SUMMARY
GPR116 is an orphan seven-pass transmembrane receptor of previously unknown function. Global disruption of the Gpr116 gene in mice revealed an unexpected, critical role for this receptor in lung surfactant homeostasis, resulting in progressive accumulation of surfactant lipids and proteins in the alveolar space, labored breathing, and a reduced lifespan. GPR116 expression analysis, bone marrow transplantation studies and characterization of conditional knockout mice revealed that GPR116 expression in ATII cells is required for maintaining normal surfactant levels. Aberrant packaging of surfactant proteins with lipids in the Gpr116 mutant mice resulted in compromised surfactant structure, function, uptake, and processing. Thus, GPR116 plays an indispensable role in lung surfactant homeostasis with important ramifications for the understanding and treatment of lung surfactant disorders.
INTRODUCTION
The vital process of mammalian breathing requires alveoli to expand and contract without collapsing, a remarkable feat that depends upon the surface-tension reducing properties of the lipid-rich surfactant film that lines the alveolus (Trapnell et al., 2003). Pulmonary surfactant, which also functions in innate immunity, is composed of 90% lipids (primarily phosphatidylcholine) and 10% protein [surfactant proteins A (SP-A), SP-B, SP-C, and SP-D]. In premature infants insufficient production of surfactant leads to neonatal respiratory distress syndrome (RDS). Surfactant deficiency also contributes to the pathogenesis of acute lung injury (ALI) and acute RDS (ARDS), disorders that can afflict patients of all ages and carry a mortality rate greater than 25% (Lewis and Veldhuizen, 2006; Raghavendran et al., 2011). Conversely, accumulation of surfactant in the alveolar space leads to pulmonary alveolar proteinosis (PAP) (Carey and Trapnell, 2010). Although important progress has been made in understanding surfactant catabolism by macrophages, a process dependent upon GM-CSF signaling (Carey and Trapnell, 2010; Sakagami et al., 2009), mechanisms regulating surfactant homeostasis in the alveolar space remain largely unexplained. Furthermore, whole-lung lavage, the standard of care for PAP, only helps a subset of patients, has numerous complications, and does not address the pathogenic mechanisms at work (Borie et al., 2011). Thus, new methods to regulate surfactant levels and activity are urgently needed and have the potential to impact a myriad of lung diseases.
RESULTS AND DISCUSSION
Genetic Disruption of Gpr116 Results in a Shortened Lifespan Associated with Pulmonary-Specific Abnormalities
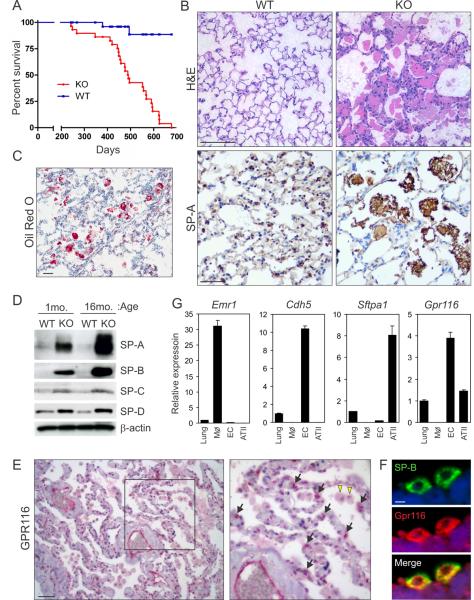
G-protein coupled receptor (GPCR) superfamily members mediate cell signaling in response to a diverse array of extracellular stimuli and comprise over one third of the drug targets of modern medicine (Tang et al., 2012). While searching a large panel of gene expression libraries for endothelial GPCRs (Seaman et al., 2007; St Croix et al., 2000) we identified Gpr116, also called Ig-Hepta in rats (Abe et al., 1999), as a panendothelial expressed gene of unknown function [Figures S1A–S1H available online; see also (Wallgard et al., 2008)]. Gpr116 was broadly expressed in normal unfractionated tissues with highest expression in lung (Abe et al., 1999) (Figure S1C). To explore the function of GPR116, we generated Gpr116−/− knockout (KO) mice by removing exon 2 containing the start codon and signal peptide (Figures S1I –S1L). Gpr116 KO mice were viable and fertile but displayed labored breathing by 4 months of age, weighed up to 25% less than wildtype (WT) littermates by 14 months, and had a shortened lifespan (Figure 1A; Figure S2A). A complete histopathological survey of 6-month-old mice revealed defects only in the KO lung, while heterozygous mice were normal. A PAP-like phenotype was observed in the KO, which included a striking accumulation of eosinophilic, periodic acid-Schiff (PAS) positive material in the alveolar space (Figure 1B; Figure S2B). Oil red O staining confirmed the buildup of lipids in the alveolar material that also stained positive for SP-A by immunohistochemistry (Figures 1B and 1C). Immunoblotting showed a large increase in SP-A and SP-B and a smaller increase in SP-C and SP-D in Gpr116 KO lung samples (Figure 1D). Supraphysiologic accumulation of alveolar SP-A was apparent by 2 weeks of age and increased with time (Figure 1D; Figure S2C).
Figure 1. Gpr116 is expressed in lung ECs and ATII cells and is important for maintaining lung surfactant homeostasis.
(A) Survival analysis of Gpr116 WT and KO mice.
(B) An accumulation of eosinophilic, SP-A+ material was observed in the alveolar space of 6-month-old lungs of KO mice by H&E and SP-A staining.
(C) The accumulated lung material stained positive with Oil Red O dye indicative of a buildup of neutral lipids in the alveolar space. Scale bar in (B) and (C) is 50μm.
(D) Immunoblotting for SP-A, SP-B, SP-C, and SP-D in KO versus WT whole lung lysates.
(E) Immunohistochemical staining of normal human lung. GPR116 protein (red) can be found in both ECs and ATII cells (arrows in inset) whereas alveolar macrophages were negative (yellow arrowheads). Scale bar is 100μm.
(F) Immunofluorescence staining of normal human lung. GPR116 protein (red) co-localizes with SP-B (Green). Scale bar is 5μm.
(G) QPCR analysis was used to assess Gpr116 expression in unfractionated lung tissue, lung ECs, and ATII cells and alveolar macrophages (MØ). Lineage specific markers were used to verify enrichment of isolated macrophages (Emr1, encoding F4/80 antigen), ECs (Cdh5), and ATII cells (Sftpa). Error bars represent SD.
See also Figures S1 and S2.
To better understand GPR116 protein expression patterns, we performed immunohistochemical staining of human lung tissues. GPR116 was strongly expressed in lung endothelial cells (ECs) that lined blood-filled vessels (Figure 1E), consistent with the earlier mRNA expression analysis (Figure S1C). GPR116 protein was also detected in sporadic epithelial cells lining the alveoli (Figure 1E, arrows) that were subsequently identified as ATII cells based on SP-B co-immunofluorescence staining (Figure 1F). Clara cells did not stain (Figure S2D). QPCR analysis confirmed Gpr116 expression in both ATII cells and ECs isolated from murine lung (Figure 1G).
Bronchoalveolar lavage fluid (BALF) had a milky appearance in the Gpr116 KO (Figure 2A), similar to that observed in PAP patients (Trapnell et al., 2003). An analysis of the BALF lipid content at 3 months revealed an 8-fold increase in phosphatidylcholine (Figure 2B) and a 13-fold increase in cholesterol, the most abundant neutral lipid in surfactant, even though blood cholesterol levels were not significantly altered (Figure S2E). H&E staining of BALF cytospins revealed numerous enlarged foamy macrophages that were occasionally multinucleated (Figure 2C). By ultrastructural microscopy, alveolar spaces of the KO lungs showed an excessive number of multi-lamellated structures that resembled the intracytoplasmic inclusions, or lamellar bodies (LB) of ATII cells (Figure 2D). Furthermore, macrophages were loaded with surfactant laden phagosomes and frequently contained cholesterol clefts (Figure 2E). Thus, Gpr116 KO mice display many of the phenotypic hallmarks of human PAP.
Figure 2. Foamy macrophages result from changes in the alveolar microenvironment and are not caused by GPR116 loss in macrophages.
(A) Clarity of BALF was evaluated in Gpr116 WT and KO mice.
(B) Phosphatidylcholine (PC) levels were quantified in KO versus WT BALF at 3 and 16 months. (p< 0.01). Error bars represent SD.
(C) H&E staining of BALF from WT and KO mice. Approximately half of the macrophages in the KO were enlarged and foamy and a smaller fraction (~3%) was binucleate (inset). Scale bar is 50μm.
(D) Ultrastructural analysis of surfactant in the alveolar space of Gpr116 KO lungs. Scale bar is 10μm.
(E) Ultrastructural analysis of macrophages with surfactant-laden phagosomes. Macrophages contained rod-shaped electron-dense inclusions (arrows) similar to those in Sftpa transgenic mice (Elhalwagi et al., 1999), possibly Ym1 crystals, as well as electron-transparent cholesterol clefts (arrowheads). Scale bar is 2μm.
(F) SP-A immunohistochemistry performed on lungs following bone marrow transplantation. The transfer of Gpr116 WT BM into KO mice failed to prevent the accumulation of SP-A+ material in the alveolar space. Scale bar is 100μm
(G) Immunofluorescence performed on BALF following bone marrow transplantation. An accumulation of enlarged, occasionally binucleate, macrophages was observed 6 months following the transfer of WT BM into the KO host. GFP expression of BALF cells confirms their origin from GFP-positive WT donor mice. To visualize all BM cells, membranes were labeled with CellMask Orange (CMO, red) and nuclei with DAPI (blue). Scale bar is 10μm.
See also Figure S3.
Lung Defects in Global Gpr116 KO Mice are not Caused by Loss of GPR116 in ECs or Macrophages
The normal alveolus is lined by ATI and ATII epithelial cells that are separated from ECs by a basement membrane. To determine if GPR116+ ECs influence surfactant levels indirectly, perhaps through paracrine regulation of neighboring ATII cells or macrophages, we generated Gpr116 conditional KO mice (Gpr116−/flox, Tie2-Cre) that contain a Gpr116 `floxed' allele and Cre driven by the Tie2-promoter that results in deletion in ECs and a fraction of hematopoietic cells. No defects were observed despite efficient deletion of Gpr116 in lung ECs (Figure S3A to S3D). These results suggest that a non-endothelial cell type may be responsible for driving the global Gpr116 KO phenotype.
Autoimmune PAP is caused primarily by defects in GM-CSF signaling that result in defective catabolism of surfactant by alveolar macrophages (Borie et al., 2011; Carey and Trapnell, 2010). To determine if GM-CSF signaling in macrophages was perturbed in the Gpr116 KO, we used QPCR to evaluate Csf2 (GM-CSF) levels, and critical downstream components required for macrophage differentiation and function including Sfpi1 (PU.1), pparg (PPARγ) and Abcg1 (Malur et al., 2011a; Malur et al., 2011b; Shibata et al., 2001) but did not observe any alterations in expression (Figure S3E–S3G). Furthermore, neither Gpr116 mRNA nor GPR116 protein were detected in macrophages (Figures 1E and 1G) and Vav-iCre conditional Gpr116 KO mice that deleted Gpr116 efficiently in macrophages (Figure S3H) and possibly other vav+ leukocytes, failed to develop a PAP-like phenotype (Figure S3I). We also performed bone marrow (BM) transplantation experiments and found that BM from the Gpr116 KO was unable to transfer the PAP-like disease to WT recipients (Figure S3J). Conversely, transfer of WT Gpr116 BM into KO mice failed to prevent the accumulation of surfactant (Figure 2F). Moreover, in the latter experiments, WT macrophages became foamy, enlarged and binucleate, similar to that in the global KO (compare figure 2G with Figure 2C), indicating that the KO lung microenvironment drives the abnormal macrophage phenotype. Consistent with this idea, foamy macrophages transplanted from KO lungs to surfactant-free cell culture rapidly reverted to WT size and exhibited normal phagocytic function (Figure S3K). Based on these data, we conclude that the PAP-like phenotype in the Gpr116 KO is not driven by an autonomous macrophage defect, but rather a distinct mechanism involving other alveolar cell type(s).
Autonomous Expression of GPR116 in ATII cells is Required for Normal Lung Surfactant Homeostasis
ATII cells produce surfactant and, along with macrophages, participate in its catabolism (Trapnell et al., 2003). No ultrastructural abnormalities were observed in ATII cells at 1 month. However, by 1 year KO ATII cells displayed mild hyperplasia, increased LB size and evidence of lamellar body fusion (Figures S4). To determine if ATII cells were driving the global KO phenotype, we generated conditional Gpr116 KO mice containing a `floxed' Gpr116 allele and a Cre transgene driven by the constitutive SFTPC promoter. Interestingly, SFTPC-Cre Gpr116 conditional KO mice showed a phenotype indistinguishable from the global null, including amorphous eosinophilic SP-A+ material in the alveoli (Figure 3A).
Figure 3. GPR116 expression in ATII cells is important for lung surfactant homeostasis.
(A) Surfactant accumulation in the alveolar space was assessed following constitutive SFTPC-Cre mediated deletion
(B) Surfactant accumulation in the alveolar space was assessed following doxycyclineinducible SFTPC-Cre mediated deletion. Scale bar in (A) and (B) is 50μm.
(C) Immunoblotting for SP-A, SP-B, SP-C, and SP-D in BALF derived from Gpr116−/flox conditional KO mice containing the indicated transgenes.
(D) Phosphatidylcholine (PC) and total cholesterol levels were quantified in WT versus KO BALF at 1 year of age (n=3, p<0.01). Error bars represent SD.
See also Figure S4.
Although the SFTPC promoter is predominantly expressed by ATII cells in adult mice, during development SFTPC-Cre is also expressed in early endoderm leading to permanent Cre-mediated deletion of floxed genes in all respiratory epithelium including ATI cells (Okubo et al., 2005). To restrict the deletion to ATII cells, we further generated doxycycline-inducible SFTPC-driven Gpr116 conditional KO mice that contained a floxed Gpr116 allele and both the tetO-Cre and SFTPC-rtTA transgenes. Following doxycycline induction we observed an accumulation of surfactant in the alveoli of SFTPC conditional Gpr116 KO mice [Gpr116−/flox; Tg(SFTPC-rtTA/tetO-Cre)], but not control mice that retained a wildtype Gpr116 allele [Gpr116−/+; Tg(SFTPC-rtTA/tetO-Cre)] (Figure 3B). Furthermore, in the BALF of the conditional KO we detected an accumulation of SPA, SP-B, SP-C and SP-D by western blotting and an elevation of PC and cholesterol by ELISA (Figure 3C and 3D), recapitulating each of the major phenotypes observed in the global KO. Taken together with the previous GPR116 expression analysis (Figures 1E–G; Figure S2D), we conclude that specific loss of GPR116 expression in ATII cells is responsible for the lung defects observed in global KO mice.
To explore the subcellular location of GPR116, we expressed a C-terminal GFP-tagged fusion protein (GPR116-GFP) in A549 lung cells, a LB-containing cell line derived from ATII cells (Lieber et al., 1976). GPR116 was found to reside predominantly in cytoplasmic vesicles with additional, limited expression at the cell surface (Figure 4A). This expression pattern was confirmed by both immunofluorescence (using GPR116 N-terminal Flag-tagged and C-terminal pyo-tagged vectors) and cell surface biotinylation studies (data not shown). Co-immunofluorescence labeling of GPR116 with ABCA3, a receptor that has been localized to the LB limiting membrane (Mulugeta et al., 2002), revealed partial co-localization in LBs (Figure 4B). Thus, GPR116 resides both in LBs and at the cell surface, consistent with KO abnormalities both in the alveolar space (Figures 1B and 1C; Figure S2B) and inside the ATII cell (Figures S4).
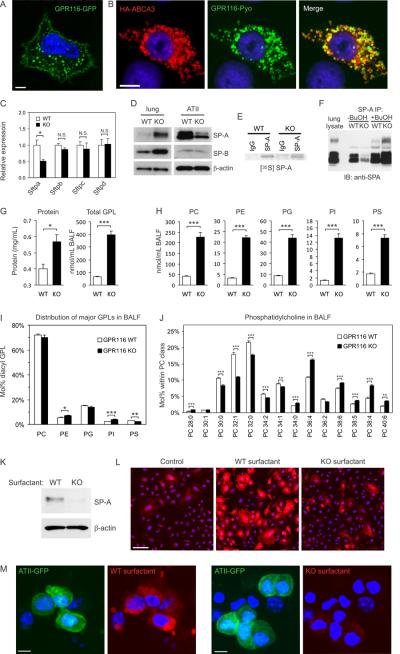
Figure 4. Gpr116 disruption leads to a defect in surfactant uptake by ATII cells.
(A) GPR116-GFP expression is localized to the cell surface and LB-like structures in A549 cells. Scale bar is 5μm.
(B) Immunofluorescence staining was used to evaluate co-localization of GPR116 (green) with ABCA3 (red) in A549 cells. The merged image shows partial co-localization (yellow) in lamellar body-like structures. In this experiment cells were permeabilized to detect ABCA3 which resulted in reduced staining of GPR116 at the cell surface. Scale bar is 10μm.
(C) QPCR analysis of purified ATII cells for SP-A, SP-B, SP-C and SP-D at 6 weeks. (n=3). Error bars represent SD.
(D) Immunoblotting of SP-A and SP-B in whole lung lysates or isolated ATII cells derived from 6-week-old Gpr116 KO versus WT mice. β-actin was used as a loading control.
(E) Metabolic labeling of SP-A protein in ATII cells derived from Gpr116 WT and KO lungs.
(F) Immunoprecipitation (IP) of SP-A with rabbit anti-SPA antibodies. Note the efficient IP of SP-A from Gpr116 KO BALF required butanol (BuOH) extraction.
(G) Total protein and total glycerophospholipid (GPL) levels were quantified in BALF from 6-week old WT or KO mice (n=5).
(H) Total PC, PE, PG, PI and PS levels were quantified in BALF from 6-week old WT or KO mice (n=5).
(I) The relative distribution of GPLs were quantified in BALF from 6-week old WT or KO mice (n=5).
(J) The individual PC species were quantified in BALF from 6-week old WT or KO mice (n=5). The PC species are labeled according to the total number of carbons in their fatty acid (FA) chains followed by the number of double bonds in the FA chains.
(K) Purified human SP-A was mixed with surfactant from WT or KO mice and uptake into isolated ATII cells was monitored by Immunoblotting with mouse anti-human SP-A antibodies. β-actin was used as a loading control.
(L) Uptake of rhodamine-phosphatidylethanolamine (rhodamine-PE) labeled WT or KO surfactant was evaluated in A549 cells. The control represents rhodamine-PE alone. Scale bar is 100μm.
(M) Uptake of rhodamine-PE labeled WT or KO surfactant was evaluated in ATII-enriched cell fractions from SPC-GFP transgenic mice. In this particular experiment, GFP-positive ATII cells (green) were not sorted by flow cytometry but a partial enrichment of ATII cells was achieved by magnetic depletion of hematopoietic cells and endothelial cells. Scale bar is 5μm.
Error bars represent SD (C) or SEM (G–J).
*p<0.05, **p<0.01, ***p<0.001
See also Tables S2 and S3.
GPR116 is Required for Surfactant Uptake into ATII Cells
To understand the basis of the alveolar surfactant protein accumulation we began by evaluating the mRNA levels of surfactant proteins in isolated ATII cells. We observed either no alteration in the KO (Sftpb, Sftpc and Sftpd), or a 50% decrease (Sftpa) in expression (Figure 4C). Similarly, QPCR failed to detect any increase in expression of genes involved in lipid synthesis or transport in the KO lung, including Abca3, Pcyt1a, Fasn, Gpam, Hmgcr and Srebf2 (data not shown). We also failed to detect any increase in radiolabeled phosphatidylcholine in the KO alveolar space following intravenous injection of 14C-choline into Gpr116 WT or KO mice (data not shown). Next, we examined SP-A and SP-B protein levels in isolated ATII cells and detected either no differences between WT and KO (SP-B) or a decrease in the KO (SP-A), in striking contrast to the clear increase observed in KO BALF and total lung lysates (Figure 4D). Furthermore, metabolic labeling of SP-A, the most overexpressed surfactant protein in the KO, revealed a slightly reduced production by Gpr116 KO ATII cells (Figure 4E).
Although immunohistochemistry revealed predominant expression of SP-A in ATII cells of WT lung, in the KO, SP-A staining localized predominantly to the alveolar space and was barely detectable inside ATII cells (Figure 1B) suggesting that re-uptake into ATII cells may be impaired. Because individual surfactant components were overexpressed to variable degrees in the KO lung (Figure 1D), we hypothesized that the altered surfactant composition could result in defective uptake. We obtained further evidence that surfactant composition was altered when we found that a polyclonal anti-SPA antibody that readily immunoprecipitated SP-A from Gpr116 WT lung lavage only immunoprecipitated a small fraction of SP-A from the KO surfactant (Figure 4F). Importantly, SP-A immunoprecipitation from the KO was rescued following lipid extraction of the surfactant with butanol, revealing an abnormal association of SP-A with lipids that presumably resulted in epitope masking (Figure 4F). These results suggested that the phospholipid composition may be altered in the Gpr116 KO. To investigate this possibility further, we isolated BALF from either 6-week old or 6-month old Gpr116 WT or KO mice and performed lipidomics analysis using electrospray ionization mass spectrometry (ESI-MS) (Figure 4G to J, and Table S2 and S3) (Ivanova et al., 2007; Myers et al., 2011). These studies revealed that the total amount of phospholipids in the BALF increased rapidly in Gpr116 KO versus WT mice (5.9-fold by 6-weeks of age), and preceded the large increase in surfactant proteins that occurred by 6 months of age (Figure 4G–4J). An analysis of the different surfactant phospholipid classes, including phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylglycerol (PG), phosphatidylinositol (PI) and phosphatidylserine (PS) revealed that the total levels of each where significantly increased in KO BALF, ranging from 4.1-fold (PS) to 9.7-fold (PI). Modest but significant changes in the ratio of PE, PI and PS were also detected by 6-weeks of age (Figure 4I). Furthermore, an analysis of the individual lipid species within each class revealed an unexpected shift from saturated or monounsaturated fatty acids (FAs) to polyunsaturated FAs (Figure 4J and Table S2). A significantly increased amount of saturated lysophospholipids was also detected in the knockout BALF (Table S3). Further studies are required to determine if reacylation of lysophospholipids is impaired or degradation of phospholipids by phospholipases is enhanced in the KO. Taken together, these studies reveal multiple and extensive changes in the phospholipid composition of Gpr116 KO surfactant that potentially impact its biophysical properties.
Next, we sought to determine if the altered surfactant composition impacts uptake of surfactant proteins or lipids into ATII cells. First, we isolated equivalent amounts of WT or KO surfactant, spiked it with purified human SP-A, and then monitored SP-A uptake into freshly isolated ATII cells by Immunoblotting using an anti-human SP-A mouse monoclonal antibody. The antibody employed does not cross-react with mouse SP-A, enabling us to track uptake of the exogenous human SP-A protein without interference from endogenous mouse SP-A. As shown if figure 4K, these studies reveal that SP-A/WT surfactant was taken up by the ATII cells more efficiently than the SPA/KO surfactant. Next, we labeled the surfactant from WT or KO BALF with rhodamine and examined uptake into both A549 cells and GFP-positive ATII cells from SPC-GFP mice. In both models, WT surfactant was taken up more rapidly than KO surfactant (Figure 4L and 4M), although uptake into alveolar macrophages was unaffected. Taken together, these results indicate that GPR116 plays a role in regulating surfactant uptake by ATII cells which is related, at least in part, to an abnormal surfactant assembly and composition.
Here, we provide evidence that GPR116 plays a highly specific, essential role in lung surfactant homeostasis and provide a mouse model for studying PAP driven by an autonomous ATII cell defect. The involvement of a GPCR family member in surfactant regulation has important implications for the understanding and treatment of lung surfactant disorders.
EXPERIMENTAL PROCEDURES
Animals
Mice were housed in a pathogen-free facility certified by the Association for Assessment and Accreditation of Laboratory Animal Care International, and the study was carried out in accordance with protocols approved by the NCI Animal Care and Use Committee. Further details on gene targeting strategies and animal experiments are described in Supplemental Experimental Procedures.
Immunoblotting
Immunoblotting was performed as previously described (Nanda et al., 2004) using antibodies from Millipore against SP-A (AB3420), SP-B (AB3780), SP-C (AB3786), and SP-D (AB3434). Additional anti-SPA antibodies were purchased from Santa Cruz (sc-7699), Abcam (ab115791) and US Biological (S8400-02).
Statistical Analysis
Unless stated otherwise, differences between two groups (for example, Gpr116 WT and KO) were presented as the average ± SEM, and statistical significance was calculated using a Student's t test. For comparisons between multiple groups, a one-way ANOVA was used with a Bonferroni posttest. p values < 0.05 were considered significant.
Supplementary Material
Highlights
Gpr116 plays a critical non-redundant role in lung surfactant homeostasis
Gpr116 deletion results in surfactant accumulation in the alveolar space
Gpr116 regulates surfactant composition and uptake by alveolar type II cells
Modulation of GPR116 activity could aid in treatment of lung surfactant disorders
ACKNOWLEDGMENTS
This manuscript is dedicated to the memory of our dear friend and colleague Dr. Jo Rae Wright, who provided helpful advice and SFTPC-GFP mice for these studies. We thank Brigid L.M. Hogan and Jason R. Rock for providing SFTPC-Cre mice. This research was supported by the intramural research program of the NCI, NIH, DHHS, and with federal funds from the NCI under Contract No. HHSN261200800001E and NIH HL34788. The content of this publication does not necessarily reflect the views or policies of the DHHS, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Partial support for the lipidomic analysis was provided by an award from the NIGMS (U54 GM069338 to HAB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes four figures, three tables, and Extended Experimental Procedures and supplemental references and can be found with this article online.
REFERENCES
- Abe J, Suzuki H, Notoya M, Yamamoto T, Hirose S. Ig-hepta, a novel member of the G protein-coupled hepta-helical receptor (GPCR) family that has immunoglobulin-like repeats in a long N-terminal extracellular domain and defines a new subfamily of GPCRs. J Biol Chem. 1999;274:19957–19964. doi: 10.1074/jbc.274.28.19957. [DOI] [PubMed] [Google Scholar]
- Borie R, Danel C, Debray MP, Taille C, Dombret MC, Aubier M, Epaud R, Crestani B. Pulmonary alveolar proteinosis. Eur Respir Rev. 2011;20:98–107. doi: 10.1183/09059180.00001311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey B, Trapnell BC. The molecular basis of pulmonary alveolar proteinosis. Clin Immunol. 2010;135:223–235. doi: 10.1016/j.clim.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elhalwagi BM, Zhang M, Ikegami M, Iwamoto HS, Morris RE, Miller ML, Dienger K, McCormack FX. Normal surfactant pool sizes and inhibitionresistant surfactant from mice that overexpress surfactant protein A. Am J Respir Cell Mol Biol. 1999;21:380–387. doi: 10.1165/ajrcmb.21.3.3676. [DOI] [PubMed] [Google Scholar]
- Ivanova PT, Milne SB, Byrne MO, Xiang Y, Brown HA. Glycerophospholipid identification and quantitation by electrospray ionization mass spectrometry. Methods Enzymol. 2007;432:21–57. doi: 10.1016/S0076-6879(07)32002-8. [DOI] [PubMed] [Google Scholar]
- Lewis JF, Veldhuizen RA. The future of surfactant therapy during ALI/ARDS. Semin Respir Crit Care Med. 2006;27:377–388. doi: 10.1055/s-2006-948291. [DOI] [PubMed] [Google Scholar]
- Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- Malur A, Baker AD, McCoy AJ, Wells G, Barna BP, Kavuru MS, Malur AG, Thomassen MJ. Restoration of PPARgamma reverses lipid accumulation in alveolar macrophages of GM-CSF knockout mice. Am J Physiol Lung Cell Mol Physiol. 2011a;300:L73–80. doi: 10.1152/ajplung.00128.2010. [DOI] [PubMed] [Google Scholar]
- Malur A, Huizar I, Wells G, Barna BP, Malur AG, Thomassen MJ. Lentivirus-ABCG1 instillation reduces lipid accumulation and improves lung compliance in GM-CSF knock-out mice. Biochem Biophys Res Commun. 2011b;415:288–293. doi: 10.1016/j.bbrc.2011.10.043. [DOI] [PubMed] [Google Scholar]
- Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H. Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3. J Biol Chem. 2002;277:22147–22155. doi: 10.1074/jbc.M201812200. [DOI] [PubMed] [Google Scholar]
- Myers DS, Ivanova PT, Milne SB, Brown HA. Quantitative analysis of glycerophospholipids by LC-MS: acquisition, data handling, and interpretation. Biochim Biophys Acta. 2011;1811:748–757. doi: 10.1016/j.bbalip.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer Res. 2004;64:817–820. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- Okubo T, Knoepfler PS, Eisenman RN, Hogan BL. Nmyc plays an essential role during lung development as a dosage-sensitive regulator of progenitor cell proliferation and differentiation. Development. 2005;132:1363–1374. doi: 10.1242/dev.01678. [DOI] [PubMed] [Google Scholar]
- Raghavendran K, Willson D, Notter RH. Surfactant therapy for acute lung injury and acute respiratory distress syndrome. Crit Care Clin. 2011;27:525–559. doi: 10.1016/j.ccc.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakagami T, Uchida K, Suzuki T, Carey BC, Wood RE, Wert SE, Whitsett JA, Trapnell BC, Luisetti M. Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med. 2009;361:2679–2681. doi: 10.1056/NEJMc0904077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11:539–554. doi: 10.1016/j.ccr.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity. 2001;15:557–567. doi: 10.1016/s1074-7613(01)00218-7. [DOI] [PubMed] [Google Scholar]
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- Tang XL, Wang Y, Li DL, Luo J, Liu MY. Orphan G protein-coupled receptors (GPCRs): biological functions and potential drug targets. Acta Pharmacol Sin. 2012;33:363–371. doi: 10.1038/aps.2011.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- Wallgard E, Larsson E, He L, Hellstrom M, Armulik A, Nisancioglu MH, Genove G, Lindahl P, Betsholtz C. Identification of a core set of 58 gene transcripts with broad and specific expression in the microvasculature. Arterioscler Thromb Vasc Biol. 2008;28:1469–1476. doi: 10.1161/ATVBAHA.108.165738. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.