Abstract
The biomechanical factors that result from the haemodynamic load on the cardiovascular system are a common denominator of several vascular pathologies. Thickening and calcification of the aortic valve will lead to reduced opening and the development of left ventricular outflow obstruction, referred to as aortic valve stenosis. The most common pathology of the aorta is the formation of an aneurysm, morphologically defined as a progressive dilatation of a vessel segment by more than 50% of its normal diameter. The aortic valve is exposed to both haemodynamic forces and structural leaflet deformation as it opens and closes with each heartbeat to assure unidirectional flow from the left ventricle to the aorta. The arterial pressure is translated into tension-dominated mechanical wall stress in the aorta. In addition, stress and strain are related through the aortic stiffness. Furthermore, blood flow over the valvular and vascular endothelial layer induces wall shear stress. Several pathophysiological processes of aortic valve stenosis and aortic aneurysms, such as macromolecule transport, gene expression alterations, cell death pathways, calcification, inflammation, and neoangiogenesis directly depend on biomechanical factors.
Keywords: Abdominal aortic aneurysm, Aortic stenosis, Inflammation, Thoracic aortic aneurysm
1. Introduction
1.1. ‘Nothing makes sense in biology except in light of evolution’1
The evolution of species was associated with a more and more organized circulatory system. Simple diffusion of extracellular liquid evolved through flow driven by an archaic heart, to a highly organized system in mammals, including blood flow related to the specific metabolic demand of an organ. The latter was made possible by heart pumping ability and regulated by peripheral resistances, which together generated the arterial blood pressure. Therefore, blood velocity (kinetic energy), heart frequency (pulsatility), and blood pressure (potential energy) are the three fundamental physical components of the haemodynamic load in arterial wall biology. In this evolutionist view, haemodynamics is a fundamental organizing principle selected for diversification and adaptation to life. Nevertheless, haemodynamics is also a common organizing principle in cardiovascular pathologies, such as aortic wall and aortic valve diseases. In fact, haemodynamic is the highest common denominator of vascular pathologies, whatever their atheromatous or non-atheromatous aetiologies. The present review will focus on the biomechanical factors involved in the development of aortic valve stenosis and aortic aneurysms.
1.2. The aorta and the aortic valve
The aorta is the first arterial segment of the body, directly connected to the heart. A unidirectional flow of the blood ejected from the left ventricle into the aorta is maintained through the aortic valve, which opens and closes with each heartbeat. The aortic valve consists of three leaflets (called cusps), corresponding to the physiological dilatations of the aortic root, which are referred to as the sinuses of Valsalva. The left and right cusps correspond to the aortic departure of the left and right coronary artery, whereas the posterior aortic valve leaflet is referred to as the non-coronary cusp. In addition to this common tricuspid anatomy of the aortic valve, a congenital bicuspid valve is found in approximately 0.5–2% of the general population giving rise to differential biomechanical forces both on the valve and the aortic wall. Each aortic valve leaflet is histologically composed of three layers, with the fibrosa towards the aortic surface, the ventricularis near the left ventricle, and the spongiosa in between these layers.
The aorta is the largest artery in the body with a diameter of ∼3 cm at its origin (ascending aorta), ∼2.5 cm in the descending portion (thoracic aorta), and 1.8–2 cm in the abdomen (abdominal aorta). Like other arterial segments, the aortic wall consists of three layers; the intima composed of a thin layer of endothelial cells, subendothelial connective tissue, and an internal elastic lamina; the tunica media of the aorta is much thicker than that of peripheral arteries, composed of several layers of packed smooth muscle cells and extracellular matrix (ECM); the adventitia is composed of connective tissue enfolding small vessels (vasa vasorum) and nerves (nervi vascularis).
As the valve opens during cardiac systole, the blood flows through the aortic valve into the aorta for distribution to the peripheral organs through its multiple branches. In addition to this conduit function, the aorta also accomplishes a buffering function through its (visco)-elastic properties (compliance). The aorta can distend at each systole to allow the reception of the blood stroke volume and this elastic energy is expended during the diastole when the aortic tube recoils, and the blood flow continues to the periphery.
As a teleonomic consequence of evolutionary haemodynamics in mammals, particularly of blood pressure, the wall of conductance arteries must withstand haemodynamic load (the content) within the arterial system (the container). As a consequence, a pressure gradient exists between the arterial circulation and the interstitial pressure in the adventitia, generating a transmural water flow radially outwards through the arterial wall. This hydraulic conductance from the lumen to the adventitia conveys substances that can be retained, modified, and activated during their mass transport through the wall. The three major biomechanical factors impact convection, namely pressure, flow, and pulsatility. The wall structural predominant modulators of convection are endothelial impermeability, elastic lamina integrity, and the physico-chemical properties of the molecules (mass, charge, hydrophilic properties, affinity for wall components).2 The most studied convection process in humans is the convection of low density lipoprotein through the arterial wall in atherosclerosis.3 In this context, the preferential localization of atheroma in the arterial bifurcation4 may be due to the lack of the washing effect of laminar longitudinal flow, and a local recirculation with concentration of plasma substances adjacent to the luminal surface and an increased radial mass transport.
2. Basics continuum mechanical definitions
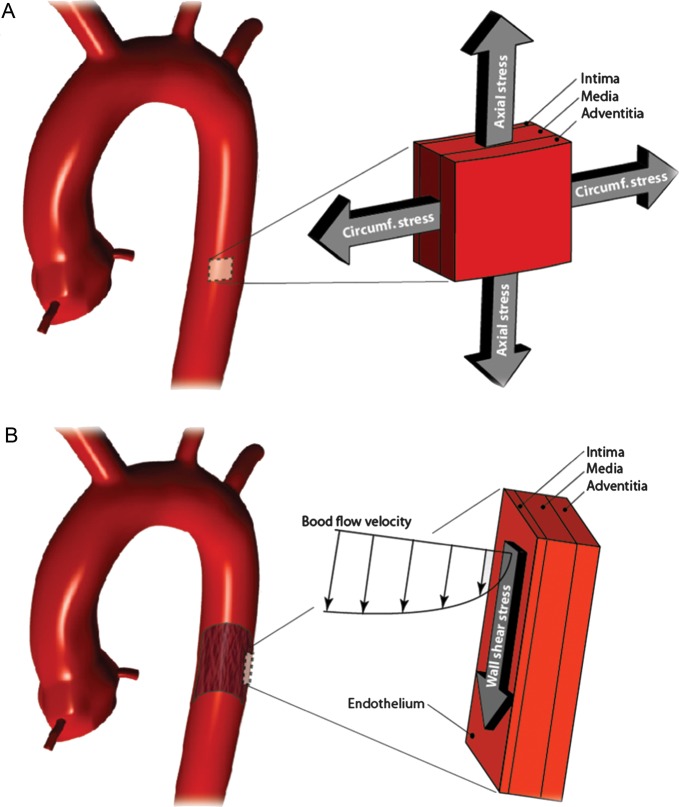
Mechanical loads on cardiovascular tissue cause stresses and strains (Table 1). Stress is defined as force per area and according to its direction, normal and shear stresses can be distinguished (Figure 1A). The actual stress components depend on the coordinate system, i.e. how the area is related with respect to the force acting on the area element. When area elements are considered such that the shear stress vanishes, the structure is only loaded by normal stresses, which are called principal stresses.
Table 1.
Terminology of aortic wall and aortic valve biomechanics
| Stress | Force per area |
|---|---|
| Normal stress | Stress perpendicular to the area |
| Shear stress | Stress parallel to the area |
| Wall shear stress (WSS) | Stress induced by blood flowing over the valve or the luminal side of the vascular wall |
| Laminar shear stress | Shear stress caused by a streamlined flow |
| Oscillatory shear stress | Shear stress caused by oscillatory flow |
| Strain | Deformation measure |
| Normal strain | Relative changes in length of a line |
| Shear strain | Relative changes in angle of a square |
| Isochoric (isovolumetric) | Constant volume |
| Isotropic | Uniform in all directions |
| Vortical structures (VS) | Spatial region, within which the fluid is at rotational (vortical) motion |
| Finite element method (FEM) | Numerical concept to solve structural or fluid-mechanical problems |
| Computational flow dynamics (CFD) | Numerical method to compute fluid flow from given initial conditions |
| Fluid structure interaction (FSI) | Method to prescribe the mechanical interaction between fluid and solid domains |
Figure 1.
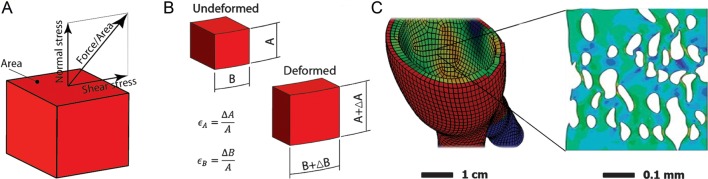
Basic continuum mechanical definitions. (A) Stress is defined as force per area and can be split into shear and normal stresses, respectively. (B) Definition of strain by relating the deformed to the undeformed configuration. (C) Stress and strains always correspond to a length scale. Left and right images show stress at the macroscopic and microscopic length scale, respectively. The specific length scales are indicated by the scaling bars.
Strain is a quantity that records change in length (normal strain) and angle (shear strain) during the deformation of a tissue (Table 1). Similar to stress, also the strain can be considered within the principal coordinate system, such that only normal strains appear (Figure 1B). The stiffness defines the tissue's mechanical properties, and relates stress and strain, which are always associated with a specific length scale (Figure 1C). Only for simple geometries, an analytically stress analysis is possible and, for more complicated (and clinically relevant) cases, numerical schemas such as the finite element method (FEM) are required (Table 1).
Like other viscous fluids, also blood induces shear stresses according to the spatial velocity gradient, i.e. the shear rate. The fluid's viscosity relates shear stress and shear rate. When flowing over a wall, blood flow causes wall shear stress (WSS), which is sensed by the endothelial layer, for example. Blood flow can be predicted with computational flow dynamics (CFD) simulations that frequently use the FEM. CFD simulations depend, to a large extent, on the boundary conditions that are prescribed at the boundaries of the analysed flow domain. Vascular structures such as the aortic wall and the valve interfere with the blood flow and vice versa. Biomechanical simulations that account for that coupling are called fluid structure interaction simulations (FSI; Table 1).
One major objective of biomechanics in the biology of aortic wall and aortic valve diseases is to study the force transmission from the macroscopic to the cellular and extracellular levels. At the macroscopic scale, the tissue can be regarded as a continuum, which constitution depends on the underlying histology. For example, the organization of ECM, cells, proteins, etc. defines a mathematical model, which in turn computes the macroscopic valve and wall stress. Since such a mathematical model characterizes the constitution of the tissue, it is also called a constitutive formulation. Finally, the stress state has to satisfy the continuum mechanical governing equations and being in equilibrium with the external loadings, which renders an (initial) boundary value problem to be solved.
3. The biomechanics of the aortic valve
The aortic valve is exposed to both haemodynamic forces and structural leaflet deformation as it opens and closes with every heartbeat. Thickening and calcification of the aortic valve will lead to reduced opening and the development of left ventricular outflow obstruction, referred to as aortic stenosis.5 The histological changes in aortic stenosis are characterized by dystrophic calcification, inflammatory cell infiltration, and heterotopic bone formation.5,6
As the valve opens during cardiac systole, the blood flows through the aortic valve with a peak velocity of 1.35 ± 0.35 m/s7 (Figure 2A). However, in calcified aortic valves the blood velocity, and hence the pressure gradient between the aorta and the left ventricle in systole, is higher. When the maximal systolic blood velocity exceeds 4 m/s (corresponding to a mean pressure gradient >40 mmHg), a severe aortic stenosis has devolved (Figure 2B).
Figure 2.
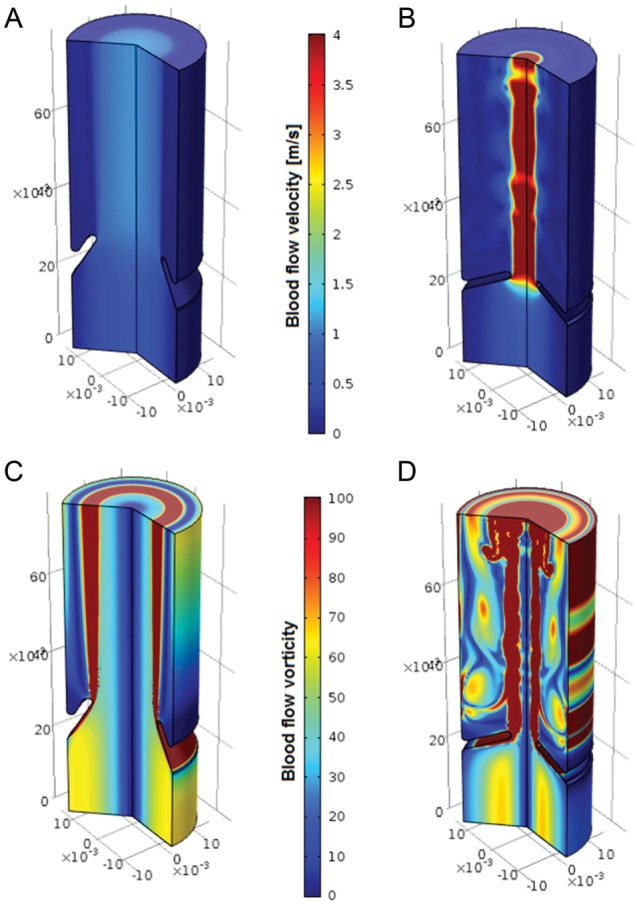
Haemodynamic differences between a normal (left ) and a stenotic (right ) aortic valve. (A) and (B) shows the velocity and (C) and (D) the vorticity of blood flow based on an axisymmetric CFD simulation. Note the perturbed flow pattern and the jet-like flow in the stenotic valve. (Illustration by J. Biasetti)
The aortic valve closes at the end of systole, mainly due to a lowering of the axial pressure difference, although also the pooling of blood into the aortic sinuses may be involved in the diastolic closing phase.7 The aortic valve is exposed to laminar shear stress on ventricular side when the blood flows past the leaflets during systole (Figure 3A), and to oscillatory shear stress on the aortic side when blood pools into the sinuses during diastole (Figure 3B). The diastolic coronary flow partially induces laminar shear stress over the left and right cusps, whereas the flow over the non-coronary cusp may be solely oscillatory (Figure 3C). Interestingly, an echocardiographic study of ∼200 patients identified the non-coronary cusp as firstly affected by thickening,8 suggesting that lower laminar shear stress, in the absence of diastolic coronary flow, may accelerate valvular thickening.
Figure 3.
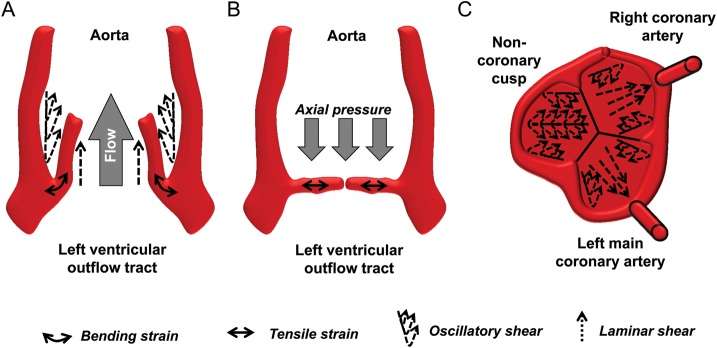
Wall shear stress (dotted arrows) and strain (solid arrows) in the aortic valve during systole (A) and diastole (B and C). During systole, the ventricular side of the valve is subjected to laminar shear stress, whereas on the aortic side the systolic shear stress is mainly oscillatory (A). The bending strain is illustrated with a curved arrow (A). In diastole, axial strain results from the diastolic pressure gradient and tensile strain from the elongation of the valve leaflets (B). At this time, the aortic side is subjected to oscillatory shear as the blood pools into the aortic sinuses, and to laminar shear by the diastolic coronary flow (C). (A) and (B) adapted from ref.7
In addition to shear stress, the leaflets are subjected to axial stress, when the valve is closed during diastole, resulting in a transverse pressure gradient over the valve (Figure 3B). The aortic cusps are longer in both circumferential and radial direction during diastole compared with systole (Figure 3B). The loss of cusp stretch during valvular calcification may prevent a complete leaflet coaptation resulting in a regurgitant flow from the aorta to the left ventricle during diastole,7 and a considerable proportion of aortic stenosis patients have concomitant aortic regurgitation.9
The strain of the aortic valve during the heart cycle is both as a result of the axial pressure, the bending forces, and of changes in length (Figure 3). During the heart cycle, there is a reversal of the curvature of the aortic cusps leading to a regional bending strain. In accordance with the above-mentioned relation between stiffness and strain, the increased stiffness in early aortic valve sclerosis would hence lead to a decreased strain, which has also been demonstrated using FEM.10 In the latter study, it was, however, modelled that commisural fusion associated with rheumatic valve disease and bicuspid valves will result in increased valve maximal curvatures both in parallel and perpendicular to the valve matrix fibre orientation.10 Taken together, aortic valve disease may hence be associated with both decreased and increased bending strain.
Early valve lesions in aortic stenosis preferentially occur on the aortic side of the valvular leaflets. The reason for this propensity of the fibrosa to valvular lesions may be related to the above-mentioned differences in terms of both haemodynamic and biomechanical forces between the ventricular and the aortic side of the valve leaflets. In addition, the valve morphology is different on the aortic and ventricular side of the valve. Whereas the fibrosa layer close to the aortic surface consists mainly of collagen fibres in circumferential direction, the ventricularis layer is mainly composed of elastin, and ex vivo studies of separated valvular layers have revealed that the fibrosa is stiffer compared with the ventricularis layer.11
The congenital bicuspid valve differs from the common tricuspid valve not only through the number of cusps, but also in terms of the ring geometry (oval vs. circular). The geometry of bicuspid valves leads to unsteady shear stresses even under physiological flow and pressure conditions, which may play a role in the accelerated calcification associated with bicuspid valve anatomy.12 Importantly, the alterations of systolic blood flow associated with bicuspid valve geometry may be responsible for the development of aneurysms in the aortic root.13
The localization of the congenital cusp fusion may affect both valve calcification and aortic root dilatation.14 Although the latter observation may result from differential biomechanical profiles, it cannot be excluded that the observed associations are linked through common genetic predispositions to disease.14 The notion of a biomechanical association between bicuspid valve anatomy and aortic root dilatation has received support from measurements of the cusp opening angle (COA), which were recently introduced based on MRI analysis, showing that a reduced COA in bicuspid compared with tricuspid valves corresponded with aortic root dilatation.15 In addition, computational assessment has shown alterations of wall-shear stress in the bicuspid fibrosa, which were associated with increased calcification.16 Finally, bicuspid and tricuspid stenotic valves exhibit differential mRNA levels despite similar degree of stenosis severity in terms of both inflammatory gene expression17 and the nuclear enzyme Poly(ADP-ribose) polymerase 1 (PARP-1).18 In the latter context, it is interesting that PARP-1 inhibition protects against low shear stress-induced inflammation,19 suggesting that changes in shear stress related to valve anatomy may alter cellular responses in terms of gene expression.
4. The biomechanics of the aorta
4.1. Wall stress and strain as a consequence of the blood pressure
The aorta can be regarded as an inflated tube-like structure, where the arterial pressure is translated into tension-dominated mechanical wall stress. Specifically, circumferential and axial stresses are the dominating principal stresses in the aorta (Figure 4). Due to inflation, the wall deforms isochorically, i.e. the tissue volume is kept constant.20 Consequently, extension in circumferential and axial directions leads to thinning of the wall.
Figure 4.
(A) Circumferential and longitudinal stresses are the dominating principal stresses in the aortic wall. (B) Wall shear stress (WSS) as a consequence of blood flow over the endothelial layer.
With increasing aortic diameter, the normal wall becomes proportionally thicker. This is realized by an increased number of medial lamellar units (MLU), which keeps the tension carried by a single MLU in the normal wall constant at about 2 ± 0.4 N/m.21 At mean arterial pressure, the circumferential wall stress in the ascending aorta is 92.51 ± 6.35 kPa.22 The aorta is dynamically pressurized, such that wall stress and strain oscillate with the cardiac cycle.
The stiffness of the aorta increases progressively with strain, i.e. the same aorta will be stiffer at hypertensive loading than at normal arterial pressure. Consequently, for a meaningful comparison of the aortic stiffness, it is important to match the strain levels. Like other vessels also the normal aorta is anisotropic,23 i.e. its stiffness along the circumferential and longitudinal directions differ. Aortic anisotropy seems to disappear with aneurysmal disease, which could be a direct consequence of the less organized collagen.24
4.2. Wall shear stress and strain as a consequence of the blood flow
Blood flow within the aorta (Figure 4) is highly unsteady and the assumptions for a Poiseuille flow are not satisfied. During a major part of the cardiac cycle, blood flows downwards, but at late diastole reversal flow is seen. Whereas a non-Newtonian description of blood may be required for resolving local blood flow features, a Newtonian model appears adequate when predicting WSS in large arteries such as the aorta.25 The Newtonian model is the simplest constitutive model for blood and assumes that the shear stress produced by the viscous blood is linear with respect to the shear rate. WSS in the normal thoracic aorta ranges from 2.2 to 3.5 dyn/cm2,26 and increases by several folds during exercise.27
Analysing the complex flow pattern in the aorta solely based on velocity information is difficult. For example, a reliable detection of vortical structures (VS), i.e. spatial region, within which the blood is at rotational (vortical) motion, is not possible. It is emphasized that VS dynamics closely determine WSS distribution and WSS peaks can be the direct consequence of VS impingement.
4.3. Aortic aneurysms
Because of its continuous haemodynamic adjustments (exposure to pulsatile flow), the aorta is particularly prone to injury and disease resulting from mechanical trauma. Typically, histologic changes occur with age in the media. These include: cystic medial necrosis, defined as pooling of mucoid material; elastin fragmentation, characterized by disruption of elastin lamellae; fibrosis, defined as an increase in collagen at the expense of smooth muscle cells; and medionecrosis, defined as areas with apparent loss of nuclei.28 These changes show a striking correlation with age and their location suggests that they appear as a consequence of injury and repair caused by haemodynamic events.
The most common pathology of the aorta is the formation of an aneurysm, morphologically defined as a progressive dilatation of a vessel segment by more than 50% of its normal diameter. In contrast, despite common pathology and aetiologies, dissections are due to acute intraparietal rupture. Whereas abdominal aortic aneurysms (AAA) mainly originate in atherosclerotic segments, the aneurysm of the ascending aorta is not related to lipid accumulation but rather to the degradation of the vascular wall components due to either intrinsic defects of the cell components or to pathological haemodynamic conditions.
Dilation of the ascending aorta preferentially occurs at the outer curvature of the vessel, which has the maximum axial stress.29 Interestingly, whereas the thoracic aortic aneurysms associated with Marfan mutations preferentially develop in sinuses of Valsalva in response to physiological diastolic vortices, aneurysms in response to the altered systolic flow associated with bicuspid valves develop in the aortic root.13
The impact of biomechanical factors on the evolution of AAA was demonstrated by the original observations of Vollmar et al.30 In this remarkable study, the authors reported an increased frequency of aneurysm of the abdominal aorta in patients with above-knee amputations compared with paired controls (5.5 vs. 1.1%), and that the side of unilateral amputation provoked an axial shifting of the infrarenal aorta, favouring aneurysmal development.
Aortic dissection is the consequence of a tear on the luminal side of the arterial wall. The tear is usually transverse, involves more than half the vessel circumference, and occurs mainly at proximal sites of the aorta (65% 1–3 cm after the origin of the coronary arteries, 10% after the origin of the left subclavian artery) which explains why dissections are rare in the abdominal aorta.31 Entry of flowing blood through the tear separates the media in two layers. The inner layer is composed of the intima and the portion of the media that contains the internal elastic lamina, whereas the external layer consists of the external elastic lamina and the adventitia. Progression of the dissection can either lead to reentry of the blood in the true lumen or complete aortic wall rupture (fatal).
4.4. Intraluminal thrombus
The intraluminal thrombus (ILT) is a neo-tissue generated at the blood/wall interface by recirculation as described above in AAA. ILT is biologically active, with spatio-temporal dynamics, including renewal (platelet activation, red blood cell, neutrophil and possible bacterial trapping, fibrin formation, fibrinolysis initiation) at the blood luminal interface and progressive degradation toward the abluminal layer.2 Since the ILT is a highly porous biomaterial,32 radial convection, and activation of blood–borne macromolecules, particles and cells are highly active through the ILT towards the arterial wall.2 Pathogenic mediators, generated at the blood/ILT interface, are thus radially conveyed towards the media and the adventitia in AAA.33 In particular, active proteases and oxidative activities convected from the ILT participate to the degradation of the wall, as will be further outlined below.
VS are much more dominant in the aneurysmatic than the normal aorta, which could have direct implications for ILT formation. Specifically, strong VS provide biomechanical and biochemical conditions that favour platelet activation, i.e. a first step of ILT formation.34 The ILT weakens35 and thinnens36 the underlying aneurysm wall. While this clearly increases the risk for aneurysm rupture, the ILT can carry mechanical stress,37 and hence also protects the wall from stress.38 These two competing effects need to be carefully evaluated in the individual patient, which is possible with a biomechanical rupture risk assessment.39
5. Cellular responses to biomechanical factors
5.1. Endothelial cells
The interface between the blood and the aortic wall and valve is represented by endothelial cells, which hence are the first to be exposed to the shear stress generated by the blood flow. Flow shear stress experienced by the endothelial cells is associated with changes in gene expression patterns through positive and negative shear stress responsive elements in their promoter regions.40 For example, whereas laminar shear stress appears to have protective effects in terms of increased superoxide dismutase and endothelial nitric oxide (NO) synthase expression in cultured human endothelial cells, oscillatory shear stress did not induce these protective genes in the same study,41 suggesting that changes in shear stress may contribute to alterations of endothelial cell function. However, as outlined above, the endothelium-blood flow interaction is lost once an ILT is formed. Based on this assumption, it has been suggested that endothelium-dependent responses to shear stress may not be involved in AAA progression.42 Nevertheless, shear stress-induced platelet aggregation may contribute to ILT growth and hence play a continued role in aneurysm progression and rupture.2
Interestingly, laminar shear stress induces differential responses in porcine endothelial cells derived from the aortic wall compared with those derived from the aortic valve. Whereas aortic endothelial cells align parallel to flow, aortic valve endothelial cells align perpendicular to flow.43 The latter observation was associated with differences in signalling pathways, and suggests important differences in the endothelial response to biomechanical factors between the aorta and the aortic valve.
As outlined above, the endothelialized aortic valve leaflets are exposed to differential biomechanical factors on the aortic and the ventricular side, which is also reflected in differential gene expression, with higher levels of calcification-associated gene expression on the aortic side, whereas inflammation-associated gene expression appears to dominate on the ventricular side.44 For example, the bone morphogenic protein (BMP) -4 is preferentially expressed on the aortic side of porcine valves,44 and is down-regulated by shear stress similar to that exerted on the valve's ventricular side.45 In addition, ex vivo exposure of the aortic side of porcine aortic valves to non-physiologic pulsatile shear stress up-regulates ICAM-1, VCAM-1, BMP-4, and TGF-β expressions as determined by immunohistochemistry, whereas the oscillatory shear stress normally encountered by this valvular surface (Figure 3C) did not alter proinflammatory gene expression.46
In addition to mediators of inflammation and calcification, also the release of endothelium-derived vasoactive factors may be involved in the biomechanics of the aortic valve. For example, either endothelial denudation or inhibition of NO synthesis increased the elastic modulus of pocine aortic valves using biaxial micromechanical testing, suggesting that the endothelium may be involved in the regulation of valvular stiffness.47
5.2. Valvular interstitial cells
The valvular interstitial cell, which is the dominating cell type in aortic valves, represents a highly plastic cell population with dynamic phenotypic features. The development of an activated myofibroblast phenotype in the valve interstitium appears to be a key process in aortic valve stenosis.5,7,48 In addition, valvular interstitial cells may develop towards an osteoblastic phenotype taking part in active heterotopic bone formation within the valvular leaflet.48 Recently, epigenetic alterations were implicated in valvular interstitial cell polarization. It has been shown that calcified regions of stenotic human valves exhibit less methylation of the 5-lipoxygenase promoter compared with non-calcified valve tissue.49 In the latter study, treatment with a hypomethylator in addition decreased promoter methylation and increased 5-lipoxygenase transcription and leukotriene production,49 which may participate in the pathological processes of aortic stenosis.17 In addition, several genes encoding osteogenic proteins associated with heterotopic bone formation in aortic valves5,50 are regulated by GC-rich promoters sensitive to methylation/hypomethylation. Interestingly, osteopontin gene methylation is decreased in response to shear stress in murine bone marrow-derived progenitor cells, associated with increased osteopontin expression.51 Although the implications for aortic stenosis pathophysiology remain to be established, those results provide a first suggestion of mechanically induced osteogenic differentiation through epigenetic alterations.
It should, however, be considered that valvular interstitial cells may be protected from shear stress by the endothelial layers. These cells are in contrast exposed to the valve's changes in stress and strain during the cardiac cycle. Whereas the opening and closing of the aortic valve imposes changes in strain on the valvular insterstitial cells under physiologic conditions, increased valvular stiffness would imply reduced strain, as outlined above. In this context, it is interesting that porcine aortic valve interstitial cells decrease mRNA expression levels of several proinflammatory genes, such as VCAM-1 and MCP-1, in response to physiologic cyclic strain compared with static conditions.52 As stated above, aortic stenosis in bicuspid valves is, however, associated with an increased bending strain, and in this context cyclic strain has also been associated with matrix metalloproteinase (MMP53,54) and osteogenic gene expression in human aortic smooth muscle and aortic valve interstitial cells.55
Finally, also the stiffness of the valve per se may influence the valvular interstitial cell phenotype, as demonstrated by studies of cells cultured on different matrices, in which substrates simulating the stiffer fibrosa layer (see above) are associated with an activated VIC phenotype and calcification.56
5.3. Vascular smooth muscle cells
In the aortic wall, vascular smooth muscle cells respond to biomechanical factors both directly through the exposure to cyclic stretch in response to the pulsatile pressure, and indirectly through mediators convected through the aortic wall with the transmural interstitial flow. In addition, under normal conditions, endothelium-derived vasoactive factors released in response to shear stress act on the aortic smooth muscle cells, although this interaction may be lost with the development of the ILT in the context of AAA, as outlined above.
The two dominating smooth muscle cell driven processes in aortic aneurysms are increased vascular smooth muscle cell apoptosis and alterations in ECM turn over.2 The direct effects of cyclic stretching of vascular smooth muscle cells on collagen production were described almost 40 years ago.57 In addition, increased strain up-regulates MMPs in vascular smooth muscle cells,58 suggesting that local increases in mechanical strain may lead to enhanced matrix degradation by smooth muscle cells.
6. Biomechanical factors and cardiovascular calcification
Vascular and valvular calcification show similarities in their molecular mechanisms in terms of the involvement of both a dystrophic calcification and osteogenic mediators.48 The process of medial calcification in aortic aneurysm is associated with a switch to a pro-calcifying vascular smooth muscle cell phenotype.2 Likewise, in valvular calcification, interstitial cells develop into an osteoblastic phenotype.48 However, calcification may be protective in the evolution of AAA through the greater resistance of calcified tissue to proteolysis,2 which is in contrast to calcification as the main driver of aortic valve stenosis.
In aortic aneurysms, calcifications are usually localized in the outer part of the media and delimit the external side of the aneurysmal dilatation.2 Histological examination of aortic valves has revealed two different patterns of calcification, arising in the coaptation area of the aortic cusps and/or the radially from the cusp attachment to the centre of the valve59 (Figure 5). These areas correspond to sites where the greatest flexion stress (and hence strain) occurs, suggesting that these biomechanical factors may be directly involved in valve calcification. In support of the latter notion, porcine valves subjected to elevated cyclic stretch in the presence of osteogenic media and TGFβ develop calcifications, which is dependent on BMP signalling and also the degree of stretch.60 Since either cyclic stretch or osteogenic stimulation alone did not induce calcification,60 the latter findings suggest that valvular calcification occurs through a synergy between biomechanical factors and osteogenic/proinflammatory signalling.
Figure 5.
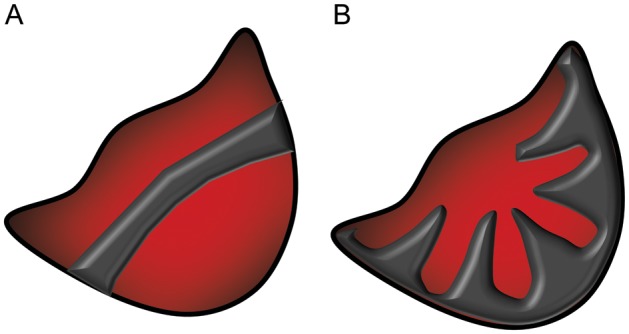
Patterns of calcification of human aortic valve cusps, along the coaptation area (A) and radially from the valve insertion (B). Adapted from ref.59
7. Convection in pathology
7.1. Convection in aneurysms
The convection of plasma enzyme precursors (zymogens and proforms) through the arterial wall is enhanced in aneurysmal pathology, due in part to the degradation of elastin. For example, plasma prothrombin13 and plasminogen61 are convected and activated on smooth muscle cell in aneurysms of the ascending aorta, participating to the degradation of the arterial wall. In addition, the convection of inflammatory mediators (such as leukotriene B4) derived from neutrophils in the luminal side of the ILT may further enhance the inflammatory circuits within AAA.33
7.2. Convection in the aortic valve
The transverse pressure gradient exerted on the closed aortic valve during diastole may allow for convection to occur also through the valve. Since valve tissue lack the internal elastic lamina, the pressure difference over the valvular leaflet could act as driving force for the convection of macromolecules. In support of the latter notion, it has been observed that intravenous injection of horseradish peroxidase (HRT) in rats results in leaky spots on the aortic side of the valve and a spread of HRT both through diffusion in parallel to the endothelial layer and through convection towards the ventricular side of the leaflets.62 In analogy to aneurysm disease, macromolecules such as proteases may hence be convected from the aortic towards the ventricular side of the leaflet. These enzymatic activities may participate in the valvular remodelling associated with early aortic valve sclerosis, leading to a stiffer valve. As a result, the reduced opening of the valve may, together with an increased ejection time, increase the axial pressure on the valve from the ventricular side, and it can be speculated that convection may change direction as the systolic forces towards the aorta will exceed the diastolic pressure on the closed valve. In support of the latter notion, the example depicted in Figure 2 has an about four to six times higher pressure gradient across the leaflet as a result of the reduced opening.
8. Immunological activation in response to biomechanical factors
Inflammatory cell components are consistently found in aortic2,33 and aortic valve pathologies,5,6,17,48 but the cause–effect relationship between the presence of inflammatory cell infiltrates and the pathologic condition in these structure remains a source of debate. For example, although it is generally accepted that infiltrated leucocytes contribute to the progression of AAA, extensive immune-histo-morphometric studies have failed to demonstrate a direct correlation between inflammatory cell count and the extent of either maximal aneurysm diameter or clinical presentation.63 Immune cells may hence rather enter in the pathogenic process of AAA in response to structural changes (disappearance of smooth muscle cells, degradation of ECM fibres) that are primarily driven by biomechanical factors.
Cellular infiltrates in the fragmented media/adventitia transition of AAA consist of lymphocytes (CD20+ B- and CD3+ T-lymphocytes) and phagocytic leucocytes (CD45+CD68+ macrophages). Interestingly, these cells are typically present in areas devoid of smooth muscle cells but are never detected in the media of healthy arteries. The circumferential distribution along the elastin sheets suggests that lymphoid and myeloid cell infiltrates follow the weakening of the wall due to the disappearance of smooth muscle cells and the degradation of ECM. Indeed, the arterial media is an immune-privileged tissue because the survival and activation of leucocytes are actively inhibited by healthy smooth muscle cells, largely through consumption of tryptophan.64
An alternative explanation for the circumferential distribution of leucocyte aggregates in AAA is the tissue pressure gradient. Leucocyte extravasation takes place at sites of low intravascular pressure, such as post-capillary venules and high endothelial venules. In the case of the aortic wall, the pressure around the venule is increasingly higher from the adventitia to the intima, suggesting that leucocytes, like anadromous fishes, must migrate against the pressure gradient to access the most luminal layers. This may explain why lymphoid aortic infiltrates appear in packets distributed along the isopressure lines of the arterial wall circumference, suggesting that these cells cannot centripetally migrate beyond a given pressure level.
9. Angiogenesis in response to biomechanical factors
Microvessels are abundant in the medial layer of histologic sections from AAA, suggesting an active neoangiogenesis in aneurysm formation. In contrast to small-sized arteries with stenotic lesions, in which luminal and adventitial microvessels connect at sites of an obliterated medial layer,65 no direct transmural vascular connection between inner and outer microvessels has yet been demonstrated in AAA. Furthermore, the microvessels at opposite sides of the aneurysmal aortic wall may have a different origin since they differ in both anatomic structure and reactivity.66
The most accepted origin of the microvessels found in the outer medial layers is an inward vascularization arising from the adventitial vasa vasorum. The angiogenic triggers involved in this process are likely to be transported radially from the lumen through the media. For example, lipid mediators generated in the intima induce medial smooth muscle cell production of VEGF-A,67 which can radially reach the adventitial vasa vasorum. In addition, products of elastin degradation may act as angiogenic triggers through upregulation of MT1-MMP leading to sprouting and tube formation of endothelial cells68 Finally, vasa vasorum may also originate from the luminal endothelium,65,66 but this has not been explored in large size arteries such as the aorta.
Interestingly, in ruptured aneurysms, microvessels are detected also in the central medial layers, at the rupture edges only, where their presence could be either a trigger or a consequence of wall dissection.69 It is possible that at smaller scale when compared with aortic dissections, luminal microtears could let some blood components enter the vessel wall from the luminal side. The recanalization of such micro-intramural thrombi would offer one possible explanation as to the lack of pericytes in luminal microvessels, suggesting another origin than by sprouting of pre-existing vessels. In support of the latter notion, carotid artery ligation in mice induces CD31+ neovessels confined to the luminal organizing thrombus and not connected to the outer arterial layers.70
This type of angiogenesis, possibly driven by the production of VEFG by the monocyte macrophages that colonize the organizing thrombus,71 is known to favour the recanalization of the intramural thrombus and, when successful, may contribute to the re-entry of blood flow in the true lumen in some cases of human aortic dissection.
10. Summary and conclusion
The evolution of an organized and regulated circulatory system led to biomechanical loads on cardiovascular tissues. The formation of aortic valve stenosis and aortic aneurysms is a multifactorial and predominantly degenerative process, resulting from a complex interplay between haemodynamic factors and the adaptive biological processes elicited. The latter constitute the basis for the remodelling that takes place throughout the entire lifetime in order to maintain the integrity and the function of tissues faced to the cyclic mechanical stress. The stress and strain exerted on the aorta and the aortic valve induce changes directly sensed by the structural cells, and also alter the ECM, both contributing to structural alterations. Associated pathologies, such as aortic aneurysms and aortic stenosis, may result from either an enhanced intrinsic propensity of the arterial wall to dilate in response to normal biomechanics (Marfan, bicuspid valve), or as a result of pathologic biomechanical load in a given arterial segment. Several disease-specific pathophysiological processes directly depend on biomechanical factors, such as macromolecule transport, gene expression alterations, cell death pathways, calcification, inflammation, and neoangiogenesis. In conclusion, it is important to take into account biomechanical factors when studying the pathophysiology of aortic wall and aortic valve diseases.
Funding
Supported by the Swedish Research Council (grant number 2011-2988), and the Swedish Heart and Lung Foundation (grant numbers 20120474 and 20120827) and by the European Union integrated project ‘Fighting Aneurysmal Disease’. (‘FAD’; FP-7, HEALTH F2-2008-200647).
Acknowledgement
The authors thank Dr Jacopo Biasetti (Royal Institute of Technology, Stockholm, Sweden) for the modelling of the blood flow in normal and stenotic aortic valves.
Conflicts of interest: none declared.
References
- 1.Dobzhansky T. Nothing in biology makes sense except in the light of evolution. Am Biol Teach. 1973;5:125–129. doi:10.2307/4444260. [Google Scholar]
- 2.Michel JB, Martin-Ventura JL, Egido J, Sakalihasan N, Treska V, Lindholt J, et al. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc Res. 2011;90:18–27. doi: 10.1093/cvr/cvq337. doi:10.1093/cvr/cvq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang N, Vafai K. Low-density lipoprotein (LDL) transport in an artery – A simplified analytical solution. Int J Heat Mass Transfer. 2008;51:497–505. doi:10.1016/j.ijheatmasstransfer.2007.05.023. [Google Scholar]
- 4.Caro CG. Discovery of the role of wall shear in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:158–161. doi: 10.1161/ATVBAHA.108.166736. doi:10.1161/ATVBAHA.108.166736. [DOI] [PubMed] [Google Scholar]
- 5.Rajamannan NM, Evans FJ, Aikawa E, Grande-Allen KJ, Demer LL, Heistad DD, et al. Calcific aortic valve disease: not simply a degenerative process. Circulation. 2011;124:1783–1791. doi: 10.1161/CIRCULATIONAHA.110.006767. doi:10.1161/CIRCULATIONAHA.110.006767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. doi:10.1161/01.CIR.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 7.Balachandran K, Sucosky P, Yoganathan AP. Hemodynamics and mechanobiology of aortic valve inflammation and calcification. Int J Inflam. 2011;2011:263870. doi: 10.4061/2011/263870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cujec B, Pollick C. Isolated thickening of one aortic cusp: preferential thickening of the noncoronary cusp. J Am Soc Echocardiogr. 1988;1:430–432. doi: 10.1016/s0894-7317(88)80025-7. [DOI] [PubMed] [Google Scholar]
- 9.Honda S, Kitai T, Okada Y, Tani T, Kim K, Kaji S, et al. Impact of aortic regurgitation on the prognosis of severe aortic stenosis. Heart. 2012;98:1591–1594. doi: 10.1136/heartjnl-2012-302089. doi:10.1136/heartjnl-2012-302089. [DOI] [PubMed] [Google Scholar]
- 10.Katayama S, Umetani N, Hisada T, Sugiura S. Bicuspid aortic valves undergo excessive strain during opening: A simulation study. J Thorac Cardiovasc Surg. doi: 10.1016/j.jtcvs.2012.05.032. Advance Access published June 12, 2012, doi:10.1016/j.jtcvs.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 11.Stella JA, Sacks MS. On the biaxial mechanical properties of the layers of the aortic valve leaflet. J Biomechem Eng. 2007;129:757–766. doi: 10.1115/1.2768111. doi:10.1115/1.2768111. [DOI] [PubMed] [Google Scholar]
- 12.Yap CH, Saikrishnan N, Tamilselvan G, Vasilyev N, Yoganathan AP. The congenital bicuspid aortic valve can experience high-frequency unsteady shear stresses on its leaflet surface. Am J Physiol Heart Circ Physiol. 2012;303:H721–H731. doi: 10.1152/ajpheart.00829.2011. doi:10.1152/ajpheart.00829.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Touat Z, Lepage L, Ollivier V, Nataf P, Hvass U, Labreuche J, et al. Dilation-dependent activation of platelets and prothrombin in human thoracic ascending aortic aneurysm. Arterioscler Thromb Vasc Biol. 2008;28:940–946. doi: 10.1161/ATVBAHA.107.158576. doi:10.1161/ATVBAHA.107.158576. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634–1638. doi: 10.1136/hrt.2007.132092. doi:10.1136/hrt.2007.132092. [DOI] [PubMed] [Google Scholar]
- 15.Della Corte A, Bancone C, Conti CA, Votta E, Redaelli A, Del Viscovo L, et al. Restricted cusp motion in right-left type of bicuspid aortic valves: a new risk marker for aortopathy. J Thorac Cardiovasc Surg. 2012;144:360–369. doi: 10.1016/j.jtcvs.2011.10.014. 369 e361 doi:10.1016/j.jtcvs.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Chandra S, Rajamannan NM, Sucosky P. Computational assessment of bicuspid aortic valve wall-shear stress: implications for calcific aortic valve disease. Biomechem Model Mechanobiol. 2012;11:1085–1096. doi: 10.1007/s10237-012-0375-x. doi:10.1007/s10237-012-0375-x. [DOI] [PubMed] [Google Scholar]
- 17.Nagy E, Andersson DC, Caidahl K, Eriksson MJ, Eriksson P, Franco-Cereceda A, et al. Upregulation of the 5-lipoxygenase pathway in human aortic valves correlates with severity of stenosis and leads to leukotriene-induced effects on valvular myofibroblasts. Circulation. 2011;123:1316–1325. doi: 10.1161/CIRCULATIONAHA.110.966846. doi:10.1161/CIRCULATIONAHA.110.966846. [DOI] [PubMed] [Google Scholar]
- 18.Nagy E, Caidahl K, Franco-Cereceda A, Bäck M. Increased transcript level of poly(ADP-ribose) polymerase (PARP-1) in human tricuspid compared with bicuspid aortic valves correlates with the stenosis severity. Biochem Biophys Res Commun. 2012;420:671–675. doi: 10.1016/j.bbrc.2012.03.064. doi:10.1016/j.bbrc.2012.03.064. [DOI] [PubMed] [Google Scholar]
- 19.Qin WD, Wei SJ, Wang XP, Wang J, Wang WK, Liu F, et al. Poly(ADP-ribose) polymerase 1 inhibition protects against low shear stress induced inflammation. Biochim Biophys Acta. 2012;1833:59–68. doi: 10.1016/j.bbamcr.2012.10.013. doi:10.1016/j.bbamcr.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Carew TE, Vaishnav RN, Patel DJ. Compressibility of the arterial wall. Circ Res. 1968;23:61–68. doi: 10.1161/01.res.23.1.61. doi:10.1161/01.RES.23.1.61. [DOI] [PubMed] [Google Scholar]
- 21.Clark JM, Glagov S. Transmural organization of the arterial media. The lamellar unit revisited. Arteriosclerosis. 1985;5:19–34. doi: 10.1161/01.atv.5.1.19. [DOI] [PubMed] [Google Scholar]
- 22.Koullias G, Modak R, Tranquilli M, Korkolis DP, Barash P, Elefteriades JA. Mechanical deterioration underlies malignant behavior of aneurysmal human ascending aorta. J Thorac Cardiovasc Surg. 2005;130:677–683. doi: 10.1016/j.jtcvs.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 23.Doyle JM, Dobrin PB. Finite deformation analysis of the relaxed and contracted dog carotid artery. Microvasc Res. 1971;3:400–415. doi: 10.1016/0026-2862(71)90042-2. doi:10.1016/0026-2862(71)90042-2. [DOI] [PubMed] [Google Scholar]
- 24.Gasser TC, Gallinetti S, Xing X, Forsell C, Swedenborg J, Roy J. Spatial orientation of collagen fibers in the abdominal aortic aneurysm's wall and its relation to wall mechanics. Acta Biomater. 2012;8:3091–3103. doi: 10.1016/j.actbio.2012.04.044. doi:10.1016/j.actbio.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 25.Biasetti J, Hussain F, Gasser TC. Blood flow and coherent vortices in the normal and aneurysmatic aortas: a fluid dynamical approach to intra-luminal thrombus formation. J R Soc Interface. 2011;8:1449–1461. doi: 10.1098/rsif.2011.0041. doi:10.1098/rsif.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reymond P, Crosetto P, Deparis S, Quarteroni A, Stergiopulos N. Physiological simulation of blood flow in the aorta: comparison of hemodynamic indices as predicted by 3-D FSI, 3-D rigid wall and 1-D models. Med Eng Phys. doi: 10.1016/j.medengphy.2012.08.009. Advance Access published September 11, 2012, doi:10.1016/j.medengphy.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Taylor CA, Cheng CP, Espinosa LA, Tang BT, Parker D, Herfkens RJ. In vivo quantification of blood flow and wall shear stress in the human abdominal aorta during lower limb exercise. Ann Biomed Eng. 2002;30:402–408. doi: 10.1114/1.1476016. doi:10.1114/1.1476016. [DOI] [PubMed] [Google Scholar]
- 28.Schlatmann TJ, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am J Cardiol. 1977;39:13–20. doi: 10.1016/s0002-9149(77)80004-0. doi:10.1016/S0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 29.Gomez D, Al Haj Zen A, Borges LF, Philippe M, Gutierrez PS, Jondeau G, et al. Syndromic and non-syndromic aneurysms of the human ascending aorta share activation of the Smad2 pathway. J Pathol. 2009;218:131–142. doi: 10.1002/path.2516. doi:10.1002/path.2516. [DOI] [PubMed] [Google Scholar]
- 30.Vollmar JF, Paes E, Pauschinger P, Henze E, Friesch A. Aortic aneurysms as late sequelae of above-knee amputation. Lancet. 1989;2:834–835. doi: 10.1016/s0140-6736(89)92999-1. doi:10.1016/S0140-6736(89)92999-1. [DOI] [PubMed] [Google Scholar]
- 31.Svensson LG, Crawford ES. Aortic dissection and aortic aneurysm surgery: clinical observations, experimental investigations, and statistical analyses. Part II. Curr Probl Surg. 1992;29:913–1057. doi: 10.1016/0011-3840(92)90003-l. [DOI] [PubMed] [Google Scholar]
- 32.Gasser TC, Martufi G, Auer M, Folkesson M, Swedenborg J. Micromechanical characterization of intra-luminal thrombus tissue from abdominal aortic aneurysms. Ann Biomed Eng. 2010;38:371–379. doi: 10.1007/s10439-009-9837-4. doi:10.1007/s10439-009-9837-4. [DOI] [PubMed] [Google Scholar]
- 33.Houard X, Ollivier V, Louedec L, Michel JB, Bäck M. Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil-derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J. 2009;23:1376–1383. doi: 10.1096/fj.08-116202. doi:10.1096/fj.08-116202. [DOI] [PubMed] [Google Scholar]
- 34.Biasetti J, Spazzini PG, Swedenborg J, Gasser TC. An integrated fluid-chemical model toward modeling the formation of intra-luminal thrombus in abdominal aortic aneurysms. Front Physiol. 2012;3:266. doi: 10.3389/fphys.2012.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vorp DA, Lee PC, Wang DH, Makaroun MS, Nemoto EM, Ogawa S, et al. Association of intraluminal thrombus in abdominal aortic aneurysm with local hypoxia and wall weakening. J Vasc Surg. 2001;34:291–299. doi: 10.1067/mva.2001.114813. doi:10.1067/mva.2001.114813. [DOI] [PubMed] [Google Scholar]
- 36.Kazi M, Thyberg J, Religa P, Roy J, Eriksson P, Hedin U, et al. Influence of intraluminal thrombus on structural and cellular composition of abdominal aortic aneurysm wall. J Vasc Surg. 2003;38:1283–1292. doi: 10.1016/s0741-5214(03)00791-2. doi:10.1016/S0741-5214(03)00791-2. [DOI] [PubMed] [Google Scholar]
- 37.Gasser TC, Gorgulu G, Folkesson M, Swedenborg J. Failure properties of intraluminal thrombus in abdominal aortic aneurysm under static and pulsating mechanical loads. J Vasc Surg. 2008;48:179–188. doi: 10.1016/j.jvs.2008.01.036. doi:10.1016/j.jvs.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 38.Mower WR, Quinones WJ, Gambhir SS. Effect of intraluminal thrombus on abdominal aortic aneurysm wall stress. J Vasc Surg. 1997;26:602–608. doi: 10.1016/s0741-5214(97)70058-2. doi:10.1016/S0741-5214(97)70058-2. [DOI] [PubMed] [Google Scholar]
- 39.Gasser TC, Auer M, Labruto F, Swedenborg J, Roy J. Biomechanical rupture risk assessment of abdominal aortic aneurysms: model complexity versus predictability of finite element simulations. Eur J Vasc Endovasc Surg. 2010;40:176–185. doi: 10.1016/j.ejvs.2010.04.003. doi:10.1016/j.ejvs.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Chen XL, Varner SE, Rao AS, Grey JY, Thomas S, Cook CK, et al. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. doi:10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 41.Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci USA. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. doi:10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasheras JC. The biomechanics of arterial aneurysms. Annu Rev Fluid Mech. 2007;39:293–319. doi:10.1146/annurev.fluid.39.050905.110128. [Google Scholar]
- 43.Butcher JT, Penrod AM, Garcia AJ, Nerem RM. Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. Arterioscler Thromb Vasc Biol. 2004;24:1429–1434. doi: 10.1161/01.ATV.0000130462.50769.5a. doi:10.1161/01.ATV.0000130462.50769.5a. [DOI] [PubMed] [Google Scholar]
- 44.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. doi:10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, et al. Transcriptional profiles of valvular and vascular endothelial cells reveal phenotypic differences: influence of shear stress. Arterioscler Thromb Vasc Biol. 2006;26:69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. doi:10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- 46.Sucosky P, Balachandran K, Elhammali A, Jo H, Yoganathan AP. Altered shear stress stimulates upregulation of endothelial VCAM-1 and ICAM-1 in a BMP-4- and TGF-beta1-dependent pathway. Arterioscler Thromb Vasc Biol. 2009;29:254–260. doi: 10.1161/ATVBAHA.108.176347. doi:10.1161/ATVBAHA.108.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Hamamsy I, Balachandran K, Yacoub MH, Stevens LM, Sarathchandra P, Taylor PM, et al. Endothelium-dependent regulation of the mechanical properties of aortic valve cusps. J Am Coll Cardiol. 2009;53:1448–1455. doi: 10.1016/j.jacc.2008.11.056. doi:10.1016/j.jacc.2008.11.056. [DOI] [PubMed] [Google Scholar]
- 48.New SE, Aikawa E. Cardiovascular calcification: an inflammatory disease. Circ J. 2011;75:1305–1313. doi: 10.1253/circj.cj-11-0395. doi:10.1253/circj.CJ-11-0395. [DOI] [PubMed] [Google Scholar]
- 49.Nagy E, Bäck M. Epigenetic regulation of 5-lipoxygenase in the phenotypic plasticity of valvular interstitial cells associated with aortic valve stenosis. FEBS Lett. 2012;586:1325–1329. doi: 10.1016/j.febslet.2012.03.039. doi:10.1016/j.febslet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 50.Nagy E, Eriksson P, Caidahl K, Yousry M, Ingelsson E, Hansson GK, et al. Valvular osteoclasts in calcification and aortic valve stenosis severity. Int J Cardiol. doi: 10.1016/j.ijcard.2013.01.207. Advance Access published February 26, 2013, doi:10.1016/j.ijcard.2013.01.207. [DOI] [PubMed] [Google Scholar]
- 51.Arnsdorf EJ, Tummala P, Castillo AB, Zhang F, Jacobs CR. The epigenetic mechanism of mechanically induced osteogenic differentiation. J Biomechem. 2010;43:2881–2886. doi: 10.1016/j.jbiomech.2010.07.033. doi:10.1016/j.jbiomech.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith KE, Metzler SA, Warnock JN. Cyclic strain inhibits acute pro-inflammatory gene expression in aortic valve interstitial cells. Biomech Model Mechanobiol. 2010;9:117–125. doi: 10.1007/s10237-009-0165-2. doi:10.1007/s10237-009-0165-2. [DOI] [PubMed] [Google Scholar]
- 53.Fondard O, Detaint D, Iung B, Choqueux C, Adle-Biassette H, Jarraya M, et al. Extracellular matrix remodelling in human aortic valve disease: the role of matrix metalloproteinases and their tissue inhibitors. Eur Heart J. 2005;26:1333–1341. doi: 10.1093/eurheartj/ehi248. doi:10.1093/eurheartj/ehi248. [DOI] [PubMed] [Google Scholar]
- 54.Ketelhuth DF, Bäck M. The role of matrix metalloproteinases in atherothrombosis. Curr Atheroscler Rep. 2011;13:162–169. doi: 10.1007/s11883-010-0159-7. doi:10.1007/s11883-010-0159-7. [DOI] [PubMed] [Google Scholar]
- 55.Ferdous Z, Jo H, Nerem RM. Differences in valvular and vascular cell responses to strain in osteogenic media. Biomaterials. 2011;32:2885–2893. doi: 10.1016/j.biomaterials.2011.01.030. doi:10.1016/j.biomaterials.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29:936–942. doi: 10.1161/ATVBAHA.108.182394. doi:10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- 57.Leung DY, Glagov S, Mathews MB. Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science. 1976;191:475–477. doi: 10.1126/science.128820. doi:10.1126/science.128820. [DOI] [PubMed] [Google Scholar]
- 58.Grote K, Flach I, Luchtefeld M, Akin E, Holland SM, Drexler H, et al. Mechanical stretch enhances mRNA expression and proenzyme release of matrix metalloproteinase-2 (MMP-2) via NAD(P)H oxidase-derived reactive oxygen species. Circ Res. 2003;92:e80–e86. doi: 10.1161/01.RES.0000077044.60138.7C. doi:10.1161/01.RES.0000077044.60138.7C. [DOI] [PubMed] [Google Scholar]
- 59.Thubrikar MJ, Aouad J, Nolan SP. Patterns of calcific deposits in operatively excised stenotic or purely regurgitant aortic valves and their relation to mechanical stress. Am J Cardiol. 1986;58:304–308. doi: 10.1016/0002-9149(86)90067-6. doi:10.1016/0002-9149(86)90067-6. [DOI] [PubMed] [Google Scholar]
- 60.Balachandran K, Sucosky P, Jo H, Yoganathan AP. Elevated cyclic stretch induces aortic valve calcification in a bone morphogenic protein-dependent manner. Am J Pathol. 2010;177:49–57. doi: 10.2353/ajpath.2010.090631. doi:10.2353/ajpath.2010.090631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Borges LF, Gomez D, Quintana M, Touat Z, Jondeau G, Leclercq A, et al. Fibrinolytic activity is associated with presence of cystic medial degeneration in aneurysms of the ascending aorta. Histopathology. 2010;57:917–932. doi: 10.1111/j.1365-2559.2010.03719.x. doi:10.1111/j.1365-2559.2010.03719.x. [DOI] [PubMed] [Google Scholar]
- 62.Zeng Z, Yin Y, Huang AL, Jan KM, Rumschitzki DS. Macromolecular transport in heart valves. I. Studies of rat valves with horseradish peroxidase. Am J Physiol Heart Circ Physiol. 2007;292:H2664–H2670. doi: 10.1152/ajpheart.01419.2006. doi:10.1152/ajpheart.01419.2006. [DOI] [PubMed] [Google Scholar]
- 63.Hellenthal FA, Geenen IL, Teijink JA, Heeneman S, Schurink GW. Histological features of human abdominal aortic aneurysm are not related to clinical characteristics. Cardiovasc Pathol. 2009;18:286–293. doi: 10.1016/j.carpath.2008.06.014. doi:10.1016/j.carpath.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 64.Cuffy MC, Silverio AM, Qin L, Wang Y, Eid R, Brandacher G, et al. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol. 2007;179:5246–5254. doi: 10.4049/jimmunol.179.8.5246. [DOI] [PubMed] [Google Scholar]
- 65.Kumamoto M, Nakashima Y, Sueishi K. Intimal neovascularization in human coronary atherosclerosis: its origin and pathophysiological significance. Hum Pathol. 1995;26:450–456. doi: 10.1016/0046-8177(95)90148-5. doi:10.1016/0046-8177(95)90148-5. [DOI] [PubMed] [Google Scholar]
- 66.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–658. doi: 10.1016/j.cardiores.2007.06.020. doi:10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ho-Tin-Noe B, Le Dall J, Gomez D, Louedec L, Vranckx R, El-Bouchtaoui M, et al. Early atheroma-derived agonists of peroxisome proliferator-activated receptor-gamma trigger intramedial angiogenesis in a smooth muscle cell-dependent manner. Circ Res. 2011;109:1003–1014. doi: 10.1161/CIRCRESAHA.110.235390. doi:10.1161/CIRCRESAHA.110.235390. [DOI] [PubMed] [Google Scholar]
- 68.Robinet A, Fahem A, Cauchard JH, Huet E, Vincent L, Lorimier S, et al. Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of MT1-MMP. J Cell Sci. 2005;118:343–356. doi: 10.1242/jcs.01613. doi:10.1242/jcs.01613. [DOI] [PubMed] [Google Scholar]
- 69.Choke E, Thompson MM, Dawson J, Wilson WR, Sayed S, Loftus IM, et al. Abdominal aortic aneurysm rupture is associated with increased medial neovascularization and overexpression of proangiogenic cytokines. Arterioscler Thromb Vasc Biol. 2006;26:2077–2082. doi: 10.1161/01.ATV.0000234944.22509.f9. doi:10.1161/01.ATV.0000234944.22509.f9. [DOI] [PubMed] [Google Scholar]
- 70.Galis ZS, Lessner SM. Will the real plaque vasculature please stand up? Why we need to distinguish the vasa plaquorum from the vasa vasorum. Trends Cardiovasc Med. 2009;19:87–94. doi: 10.1016/j.tcm.2009.06.001. doi:10.1016/j.tcm.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Modarai B, Humphries J, Burnand KG, Gossage JA, Waltham M, Wadoodi A, et al. Adenovirus-mediated VEGF gene therapy enhances venous thrombus recanalization and resolution. Arterioscler Thromb Vasc Biol. 2008;28:1753–1759. doi: 10.1161/ATVBAHA.108.170571. doi:10.1161/ATVBAHA.108.170571. [DOI] [PubMed] [Google Scholar]