Abstract
This was the first randomized, controlled smoking cessation trial assessing the efficacy of an exercise intervention as an adjunct to nicotine gum therapy in comparison to both equal contact control and standard care control conditions. Sedentary female smokers aged 18-55 were provided with nicotine gum treatment along with brief behavioral counseling and were randomized into one of these three behavioral adjunct conditions. In the “intent-to-treat” sample (N=182), at end of treatment and at one-year follow up, there were clear, but non-significant, trends in univariate analyses in which the exercise and equal contact control conditions both had higher rates of abstinence than the standard care control. However, when adjusting for other predictors of relapse in a multiple logistic regression, both exercise and equal contact control showed an advantage over standard care control in avoiding early relapse (i.e., after 1 week). In a multivariate survival model adjusting for other predictors, the equal contact condition had a significantly lower likelihood of relapse compared to the standard care condition and there was a near significant trend in which exercise offered an advantage over standard care as well. While these findings suggest a slightly improved likelihood of abstinence with exercise compared with standard care, exercise did not differ from equal contact control in its efficacy. Potential explanations for these equivalent levels of efficacy and implications for the findings are discussed.
Introduction
Annually, tobacco-related illnesses prematurely claim the lives of nearly a million women worldwide, and smoking-related illnesses are becoming more prevalent among women (USDHHS, 2001). The majority of these deaths are due to cardiovascular diseases and increasingly to lung cancer. When analyzing the risk for heart disease and prevalence of associated conditions, smoking is the key preventable and modifiable risk factor for women, although physical inactivity and obesity also play a role (Schnohr, Jensen, Scharling & Nordestgaard, 2002). Moreover, smoking may increase women's vulnerability to gender-specific health consequences such as osteoporosis and pregnancy complications (USDHHS, 2001).
While tobacco use carries serious health risks and all smokers would benefit from quitting, smoking cessation is difficult. On average, 70% of tobacco users would like to quit, but less than 10% succeed in a given year. Further, long term or permanent abstinence may require several attempts (Fiore et al. 2000). The Public Health Service Clinical Practice Guidelines (Fiore et al. 2000) recommend that pharmacotherapy and behavioral counseling are effective smoking cessation treatments for both men and women, although there have been inconsistent reports of nicotine replacement therapy (NRT) efficacy among women (Perkins, 2001). It also has been suggested that women face different stressors and barriers to quitting than men. Female smokers are more likely to report co-morbid depression (Gritz et al., 1998), which has been found to decrease the likelihood of cessation (Covey, Glassman, & Stetner, 1999; Hall et al., 1998). Perkins (2001) has contended that nicotine itself may be less responsible for nicotine dependence among females and that conditioned cues related to smoking may take on greater importance for women. If true, this gender difference would help to explain findings that NRT is less efficacious among females than males (Cepeda-Benito, Reynoso, & Erath, 2004). Concerns about post-cessation weight gain likely affect women to a greater extent than men (Levine, Perkins, & Marcus, 2001; Pomerleau, Zucker, & Stewart, 2001). These concerns have been associated with higher rates of attrition in some clinical trials (Borrelli, Spring, Niaura, Hitsman, & Papandonatos, 2001; Mizes, Sloan, & Segraves, 1998; Pirie et al., 1992) and treatment failure in other trials (Jeffery, Hennrikus, Lando, Murray, & Liu, 2000; Meyers et al., 1997). Although suggestions have been made that women may benefit from tobacco dependence treatments that address these topics (Fiore et al., 2000), relatively few studies have examined programs targeted to one gender.
There are several aspects of exercise that suggest it may target some of these gender issues in smoking cessation. Exercise may help to offset post-cessation weight gain, thus increasing the likelihood that smokers can quit successfully despite their concerns about gaining weight. Exercise has been found to elevate mood and to reduce anxiety (DiLorenzo et al., 1999; Gauvin, Rejeski, & Norris, 1996) and drug cravings (Bock, Marcus, King, Borrelli, & Roberts, 1999; Ussher, Nunziata, Cropley, & West, 2001). These findings suggest that exercise may lessen the effects of nicotine withdrawal during the cessation process. This may be particularly beneficial to women, given findings that women report less withdrawal relief from NRT than men do (Hatsukami, Skoog, Allen, & Bliss, 1995).
Accordingly, there is research evidence to support the efficacy of exercise in smoking cessation. In a study where no pharmacotherapy was offered, a three times per week supervised vigorous exercise program was associated with significantly higher abstinence, lower weight gain, fewer withdrawal symptoms and cravings in comparison with an equal contact control condition (Marcus et al., 1999). Similarly, another study that included NRT as a treatment component (Martin et al., 1997) found that exercise facilitated cessation. However, the study had several methodological limitations and was conducted among problem drinkers (Ussher, Taylor, West, & McEwen, 2000). Later, Marcus et al. (2005) tested whether the results from vigorous intensity exercise could be extended to moderate intensity exercise. They found that the moderate exercise intervention together with behavioral counseling was not superior to equal contact plus a behavioral counseling group. Although a sub-sample of participants received NRT as part of the treatment, the role of NRT was not systematically evaluated. Prapavessis et al. (2007) investigated whether exercise and cognitive behavioral therapy produced similar effects on cessation and whether NRT increased the effects of exercise. Although the effect did not reach statistical significance, their findings suggested that exercise facilitated smoking cessation.
Taken together, there is evidence supporting the benefits of exercise as a component of tobacco dependence treatment, although the degree of efficacy and the role of exercise as an adjunct to NRT remain unclear. Given the established Clinical Practice Guidelines it is essential to examine whether and how much exercise may add to the suggested standard treatment consisting of behavioral counseling and pharmacotherapy (Fiore et al., 2000).
A randomized clinical trial was conducted with a sample of sedentary female smokers to examine whether exercise offers a significant advantage over equal contact with research staff as an adjunct to NRT and behavioral counseling. A standard care control group receiving only NRT and brief behavioral counseling was also included for comparison purposes. Nicotine gum therapy was chosen given its demonstrated efficacy in facilitating smoking cessation (Fiore et al, 2000; Silagy, Lancaster, Stead, Mant & Fowler, 2004) and also given findings that nicotine gum has helped to attenuate postcessation weight gain and ameliorate the effects of depressive symptoms on smoking cessation (Kinnunen, Doherty, Militello, & Garvey, 1996; Nordström, Kinnunen, Utman, & Garvey, 1999).
Method
Participants
Female smokers were recruited from the greater Boston, MA area using mainly radio, newspaper and television advertising. In these advertisements, female smokers were invited to participate in a quit smoking study in which they would receive free nicotine gum and additional treatment should they qualify. The primary inclusion criteria were that participants be sedentary (i.e., exercising fewer than 3 times per week with exercise being defined as a total of 20 minutes without stopping or at least 30 minutes broken down into 10 minute intervals throughout the day), currently smoking at least 5 cigarettes per day and between the ages of 18-55. The main exclusion criteria were active and severe psychiatric illness, a history of a serious vascular or cardiac condition and insulin-dependent diabetes mellitus. Participants with conditions contraindicated with nicotine gum use such as temporomandibular joint disorder, bleeding peptic ulcers, pregnancy or lactation were excluded as well. Information regarding exclusion criteria was obtained from a preliminary telephone screen and a baseline clinical diagnostic graded exercise test.
Research design and general procedures
Participants in this trial were followed from 3 weeks before cessation to 1 year post-cessation. All participants, independent of treatment condition, were provided with nicotine gum treatment along with brief behavioral smoking cessation counseling. Gum was first provided at the week -1 pre-quit visit. In assigning participants to a gum dose (2mg or 4mg), we followed the recommendation in the package insert that smokers reporting use of 25 cigarettes per day or more take 4mg gum with those smoking less than this level taking 2mg gum. Brief behavioral counseling was similar to counseling used in a prior nicotine gum trial conducted by our group (Garvey et al., 2000). Dr. Kinnunen trained all research staff in delivering this counseling, which adhered closely to handouts, the nicotine gum package insert and pamphlets such as “Clearing the Air” published by the National Cancer Institute. Nicotine gum treatment guidance included detailed instructions on how to chew nicotine gum and recommendations to use approximately one piece of gum per hour with a minimum of 12 pieces per day and a maximum of 24 pieces per day. Toward the end of the treatment period, participants were given instructions for weaning off the gum. The brief behavioral counseling also included guidance on possible withdrawal symptoms and how to deal with them, identification of and coping plans for urges, cravings and high risk situations. These counseling sessions included practical advice and social support, were conducted in the same manner for all participants and took approximately 10 minutes at each visit.
After the baseline visit, the participants were randomized into one of three conditions each lasting a total of 19 weeks (three weeks prequit and 16 weeks post-quit). (1) The first condition consisted of supervised exercise sessions taking place at a hospital cardiac rehabilitation center under the direction of an exercise physiologist. These 40-minute sessions included a five-minute warm-up routine, 30 minutes of moderate intensity (60%-80% of maximal heart rate – HR max) aerobic exercise, followed by a five-minute cool down and stretching. Intensity was monitored both by heart rate monitoring and the Ratings of Perceived Exertion (RPE) scale. These sessions were administered twice a week from 3 weeks precessation through 2 weeks postcessation. After the first five weeks and continuing through the end of the treatment period, supervised exercise sessions were reduced to one per week. The American College for Sports Medicine (ACSM) recommends an exercise session duration of 20-30 minutes at 60-80% of the heart rate reserve excluding a warm up and cool down period. This enables most individuals to achieve health and fitness goals. ACSM states that for deconditioned persons 2 exercise sessions per week may improve cardiorespiratory fitness although 3 to 5 sessions per week is optimal (ACSM, 2000). The main modality of the exercise during the supervised exercise sessions was walking or running on a treadmill. In addition, participants in this condition were strongly encouraged to take part in home-based exercise sessions to bring their total number of weekly exercise sessions to at least three as suggested by the ASCM. Several suggestions (e.g., walking, exercise tapes) were offered. (2) The second group was an equal contact control condition consisting of health and wellness lectures and discussions. These sessions were of equal duration and frequency as the supervised exercise sessions. The wellness sessions included no tangible cessation help but instead covered topics including aromatherapy, self-defense, first aid and facial massage (complete wellness manual is available by request). (3) The third group was a standard care control condition including only the same nicotine gum and brief behavioral counseling that participants in the other two groups received.
Those randomized into the exercise intervention and equal contact control were asked to attend twice weekly sessions for five weeks followed by weekly sessions for the remaining 14 weeks. Participants in the standard care control were provided eight sessions over the course of the 19-week study. All but one of these sessions took place following the quit date. Participants completed a total of eight assessments throughout the course of the pre-cessation/treatment period regardless of group assignment. A flow-chart of the research process is displayed in Figure 1. Nicotine gum was provided at no cost and a payment of $50 was given to participants upon the completion of the study. This trial was approved by the Harvard Medical School Office for Research Subject Protection and by the Brigham and Women's Hospital's Human Research Committee.
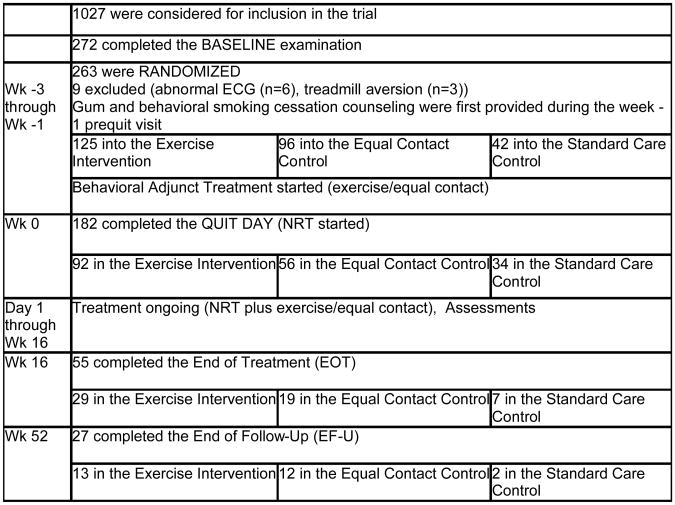
Figure 1.
Flow-chart of the procedures and number of participants in each stage of the trial.
Measures
Smoking cessation outcome
We started to measure abstinence from cigarettes immediately after quitting. At each follow-up visit a daily smoking log was recorded retrospectively indicating the number of cigarettes smoked each day. A participant was considered relapsed if she had done any smoking for 7 consecutive days or 2 consecutive episodes, such as weekends (Hughes et al., 2003). The definition of relapse implemented in this study was taken from the recommendations of the Society for Research on Nicotine Tobacco Task Force (Hughes et al., 2003) and has been used successfully in our previous reports (Korhonen et al., 2005). For this study, abstinence was recorded through 12 months. Self-reported abstinence was verified by expired carbon monoxide at each visit and salivary cotinine levels after nicotine gum use was discontinued. Abstinence was determined on an intent-to-treat basis, in that participants who reported for the baseline and the week -1 pre-quit visit (when the NRT was first provided), but at some subsequent time became lost to follow-up were considered to have relapsed.
Adherence to treatment
Treatment adherence was measured at two levels. First, among all participants adherence to pharmacological treatment (NRT) was assessed. Second, adherence to behavioral treatment was assessed among participants in the exercise and equal contact control conditions only. At each follow-up visit participants were asked to report how many pieces of nicotine gum they had used per day since the previous visit. To get a representative assessment of NRT based on self-reported daily gum use, we calculated the average number of gum pieces used at one-week follow-up and compared use between the conditions. Results from a comprehensive study involving nicotine gum suggested that gum usage during the first week of cessation was the most representative of participants’ ability to adhere to treatment, given that they are more likely to comply early in the study and less likely to return to smoking (Garvey et al., 2000). To assess adherence to adjunct behavioral treatment (i.e., attendance at exercise or equal contact sessions), we used two different measures. The first measure was prequit adherence calculated as the percentage of treatment sessions attended before the quit date. The second measure was postquit adherence calculated as the percentage of treatment sessions attended after the quit date and prior to relapse. We assessed adherence to treatment sessions in only the exercise and equal contact control conditions since they had the same number of sessions offered. Both of these measures were classified into 2 categories of “relatively high” and “relatively low” using a median split, which was 83% in prequit adherence and 66% in postquit adherence. Additionally, participants in the exercise group were asked to record on a weekly calendar the timing and the type of exercise they had engaged in at home, as well as the duration and the intensity of that exercise. For each participant in the exercise condition, the number of home-based exercised sessions that met the criteria for moderate intensity and sufficient duration (i.e., at least 30 minutes long) was entered into the analysis. Adherence to intensity of the exercise was monitored by heart rate using the Polar non-coded chest transmitter (supervised exercise sessions only) and the Ratings of Perceived Exertion (RPE) scale (supervised and home exercise). The RPE is a widely used and well validated scale to evaluate perceptions of effort during exercise (Borg & Noble, 1974).
Socio-demographic variables
These data were obtained using a semistructured questionnaire like those we have used in our previous studies (Garvey, Bliss, Hitchcock, Heinold & Rosner, 1992; Garvey et al., 2000; Kinnunen et al., 1996). We assessed age, race, education level, and marital status. Many of these pre-cessation factors have been found to be associated with likelihood of relapse (Garvey et al., 1992; 2000). Education was analyzed as a dichotomy of “low educated” (less than college degree) and “high educated” (at least a college degree).
Variables related to smoking and other substance use
For smoking-related variables we used cigarettes per day (CPD), Fagerström Test for Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker & Fagerstrom, 1991) and length of longest previous quit attempt (Ockene et al., 2000). Other substance-use related variables that have been shown to predict abstinence and may also relate to exercise effects include the presence of other smokers in the household (Garvey et al., 2000) and alcohol consumption (Shiffman et al., 1986). Weekly alcohol consumption was assessed at baseline by asking each participant how many beers, glasses of wine and other drinks (e.g., cocktails, mixed drinks) on average they consumed during a week.
Psychological variables
At baseline we measured weight concerns related to smoking cessation with a 6-item scale developed by Borelli and Mermelstein (1998). The participants replied to questions about importance of weight control, smoking for weight control, cigarettes helping in weight control, concerns about and perceived likelihood of post-cessation weight gain and likelihood of resuming smoking if one gained too much weight. Items were rated on a 0 to 4 scale (ranging from very slightly to extremely). To measure participants' depression status at baseline we used the Center for Epidemiological Studies Depression Scale (CES-D) (Radloff, 1977). The CES-D is an established self-report measure of both the frequency and severity of depressive symptoms (Kinnunen et al., 1996; Radloff, 1977). Positive and negative mood were measured using the Positive and Negative Affect Schedule (PANAS) (Watson, Clark & Tellegen, 1988). This measure consists of two 10-item scales measuring positive and negative affect. Each item is rated on a 1 to 5 scale. Self confidence in smoking cessation was measured with a single item: “How confident are you that you will be able to quit smoking for the next 3 months?” The participant rated her confidence on a 5-point scale. Psychosocial stress was measured using the Perceived Stress Scale by Cohen, Kamark & Mermelstein (1983). The four items concern inability to control important things; confidence in handling personal problems; feeling that things were going her way and feeling that difficulties were piling up. The responses followed a Likert-type scale ranging from 0 (never) to 4 (very often).
Withdrawal symptoms
Self-reported withdrawal symptoms were assessed using a modified version of a scale developed by Hughes and Hatsukami (1986). Participants used a 5-point scale to rate the frequency of a series of withdrawal symptoms (i.e., depressed mood, insomnia, irritability, anxiety, difficulty concentrating, restlessness, and increased appetite), plus craving for a cigarette.
Fitness-related variables
Body weight was measured at each visit with a standard upright scale that was calibrated frequently. Participants were weighed with shoes off and with heavy outer clothing removed. In addition, height was measured at the first pre-cessation visit to allow calculation of Body Mass Index (BMI) at each assessment. Self-reported physical activity level was assessed at baseline, at the end of treatment and at the end of the follow-up period with four Likert-type scale survey questions concerning frequency and duration of daily work and leisure activities (See Appendix 1) (Helasoja, Prättälä, Dregval, Pudule & Kasmel, 2002; Prättälä, Helasoja, & the Finbalt Group, 1999). We used a sum score of these physical activity items at baseline as a predictor of smoking cessation outcomes. Additionally, in order to assess adherence and changes in self-reported physical activity at subsequent assessments we used the first question, i.e. ”How often do you do physical exercise lasting at least 30 minutes, making you at least mildly short of breath or perspire?” (‘daily’;‘4-6 times a week’; ‘2-3 times a week’; ‘once a week’; ‘2-3 times a month’; ‘a few times a year or less’; ‘I cannot exercise because of an illness or disability’). This question best captures the exercise instructions (duration, frequency, and intensity) given to participants in the exercise group. Functional fitness level: VO2max. To establish a baseline functional capacity (fitness level), each participant underwent a clinical diagnostic exercise test during her first pre-cessation visit using a modified Bruce protocol (ACSM, 2000). As a result of this testing procedure and based on exercise intensity and duration, the estimate of maximum oxygen uptake (VO2max) was obtained. VO2max is an indicator of cardio respiratory fitness.
With the exception of depression, the baseline psychological measures and withdrawal symptoms were repeated during most follow-up assessments (i.e., 3 days and 1, 2, 4, 8, 16, 26, 39, and 52 weeks post-cessation) while fitness related variables were repeated at the end of treatment (16 weeks) and at the end of the follow-up (52 weeks). We analyzed changes in withdrawal symptoms from baseline to week 1. According to the DSM-IV-TR of the American Psychiatric Association, withdrawal symptoms generally peak at week 1 post-cessation (APA, 2000). The effects of the exercise condition on fitness (VOwmax and self-reported physical activity on frequency, intensity, and duration) were examined at the end of treatment.
Statistical analyses
The analyses were performed using SAS v. 9.1 for Windows and Statistica v. 6.1 for Windows. The goal of the univariate analyses was to assess differences in likelihood of abstinence by treatment condition. Univariate statistics included chi-square for categorical variables. T-tests and ANOVAs with Tukey's HSD post hoc tests for unequal sample sizes were used with continuous variables. For multivariate analysis, survival analyses (proportional hazards models) were performed to examine relationships among treatment condition and relapse during the one year follow-up period. First, we computed the unadjusted survival model by treatment condition (crude hazard ratios). Second, baseline variables differing across treatment condition at a minimum significance level of p < .10 in separate univariate analyses were included in the multivariate model. A stepwise procedure was used with the multivariate model, such that, variables not reaching a minimum significance level of p ≤ .05 were eliminated from the model. Thus, the final multivariate survival model included only those baseline variables significantly predicting relapse and treatment condition, which was forced into the model (adjusted hazard ratios). In addition to survival analysis, we examined treatment condition and predictors of early relapse at 3 different time points - one week, one month, and four months (end of treatment) after cessation - by conducting a series of logistic regressions. Three stepwise logistic regression models were conducted with treatment condition always forced in. For each follow-up, we assessed which predictor variables (listed in Table 1) were associated with continuous abstinence at a minimum level of p < .10. These variables were entered into the model and then subsequently eliminated if they did not reach a minimum significance level of p ≤ .05. Thus, the predictor variables included in the final model at each follow-up were significant predictors of abstinence at that particular follow-up point.
Table 1. Baseline characteristics of the study groups.
| Exercise Intervention Condition | Equal Contact Condition | Standard Care Control Condition | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Categorical variables | (%) | n | (%) | n | (%) | n | p valuea |
|
| |||||||
| Race | .30 | ||||||
| White | 81.5 | 75 | 77.8 | 42 | 75.8 | 25 | |
| African American | 13.0 | 12 | 11.1 | 6 | 12.1 | 4 | |
| Hispanic | 2.2 | 2 | 9.3 | 5 | 3.0 | 1 | |
| Other | 3.3 | 3 | 1.8 | 1 | 9.1 | 3 | |
|
| |||||||
| Education | .13 | ||||||
| Less than college | 59.8 | 55 | 44.6 | 25 | 45.5 | 15 | |
| College or more | 40.2 | 37 | 55.4 | 31 | 54.5 | 18 | |
|
| |||||||
| Marital status | .75 | ||||||
| Married | 18.5 | 17 | 21.8 | 12 | 24.2 | 8 | |
| Single/divorced/separated/widowed | 81.5 | 75 | 78.2 | 43 | 75.8 | 25 | |
|
| |||||||
| Longest Quit Attempt | .59 | ||||||
| ≤ 90 days | 50.0 | 46 | 46.4 | 26 | 57.6 | 19 | |
| > 90 days | 50.0 | 46 | 53.6 | 30 | 42.4 | 14 | |
|
| |||||||
| Alcohol consumption | .18 | ||||||
| ≤ 7 drink per week | 80.2 | 73 | 75.0 | 42 | 90.9 | 30 | |
| > 7 drinks per week | 19.8 | 18 | 25.0 | 14 | 9.1 | 3 | |
|
| |||||||
| Continuous variables | Mean | S.D. | Mean | S.D. | Mean | S.D. | p valuea |
|
| |||||||
| Age (years) | 38.3 | 9.9 | 37.9 | 9.1 | 39.9 | 9.9 | .61 |
|
| |||||||
| Number of cigarettes/day | 18.5 | 8.0 | 17.8 | 8.3 | 19.7 | 10.5 | .59 |
|
| |||||||
| Number of other smokers in the household | 1.3 | 0.5 | 1.4 | 0.6 | 1.3 | 0.6 | .27 |
|
| |||||||
| Nicotine dependencec | 4.9 | 2.4 | 4.7 | 2.4 | 5.0 | 2.0 | .79 |
|
| |||||||
| Weight concernsd | 11.5 | 5.6 | 11.2 | 5.8 | 12.9 | 5.9 | .35 |
|
| |||||||
| Depressive symptomse | 13.8 | 9.8 | 14.9 | 10.1 | 13.0 | 8.2 | .63 |
|
| |||||||
| Positive affectivityf | 25.3 | 7.8 | 21.6 | 8.8 | 23.0 | 7.5 | .02 |
|
| |||||||
| Negative affectivityf | 4.5 | 4.2 | 4.1 | 5.2 | 4.9 | 5.7 | .70 |
|
| |||||||
| Self confidenceg | 2.6 | 0.9 | 2.3 | 0.9 | 2.5 | 1.0 | .25 |
|
| |||||||
| Perceived stressh | 5.4 | 3.1 | 6.2 | 2.9 | 5.2 | 3.3 | .21 |
|
| |||||||
| Withdrawal symptomsi | 10.2 | 6.3 | 10.2 | 6.4 | 10.6 | 7.8 | .94 |
|
| |||||||
| Physical activityi | 5.1 | 2.3 | 5.1 | 2.2 | 5.5 | 2.6 | .74 |
|
| |||||||
| Weight (pounds) | 158.4 | 36.5 | 153.0 | 29.2 | 151.4 | 23.4 | .44 |
|
| |||||||
| Body mass index (kg/m2) | 26.6 | 6.1 | 25.7 | 5.3 | 25.3 | 4.3 | .42 |
|
| |||||||
| VO2 max(ml/kg/min) | 28.7 | 7.2 | 31.3 | 5.0 | 30.7 | 6.0 | .04 |
analysis of variance,
Chi-square test,
Fagerström Test for Nicotine Dependence (FTND)sum score,
Weight concerns scale sum score,
Center for Epidemiological Studies Depression Scale (CES-D) sum score,
Positive and Negative Affect Schedule (PANAS) sum score,
score based on a single item,
Perceived Stress Scale sum score,
sum score
Finally, we conducted adherence analyses among the participants in the exercise and equal contact control conditions (n = 148). We used prequit adherence and postquit adherence prior to relapse as two explanatory variables. These variables were analyzed as dichotomies (relatively high/relatively low adherence) and entered as explanatory factors in the logistic regression models. We conducted similar models as previously explained for predictors of relapse at one week, one month, four months and one year. Here, we included those significant predictors earlier detected, plus added one of the adherence variables and tested interactions between treatment condition and adherence.
The impact of the exercise condition on withdrawal symptoms at 1 week postcessation and VO2max variables was analyzed with a mixed design ANOVA (group × time) plus Tukey's HSD post hoc tests for unequal sample sizes. Chi-square tests were used for the categorical variable of self reported physical activity (Question 1 in Appendix 1).
Results
Participants
A total of 1027 prospective participants screened by telephone were considered for possible inclusion in the trial. Of those, 272 completed the baseline exercise test/medical screen at which point nine were excluded (six due to cardiac abnormalities detected with an EKG and three due to reported aversion to using a treadmill). The remaining 263 participants met full inclusion criteria and were immediately randomized (exercise n = 125, equal contact control n = 96, standard care control n = 42). Randomization into groups took place in waves of approximately 20-30 participants each. Relapse rates were monitored by treatment condition throughout the study. Early results indicated that participants randomized into standard care fared considerably worse than either of the other two groups in terms of their likelihood of smoking cessation. This ethical concern in addition to the distinction of greatest clinical relevance being between the exercise and equal contact control conditions prompted a decision to cease randomization into standard care early. Availability of facilities allowed for a greater number of participants to be randomized into the exercise intervention than into the equal contact condition.
Of those 263 randomized, 182 remained in the study for the entire three-week quit period, including the week -1 prequit visit, when they were first administered nicotine gum. These individuals were considered to have made a quit attempt and were included in the intent-to-treat sample (exercise n = 92, equal contact n = 56, standard care n = 34). However, because one of the 182 participants, randomized into the standard care control, did not provide complete baseline data, the number of participants in the analyses with baseline data was 181.
Table 1 shows the distributions of baseline variables by treatment condition. Among the categorical variables, there were no significant differences between the groups. Concerning the continuous variables, there were significant differences in baseline VO2 max (p = .04) such that the mean was lowest in the exercise and highest in the equal contact control condition. Positive affectivity scores were also significantly different (p = .02), being highest in the exercise intervention and lowest in the equal contact control condition.
Drop-out analysis
Leeman et al. (2006) reported a number of significant predictors of dropping out prior to the quit attempt in the present sample. Non-White participants, those with children living at home and those who reported greater weight concerns and higher levels of guilt were significantly more likely to drop out before making a quit attempt, while age and duration of one's longest prior quit attempt were inversely associated with likelihood of dropping out early. Treatment assignment was not a significant predictor of attrition in this analysis.
Smoking Cessation Outcomes
Abstinence rates by group
The one week, one month, four months (end of treatment) and one year (end of follow up) continuous abstinence rates are presented by treatment condition in Table 2. P-values are reported for the overall Chi-square for all three treatment groups. None of the comparisons reached statistical significance.
Table 2. Percentage and number of continuous abstainers at each follow-up by treatment condition.
| Exercise Intervention (n=92) | Equal Contact Control (n=56) | Standard Care Control (n=34) | Overall | ||||
|---|---|---|---|---|---|---|---|
| Follow-up | (%) | n | (%) | n | (%) | n | p valuea |
| One Week | 59.8 | 55 | 53.6 | 30 | 38.2 | 13 | .10 |
| One Month | 41.3 | 38 | 39.3 | 22 | 26.5 | 9 | .30 |
| Four Months = End of Treatment | 24.2 | 22 | 23.2 | 13 | 14.7 | 5 | .51 |
| Twelve Months = End of Follow-up | 9.8 | 9 | 12.5 | 7 | 5.9 | 2 | .59 .39 |
Overall Chi-square test df=2, 2-tailed
Survival Analyses
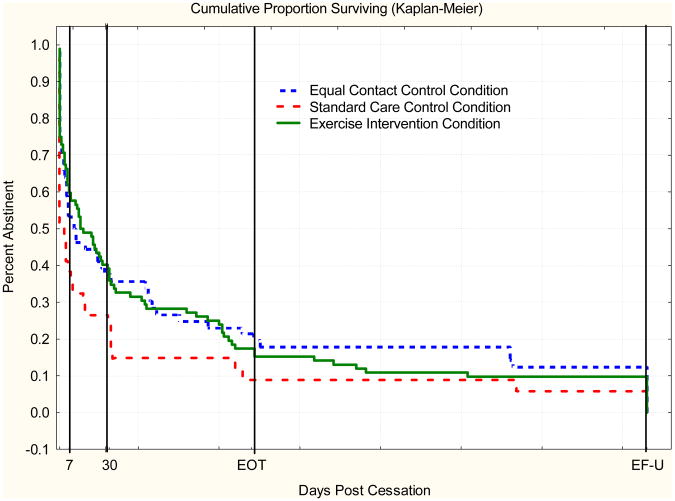
The survival curves by treatment condition are seen in Figure 2. The unadjusted survival curves suggest that both the exercise and the equal contact condition had lower relapse rates over time compared to the standard care condition. Based on the Log rank tests, neither the exercise (p = .11) nor the equal contact control group (p = .11) was significantly different from the standard care control group. However, the final multivariate survival model, including all significant predictor variables in addition to treatment condition, showed that participants in the equal contact control group (p = .04) had significantly lower relapse compared to the standard care control condition. There was also a near significant trend in which the exercise group had a lower likelihood of relapse compared with standard care (p = .05) (Table 3).
Figure 2.
Percent abstinent during 365 days postcessation by treatment condition.
Table 3.
Survival analyses of likelihood of relapse: Hazard Ratios (HR) and 95% Confidence Intervals (CI).
| Treatment Condition | HR | 95% CI | Chi-square | p valuea |
|---|---|---|---|---|
|
| ||||
| Unadjusted model (N=182) | ||||
|
| ||||
| Treatment Condition | ||||
| Exercise Intervention | 0.73 | 0.49-1.08 | 2.48 | .11 |
| Equal Contact Control | 0.70 | 0.46-1.08 | 2.59 | .11 |
| Standard Care Control | 1.00 | |||
|
| ||||
| Adjusted modela (n=179) | ||||
|
| ||||
| Treatment Condition | ||||
| Exercise Intervention | 0.67 | 0.45-1.01 | 3.71 | .05 |
| Equal Contact Control | 0.62 | 0.40-0.97 | 4.36 | .04 |
| Standard Care Control | 1.00 | |||
| Alcohol consumption | ||||
| ≤ 7 drinks/week | 1.00 | |||
| > 7 drinks/week | 1.48 | 1.01-2.17 | 4.1 | .04 |
| Longest Quit Attempt | ||||
| ≤ 90 days | 1.00 | |||
| > 90 days | 0.68 | 0.51-0.92 | 6.3 | .01 |
Adjusted for alcohol use and longest earlier quit attempt
Predictors of early relapse
The results of the final multiple logistic regression models, including treatment condition plus significant predictors at each follow-up, are shown in Table 4. One week. Significant predictors of lower relapse risk were exercise condition and baseline VO2 max, whereas higher nicotine dependence and a higher number of other smokers in the household increased the risk of relapse. One month. For one month relapse the only significant predictor was longest earlier quit attempt. A prior quit attempt longer than 90 days was associated with lower relapse risk. Exercise intervention condition did not remain a statistically significant predictor of relapse. Four months. At end of treatment, there was one significant predictor of reduced relapse risk - longest earlier quit attempt – and two significant predictors of elevated risk –consuming alcohol at least seven drinks per week and having a higher number of other smokers in the household. Treatment condition did not reach statistical significance.
Table 4.
Final multiple logistic regression models on baseline predictors of relapse: Odds Ratios (OR) and 95% Confidence Intervals (CI).
| One Week (n = 178): Abstinent n = 83/Relapsed n = 95 | ||||
|---|---|---|---|---|
|
| ||||
| Predictor of Relapse | OR | 95% CI | Wald Chi-square | p value |
|
| ||||
| Treatment Condition | ||||
| Exercise Intervention | 0.27 | 0.11-0.67 | 7.93 | .005 |
| Equal Contact Control | 0.42 | 0.16-1.11 | 3.06 | .08 |
| Standard Care Control | 1.00 | |||
| Nicotine Dependence | ||||
| FTND scorea | 1.24 | 1.06-1.45 | 7.33 | .007 |
| Number of other smokers in the householda | ||||
| 2.21 | 1.17-4.16 | 5.97 | .01 | |
| VO2maxa | 0.94 | 0.89-0.99 | 4.79 | .03 |
|
| ||||
| One Month (n = 181): Abstinent n = 69/Relapsed n = 112 | ||||
|
| ||||
| Predictor of Relapse | OR | 95% CI | Wald Chi-square | p value |
|
| ||||
| Treatment Condition | ||||
| Exercise Intervention | 0.55 | 0.23-1.35 | 1.76 | .18 |
| Equal Contact Control | 0.61 | 0.24-1.59 | 1.02 | .31 |
| Standard Care Control | 1.00 | |||
| Longest Quit Attempt | ||||
| ≤90 days | 1.00 | |||
| > 90 days | 0.54 | 0.54-1.00 | 3.86 | .05 |
|
| ||||
| Four Months (End of Treatment) (n = 179): Abstinent n = 40/Relapsed n = 139 | ||||
|
| ||||
| Predictor of Relapse | OR | 95% CI | Wald Chi-square | p value |
|
| ||||
| Treatment Condition | ||||
| Exercise Intervention | 0.52 | 0.17-1.60 | 1.33 | .25 |
| Equal Contact Control | 0.46 | 0.14-1.54 | 1.62 | .20 |
| Standard Care Control | 1.00 | |||
| Alcohol consumption | ||||
| ≤ 7 drinks/week | 1.00 | |||
| > 7 drinks/week | 4.10 | 1.12-15.00 | 4.62 | .03 |
| Longest Quit Attempt | ||||
| ≤ 90 days | 1.00 | |||
| > 90 days | 0.31 | 0.14-0.69 | 8.27 | .004 |
| Number of other smokers in the householda | 2.87 | 1.20-6.90 | 5.67 | .02 |
Continuous variable
Adherence to treatment
Adherence to pharmacological treatment was assessed in all conditions as average number of nicotine gum pieces used per day at one-week follow-up. The average use of nicotine gum after one week was 7.98 pieces per day [7.91 in exercise (SD = 5.39), 8.17 pieces in equal contact (SD = 3.92) and 7.83 pieces in standard care (SD = 4.75)]. According to an analysis of variance, there were no significant differences in average gum use between three groups, F(2, 123) = .048, p = .95).
Attendance at prequit and postquit treatment sessions was compared between the exercise and equal contact control groups. The average prequit adherence rate was 0.79 (SD = 0.17) in the exercise condition (79% of sessions attended), and 0.87 (SD = 0.17) in the equal contact condition. Based on a t- test, the prequit adherence was significantly higher in the equal contact control group, t(136) = 2.66; p = .009. The average postquit adherence rate, adjusted for time of relapse, was 0.53 (SD = 0.37) in the exercise group and 0.61 (SD = 0.42) in the equal contact control group. Based on a t- test, the difference in the postquit adherence was not significant, t(146) = 1.19; p = .25. The combined pre- and post-cessation adherence was significantly higher in the equal contact control group (M = 0.85; SD = .15) than in the exercise group (M = 0.74; SD =.17), t(146) = 3.95; p < .001).
Prequit adherence was not associated with relapse at any point (one week, one month, end of treatment, or end of follow-up.) Postquit adherence showed significant associations with one week (p = .008), one month (p = .009), end of treatment (p = .006) and end of follow-up (p = .01) relapse. When adjusted for other significant predictors at each follow-up the association of postquit adherence remained significant. The Odds Ratios (OR) indicated that those who had relatively high postquit adherence had significantly lower risk for relapse at any follow-up compared to those with relatively low adherence.
Each week participants returned weekly calendars reporting the type and duration of exercise they had engaged in at home. During the early weeks of the study the compliance to exercise recording as well as exercise frequency (at least 2 times a week) and duration (at least 30 minutes) with moderate intensity (determined by RPE) was relatively high. We have presented findings regarding the home based exercise in Table 5. During the first five weeks of the study, when participants were provided with 2 supervised exercise sessions, 49.0%, 45.7%, 47.8%, 43.5%, and 38.0% of participants, respectively, reported exercising at least twice a week for 30 minutes or more in addition to the supervised sessions. During subsequent weeks this rate reduced in linear fashion from 34.5% down to 6.5% at the end of treatment. The rates of compliance are confounded by relapse to smoking and attrition particularly in the later portions of the treatment period. We have reported the number of missing home exercise calendars at each study week and number of participants who relapsed at weeks 1, 4, 8, 12, and 16 post-cessation.
Table 5.
Participants in the Exercise group classified by number of times a week they met the criterion for home exercise (at least 30 minutes on moderate intensity.
| Study Week | Number of times Home exercise conducted each week | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 ≥ | N of Smoking Relapsers | N of Missing Ex Calendars | |
| Precessation | ||||||
| Week 1 | 21.7 | 29.4 | 29.4 | 19.6 | - | 1 |
| Week 2 | 18.5 | 35.9 | 20.7 | 25.0 | - | 1 |
| Week 3 | 16.3 | 23.9 | 30.4 | 17.4 | - | 11 |
| Postcessation | ||||||
| Week4 | 14.3 | 19.6 | 17.4 | 26.1 | 37 | 21 |
| Week 5 | 14.1 | 18.5 | 16.3 | 21.7 | 27 | |
| Week 6 | 15.2 | 12.0 | 14.1 | 21.0 | 35 | |
| Week 7 | 11.0 | 8.5 | 14.1 | 14.1 | 54 | 41 |
| Week 8 | 14.1 | 8.7 | 10.9 | 15.2 | 47 | |
| Week 9 | 12.0 | 7.6 | 7.6 | 15.2 | 53 | |
| Week 10 | 6.5 | 12.0 | 6.5 | 16.3 | 54 | |
| Week 11 | 8.7 | 3.3 | 16.3 | 6.4 | 66 | 60 |
| Week 12 | 7.6 | 10.9 | 6.5 | 7.6 | 62 | |
| Week 13 | 6.5 | 7.6 | 8.7 | 9.8 | 62 | |
| Week 14 | 6.5 | 7.6 | 7.6 | 9.8 | 63 | |
| Week 15 | 6.5 | 4.3 | 7.6 | 10.9 | 68 | 65 |
| Week 16 | 5.4 | 5.4 | 8.7 | 6.5 | 68 | |
| Week 17 | 4.4 | 8.7 | 6.5 | 8.7 | 66 | |
| Week 18 | 3.3 | 5.4 | 7.6 | 6.5 | 71 | |
| Week 19 | 0.0 | 5.4 | 3.2 | 3.3 | 70 | 81 |
Changes in VO2max, self-reported physical activity, and withdrawal symptoms
A mixed ANOVA (group × time) was conducted to examine differences in VO2max between baseline and end of the treatment. A time main effect was found to be significant F(1, 26) = 4.06, p=.05. Although the Exercise group had the largest increase of 4.1 ml/kg/min (28.8, SD = 8.5 at baseline and 32.9, SD =7.7 at the end of the treatment), the other two groups also showed an increase. The standard care group increased 2.1 ml/kg/min (from 28.0, SD = 4.2 to 30.1, SD = 2.9) and the equal contact control group increased 1.1 ml/kg/min (from 34.2, SD = 5.8 to 35.3, SD = 6.9).
To be consistent with the ACSM guidelines, we examined responses for question 1 on self-reported physical activity. More specifically, we calculated the percent of participants who reported that they exercised at least “2-3” times a week (ACSM, 2000). Among the Exercise group participants, agreement that they exercise “2-3 or more times a week” was reported at baseline by 14.1% and at the end of treatment by 25.0% of participants (p = .01). In the Equal contact control group, 7.1% reported this frequency of exercise at baseline compared with 16.0% at the end of treatment (p = .26). The standard care group had 20.6% at baseline and 12.0% at the end of treatment who exercised at that frequency (p = .59). It should be noted that there were only 7 participants in the standard care at the end of treatment.
We also examined seven withdrawal symptoms and craving at one week post-cessation, also using mixed ANOVAs (group × time). A time main effect was found for restlessness, F(1,124) = 5.0, p = .03, anger, F(1,123) = 10.7, p < .001, difficulty concentrating, F(1,124) = 4.9, p = .03, increased appetite F(1,124) = 14.9, p < .001, and craving, F(1,124) = 8.9, p = .003. With the exception of craving, the above mentioned withdrawal symptoms increased from baseline. No significant differences were found for anxiety, depression, and difficulty sleeping.
Discussion
The aim of this randomized controlled trial was to examine exercise as an adjunct to standard care in smoking cessation treatment while also controlling for equal contact. Overall, the relapse rates were very high. At the end of treatment only 24.2% of the exercise group, 23.2% of the equal contact control group and 14.7% of the standard care group remained abstinent. At the end of the one-year follow-up, the abstinence rates were 9.8%, 12.5% and 5.9%, respectively. Over the past several years, researchers have observed that the remaining smokers in the population are primarily heavily addicted, long-term users (Curtin, Brown, & Sales, 2000; Hughes & Brennan, 1996), who are resistant to current treatments (Irvin & Brandon, 2000; Irvin, Hendricks & Brandon, 2003). The rates of relapse in our study appeared to follow the same trend that these researchers have described.
However, we found that both the exercise and equal contact control conditions almost doubled the cessation rates over the standard care condition. Although the univariate results did not reach statistical significance, the multivariate survival analysis model adjusted for all significant baseline predictors showed that participants in the equal contact condition had a significantly lower likelihood of relapse compared to the standard care control. There was also a near significant trend in this analysis where exercise offered an advantage over standard care. An adjusted logistic regression model for one week abstinence showed both the exercise and equal contract groups remaining abstinent at higher rates than the standard care group. These findings suggest that adjunct treatment proved to be effective particularly in prevention of very early relapse. Our inability to show an advantage for exercise in smoking abstinence in univariate analyses has precedent in the literature. Marcus et al. (2005) and Prapavessis et al. (2007) also reported findings that suggested benefits associated with exercise but showed no significant advantage for exercise over alternate treatment conditions. One study showing a significant advantage for exercise offered no NRT (Marcus et al., 1999) and another suffered from methodological limitations (Martin et al., 1997).
Adherence to exercise intervention
Despite inconsistencies in achieving statistically significant results, these studies all suggest a beneficial effect of exercise on smoking cessation. In the present study, although the standard care control condition was less effective than two other conditions, the exercise condition did not show higher abstinence rates than the equal contact control condition. One issue to take into account is adherence to sessions (supervised exercise and wellness lectures, respectively) in these two groups. We evaluated both pre- and postquit adherence and found that both were higher in the equal contact control condition, although the difference was significant only in prequit adherence. Concerning smoking cessation outcome, only postquit adherence was a significant predictor. Those who had relatively high postquit adherence had significantly lower risk for relapse at all follow-ups compared to those with relatively low adherence. In general, adherence to exercise was low and may have diminished the beneficial effects of exercise. Thus, it is possible that, to some degree, better adherence in the equal contact group may have contributed to abstinence in this condition. Marcus et al. (2005) found that adherence to exercise sessions was 70.5% during their 8 week program while Prapavessis et al. (2007) reported 62.4 % adherence during their 12 week program. However, as a predictor of abstinence Marcus reported that only the number of weeks meeting the criteria for the exercise prescription predicted abstinence, while Prapavessis did not report the predictive value of adherence.
Exercise in smoking cessation: Intensity of exercise
While vigorous exercise intensity (Marcus et al., 1999; Prapavessis et al., 2007) has been associated with better smoking cessation rates, the terminology used to characterize exercise intensity has not been consistent. The American College of Sports Medicine (ACSM) (2000) defines the exercise-training zone with intensity between 60% and 85% of maximum heart rate as being moderate-to-vigorous and more specifically, “moderate” intensity as being between 55%-69% of HR max and “hard” as being between 70%-89% of HR max. Marcus and co-authors (1999) used the term “vigorous intensity” to refer to 60%-85%, whereas Prapavessis and colleagues (2007) defined “vigorous” as 67%-83%. In the subsequent study by Marcus and co-authors (2005) the goal was 50%-69% for moderate exercise. In our study we had a target heart rate 60%-80% of the HR max. Although the smoking cessation rates were not as high, there are clearly benefits to moderate intensity exercise, and the ACSM recommends that previously sedentary individuals progress from moderate to more vigorous exercise intensity. Moreover, as long as participants are within their cardiovascular fitness training zone, enjoyment may be the most important factor in an exercise-training program. Given the smoking cessation context it is also important to remember that moderate intensity exercise may promote better adherence (Blair & Connelly, 1996), has a higher feasibility of adoption (as even walking can be considered as exercise) (Manson et al., 1999), requires less need for supervision and has lower risk of injury (Asikainen, Kukkonen-Harjula, & Miilunpalo, 2004).
Timing of the exercise
Our exercise intervention included twice a week supervised sessions for the prequit period and for 2 weeks post-cessation. After that, supervised exercise was reduced to once a week but the total expectation remained at a minimum of three times a week. The reduction in the number of supervised exercise sessions after five weeks in this study was done in an attempt to make attendance more feasible and thereby increase adherence, however this change did not have the intended effect. It remains as a challenge to keep people engaged in an exercise routine, particularly in a group that was previously sedentary as in the present study. Increasing adherence to an exercise program might be accomplished in a number of ways. First, cognitive-behavioral interventions have been shown to increase exercise adherence among medical populations (Dishman & Buckworth, 1996). Secondly, cost-effective interventions that are easily integrated into everyday life may also have positive effects on adherence. For example, exercise regimens that can be performed easily at home, as opposed to a supervised hospital setting, may be used more frequently and maintained for a longer time. We are currently investigating whether these factors (i.e., cognitive-behavioral interventions and home exercise) increase adherence as it seems that increasing adherence to exercise should lead to higher abstinence rates in smoking cessation.
Psychosocial factors related to smoking cessation outcome
As expected, several baseline variables predicted both early and late relapse. Nicotine dependence, presence of other smokers in the household, previous quitting experience and alcohol use are examples of factors that have been found in the literature to be associated with smoking cessation (Garvey et al., 2000; Korhonen et al., 2005; Ockene et al., 2000; Shiffman et al., 1986; Strecher, Shiffman & West, 2006). In our study, when we statistically accounted for these and other predictor variables, the effect of exercise did not decrease, but on the contrary, it increased.
There are several potential explanations for the equivalent effects on smoking abstinence observed between the exercise and equal contact control conditions. There is evidence that social support is an important factor contributing to smoking cessation in interventions (Fiore et al., 2000; Korhonen, Sun, Korhonen, Uutela & Puska, 1997; Lichtenstein, Glasgow & Abrams, 1986). It is possible that participants in the exercise and equal contact control conditions received similar levels of social support from staff, which influenced their likelihood of abstinence. Links between social support and smoking cessation have been well established in women. For instance, in a large population-based survey in Scandinavia, former female smokers reported better emotional support than current smokers (Janzon et al., 2005). Unfortunately, we cannot make definitive statements about effects of social support. Although we measured expectation of social support at baseline and at end of treatment, we did not measure actual social support received. All participants received recommended standard treatment, i.e., at least NRT and behavioral counseling, which have known efficacy (Fiore et al., 2000; Silagy et al., 2004). This fact may make it more difficult to detect a significant effect of exercise. Another possibility is that participants in the equal contact control condition gleaned some benefit from the health and wellness lectures and made positive lifestyle changes which indirectly enhanced their ability to remain abstinent.
Limitations
One limitation of the study is the drop out rate (see Leeman et al., 2006 for a detailed analysis of attrition in the present trial). While higher than rates typically reported in the literature, similar attrition rates are not unheard of in smoking cessation studies. For instance, in a 13-week gum plus cognitive-behavioral group counseling trial for women, approximately one-third of participants dropped out by the fifth week of the trial (Ginsberg et al., 1997). Treatment condition was not a statistically significant predictor of dropout before the quit date in this trial according to the Leeman et al. (2006) analysis, thus dropout by treatment condition was not likely to bias our results. However, it is possible that those with higher weight concerns, who tended to drop out earlier, might have benefited from the exercise intervention.
Another limitation is that we used only one exercise modality – aerobic exercise – namely walking. However, various types of exercise programs also have the potential to promote smoking cessation via other mechanisms. Among commonly reported causes for failed smoking cessation attempts are withdrawal symptoms and cravings for cigarettes. Although exercising several times a week certainly elevates mood, it may not alleviate acute withdrawal symptoms. To address this problem, some ongoing interventions (Ussher, Nunziata, Cropley, & West, 2001; Ussher, West, Doshi, & Sampuran, 2006), are investigating the effects of isometric and short bouts of exercise on withdrawal symptoms. These laboratory studies are suggestive that perhaps isometric or short bouts of exercise could be used as a behavioral coping mechanism during tempting situations.
In sum, it appears that exercise does aid cessation, but it seems critical to create ways to increase adherence to exercise. Similarly, it seems important to ascertain the level and type of structure needed to produce exercise effects, smoking cessation effects, and short- and long-term adherence to exercise. Smoking cessation and regular exercise independently have many health promoting effects, such as reduced risk of cardiovascular diseases (USDHHS 1996USDHHS 2001). Both smoking and a sedentary lifestyle are predisposing factors for hypercholesterolemia, hypertension, as well as metabolic syndrome (Schnohr, Jensen, Scharling & Nordestgaard, 2002). Thus, combining exercise with smoking cessation interventions could provide an additive cardiovascular benefit, especially if exercise contributes to successful smoking cessation. Even in lieu of smoking cessation, introducing regular exercise into one's lifestyle may act as a harm reduction approach. Accordingly, physically active smokers demonstrate a greater active and shorter disabled life expectancy compared with smokers who are sedentary (Ferrucci et al., 1999). Thus, even if exercise has modest effects on tobacco abstinence, the additive effects of regular exercise on weight control, improved lipid- and sugar metabolism are all important in decreasing the total risk of morbidity, such as cardiovascular problems and diabetes.
Acknowledgments
Support was provided by NIH/NIDA-12503 grant to Taru Kinnunen and by grants from the Academy of Finland (200075, 103650) to Tellervo Korhonen. The authors would like to thank GlaxoSmithKline (formerly SmithKlineBeecham) for supplying the nicotine gum used in the study. We wish to extend our appreciation to Drs. Beth Nordstrom and Nolwenn Regnault as well as Mr. Laurence Molinelli for their contributions to the study.
Appendix 1.
Self-Reported Physical Activity Level Questions (Helasoja, Prättälä, Dregval et al. 2002; Prättälä, Helasoja & the Finbalt Group, 1999)
-
During your leisure time, how often do you do physical exercise lasting at least 30 minutes, making you at least mildly short of breath or perspire?”
“daily”
“4-6 times a week”
“2-3 times a week”
“once a week”
“2-3 times a month”
“a few times a year or less”
“I cannot exercise because of an illness or disability”
-
To what extent do you engage in moderate physical activity or exercise during your leisuretime?
“I enjoy reading, watching TV, and other non-physical activities during my leisure time.”
“I walk, ride a bicycle, do gardening, or some other moderate physical activity half an hourto 1 hour per week during my leisure time.”
“I engage in moderate exercise 11/2 to 21/2 hours per week during my leisure time.”
“I engage in moderate exercise 3 to 4 hours per week during my leisure time.”
“I engage in moderate exercise more than 4 hours per week during my leisure time.”
-
“How many minutes a day you spend walking or riding a bicycle on your way to and fromwork? (Including going to and coming back)”
“I work at home or don’t work at all”
”I go to work by car or by bus”
“less than 15 minutes a day”
“15-30 minutes a day”
“30-60 minutes a day”
“more than an hour a day”
-
“Which one of the following descriptions is best suited to your work?”
“very largely sedentary work (e.g. desk work, studies, light assembly work)”,
“sedentary or standing work involving some walking, lifting, or carrying”
“work involving a lot of walking, lifting, or carrying (e.g. heavy industrial work, building and construction work, heavier housework)”
“heavy manual work (e.g. forestry work, heavy agricultural work, heavy building and construction work, road work)”
Contributor Information
Taru Kinnunen, Tobacco Dependence Treatment and Research, Harvard School of Dental Medicine, Boston, Massachusetts
Robert F. Leeman, Department of Psychiatry, Yale University School of Medicine, New Haven, CT
Tellervo Korhonen, University of Helsinki, Department of Public Health, Finland
Donna M. Terwal, Tobacco Dependence Treatment and Research, Harvard School of Dental Medicine, Boston, Massachusetts
Arthur J. Garvey, Tobacco Dependence Treatment and Research, Harvard School of Dental Medicine, Boston, Massachusetts
Zandra N. Quiles, Department of Psychology, Lehman College, City University of New York, New York, NY
L. Howard Hartley, Brigham and Women's Hospital, Boston, MA
References
- American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. 6th. New York: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual. 4th. Arlington, VA: American Psychiatric Publishing; 2000. text revision. [Google Scholar]
- Asikainen TM, Kukkonen-Harjula K, Miilunpalo S. Exercise for health for early postmenopausal women. A systematic review of randomized controlled trials. Sports Medicine. 2004;34:753–778. doi: 10.2165/00007256-200434110-00004. [DOI] [PubMed] [Google Scholar]
- Blair SN, Connelly JC. How much physical activity should we do? The case for moderate amounts and intensities of physical activity. Research Quarterly for Exercise and Sport. 1996;67:193–205. doi: 10.1080/02701367.1996.10607943. [DOI] [PubMed] [Google Scholar]
- Bock BC, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addictive Behaviors. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- Borg GAV, Noble BJ. Perceived exertion. In: Wilmore JH, editor. Exercise and sport sciences reviews. New York: Academic Press; 1974. pp. 131–153. [PubMed] [Google Scholar]
- Borrelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based cessation program. Addictive Behaviors. 1998;23:609–622. doi: 10.1016/s0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- Borrelli B, Spring B, Niaura R, Hitsman B, Papandonatos G. Influences of gender and weight gain on short-term relapse to smoking in a cessation trial. Journal of Consulting & Clinical Psychology. 2001;69:511–515. doi: 10.1037//0022-006x.69.3.511. [DOI] [PubMed] [Google Scholar]
- Cepeda-Benito A, Reynoso JT, Erath S. Meta-analysis of the efficacy of nicotine replacement therapy for smoking cessation: differences between men and women. Journal of Consulting and Clinical Psychology. 2004;72:712–722. doi: 10.1037/0022-006X.72.4.712. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Naltrexone effects on short-term and long-term smoking cessation. Journal of Addictive Diseases. 1999;18:31–40. doi: 10.1300/J069v18n01_04. [DOI] [PubMed] [Google Scholar]
- Curtin L, Brown RA, Sales SD. Determinants of attrition from cessation treatment in smokers with a history of major depressive disorder. Psychology of Addictive Behaviors. 2000;14:134–142. doi: 10.1037//0893-164x.14.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLorenzo TM, Bargman EP, Stucky-Ropp RE, Brassington GS, Frensch PA, LaFontaine T. Long-term effects of aerobic exercise on psychological outcomes. Preventive Medicine. 1999;28:75–85. doi: 10.1006/pmed.1998.0385. [DOI] [PubMed] [Google Scholar]
- Dishman RK, Buckworth J. Increasing physical activity: a quantitative synthesis. Medicine and Science in Sports and Exercise. 1996;28:706–719. doi: 10.1097/00005768-199606000-00010. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Izmirlian G, Leveille S, Phillips CL, Corti MC, Brock DB, Guralnik JM. Smoking, physical activity, and active life expectancy. American Journal of Epidemiology. 1999;149:645–653. doi: 10.1093/oxfordjournals.aje.a009865. [DOI] [PubMed] [Google Scholar]
- Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating tobacco use and dependence Clinical practice guideline. Rockville, MD: U.S. Department of Health and Human Services; 2000. [Google Scholar]
- Garvey AJ, Bliss RE, Hitchcock JL, Heinold JW, Rosner B. Predictors of smoking relapse among self-quitters: a report from the Normative Aging Study. Addictive Behaviors. 1992;17:367–377. doi: 10.1016/0306-4603(92)90042-t. [DOI] [PubMed] [Google Scholar]
- Garvey AJ, Kinnunen T, Nordstrom BL, Utman CH, Doherty K, Rosner B, Vokonas PL. Effects of nicotine gum dose by level of nicotine dependence. Nicotine & Tobacco Research. 2000;2:53–63. doi: 10.1080/14622200050011303. [DOI] [PubMed] [Google Scholar]
- Gauvin L, Rejeski WJ, Norris JL. A naturalistic study of the impact of acute physical activity on feeling states and affect in women. Health Psychology. 1996;15:391–397. doi: 10.1037//0278-6133.15.5.391. [DOI] [PubMed] [Google Scholar]
- Ginsberg JP, Klesges RC, Johnson KC, Eck LH, Meyers AW, Winders SA. The relationship between a history of depression and adherence to a multicomponent smoking-cessation program. Addictive Behaviors. 1997;22:783–787. doi: 10.1016/s0306-4603(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Gritz ER, Thompson B, Emmons K, Ockene JK, McLerran DF, Nielsen IR. Gender differences among smokers and quitters in the Working Well Trial. Preventive Medicine. 1998;27:553–561. doi: 10.1006/pmed.1998.0325. [DOI] [PubMed] [Google Scholar]
- Hall SM, Reus VI, Munoz RF, Sees KL, Humfleet GL, Hartz DT. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Archives of General Psychiatry. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- Hatsukami D, Skoog K, Allen S, Bliss R. Gender and the effects of different doses of nicotine gum on tobacco withdrawal symptoms. Experimental and Clinical Psychopharmacology. 1995;3:163–173. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Helasoja V, Prättälä R, Dregval L, Pudule I, Kasmel A. Late response and item nonresponse in the Finbalt Health Monitor survey. European Journal of Public Health. 2002;12:117–123. doi: 10.1093/eurpub/12.2.117. [DOI] [PubMed] [Google Scholar]
- Hughes EG, Brennan BG. Does cigarette smoking impair natural or assisted fecundity? Fertility and Sterility. 1996;66:679–689. doi: 10.1016/s0015-0282(16)58618-x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine & Tobacco Research. 2003;5:13–25. [PubMed] [Google Scholar]
- Irvin JE, Brandon TH. The increasing recalcitrance of smokers in clinical trials. Nicotine & Tobacco Research. 2000;2:79–84. doi: 10.1080/14622200050011330. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Hendricks PS, Brandon TH. The increasing recalcitrance of smokers in clinical trials II: Pharmacotherapy trials. Nicotine & Tobacco Research. 2003;5:27–35. doi: 10.1080/1462220031000070534. [DOI] [PubMed] [Google Scholar]
- Janzon E, Engstrom G, Lindstrom M, Berglund G, Hedblad B, Janzon L. Who are the “quitters”? A cross-sectional study of circumstances associated with women giving up smoking. Scandinavian Journal of Public Health. 2005;33:175–182. doi: 10.1080/14034940410019244. [DOI] [PubMed] [Google Scholar]
- Jeffery RW, Hennrikus DJ, Lando HA, Murray DM, Liu JW. Reconciling conflicting findings regarding postcessation weight concerns and success in smoking cessation. Health Psychology. 2000;19:242–246. doi: 10.1037//0278-6133.19.3.242. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Doherty K, Militello FS, Garvey AJ. Depression and smoking cessation: characteristics of depressed smokers and effects of nicotine replacement. Journal of Consulting and Clinical Psychology. 1996;64:791–798. doi: 10.1037//0022-006x.64.4.791. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Sun S, Korhonen HJ, Uutela A, Puska P. Evaluation of a national Quit and Win contest: determinants for successful quitting. Preventive Medicine. 1997;26:556–564. doi: 10.1006/pmed.1997.0173. [DOI] [PubMed] [Google Scholar]
- Korhonen T, Kinnunen T, Quiles Z, Leeman RF, Medaglia D, Garvey AJ. Cardiovascular risk behavior among sedentary female smokers and smoking cessation outcomes. Tobacco Induced Diseases. 2005;3:7–26. doi: 10.1186/1617-9625-3-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, Quiles ZN, Molinelli LA, Medaglia Terwal D, Nordstrom BL, Garvey AJ, Kinnunen T. Attrition in a multi-component smoking cessation study for females. Tobacco Induced Diseases. 2006;3:59–71. doi: 10.1186/1617-9625-3-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine MD, Perkins KA, Marcus MD. The characteristics of women smokers concerned about postcessation weight gain. Addictive Behaviors. 2001;26:749–756. doi: 10.1016/s0306-4603(00)00156-8. [DOI] [PubMed] [Google Scholar]
- Lichtenstein E, Glasgow RE, Abrams DB. Social support in smoking cessation: in search of effective interventions. Behavior Therapy. 1986;17:607–619. [Google Scholar]
- Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. The New England Journal of Medicine. 1999;341:650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Archives of Internal Medicine. 1999;159:1229–1234. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate-intensity exercise as an aid for smoking cessation in women: a randomized controlled trial. Nicotine & Tobacco Research. 2005;7:871–80. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- Martin JE, Calfas KJ, Patten CA, Polarek M, Hofsettler JN, Beach D. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: One-year results of project SCRAP-Tobacco. Journal of Consulting and Clinical Psychology. 1997;65:190–194. doi: 10.1037//0022-006x.65.1.190. [DOI] [PubMed] [Google Scholar]
- Meyers AW, Klesges RC, Winders SE, Ward KD, Peterson BA, Eck LH. Are weight concerns predictive of smoking cessation? A prospective analysis. Journal of Consulting & Clinical Psychology. 1997;65:448–452. doi: 10.1037//0022-006x.65.3.448. [DOI] [PubMed] [Google Scholar]
- Mizes JS, Sloan DM, Segraves K. The influence of weight-related variables on smoking cessation. Behavior Therapy. 1998;29:371–385. [Google Scholar]
- Nordstrom BL, Kinnunen T, Utman CH, Garvey AJ. Long-term effects of nicotine gum on weight gain after smoking cessation. Nicotine & Tobacco Research. 1999;1:259–268. doi: 10.1080/14622299050011381. [DOI] [PubMed] [Google Scholar]
- Ockene J, Emmons K, Mermelstein RJ, Perkins KA, Bonollo DS, Voorhees CC, Hollis JF. Relapse and maintenance issues for smoking cessation. Health Psychology. 2000;19(Suppl):17–31. doi: 10.1037/0278-6133.19.suppl1.17. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Smoking cessation in women. Special considerations. CNS Drugs. 2001;15:391–411. doi: 10.2165/00023210-200115050-00005. [DOI] [PubMed] [Google Scholar]
- Pirie PL, McBride CM, Hellerstedt W, Jeffery RW, Hatsukami D, Allen S, Lando H. Smoking cessation in women concerned about weight: 12 month outcomes of a randomized trial. American Journal of Public Health. 1992;82:1238–1243. doi: 10.2105/ajph.82.9.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Zucker AN, Stewart AJ. Characterizing concerns about post-cessation weight gain: results from a national survey of women smokers. Nicotine & Tobacco Research. 2001;3:51–60. doi: 10.1080/14622200020032105. [DOI] [PubMed] [Google Scholar]
- Prapavessis H, Cameron L, Baldi JC, Robinson S, Borrie K, Harper T, Grove JR. The effects of exercise and nicotine replacement therapy on smoking rates in women. Addictive Behaviors. 2007;32:1416–1432. doi: 10.1016/j.addbeh.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Prättälä R, Helasoja V the Finbalt-group. Finbalt Health Monitor: Feasibility of a collaborative system for monitoring health behaviour in Finland and the Baltic countries. Helsinki: National Public Health Institute; 1999. Publications of the National Public Health Institute B21/1999. Report No. B21/1999. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Schnohr P, Jensen JS, Scharling H, Nordestgaard BG. Coronary heart disease risk factors ranked by importance for the individual and community. A 21 year follow-up of 12 000 men and women from The Copenhagen City Heart Study. European Heart Journal. 2002;23:620–626. doi: 10.1053/euhj.2001.2842. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Shumaker SA, Abrams DB, Cohen S, Garvey A, Grunberg NE, Swan GE. Models of smoking relapse. Health Psychology. 1986;5:13–27. [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. The Cochrane Database of Systematic Reviews. 2004 doi: 10.1002/14651858.CD000146.pub2. Issue 3. Art. No.: CD000146.pub2. [DOI] [PubMed] [Google Scholar]
- Strecher VJ, Shiffman S, West R. Moderators and mediators of a Web-based computer-tailored smoking cessation program among nicotine patch users. Nicotine & Tobacco Research. 2006;8:S95–S101. doi: 10.1080/14622200601039444. [DOI] [PubMed] [Google Scholar]
- U. S. Department of Health and Human Services. Physical activity and health: Report of the surgeon general. Atlanta, GA: Author, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- U. S. Department of Health and Human Services. Women and smoking: A report of the surgeon general. Atlanta, GA: Author, Public Health Service, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health; 2001. [Google Scholar]
- Ussher M, Nunziata P, Cropley M, West R. Effects of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology. 2001;158:66–72. doi: 10.1007/s002130100846. [DOI] [PubMed] [Google Scholar]
- Ussher M, Taylor A, West R, McEwen A. Does exercise aid smoking cessation? a systematic review. Addiction. 2000;95:199–208. doi: 10.1046/j.1360-0443.2000.9521996.x. [DOI] [PubMed] [Google Scholar]
- Ussher M, West R, Doshi R, Sampuran AK. Acute effects of exercise on desire to smoke and tobacco withdrawal symptoms. Human Psychopharmacology. 2006;21:39–46. doi: 10.1002/hup.744. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]