like growth factor 1, MEK mitogen-activated protein–ERK, O2 oxygen, pAKT phosphorylated protein kinase B, PI3 phosphatidylinositol 3, pJAK phosphorylated Janus kinase, pSTAT3 phosphorylated signal transducer and activator of transcription 3, ROS reactive oxygen species, VEGF vascular endothelial growth factor, and VEGFR vascular endothelial growth factor receptor.
STUDIES OF MODELS OF RETINOPATHY OF PREMATURITY
It is unsafe (and virtually impossible) to study heterotypic cell interactions and signaling events within the human preterm retina that cause the biologic features of severe retinopathy of prematurity. Because many newborn nonhuman mammals complete their retinal vascularization postnatally, animal models were developed to test the role of stresses in preterm infants on the pathogenesis of retinopathy of prematurity. The common neonatal animal models of oxygen-induced retinopathy use varying amounts of oxygen to examine the cellular and molecular mechanisms that drive the progression of pathologic changes in retinopathy of prematurity. All models of oxygen-induced retinopathy have limitations, because the animals in such models are not premature. Nonetheless, these models have substantially enhanced our understanding of the pathogenesis of retinopathy of prematurity.
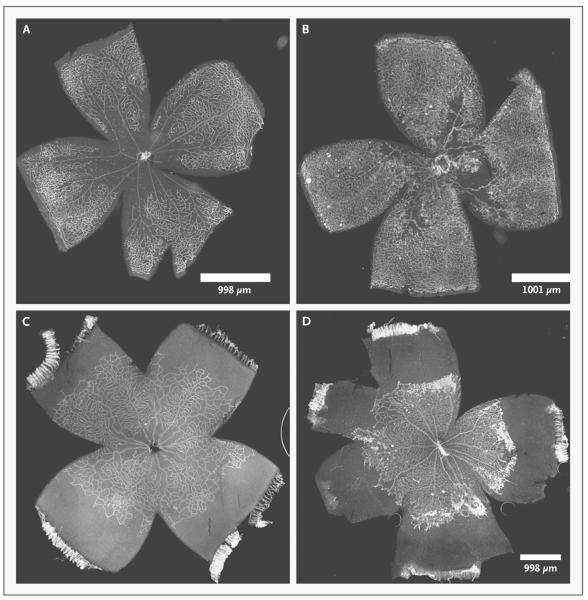
Some current models of oxygen-induced retinopathy involve high levels of oxygenation, similar to those used in the 1940s when retrolental fibroplasia was first described. However, the oxygen stresses in preterm infants have changed greatly since those early days.4 The mouse model of oxygen-induced retinopathy13 is the most widely used, because genetically altered transgenic or knockout mice can be used to study the pathways involved in angiogenesis. However, the mouse model has limitations. First, 7-day-old mice are exposed to high oxygen levels continuously for 5 days, which can cause a partial pressure of arterial oxygen (PaO2) of 500 mm Hg or more.14 The Extremely Low Gestational Age Newborns study15 tested the hypothesis that preterm infants who had blood gas disturbances on 2 of the first 3 postnatal days of life might be at risk for severe retinopathy of prematurity. That study showed that severe retinopathy of prematurity was more likely to develop in infants with a PaO2 in the highest quartile as compared with the lowest quartile. However, the median PaO2 was approximately 100 mm Hg on day 1 for all stages of retinopathy of prematurity finally analyzed, and on subsequent days, no infant had a PaO2 level as high as 400 mm Hg. Second, the oxygen level in preterm infants fluctuates on a minute-to-minute basis, but in the mouse model of oxygen-induced retinopathy, oxygen exposure is constant.16 Finally, the mouse model of oxygen-induced retinopathy causes vaso-obliteration (destruction of newly formed capillaries) in the central retina, followed by endothelial budding into the vitreous, and the retinopathy does not resemble that in most cases of severe retinopathy of prematurity seen today (Fig. 3).
Figure 3. Retinal Flat Mounts Stained with Griffonia Lectin to Visualize the Retinal Vasculature in Mouse and Rat Models of Oxygen-Induced Retinopathy.
The center of the cloverleaf is the optic nerve, and the farthest extent of each leaf of the cloverleaf is the ora serrata. There is no macula in the mouse or rat retina. The panels on the left show phase 1 retinopathy of prematurity, and the panels on the right show phase 2 retinopathy of prematurity. In Panel A, a p12 mouse model shows central hyperoxia-induced vaso-obliteration after 5 days of 75% oxygen. In Panel B, a p17 mouse model after an additional 5 days in room air shows vasoproliferation at the junctions of the vascularized and avascularized retina. This model may represent retinopathy of prematurity in the United States in the 1950s or possibly in regions lacking resources to provide oxygen regulation and monitoring. In Panel C, a p14 rat model shows delayed physiologic retinal vascular development with peripheral avascularized retina after seven cycles of fluctuations between 50% and 10% oxygen. In Panel D, a p18 rat model after 4 days in ambient air shows vasoproliferation at the junction of the vascularized and avascularized retina; this model represents retinopathy of prematurity as currently seen in the United States and other countries where oxygen is regulated.
Several current models of retinopathy of prematurity recreate fluctuations in oxygen tension, which is recognized as a risk factor for severe retinopathy of prematurity.16-18 The most widely used model of oxygen fluctuations is in the rat, in which oxygen levels fluctuate between 50% and 10% every 24 hours.19 The advantage of the rat model is that it results in fluctuations in arterial oxygen concentrations in rat pups, the extremes of which mimic measured oxygen levels in infants in whom severe retinopathy of prematurity developed.16 The rat model recreates the appearance of severe retinopathy of prematurity with delayed physiologic retinal vascular development and subsequent vasoproliferation. The rat model also causes extrauterine growth restriction, another known risk factor for severe retinopathy of prematurity.20 A limitation of the rat model is that it is relatively difficult to manipulate the rat genome. Thus, most studies of oxygen-induced retinopathy in rats use pharmacologic methods or introduce viral vectors that contain nucleic acid sequences to silence or overexpress genes in order to study signaling pathways involved in the pathogenesis of retinopathy of prematurity. Despite this limitation, the rat model of oxygen-induced retinopathy remains the most clinically relevant model of retinopathy of prematurity, since its biologic features are most like those of severe retinopathy of prematurity in preterm infants (Fig. 3).
The development of the retinal vasculature in humans differs from that in many other mammalian species used as models of oxygen-induced retinopathy.21-24 Vasculogenesis in the human infant eye is ongoing until at least 22 weeks of gestation.25 After that time, it is unknown how retinal vascularization proceeds. On the basis of studies in animals, vascularization has been thought to progress by means of angiogenesis and the extension of existing blood vessels by proliferating endothelial cells that migrate toward a gradient of vascular endothelial growth factor (VEGF).26
Thus, several reasons support revisiting the two-phase hypothesis regarding the pathogenesis of retinopathy of prematurity in terms of vaso-obliteration and vasoproliferation, as described by Ashton in 1954. In human retinopathy of prematurity, there is first a delay in physiologic retinal vascular development rather than vaso-obliteration, with subsequent vasopro-liferation in some infants with severe retinopathy of prematurity (Fig. 2). Therefore, the delayed physiologic retinal vascular development of phase 1 reflects the zone of human retinopathy of prematurity, and the vasoproliferation of phase 2 reflects stage 3 of human retinopathy of prematurity (Fig. 1, 2, and 3).
SIGNALING PATHWAYS INVOLVED IN OXYGEN-INDUCED RETINOPATHY
We study models of oxygen-induced retinopathy to identify signaling pathways involved in the pathogenesis of the phases of retinopathy of prematurity in order to determine potential interventions in human retinopathy of prematurity. In our discussion of studies in animal models, phase 1 signifies vaso-obliteration in the mouse model of oxygen-induced retinopathy and delayed physiologic retinal vascular development in the rat model of oxygen-induced retinopathy. Phase 2 signifies vasoproliferation in both mouse and rat models of oxygen-induced retinopathy. Pathways affected by oxygen stresses in cell culture and oxygen-induced retinopathy include those involving hypoxia, oxidative signaling,27,28 inflammation,29,30 and poor postnatal growth or extrauterine growth restriction.20 Interactions and overlap among the pathways, especially those that involve hypoxia, oxidative signaling, and inflammation, affect angiogenesis and the occurrence of oxygen-induced retinopathy.31 Other stresses, such as hypercapnia, acidosis, and systemic infection, cause retinopathy in the absence of oxygen stress and have also been studied.18,32,33
In phase 1 retinopathy of prematurity, a concern is that the expressed goal to use strategies that enhance physiologic retinal vascular development might worsen the second, vasoproliferative phase, depending on the timing of treatment. In addition, inhibition of vasoproliferation in phase 2 can lead to persistent avascular retina, which itself can stimulate subsequent vasoproliferation, as evidenced in studies of preterm infant eyes treated with bevacizumab for severe retinopathy of prematurity.34
RETINAL HYPOXIA
Retinal hypoxia is a major inciting feature in rat and mouse models of oxygen-induced retinopathy. Hypoxia leads to stabilization and translocation of hypoxia-inducible factors (HIFs), resulting in transcription of angiogenic genes, including those that encode VEGF, cyclooxygenase, erythropoietin,35 and angiopoietin 2.36 In the mouse model, prolyl hydroxylase inhibitors administered during phase 1 to stabilize HIFs provided protection against vaso-obliteration and subsequent vasoproliferation in phase 237 but did not reduce vasoproliferation if administered during phase 2.38
VEGF, an important survival factor,39 is critical for retinal vascular development. However, VEGF causes vasoproliferation in phase 2 in models of oxygen-induced retinopathy,38,40-43 characterized by blood-vessel growth into the vitreous rather than into the hypoxic avascular retina, which itself produces VEGF. Both the mouse44 and rat45,46 models of oxygen-induced retinopathy — especially the latter, in which the peripheral avascular retinal area was measured and VEGF signaling inhibited — have aided in the understanding of the role of VEGF in retinopathy of prematurity. Results of studies in such models led to speculation that “excessive” VEGF signaling not only caused phase 2 vasoproliferation but also appeared to contribute to avascular retina in phase 1.45,46 The use of a flt-1−/− (VEGF receptor 1 knockout) embryonic stemcell model47 and subsequent use of VEGF-neutralizing antibodies or VEGF receptor 2 (VEGFR-2) tyrosine kinase inhibitors in the rat model of oxygen-induced retinopathy showed that VEGF signaling through VEGFR-2 caused disordered divisions of endothelial cells and contributed to tortuosity and dilatation of retinal vessels, as seen in plus disease in severe retinopathy of prematurity.47,48 It is possible that the resultant disordered angiogenesis might then allow endothelial cells to proliferate outside the plane of the retina into the vitreous and that the inhibition of VEGF would reorient proliferating endothelial cells and facilitate physiologic retinal vascular development. However, the dose is critical. A later study that used a beagle model of oxygen-induced retinopathy showed that a high-dose, high-affinity antibody-based VEGF inhibitor led to persistent retinal avascularization.49
In other studies in the rat model of oxygen-induced retinopathy, the JAK-STAT (Janus-associated kinase–signal transducers and activators of transcription) signaling pathway was activated by VEGF in phase 150 and contributed to delayed physiologic retinal vascular development by reducing the expression of erythropoietin.50 Intraperitoneal delivery of the Janus kinase 2 inhibitor AG490 or erythropoietin during the early postnatal period improved physiologic retinal vascularization in a rat model of phase 1 oxygen-induced retinopathy.50 Activation of STAT3 by the oxidative enzyme NADPH oxidase occurred in the rat model of oxygen-induced retinopathy after exposure to supplemental oxygen in phase 2.51 Inhibition of NADPH oxidase with apocynin52 or of STAT3 with AG49051 inhibited vasoproliferation in phase 2 in a rat model of oxygen-induced retinopathy after rescue in supplemental oxygen. The results of such studies suggest that inhibition of the JAK-STAT pathway may reduce pathologic features in both phases 1 and 2. However, JAK-STAT signaling protects photoreceptors from light-induced damage53; therefore, additional studies are needed and may require targeted inhibition of JAK-STAT signaling.
NUTRITION AND EXTRAUTERINE GROWTH RESTRICTION
Insulin-like growth factor 1 (IGF-1) is important in fetal growth, particularly during the third trimester of pregnancy.54 Premature infants have insufficient production of IGF-1; without a placental supply, extrauterine growth restriction and delayed physiologic retinal vascularization can occur. Infants with extrauterine growth restriction are prone to severe retinopathy of prematurity.55 Extrauterine growth restriction also exacerbates oxygen-induced retinopathy.56 Administration of IGF-1 in growth-restricted mice reduced oxygen-induced retinopathy.57 These findings support the possible role of IGF-1 in reducing severe retinopathy of prematurity.
Other substances may also affect the development of retinopathy. For example, in a rat model of oxygen-induced retinopathy, ghrelin, the appetite-stimulating hormone, reduced retinopathy if it was administered during phase 1,58 possibly through the induction of IGF-1 and VEGF. The use of n–3 fatty acid supplementation during phase 1 may have provided protection against retinopathy in the mouse model of oxygen-induced retinopathy through suppression of microglia-produced tumor necrosis factor α.59 Studies in rats with oxygen-induced retinopathy have shown that vitamin C and vitamin E supplementation improves retinal vascularization in phase 1.60 Finally, the dipeptide arginyl–glutamine administered in phase 2 reduced vasoproliferation by 82% in mice in association with reduced VEGF expression, suggesting that amino acid deprivation might be considered as a contributor to oxygen-induced retinopathy.60,61
CLINICAL IMPLICATIONS
On the basis of molecular mechanisms identified in animal models of oxygen-induced retinopathy, some translational considerations for retinopathy of prematurity are presented below.
ANTIOXIDANTS
Oxidative stress has long been associated with the development of retinopathy of prematurity, because the retina is rich in polyunsaturated fatty acids that are vulnerable to reactive oxygen and nitrogen,62 and in preterm infants, the retinal antioxidant reserve is not sufficient to provide protection against reactive compounds.63-65 However, clinical trials that tested the efficacy of various antioxidants, including vitamin E, N-acetylcysteine, and lutein, have been inconclusive or have shown unacceptable side effects in infants with retinopathy of prematurity.66,67 Studies of vitamin E supplementation in preterm infants were stopped because of sepsis and necrotizing enterocolitis, but a later meta-analysis of some studies suggested that vitamin E supplementation was associated with reduced stage 3 retinopathy of prematurity.68 Thus, although it appears that oxidative stress promotes some aspects of severe retinopathy of prematurity, broad inhibition by antioxidants may not be safe.
ERYTHROPOIETIN
Very-low-birth-weight infants are at high risk not only for retinopathy of prematurity but also for subsequent neurodevelopmental impairment. Interest in erythropoietin as a neuroprotective agent is increasing. When administered in preterm infants, erythropoietin was associated with improved cognition in childhood.69 Laboratory studies have shown that early administration of erythropoietin reduced phase 1 avascularization in both mouse and rat models of oxygen-induced retinopathy.50,70 However, retrospective studies have shown an association between erythropoietin and severe oxygen-induced retinopathy in preterm infants.71,72 Erythropoietin was also found to promote intravitreal angiogenesis in a transgenic mouse model of oxygen-induced retinopathy.73 Some investigators have proposed administering erythropoietin early in preterm infants to promote physiologic retinal vascular development and attempt to reduce the risk of development of stage 3 retinopathy of prematurity, but additional studies are needed to determine the window of time for relatively safe administration.
ANTI-VEGF AGENTS
The Bevacizumab Eliminates the Angiogenic Threat of Retinopathy of Prematurity study, which compared intravitreal administration of the monoclonal anti-VEGF antibody bevacizumab (0.625 mg in 0.025 ml of solution) with laser therapy, showed improved outcomes with bevacizumab only for zone 1, stage 3 retinopathy of prematurity with plus disease.12 Since publication of that report, other studies have shown serious side effects of anti-VEGF agents in some patients with retinopathy of prematurity, including progression to stage 5 disease (total retinal detachment), persistent peripheral retinal avascularization, and recurrent intravitreal angiogenesis observed even 1 year after treatment.34 The dose of anti-VEGF agent that can reduce severe retinopathy of prematurity without adversely affecting ocular development or the development of other organs in the preterm infant remains unknown. Intravitreal bevacizumab at a dose of 0.25 mg or 0.5 mg can enter the bloodstream of preterm infants and has been reported to depress serum VEGF levels for 2 weeks, raising concern about potential adverse effects on developing organs.74 Side effects are difficult to assess, because infants in whom severe retinopathy of prematurity develops often have neurologic and other developmental issues. Thus, the use of anti-VEGF agents to reduce severe retinopathy of prematurity may be promising, but additional studies regarding drug doses and their timing, the type of anti-VEGF agent, and safety are needed.
NUTRITION
The algorithm WINROP (weight, IGF, neonatal retinopathy of prematurity) uses several factors, including serum IGF-1 levels and sequential postnatal weight gain, to evaluate the individual risk of severe retinopathy of prematurity. WINROP has been simplified to study poor postnatal weight gain as an indicator of a high risk of severe retinopathy of prematurity.75 In the United States, the WINROP algorithm was reported to have 98% sensitivity for identifying high-risk infants.76 However, in a Mexican patient population, the WINROP algorithm correctly predicted severe retinopathy of prematurity in 84.7% of extremely preterm infants and correctly identified only 26.6% of infants in whom severe retinopathy of prematurity did not develop,77 findings that highlight potential differences among preterm infants with retinopathy of prematurity in different regions of the world.78 Nonetheless, in populations in which WINROP has been validated, its use may reduce the burden of screening. This is an important consideration, given the growing number of preterm births worldwide and the insufficient number of ophthalmologists trained to screen infants for retinopathy of prematurity.78
SUMMARY
Models of oxygen-induced retinopathy have elucidated how oxygen stresses may lead to the development of retinopathy of prematurity through activated signaling pathways. Screening is currently carried out according to the guidelines in Table 1. Current treatment for severe retinopathy of prematurity focuses on laser therapy and visual rehabilitation, and potential new treatment strategies include targets within oxidative pathways, erythropoietin, and anti-VEGF agents.
Acknowledgments
Dr. Hartnett reports receiving consulting fees and travel support from Genentech, royalties from Lippincott Williams & Wilkins, and consulting fees from Axikin Pharmaceuticals through her institution. Dr. Penn reports receiving payment for board membership from Janssen; consulting fees, as well as grant support through his institution, from Alcon; and consulting fees, as well as grant support through his institution, from PanOptica.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Good WV, Hardy RJ, Dobson V, et al. The incidence and course of retinopathy of prematurity: findings from the Early Treatment for Retinopathy of Prematurity Study. Pediatrics. 2005;116:15–23. doi: 10.1542/peds.2004-1413. [DOI] [PubMed] [Google Scholar]
- 2.Lad EM, Hernandez-Boussard T, Morton JM, Moshfeghi DM. Incidence of retinopathy of prematurity in the United States: 1997 through 2005. Am J Ophthalmol. 2009;148:451–8. doi: 10.1016/j.ajo.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Section on Ophthalmology American Academy of Pediatrics. American Academy of Ophthalmology. American Association for Pediatric Ophthalmology and Strabismus Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2006;117:572–6. doi: 10.1542/peds.2005-2749. [DOI] [PubMed] [Google Scholar]; Pediatrics. 2006;118:1324. Erratum. [Google Scholar]
- 4.Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens: Preliminary report. Am J Ophthalmol. 1942;25:203–4. doi: 10.1016/j.ajo.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 5.Michaelson IC. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK. 1948;68:137–80. [Google Scholar]
- 6.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patz A, Eastham A, Higginbotham DH, Kleh T. Oxygen studies in retrolental fibroplasia. Am J Ophthalmol. 1953;36:1511–22. [PubMed] [Google Scholar]
- 8.The STOP-ROP Multicenter Study Group Supplemental Therapeutic Oxygen for Prethreshold Retinopathy of Prematurity (STOP-ROP), a randomized, controlled trial. I: primary outcomes. Pediatrics. 2000;105:295–310. doi: 10.1542/peds.105.2.295. [DOI] [PubMed] [Google Scholar]
- 9.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362:1959–69. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenson B, Rocklehurst P, Tarnow-Mordi W. Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Engl J Med. 2011;364:1680–2. doi: 10.1056/NEJMc1101319. [DOI] [PubMed] [Google Scholar]
- 11.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity: Snellen visual acuity and structural outcome at 5 ½ years after randomization. Arch Ophthalmol. 1996;114:417–24. doi: 10.1001/archopht.1996.01100130413008. [DOI] [PubMed] [Google Scholar]
- 12.Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–15. doi: 10.1056/NEJMoa1007374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith LEH, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–11. [PubMed] [Google Scholar]
- 14.Penn JS, Henry MM, Wall PT, Tolman BL. The range of PaO2 variation determines the severity of oxygen induced retinopathy in newborn rats. Invest Ophthalmol Vis Sci. 1995;36:2063–70. [PubMed] [Google Scholar]
- 15.Hauspurg AK, Allred EN, Vanderveen DK, et al. Blood gases and retinopathy of prematurity: the ELGAN Study. Neonatology. 2011;99:104–11. doi: 10.1159/000308454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham S, Fleck BW, Elton RA, Mclntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–5. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 17.York JR, Landers S, Kirby RS, Arbogast PG, Penn JS. Arterial oxygen fluctuation and retinopathy of prematurity in very-low-birth-weight infants. J Perinatol. 2004;24:82–7. doi: 10.1038/sj.jp.7211040. [DOI] [PubMed] [Google Scholar]
- 18.McColm JR, Hartnett ME. Retinopathy of prematurity: current understanding based on clinical trials and animal models. In: Hartnett ME, Trese MT, Capone A Jr, Steidl SM, Keats BK, editors. Pediatric retinal diseases: medical and surgical approaches. Lippincott Williams & Wilkins; Philadelphia: 2005. pp. 387–409. [Google Scholar]
- 19.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–31. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]; Pediatr Res. 1995;37:353. Erratum. [Google Scholar]
- 20.Hellström A, Hård AL, Engström E, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123(4):e638–e645. doi: 10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- 21.Hughes S, Yang H, Chan-Ling T. Vascularisation of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci. 2000;41:1217–28. [PubMed] [Google Scholar]
- 22.Jiang B, Liou GI, Behzadian MA, Caldwell RB. Astrocytes modulate retinal vasculogenesis: effects on fibronectin expression. J Cell Sci. 1994;107:2499–508. doi: 10.1242/jcs.107.9.2499. [DOI] [PubMed] [Google Scholar]
- 23.Gariano RF. Cellular mechanisms in retinal vascular development. Prog Retin Eye Res. 2003;22:295–306. doi: 10.1016/s1350-9462(02)00062-9. [DOI] [PubMed] [Google Scholar]
- 24.Lutty GA, Merges C, Grebe R, Prow T, McLeod DS. Canine retinal angioblasts are multipotent. Exp Eye Res. 2006;83:183–93. doi: 10.1016/j.exer.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Hasegawa T, McLeod DS, Prow T, Merges C, Grebe R, Lutty GA. Vascular precursors in developing human retina. Invest Ophthalmol Vis Sci. 2008;49:2178–92. doi: 10.1167/iovs.07-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]; Invest Ophthalmol Vis Sci. 2008;49:2342. Erratum. [Google Scholar]
- 26.Chan-Ling T, Gock B, Stone J. The effect of oxygen on vasoformative cell division: evidence that ‘physiological hypoxia’ is the stimulus for normal retinal vasculogenesis. Invest Ophthalmol Vis Sci. 1995;36:1201–14. [PubMed] [Google Scholar]
- 27.Brooks SE, Gu X, Samuel S, et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:222–8. [PubMed] [Google Scholar]
- 28.El-Remessy AB, Al-Shabrawey M, Platt DH, et al. Peroxynitrite mediates VEGF’s angiogenic signal and function via a nitration-independent mechanism in endothelial cells. FASEB J. 2007;21:2528–39. doi: 10.1096/fj.06-7854com. [DOI] [PubMed] [Google Scholar]
- 29.Hardy P, Beauchamp M, Sennlaub F, et al. New insights into the retinal circulation: inflammatory lipid mediators in ischemic retinopathy. Prostaglandins Leukot Essent Fatty Acids. 2005;72:301–25. doi: 10.1016/j.plefa.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Yanni SE, Barnett JM, Clark ML, Penn JS. The role of PGE2 receptor EP4 in pathologic ocular angiogenesis. Invest Ophthalmol Vis Sci. 2009;50:5479–86. doi: 10.1167/iovs.09-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling: role of NAD(P)H oxidase. Mol Cell Biochem. 2004;264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 32.Holmes JM, Zhang S, Leske DA, Lanier WL. Metabolic acidosis-induced retinopathy in the neonatal rat. Invest Ophthalmol Vis Sci. 1999;40:804–9. [PubMed] [Google Scholar]
- 33.Holmes JM, Zhang S, Leske DA, Lanier WL. Carbon-dioxide induced retinopathy in the neonatal rat. Curr Eye Res. 1998;17:608–16. [PubMed] [Google Scholar]
- 34.Hu J, Blair MP, Shapiro MJ, Lichtenstein SJ, Galasso JM, Kapur R. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000–6. doi: 10.1001/archophthalmol.2012.592. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh MM, Linde NS, Wynter A, et al. HIF–prolyl hydroxylase inhibition results in endogenous erythropoietin induction, erythrocytosis, and modest fetal hemoglobin expression in rhesus macaques. Blood. 2007;110:2140–7. doi: 10.1182/blood-2007-02-073254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rey S, Semenza GL. Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc Res. 2010;86:236–42. doi: 10.1093/cvr/cvq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sears JE, Hoppe G, Ebrahem Q, Anand-Apte B. Prolyl hydroxylase inhibition during hyperoxia prevents oxygeninduced retinopathy. Proc Natl Acad Sci U S A. 2008;105:19898–903. doi: 10.1073/pnas.0805817105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92:10457–61. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nishijima K, Ng YS, Zhong L, et al. Vascular endothelial growth factor-A is a survival factor for retinal neurons and a critical neuroprotectant during the adaptive response to ischemic injury. Am J Pathol. 2007;171:53–67. doi: 10.2353/ajpath.2007.061237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pierce EA, Avery RL, Foley ED, Aiello LP, Smith LEH. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995;92:905–9. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LEH. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci U S A. 1996;93:4851–6. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werdich XQ, McCollum GW, Rajaratnam VS, Penn JS. Variable oxygen and retinal VEGF levels: correlation with incidence and severity of pathology in a rat model of oxygen-induced retinopathy. Exp Eye Res. 2004;79:623–30. doi: 10.1016/j.exer.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Ozaki H, Seo M-S, Ozaki K, et al. Blockade of vascular endothelial cell growth factor receptor signalling is sufficient to completely prevent retinal neovascularization. Am J Pathol. 2000;156:697–707. doi: 10.1016/S0002-9440(10)64773-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sone H, Kawakami Y, Kumagai AK, et al. Effects of intraocular or systemic administration of neutralizing antibody against vascular endothelial growth factor on the murine experimental model of retinopathy. Life Sci. 1999;65:2573–80. doi: 10.1016/s0024-3205(99)00526-3. [DOI] [PubMed] [Google Scholar]
- 45.Budd S, Byfield G, Martiniuk D, Geisen P, Hartnett ME. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Exp Eye Res. 2009;89:718–27. doi: 10.1016/j.exer.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geisen P, Peterson LJ, Martiniuk D, Uppal A, Saito Y, Hartnett ME. Neutralizing antibody to VEGF reduces intravitreous neovascularization and does not interfere with vascularization of avascular retina in a rat model of retinopathy of prematurity. Mol Vis. 2008;14:345–57. [PMC free article] [PubMed] [Google Scholar]
- 47.Zeng G, Taylor SM, McColm JR, et al. Orientation of endothelial cell division is regulated by VEGF signaling during blood vessel formation. Blood. 2007;109:1345–52. doi: 10.1182/blood-2006-07-037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartnett ME, Martiniuk DJ, Byfield GE, Geisen P, Zeng G, Bautch VL. Neutralizing VEGF decreases tortuosity and alters endothelial cell division orientation in arterioles and veins in rat model of ROP: relevance to plus disease. Invest Ophthalmol Vis Sci. 2008;49:3107–14. doi: 10.1167/iovs.08-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lutty GA, McLeod DS, Bhutto I, Wiegand SJ. Effect of VEGF trap on normal retinal vascular development and oxygeninduced retinopathy in the dog. Invest Ophthalmol Vis Sci. 2011;52:4039–47. doi: 10.1167/iovs.10-6798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, Byfield G, Jiang Y, Smith GW, McCloskey M, Hartnett ME. VEGF-mediated STAT3 activation inhibits retinal vascularization by down-regulating erythropoietin expression. Am J Pathol. 2012;180:1243–53. doi: 10.1016/j.ajpath.2011.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Byfield GE, Budd S, Hartnett ME. The role of supplemental oxygen and JAK/STAT signaling in intravitreous neovascularization in a ROP rat model. Invest Ophthalmol Vis Sci. 2009;50:3360–5. doi: 10.1167/iovs.08-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008;49:1591–8. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ueki Y, Wang J, Chollangi S, Ash JD. STAT3 activation in photoreceptors by leukemia inhibitory factor is associated with protection from light damage. J Neurochem. 2008;105:784–96. doi: 10.1111/j.1471-4159.2007.05180.x. [DOI] [PubMed] [Google Scholar]
- 54.Randhawa R, Cohen P. The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab. 2005;86:84–90. doi: 10.1016/j.ymgme.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 55.Hellström A, Engström E, Hård A-L, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics. 2003;112:1016–20. doi: 10.1542/peds.112.5.1016. [DOI] [PubMed] [Google Scholar]
- 56.Holmes JM, Duffner LA. The effect of postnatal growth retardation on abnormal neovascularization in the oxygen exposed neonatal rat. Curr Eye Res. 1996;15:403–9. doi: 10.3109/02713689608995831. [DOI] [PubMed] [Google Scholar]
- 57.Vanhaesebrouck S, Daniëls H, Moons L, Vanhole C, Carmiliet P, De Zegher F. Oxygen-induced retinopathy in mice: amplification by neonatal IGF-I deficit and attenuation by IGF-I administration. Pediatr Res. 2009;65:307–10. doi: 10.1203/PDR.0b013e3181973dc8. [DOI] [PubMed] [Google Scholar]
- 58.Zaniolo K, Sapieha P, Shao Z, et al. Ghrelin modulates physiologic and pathologic retinal angiogenesis through GHSR-1a. Invest Ophthalmol Vis Sci. 2011;52:5376–86. doi: 10.1167/iovs.10-7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–73. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Penn JS, Tolman BL, Bullard LE. Effect of a water-soluble vitamin E analog, Trolox C, on retinal vascular development in an animal model of retinopathy of prematurity. Free Radic Biol Med. 1997;22:977–84. doi: 10.1016/s0891-5849(96)00479-0. [DOI] [PubMed] [Google Scholar]
- 61.Neu J, Afzal A, Pan H, et al. The dipeptide Arg-Gln inhibits retinal neovascularization in the mouse model of oxygeninduced retinopathy. Invest Ophthalmol Vis Sci. 2006;47:3151–5. doi: 10.1167/iovs.05-1473. [DOI] [PubMed] [Google Scholar]
- 62.Kermorvant-Duchemin E, Sennlaub F, Sirinyan M, et al. Trans-arachidonic acids generated during nitrative stress induce a thrombospondin-1-dependent microvascular degeneration. Nat Med. 2005;11:1339–45. doi: 10.1038/nm1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Buonocore G, Perrone S, Bracci R. Free radicals and brain damage in the newborn. Biol Neonate. 2001;79:180–6. doi: 10.1159/000047088. [DOI] [PubMed] [Google Scholar]
- 64.Rogers S, Witz G, Anwar M, Hiatt M, Hegyi T. Antioxidant capacity and oxygen radical diseases in the preterm newborn. Arch Pediatr Adolesc Med. 2000;154:544–8. doi: 10.1001/archpedi.154.6.544. [DOI] [PubMed] [Google Scholar]
- 65.Nielsen JC, Naash MI, Anderson RE. The regional distribution of vitamins E and C in mature and premature human retinas. Invest Ophthalmol Vis Sci. 1988;29:22–6. [PubMed] [Google Scholar]
- 66.Dani C, Lori I, Favelli F, et al. Lutein and zeaxanthin supplementation in preterm infants to prevent retinopathy of prematurity: a randomized controlled study. J Matern Fetal Neonatal Med. 2012;25:523–7. doi: 10.3109/14767058.2011.629252. [DOI] [PubMed] [Google Scholar]
- 67.Soghier LM, Brion LP. Cysteine, cystine or N-acetylcysteine supplementation in parenterally fed neonates. Cochrane Database Syst Rev. 2006;4:CD004869. doi: 10.1002/14651858.CD004869.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raju TNK, Langenberg P, Bhutani V, Quinn GE. Vitamin E prophylaxis to reduce retinopathy of prematurity: a reappraisal of published trials. J Pediatr. 1997;131:844–50. doi: 10.1016/s0022-3476(97)70031-3. [DOI] [PubMed] [Google Scholar]
- 69.Brown MS, Eichorst D, LaLa-Black B, Gonzalez R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2009;124(4):e681–e687. doi: 10.1542/peds.2008-2701. [DOI] [PubMed] [Google Scholar]
- 70.Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LEH. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009;50:1329–35. doi: 10.1167/iovs.08-2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohlsson A, Aher SM. Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev. 2006;3:CD004863. doi: 10.1002/14651858.CD004863.pub2. [DOI] [PubMed] [Google Scholar]
- 72.Brown MS, Barón AE, France EK, Hamman RF. Association between higher cumulative doses of recombinant erythropoietin and risk for retinopathy of prematurity. J AAPOS. 2006;10:143–9. doi: 10.1016/j.jaapos.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 73.Morita M, Ohneda O, Yamashita T, et al. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003;22:1134–46. doi: 10.1093/emboj/cdg117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato T, Wada K, Arahori H, et al. Serum concentrations of bevacizumab (Avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–33. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Löfqvist C, Hansen-Pupp I, Andersson E, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch Ophthalmol. 2009;127:622–7. doi: 10.1001/archophthalmol.2009.69. [DOI] [PubMed] [Google Scholar]
- 76.Wu C, Löfqvist C, Smith LH, VanderVeen DK, Hellström A. Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants: a multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch Ophthalmol. 2012;130:992–9. doi: 10.1001/archophthalmol.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zepeda-Romero LC, Hård AL, Gomez-Ruiz LM, et al. Prediction of retinopathy of prematurity using the screening algorithm WINROP in a Mexican population of preterm infants. Arch Ophthalmol. 2012;130:720–3. doi: 10.1001/archophthalmol.2012.215. [DOI] [PubMed] [Google Scholar]
- 78.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84:77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]