Abstract
Low and oscillatory wall shear stress is widely assumed to play a key role in the initiation and development of atherosclerosis. Indeed, some studies have relied on the low shear theory when developing diagnostic and treatment strategies for cardiovascular disease. We wished to ascertain if this consensus is justified by published data. We performed a systematic review of papers that compare the localization of atherosclerotic lesions with the distribution of haemodynamic indicators calculated using computational fluid dynamics. The review showed that although many articles claim their results conform to the theory, it has been interpreted in different ways: a range of metrics has been used to characterize the distribution of disease, and they have been compared with a range of haemodynamic factors. Several studies, including all of those making systematic point-by-point comparisons of shear and disease, failed to find the expected relation. The various pre- and post-processing techniques used by different groups have reduced the range of shears over which correlations were sought, and in some cases are mutually incompatible. Finally, only a subset of the known patterns of disease has been investigated. The evidence for the low/oscillatory shear theory is less robust than commonly assumed. Longitudinal studies starting from the healthy state, or the collection of average flow metrics derived from large numbers of healthy vessels, both in conjunction with point-by-point comparisons using appropriate statistical techniques, will be necessary to improve our understanding of the relation between blood flow and atherogenesis.
Keywords: Atherosclerosis, Haemodynamics, Blood flow, Arterial disease, Shear stress
1. Introduction
The non-uniform distribution of atherosclerotic lesions in the arterial system had been attributed to variation in blood flow characteristics as early as the nineteenth century.1 Today, the concept that local haemodynamic factors account for the patchiness of the disease has become almost universally accepted by researchers in the field.2 Acceptance emerged after several decades during which the nature of the flow features that drive atherogenesis was the subject of considerable debate. While Fry3 suggested that high wall shear stress (WSS) leads to endothelial damage and a subsequent pathological response, Caro et al.4 proposed that high WSS was protective, lesions instead occurring in regions of low WSS. In the 1980s, flow reversal was identified as another key factor, and an oscillatory shear index (OSI) was introduced by Ku et al.5 to quantify it. This index has the time-averaged magnitude of WSS in the denominator, so high OSI tends to be associated with low mean shear; the low and oscillatory shear theories have become fused to some extent.
In their 1992 review of fluid dynamics at arterial bifurcations, Lou and Yang6 noted, ‘The theory of low shear stress prevails at the moment, although the correlation between high-atherosclerosis probability areas and expected regions of low wall shear stress is far from perfect. It is, therefore, not prudent to accept one theory over another’. Twenty years later, the low and/or oscillatory WSS hypothesis has become the consensus mechanism for the initiation of atherosclerosis.7,8 Some studies even rely on the theory for the development or assessment of potential diagnostic or treatment strategies.9,10 We wished to ascertain if the literature really does justify the consensus.
Blood flow has been intensively studied in recent years. The techniques of computational fluid dynamics (CFD) have commonly been employed.2,11–14 In this approach, numerical methods are used to approximate the flow fields in arteries, which are too complex for analytic solution; haemodynamic factors such as WSS are derived in turn from the flow fields. The increasing power:cost ratio of computers and the ready availability of techniques for incorporating image-derived geometries into CFD models have made this approach more reliable than methods based solely on in vivo measurement.
The aims of the review presented here were to identify articles that compared CFD-derived metrics with lesion distributions, to collate their conclusions, and to gauge the strength of the evidence for the consensus view of the relationship between WSS and atherosclerosis. To increase the rigour of the review, the literature search was conducted in a systematic fashion.
2. Methods
A systematic search was conducted on the PubMed database15 for articles published online before 21 March 2012 whose abstract and/or title contained at least one keyword from each of the following three groups:
athero, atheroma, atheromata, atheromatous, atherosclerosis, atherosclerotic, atherogenesis, atherogenic, intima-media thickness, intimal thickness, wall thickness;
shear;
CFD, computational, computation, compute, computed, computing, numerical, calculation, calculate, calculated, calculating, simulation, simulate, simulated, simulating.
The articles were then evaluated against a set of inclusion and exclusion criteria. To be included, compliance with the following criteria was required:
the work was an original contribution;
CFD was used to simulate blood flow in one or more vascular geometries;
at least one of these vascular geometries was anatomically realistic—i.e. it was based on measurements or images, was sufficiently extended and included the features necessary to capture the physiologically relevant 3D flow dynamics;
the localization of atherosclerotic lesions in the region of interest was based on the distribution of an established marker of the disease, presented either as an original contribution in the article, in a companion paper from the same group, or in a cited article from a different research group. The species used for the disease localization study and CFD analysis had to match;
the patterns of shear-related parameters in the region of interest were shown or described in detail, and compared with the localization of disease in the same region;
the relation between blood flow and the initiation of atherosclerosis was discussed.
Excluded from the review were:
studies that performed CFD on severely stenosed geometries and compared the resulting WSS maps with the spatial distribution of occlusion in these geometries. With such advanced disease, it cannot be assumed that variations in shear are a cause rather than an effect of the lesion;
studies in which the geometries for the CFD analysis were obtained after angioplasty or endarterectomy in the region of interest, since these techniques cannot guarantee an accurate reproduction of the original ‘healthy’ vascular geometry;
studies in which a stent, cuff, graft, or other foreign object was introduced in the region of interest, since contact with the foreign material or stresses induced by the presence of the object may trigger mechanisms unrelated to normal atherogenesis. Studies in which the geometry of the vascular region of interest was itself surgically altered, e.g. by anastomosis, were also discarded since effects related to the surgery may act as confounding factors;
studies in which the disease was mechanically induced, e.g. by denudation of the endothelium. Again, these studies may not replicate the normal initiation of the disease;
studies in which gene expression, white blood cell density, or low-density lipoprotein (LDL) permeability were used as surrogates for lesions, since these properties are not established markers of the disease.
Supplementary material online, Figure S1 charts the search criteria and explains the number of articles eliminated at each stage.
3. The selected literature
Twenty-seven articles12,14,16–40 (see Supplementary material online, Table S1) were selected from an original set of 406. (Supplementary material online, Table S1 also lists eight associated papers41–48 containing lesion data that were not presented in the selected articles themselves.) The selected articles originated from 15 different institutions and studied human, porcine, rabbit, and murine blood vessels; coronary arteries were the most frequently studied vessels, but the carotid bifurcation, aorta, and internal common carotid were also investigated (Table 1). In the animal studies, interventions were required to initiate or accelerate the disease process: all animals were cholesterol-fed, mice had an apolipoprotein E or low-density lipoprotein receptor (LDLR−/−) null mutation, and, in one case, the flow conditions were modified by surgical induction of aortic valve regurgitation.35
Table 1.
Species and vascular regions examined in the reviewed articles
| Human | Porcine | Leporine | Murine | |
|---|---|---|---|---|
| Coronary arteries | 12 | 4 | — | — |
| Carotid bifurcation | 5 | — | — | |
| Aorta | 1 | — | 2 | 2 |
| Internal carotid | 1 | — | — | — |
4. Findings
Section 1 uncritically presents the results for the association between blood flow and atherosclerosis given in the reviewed articles. Subsequent sections also consider the methods that were used. Since different research groups applied different levels of data reduction, Section 2 investigates how data-processing methodologies differed between articles. Given the high sensitivity of blood flow to geometric changes, Section 3 details at what stage of the disease process the flow was characterized. Finally, Section 4 gives an overview of the different techniques used to assess disease location. In each of these three sections, implications for the evidence concerning the role of blood flow in early atherosclerosis are discussed.
4.1. Many but not all articles interpret their results as supporting the low/oscillatory shear theory
4.1.1. Studies of steady flow
Based on investigations of steady-state flow, in which the time-varying character of the flowing blood was not simulated, nine papers concluded that their results confirm the theory that low WSS promotes atherogenesis:
Krams et al.17 and Wentzel et al.21,24 reported an inverse correlation between wall thickness and steady WSS;
Goubergrits et al.20 saw a good visual correlation between locations of fibrous plaques and regions of low WSS;
Chatzizisis et al.27 found that fibroatheromata and intermediate lesions develop in vascular segments with lower baseline WSS than segments with minimal lesions;
Koskinas et al.33 observed a greater increase in maximum intima-medial thickness in vascular segments with low baseline WSS compared to vascular segments with high baseline WSS;
Olgac et al.29 reported a region of low WSS that coincided with the location of a plaque;
Siogkas et al.38 noted the largest plaque formation in a region of low WSS;
Chatzizisis et al.36 found that lipid accumulation was enhanced in segments with low baseline WSS.
In contrast, a quantitative comparison by Gijsen et al.25 did not result in an overall relationship between WSS and wall thickness.
4.1.2. Studies of time-varying flow
The first papers to report on time-varying flow, by Perktold and Resch12 and Perktold et al.,16 concluded that the location of atherosclerotic plaques correlated with the extent and location of a recirculation zone (and consequent low WSS) occurring during part of the cardiac cycle. However, subsequent studies focused on time-integrated effects.
4.1.2.1. Time-averaged WSS
Three papers concluded that low time-averaged wall shear stress (TAWSS; WSS magnitude averaged over the cardiac cycle) co-located with atherosclerosis or was associated with its rate of progression:
Suo et al.28 concluded that low TAWSS occurred at sites with an increased incidence of lesions;
Zhu et al.31 compared TAWSS magnitudes in two mouse strains and found lower values in the strain having greater athero-susceptibility;
Samady et al.37 found that plaque area increased over time in vascular segments with low TAWSS, but decreased in intermediate- and high-TAWSS segments.
However, two studies did not support the low TAWSS theory: Joshi et al.23 could not find a significant correlation between intimal thickness in three out of four arteries, and van der Giessen et al.32 could not establish a correlation with plaque thickness in a mildly diseased artery.
4.1.2.2. Other shear-derived metrics
The OSI, first proposed5 to characterize flow pulsatility and later generalized to three-dimensional flow,49 was computed alongside TAWSS in many of the reviewed papers.
The conclusions for TAWSS and OSI were in agreement in four papers that found an association with early atherosclerosis: Taylor et al.14 and Buchanan et al.18,22 noted that regions of low TAWSS and high OSI both coincide with a high probability-of-occurrence of early atherosclerotic lesions, and Olgac et al.30 reported that a region of low TAWSS and high OSI coincided with the location of a plaque.
One paper found agreement between TAWSS and OSI but no association with atherosclerosis: Steinman et al.19 could not find a quantitative general relationship between TAWSS or OSI and wall thickness.
In other cases, the conclusions for TAWSS, OSI, and other metrics disagreed, or at least showed discrepancies:
Augst et al.26 computed TAWSS, OSI, and wall shear stress angle gradients (WSSAG). They reported a significant relationship between WSS or WSSAG and intima-medial thickness in one part of the carotid but not another, and no relation with OSI in either region;
Hoi et al.35 concluded that OSI and the relative residence time (RRT) could explain changes in plaque distribution as a result of changes in flow conditions, whereas TAWSS could not;
According to Zhang et al.,39 an OSI-based risk factor was a better predictor than a TAWSS-based one of subsequent stenosis;
Knight et al.34 and Rikhtegar et al.40 found that the largest number of plaque locations could be predicted using a low TAWSS threshold, but that OSI and RRT produced fewer false negatives.
4.1.3. Summary
Table 2 provides an overview of the conclusions presented in the selected articles. Most stated that their results were in agreement with the low/oscillatory shear theory, but five19,23,25,26,32 did not find a significant overall relationship.
Table 2.
Overview of the conclusions presented by the 27 reviewed articles
| Theory | Supported | Not supported |
|---|---|---|
| Low WSS (steady flow) | 9 | 2 |
| Low instantaneous WSS | 2 | 0 |
| Low TAWSS | 3 | 1 |
| TAWSS and OSI | 4 | 2 |
| TAWSS or OSI, or RRT | 4 | 0 |
4.2. Different and sometimes conflicting levels of data reduction were required to reach a conclusion
4.2.1. Descriptive analysis
In the two earliest papers that satisfied all selection criteria,12,16 the comparison of flow and disease was essentially descriptive; differences in plaque location in carotids with different bifurcation angles43 could be explained by differences in the extent and location of a recirculation zone. Such analysis does not provide any statistical evidence for the relation between WSS and atherogenesis, in part because of the qualitative nature of the clinical observations on which these studies were based. (The clinical observations were quantified later50).
4.2.2. Visual comparison of maps
Eleven articles14,18,20,22,25,28–32,35 presented a visual comparison between maps of flow-related parameters and disease indicators. In all cases, this approach led to a conclusion in favour of the low/oscillatory shear theory. However, anomalies that run counter to this trend are readily visible in the maps presented in at least three of the articles.18,20,22 Additionally, although Hoi et al.35 advocated the use of RRT as a surrogate marker of atherosclerosis, they conceded that this metric failed to predict the absence of plaques in the proximal descending thoracic aorta of LDLR−/− mice. Another of these studies28 inadvertently transposed the labelling of branches in one map but not in the other; the degree of correlation needs re-examination. Thus, the weight of evidence obtained with this method is open to question in at least 45% of the studies.
4.2.3. Point-by-point comparison
Quantitative point-by-point comparison is a more rigorous form of analysis. Steinman et al.,19 Joshi et al.,23 Wentzel et al.,24 Gijsen et al.,25 and Augst et al.26 performed point-wise comparisons of WSS with intimal, intima-medial, or wall thickness. Spatial resolution varied from summary measures for each 1.5-mm wide rectangular patch26 to one data point per node on the surface of the finite element mesh.19 Importantly, none of these studies found a significant correlation.
4.2.4. Axial averaging
A study by Wentzel et al.24 was one of those in which a point-by-point comparison did not yield the inverse relationship between wall thickness and WSS expected according to the consensus view. The authors went on to average the data in the axial direction.21,24 They argued that this approach should be used in studies (such as theirs) where WSS is computed for the geometry of the diseased vessel but used to explain the initiation of disease, since it will reduce the effect of variations in luminal shape or size which would not have occurred in the disease-free vessel. An inverse relation between WSS and disease was obtained.
This technique also resulted in a significant inverse WSS-intimal thickness relation for Krams et al.17 but not for van der Giessen et al.32 In a related technique, Joshi et al.23 eliminated axial variation by normalizing the data per cross-section; as already noted, they found a significant inverse WSS-intimal thickness correlation in only one out of four cases.
4.2.5. Circumferential averaging
Although Wentzel et al.21 advised against the use of circumferential averaging, since it obscures curvature-induced circumferential variations in WSS, Chatzizisis et al.,27,36 Koskinas et al.,33 and Samady et al.37 obtained a significant inverse correlation using this technique.
4.2.6. Selective analysis
A number of articles limited their analysis to a subset of the data. The pre-selection was based either on geometrical features or on values of shear- or disease-metrics.
Wentzel et al.,21,24 Chatzizisis et al.,27,36 and Koskinas et al.33 excluded data from regions of branching;
Zhang et al.39 visually preselected locations of low TAWSS and high OSI, and then performed a more quantitative analysis on the subset;
Chatzizisis et al.27,36 and Koskinas et al.33 analysed only segments with uniform intima-medial thickness and WSS.
In these cases, the pre-selection will have restricted variation in shear stress or disease severity and may, therefore, have obscured important aspects of the relation between WSS and disease.
4.2.7. Thresholding
Although most articles did not specify a threshold below or above which a haemodynamic factor was believed to promote atherosclerotic disease, five articles presented their haemodynamic results in such a categorical sense.
Koskinas et al.,33 who studied the flow in porcine coronary arteries, fixed the WSS threshold at 1.2 Pa, a value previously used in a study of flow in human coronaries;51
In a second study of porcine coronary arteries by the same group,36 a threshold of 1 Pa was chosen;
Samady et al.,37 in a study of human coronary arteries, defined low TAWSS as <1 Pa and high TAWSS as ≥2.5 Pa.
The significance of the results obtained in such studies will obviously depend on the choice of threshold. Although it is commonly assumed, as in these studies, that normal arterial shear lies in the 1.0–1.5 Pa range, allometric arguments demonstrate that this is not a universal property.52 A better approach might be that of Knight et al.34 and Rikhtegar et al.40 who dichotomized haemodynamic variables by balancing the number of correctly predicted plaque locations and false positives for 10 patients.
4.2.8. Summary
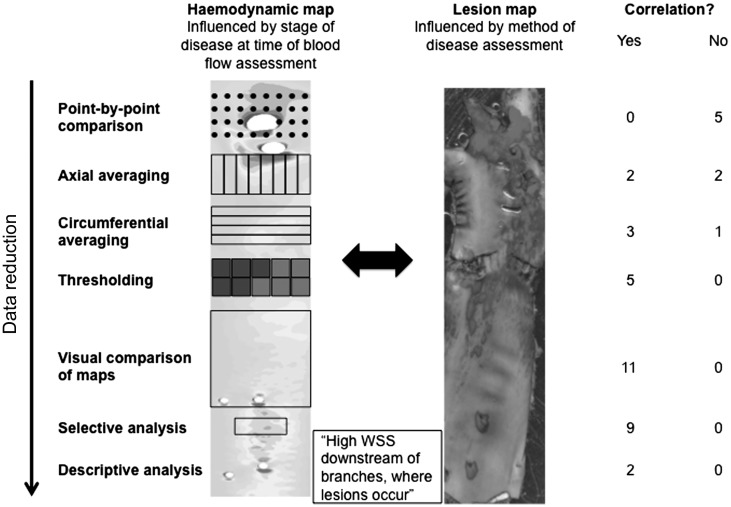
Figure 1 summarizes the various data-processing methods employed in the reviewed articles. Straightforward point-by-point comparisons did not yield results consistent with the consensus low shear theory. Although averaging did produce some significant results, averaging techniques advised against by some groups were essential for others' analyses. Pre-selection of data may have restricted the range of shears and disease severities that were considered and hence limited the validity of the conclusions. Thresholding of haemodynamic parameters needs to be based on objective criteria; furthermore, the categorical approach and the continuum approach represent different interpretations of the low shear theory—only mechanistic studies can determine which (if either) is correct.
Figure 1.
Schematic overview of the various data-processing methods employed in the reviewed articles, illustrated on lesion and WSS maps taken from Cremers et al.72 and Peiffer et al.,73 respectively. The summarizing table on the right indicates that the correlation between the lesion and haemodynamic distributions, as perceived by the authors of the reviewed articles, increased for increasing levels of data reduction. Note that articles were double counted if multiple analysis techniques were reported.
4.3. Blood flow was assessed at different stages in the disease process
Small changes in geometry can induce significant alterations of WSS.53,54 The validity of studies which compute patterns of shear therefore depends on the anatomical fidelity of the reconstructed vascular geometry.
4.3.1. Subject-specific studies
4.3.1.1. Single geometry per subject
Studies of human vessels have often used the same geometry for the assessment of disease and for the computation of WSS. Obviously, lesions have to be present in the segment in order to allow such an investigation. In some cases, the geometry was obtained from individuals who were free of cardiovascular symptoms or, post-mortem, from individuals whose primary cause of death was not cardiac related.19,23,26 On the other hand, many studies used subjects with known atherosclerosis, such as patients who went to clinic with a lesion requiring evaluation or treatment.17,20,21,24,25,32
In studies of this type, there is an explicit or implicit reliance on compensatory remodelling55,56 to ensure that the presence of lesions did not alter the disease-free geometry. In the absence of compensatory remodelling, this approach could obscure an inverse correlation between early atherosclerosis and WSS since a narrowed lumen leads to an increase in velocity (for the same flow rate).
4.3.1.2. Virtual plaque removal
The concept of removing plaques virtually from diseased geometries is attractive. Olgac et al.,29,30 Knight et al.,34 and Rikhtegar et al.40 used an image-based approach for virtual plaque removal, but acknowledged the potential for under- or overestimating the size of the healthy lumen.
4.3.1.3. Longitudinal studies
Samady et al.37 were the only authors in the final selection to report a longitudinal study in human subjects: they re-scanned each person 6 months after the first examination. However, this study focused on progression rather than initiation of disease: the baseline geometries were taken from patients rather than healthy volunteers. Longitudinal studies are easier in animals, but again the focus has been on progression rather than initiation.27,33,36
4.3.2. Multiple group studies
A different option is to abandon the subject-specific approach. Instead, the distribution of lesions is assessed in a group of diseased vessels, blood flow is simulated in geometries obtained from a group of healthy vessels, and the comparison is performed between group means. Each group needs to be big enough to make inter-subject variation unimportant. In most such studies, however, the haemodynamic group has been limited to just one geometry;12,14,16,18,22,28,35 only Zhu et al.,31 using mice, performed a full statistical comparison.
4.3.3. Summary
There have been no longitudinal studies that examined the initiation of disease in people. Instead, researchers have focused on progression, or have made assumptions about the nature of vascular remodelling, about how to remove lesions virtually, or about inter-subject variation. The high sensitivity of WSS stress to geometry makes the validity of these assumptions critical. All five studies that failed to find a relation between flow metrics and early lesions used the diseased geometry for flow mapping. However, not all studies using this technique failed to find a relation.17,20,21,24
4.4. Disease localization was assessed at different stages in the disease process
Atherosclerosis was mapped through the identification of one or more of its constituents or by measuring geometric anomalies in wall and/or luminal dimensions. Some studies investigated the likelihood that lesions will occur at a particular level of WSS, whereas others investigated the severity of the disease in relation to shear.
4.4.1. Disease assessment based on lesion composition
4.4.1.1. Lipid staining
Recognizing that the onset of atherosclerosis is characterized by lipid accumulation in the inner wall,57 a number of research groups made use of lipid staining with oil red O or Sudan IV to identify the earliest stages of the disease. This technique was applied in the studies of Taylor et al.,14 Goubergrits et al.,20 Suo et al.,28 Chatzizisis et al.,27,36 and Hoi et al.,35 or their companion studies.
4.4.1.2. Calcium scoring
As the disease progresses, other plaque components appear. Olgac et al.,29,30 Knight et al.,34 and Rikhtegar et al.40 focused on the calcium content of the wall, which they obtained by computed tomography. Disadvantages of this method are that it cannot locate non-calcified plaques and that the calcified areas may not exactly match the locations where the disease was initiated if there is a continuous two-way interaction between flow and the development of the disease. In addition, calcification without atherosclerosis is possible; confirmation of plaques by an experienced clinician, as was carried out in the studies by Knight et al.34 and Rikhtegar et al.40 is, therefore, important.
4.4.2. Disease assessment based on geometric changes
Early wall thickening might not be detectable from an assessment of luminal dimensions, due to compensatory remodelling. Hence, a large number of the selected articles opted instead to use intimal, intima-medial, or wall thickness as a measure of disease.
Joshi et al.,23 Zhu et al.,31 and Chatzizisis et al.,36 or their companion papers made use of post-mortem preparations to assess thickness. Imaging techniques, such as magnetic resonance imaging (MRI),19,39 ultrasound,26 and intravascular ultrasound,17,21,24,25,27,32,33,37,38 have been used for the same purpose in vivo. There are disadvantages, however. With intima-medial and wall thickness, it is not possible to distinguish between intimal thickening due to atherosclerosis, medial thickening in response to elevated blood pressure26 or adaptive intimal thickening unrelated to atherosclerosis; medial thinning under lesions may also cause problems. Imaging methods that combine the measurement of intimal thickness with the identification of intimal composition in vivo would be ideal.
4.4.3. Probability-of-occurrence vs. plaque severity
Studies of the relation between flow and disease have variously investigated whether or not atherosclerotic lesions occur in certain haemodynamic environments,18,20,22,29,30,35,38 or how likely it is to find lesions in these regions,14,28,34,39 or have interpreted the low/oscillatory shear theory in a dose-dependent way by asking whether disease is more advanced in these regions.17,19,21,23–27,31–33,36,37
For completeness, we note that an additional category of articles have addressed a slightly different question by asking if certain haemodynamic conditions can protect against the disease.34,40
4.4.4. Summary
A variety of techniques were used to assess the location of atherosclerotic disease. Whereas lipid staining will have picked up early signs of atherosclerosis, this is not the case for approaches involving calcium scoring or luminal narrowing. Moreover, measurements of intimal, intima-medial, and wall thickness cannot specifically identify intimal thickening due to atherosclerosis; the five articles which did not find a correlation between WSS and atherogenesis used these methods, although some articles using these methods17,21,24,27,31,33,36–39 did find a relation. Finally, it is not clear if the low/oscillatory shear theory should be interpreted in terms of the frequency or severity of disease, or whether both measures are satisfactory.
5. Discussion
The low shear stress theory was heterodox when first proposed. It challenged the prevailing high shear stress theory, which appeared to account for the pattern of disease observed in various non-human species and which was associated with the intuitively appealing mechanistic hypothesis that high shear stress damages the endothelium, allowing excessive entry of plasma lipoproteins into the wall.
The involvement of oscillatory shear emerged from the studies of Ku et al.,5 who examined lesion distributions in post-mortem human arteries and used laser Doppler anemometry to measure velocity profiles in anatomically accurate models of those arteries. Indeed, the relation between intimal thickness and OSI was much stronger than that between intimal thickness and mean shear. Today the low/oscillatory shear theory is widely regarded as established, and it is contrary views that are regarded as heterodox.
The present systematic review considers whether the evidence justifies that consensus. It focused on studies that employed CFD to assess patterns of wall shear because these are currently the most reliable methods. (Recent improvements in the accuracy of flow measurement by MRI58 and other techniques59 may change this in the near future.)
We cannot exclude having overlooked some relevant papers, despite the rigorous methodology. Nevertheless, reliable conclusions can be drawn from those that were reviewed. First, the majority of the studies supported the low/oscillatory shear theory but some did not. Secondly, some found the same relation with disease for low WSS and OSI, but others did not. Thirdly, close inspection shows that the theory has been interpreted and tested in different ways. This third point could be regarded as evidence that the theory is robust and insensitive to precise formulation—supporting evidence was repeatedly obtained despite the following methodological issues: a variety of metrics were used to represent the disease distribution; blood flow was assessed at different stages in the disease process; data reduction techniques limited the range of shears or disease severities under consideration, or had mutually contradictory rationales; and WSS was treated as a continuous or a categorical variable. An alternative reading is that there is a lack of unanimity between studies, and that the diversity of methods weakens rather than strengthens support for a single, precise hypothesis. According to this viewpoint, the low/oscillatory theory may very well be true but—taken together—the evidence for it is less clear-cut than might at first appear.
The five studies19,23,25,26,32 that failed to find a significant overall relation between low WSS and disease are naturally of particular interest, since they contradict the current consensus. They all computed flow in the geometry that was used to assess disease, and they all used a wall thickness-related metric as an index of disease severity. It is tempting to discount them on these grounds, but many studies using one or other of these methods did find a correlation, as did two studies17,21 that used both methods. It could equally be claimed that four of the five are methodologically the best studies because they used a quantitative, point-by-point comparison between the two variables (and the only other study24 using point-by-point analysis also failed to obtain a correlation with this method, even though a correlation was obtained after axial averaging of the data). The fact that no point-by-point analysis supported the consensus is rendered even more striking by the fact that such methods tend to over-estimate correlations (see below).
Since this review did not distinguish between different vascular beds, it is important that the five studies were not restricted to a single-vessel type but included coronary and carotid arteries. Equally, since this review did not distinguish between human disease and lesions occurring in hypercholesterolaemic animals, with or without genetic modification, it is important that all five negative studies used human vessels; the investigations which used animal models conformed to the consensus, and that is also true of a study not meeting our inclusion criteria, where a flow-modifying cuff was placed around the carotid artery of hypercholesterolaemic mice—lesions developed in regions of low TAWSS.60,61 (Interestingly, lesion type appeared to depend on the degree of oscillation of the shear.)
A number of issues will need to be addressed by future studies in order to improve and refine the existing evidence.
5.1. Are we capturing the relevant flow metrics?
Do the conventional metrics—WSS, TAWSS, OSI, and RRT—capture physiologically relevant features of the flow? And do they quantify the essence of the expression ‘disturbed flow’ that is so commonly used in the biological literature? Or should we explore other parameters, such as the directional OSI suggested by Chakraborty et al.?62
A number of authors have suggested an indirect link between flow and disease that depends on flow affecting fluid-phase mass transport of solutes (oxygen, LDL) near the wall, rather than direct effects of flow on the wall itself.29,30,63,64 WSS and transport are related, but idealized models show that their patterns need not be identical.65,66
5.2. What level of data reduction is required and justifiable?
The use of different data reduction techniques to decrease the amount of data-processing (e.g. by limiting the analysis to a subset of the available data, averaging to combine data, or thresholding) complicates the evaluation of the evidence. Authors should make it possible to evaluate results after minimal processing, for example, by providing figures that allow a visual comparison, even when statistics are applied only to reduced measures.
Point-by-point comparisons represent an ideal. Nevertheless, there remain limits to the spatial resolution that can be attained. In particular, cross-correlation between adjacent data points must be minimized to prevent over estimation of statistical significance.67 Errors in image registration are also limiting.
5.3. Are subject-specific studies introducing errors?
Studies of the effects of WSS on the initiation of atherosclerosis should compute flows using disease-free geometries. On the other hand, the large effect that small changes in geometry can have on WSS patterns suggests that subject-specific investigations are best. Currently, these two requirements are hard to reconcile: they require either that effects of disease on geometry are negated naturally (by compensatory remodelling) or removed artificially (by hard-to-validate ‘virtual’ techniques), or that longitudinal studies are carried out over periods—at least for human disease—of many years.
These problems could be resolved by the development of techniques that allow disease to be mapped at a stage before intimal thickening occurs, and by methods that could ethically be used in such minimally affected subjects. Until such techniques are available, it may be more appropriate to drop the subject-specific approach and instead use different study groups: a diseased one for the assessment of lesion patterns and a disease-free one for the assessment of flow. The essential requirement of sufficiently large samples in the haemodynamic group has been fulfilled in only one study of those reviewed, and in none of those that considered human vessels.
5.4. Have all disease distributions been considered?
Disease distributions sometimes seem inconsistent. This is illustrated by the original debate over the high and low shear theories: Fry3 observed lesions at the downstream margin of aortic side branches, a site that Caro et al.4 found to be spared of disease. As both theories assumed that this region experiences high WSS, the debate concerned the distribution of lesions rather than characteristics of the blood flow.
Since the high shear theory was predicated on the distribution seen in animal models while the low shear theory was based on a study of human post-mortem material, the discrepancy was attributed to differences in plasma cholesterol levels.4,68 However, it has subsequently emerged that lesion patterns change with age in rabbit and human aortas, and in a parallel way;69 the discrepancy might be explained better by such changes. Lesions can also show different patterns in one species at a single age. In the mouse, they occur upstream of branches originating in the aortic arch, all around branches of the thoracic aorta, and downstream of branches in the abdominal aorta.70,71
The different patterns provide an additional challenge for haemodynamic theories of atherogenesis. The fact that the large majority of studies concerning shear and atherosclerosis have concentrated on relatively few locations in mature arteries may have led to an underestimate of the variety of mechanical factors that can trigger disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This work was supported by the British Heart Foundation and the BHF Centre of Research Excellence.
Supplementary Material
Acknowledgements
The authors are grateful for clarifications from corresponding authors of a number of the reviewed articles. Emma Bailey is gratefully acknowledged for her advice in the preparation of the manuscript.
Conflict of interest: none declared.
References
- 1.von Rindfleisch GE. A Text-book of Pathological Histology: An Introduction to the Study of Pathological Anatomy. Philadelphia: Lindsay and Blakiston; 1872. [Google Scholar]
- 2.Steinman DA. Image-based computational fluid dynamics: a new paradigm for monitoring hemodynamics and atherosclerosis. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:183–197. doi: 10.2174/1568006043336302. [DOI] [PubMed] [Google Scholar]
- 3.Fry DL. Certain chemorheologic considerations regarding the blood vascular interface with particular reference to coronary artery disease. Circulation. 1969;40:38–57. [Google Scholar]
- 4.Caro CG, Fitz-Gerald JM, Schroter RC. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci. 1971;177:109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- 5.Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 6.Lou Z, Yang WJ. Biofluid dynamics at arterial bifurcations. Crit Rev Biomed Eng. 1992;19:455–493. [PubMed] [Google Scholar]
- 7.Cecchi E, Giglioli C, Valente S, Lazzeri C, Gensini GF, Abbate R, et al. Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis. 2011;214:249–256. doi: 10.1016/j.atherosclerosis.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Wentzel JJ, Chatzizisis YS, Gijsen FJ, Giannoglou GD, Feldman CL, Stone PH. Endothelial shear stress in the evolution of coronary atherosclerotic plaque and vascular remodelling: current understanding and remaining questions. Cardiovasc Res. 2012;96:234–243. doi: 10.1093/cvr/cvs217. [DOI] [PubMed] [Google Scholar]
- 9.Sawchuk AP, Unthank JL, Dalsing MC. Drag reducing polymers may decrease atherosclerosis by increasing shear in areas normally exposed to low shear stress. J Vasc Surg. 1999;30:761–764. doi: 10.1016/s0741-5214(99)70116-3. [DOI] [PubMed] [Google Scholar]
- 10.Redaelli A, Maisano F, Ligorio G, Cattaneo E, Montevecchi FM, Alfieri O. Flow dynamics of the St Jude Medical Symmetry aortic connector vein graft anastomosis do not contribute to the risk of acute thrombosis. J Thorac Cardiovasc Surg. 2004;128:117–123. doi: 10.1016/j.jtcvs.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 11.Friedman MH, O'Brien V, Ehrlich LW. Calculations of pulsatile flow through a branch: implications for the hemodynamics of atherogenesis. Circ Res. 1975;36:277–285. doi: 10.1161/01.res.36.2.277. [DOI] [PubMed] [Google Scholar]
- 12.Perktold K, Resch M. Numerical flow studies in human carotid artery bifurcations: basic discussion of the geometric factor in atherogenesis. J Biomed Eng. 1990;12:111–123. doi: 10.1016/0141-5425(90)90131-6. [DOI] [PubMed] [Google Scholar]
- 13.Giddens DP, Zarins CK, Glagov S. The role of fluid mechanics in the localization and detection of atherosclerosis. J Biomech Eng. 1993;115:588–594. doi: 10.1115/1.2895545. [DOI] [PubMed] [Google Scholar]
- 14.Taylor CA, Hughes TJ, Zarins CK. Finite element modeling of three-dimensional pulsatile flow in the abdominal aorta: relevance to atherosclerosis. Ann Biomed Eng. 1998;26:975–987. doi: 10.1114/1.140. [DOI] [PubMed] [Google Scholar]
- 15.Pubmed. http://www.ncbi.nlm.nih.gov/pubmed/ (21 March 2012)
- 16.Perktold K, Peter RO, Resch M, Langs G. Pulsatile non-Newtonian blood flow in three-dimensional carotid bifurcation models: a numerical study of flow phenomena under different bifurcation angles. J Biomed Eng. 1991;13:507–515. doi: 10.1016/0141-5425(91)90100-l. [DOI] [PubMed] [Google Scholar]
- 17.Krams R, Wentzel JJ, Oomen JA, Vinke R, Schuurbiers JC, de Feyter PJ, et al. Evaluation of endothelial shear stress and 3D geometry as factors determining the development of atherosclerosis and remodeling in human coronary arteries in vivo. Combining 3D reconstruction from angiography and IVUS (ANGUS) with computational fluid dynamics. Arterioscler Thromb Vasc Biol. 1997;17:2061–2065. doi: 10.1161/01.atv.17.10.2061. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan JR, Kleinstreuer C, Truskey GA, Lei M. Relation between non-uniform hemodynamics and sites of altered permeability and lesion growth at the rabbit aorto-celiac junction. Atherosclerosis. 1999;143:27–40. doi: 10.1016/s0021-9150(98)00264-0. [DOI] [PubMed] [Google Scholar]
- 19.Steinman DA, Thomas JB, Ladak HM, Milner JS, Rutt BK, Spence JD. Reconstruction of carotid bifurcation hemodynamics and wall thickness using computational fluid dynamics and MRI. Magn Reson Med. 2002;47:149–159. doi: 10.1002/mrm.10025. [DOI] [PubMed] [Google Scholar]
- 20.Goubergrits L, Affeld K, Fernandez-Britto J, Falcon L. Atherosclerosis and flow in carotid arteries with authentic geometries. Biorheology. 2002;39:519–524. [PubMed] [Google Scholar]
- 21.Wentzel JJ, Janssen E, Vos J, Schuurbiers JC, Krams R, Serruys PW, et al. Extension of increased atherosclerotic wall thickness into high shear stress regions is associated with loss of compensatory remodeling. Circulation. 2003;108:17–23. doi: 10.1161/01.CIR.0000078637.21322.D3. [DOI] [PubMed] [Google Scholar]
- 22.Buchanan JR, Kleinstreuer C, Hyun S, Truskey GA. Hemodynamics simulation and identification of susceptible sites of atherosclerotic lesion formation in a model abdominal aorta. J Biomech. 2003;36:1185–1196. doi: 10.1016/s0021-9290(03)00088-5. [DOI] [PubMed] [Google Scholar]
- 23.Joshi AK, Leask RL, Myers JG, Ojha M, Butany J, Ethier CR. Intimal thickness is not associated with wall shear stress patterns in the human right coronary artery. Arterioscler Thromb Vasc Biol. 2004;24:2408–2413. doi: 10.1161/01.ATV.0000147118.97474.4b. [DOI] [PubMed] [Google Scholar]
- 24.Wentzel JJ, Gijsen FJ, Schuurbiers JC, Krams R, Serruys PW, De Feyter PJ, et al. Geometry guided data averaging enables the interpretation of shear stress related plaque development in human coronary arteries. J Biomech. 2005;38:1551–1555. doi: 10.1016/j.jbiomech.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 25.Gijsen FJ, Wentzel JJ, Thury A, Lamers B, Schuurbiers JC, Serruys PW, et al. A new imaging technique to study 3-D plaque and shear stress distribution in human coronary artery bifurcations in vivo. J Biomech. 2007;40:2349–2357. doi: 10.1016/j.jbiomech.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Augst AD, Ariff B, McG Thom SA, Xu XY, Hughes AD. Analysis of complex flow and the relationship between blood pressure, wall shear stress, and intima-media thickness in the human carotid artery. Am J Physiol Heart Circ Physiol. 2007;293:H1031–H1037. doi: 10.1152/ajpheart.00989.2006. [DOI] [PubMed] [Google Scholar]
- 27.Chatzizisis YS, Jonas M, Coskun AU, Beigel R, Stone BV, Maynard C, et al. Prediction of the localization of high-risk coronary atherosclerotic plaques on the basis of low endothelial shear stress: an intravascular ultrasound and histopathology natural history study. Circulation. 2008;117:993–1002. doi: 10.1161/CIRCULATIONAHA.107.695254. [DOI] [PubMed] [Google Scholar]
- 28.Suo J, Oshinski JN, Giddens DP. Blood flow patterns in the proximal human coronary arteries: relationship to atherosclerotic plaque occurrence. Mol Cell Biomech. 2008;5:9–18. [PubMed] [Google Scholar]
- 29.Olgac U, Kurtcuoglu V, Saur SC, Poulikakos D. Identification of atherosclerotic lesion-prone sites through patient-specific simulation of low-density lipoprotein accumulation. Med Image Comput Comput Assist Interv. 2008;11:774–781. doi: 10.1007/978-3-540-85990-1_93. [DOI] [PubMed] [Google Scholar]
- 30.Olgac U, Poulikakos D, Saur SC, Alkadhi H, Kurtcuoglu V. Patient-specific three-dimensional simulation of LDL accumulation in a human left coronary artery in its healthy and atherosclerotic states. Am J Physiol Heart Circ Physiol. 2009;296:H1969–H1982. doi: 10.1152/ajpheart.01182.2008. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Zhang J, Shih J, Lopez-Bertoni F, Hagaman JR, Maeda N, et al. Differences in aortic arch geometry, hemodynamics, and plaque patterns between C57BL/6 and 129/SvEv mice. J Biomech Eng. 2009;131:121005. doi: 10.1115/1.4000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Giessen AG, Schaap M, Gijsen FJ, Groen HC, van Walsum T, Mollet NR, et al. 3D fusion of intravascular ultrasound and coronary computed tomography for in-vivo wall shear stress analysis: a feasibility study. Int J Cardiovasc Imaging. 2010;26:781–796. doi: 10.1007/s10554-009-9546-y. [DOI] [PubMed] [Google Scholar]
- 33.Koskinas KC, Feldman CL, Chatzizisis YS, Coskun AU, Jonas M, Maynard C, et al. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: a serial, in vivo intravascular ultrasound study. Circulation. 2010;121:2092–2101. doi: 10.1161/CIRCULATIONAHA.109.901678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight J, Olgac U, Saur SC, Poulikakos D, Marshall W, Cattin PC, et al. Choosing the optimal wall shear parameter for the prediction of plaque location-A patient-specific computational study in human right coronary arteries. Atherosclerosis. 2010;211:445–450. doi: 10.1016/j.atherosclerosis.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Hoi Y, Zhou YQ, Zhang X, Henkelman RM, Steinman DA. Correlation between local hemodynamics and lesion distribution in a novel aortic regurgitation murine model of atherosclerosis. Ann Biomed Eng. 2011;39:1414–1422. doi: 10.1007/s10439-011-0255-z. [DOI] [PubMed] [Google Scholar]
- 36.Chatzizisis YS, Baker AB, Sukhova GK, Koskinas KC, Papafaklis MI, Beigel R, et al. Augmented expression and activity of extracellular matrix-degrading enzymes in regions of low endothelial shear stress colocalize with coronary atheromata with thin fibrous caps in pigs. Circulation. 2011;123:621–630. doi: 10.1161/CIRCULATIONAHA.110.970038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samady H, Eshtehardi P, McDaniel MC, Suo J, Dhawan SS, Maynard C, et al. Coronary artery wall shear stress is associated with progression and transformation of atherosclerotic plaque and arterial remodeling in patients with coronary artery disease. Circulation. 2011;124:779–788. doi: 10.1161/CIRCULATIONAHA.111.021824. [DOI] [PubMed] [Google Scholar]
- 38.Siogkas P, Sakellarios A, Exarchos T, Athanasiou L, Karvounis E, Stefanou K, et al. Multi-scale patient-specific artery and atherogenesis models. IEEE Trans Biomed Eng. 2011;58:3464–3468. doi: 10.1109/TBME.2011.2164919. [DOI] [PubMed] [Google Scholar]
- 39.Zhang C, Xie S, Li S, Pu F, Deng X, Fan Y, et al. Flow patterns and wall shear stress distribution in human internal carotid arteries: the geometric effect on the risk for stenoses. J Biomech. 2012;45:83–89. doi: 10.1016/j.jbiomech.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Rikhtegar F, Knight JA, Olgac U, Saur SC, Poulikakos D, Marshall W, et al. Choosing the optimal wall shear parameter for the prediction of plaque location-A patient-specific computational study in human left coronary arteries. Atherosclerosis. 2012;221:432–437. doi: 10.1016/j.atherosclerosis.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Maeda N, Johnson L, Kim S, Hagaman J, Friedman M, Reddick R. Anatomical differences and atherosclerosis in apolipoprotein E-deficient mice with 129/SvEv and C57BL/6 genetic backgrounds. Atherosclerosis. 2007;195:75–82. doi: 10.1016/j.atherosclerosis.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou YQ, Zhu SN, Foster FS, Cybulsky MI, Henkelman RM. Aortic regurgitation dramatically alters the distribution of atherosclerotic lesions and enhances atherogenesis in mice. Arterioscler Thromb Vasc Biol. 2010;30:1181–1188. doi: 10.1161/ATVBAHA.110.204198. [DOI] [PubMed] [Google Scholar]
- 43.De Syo D. Radiogrametric analysis of carotid bifurcation: hemodynamic-atherogenetic repercussions on surgical patients. In: Liepsch D, editor. Biofluid Mechanics. Blood Flow in Large Vessels. Proceedings of the 2nd International Symposium, Biofluid Mechanics and Biorheology. Heidelberg: Springer-Verlag Berlin;; 1990. [Google Scholar]
- 44.Cornhill JF, Roach MR. A quantitative study of the localization of atherosclerotic lesions in the rabbit aorta. Atherosclerosis. 1976;23:489–501. doi: 10.1016/0021-9150(76)90009-5. [DOI] [PubMed] [Google Scholar]
- 45.Svindland A. The localization of sudanophilic and fibrous plaques in the main left coronary bifurcation. Atherosclerosis. 1983;48:139–145. doi: 10.1016/0021-9150(83)90100-4. [DOI] [PubMed] [Google Scholar]
- 46.Malinauskas RA, Herrmann RA, Truskey GA. The distribution of intimal white blood cells in the normal rabbit aorta. Atherosclerosis. 1995;115:147–163. doi: 10.1016/0021-9150(94)05497-7. [DOI] [PubMed] [Google Scholar]
- 47.Herrmann RA, Malinauskas RA, Truskey GA. Characterization of sites with elevated LDL permeability at intercostal, celiac, and iliac branches of the normal rabbit aorta. Arterioscler Thromb. 1994;14:313–323. doi: 10.1161/01.atv.14.2.313. [DOI] [PubMed] [Google Scholar]
- 48.Zeindler CM, Kratky RG, Roach MR. Quantitative measurements of early atherosclerotic lesions on rabbit aortae from vascular casts. Atherosclerosis. 1989;76:245–255. doi: 10.1016/0021-9150(89)90108-1. [DOI] [PubMed] [Google Scholar]
- 49.He X, Ku DN. Pulsatile flow in the human left coronary artery bifurcation: average conditions. J Biomech Eng. 1996;118:74–82. doi: 10.1115/1.2795948. [DOI] [PubMed] [Google Scholar]
- 50.De Syo D, Franjic BD, Lovricevic I, Vukelic M, Palenkic H. Carotid bifurcation position and branching angle in patients with atherosclerot0ic carotid disease. Coll Antropol. 2005;29:627–632. [PubMed] [Google Scholar]
- 51.Stone PH, Coskun AU, Kinlay S, Popma JJ, Sonka M, Wahle A, et al. Regions of low endothelial shear stress are the sites where coronary plaque progresses and vascular remodelling occurs in humans: an in vivo serial study. Eur Heart J. 2007;28:705–710. doi: 10.1093/eurheartj/ehl575. [DOI] [PubMed] [Google Scholar]
- 52.Weinberg PD, Ethier CR. Twenty-fold difference in hemodynamic wall shear stress between murine and human aortas. J Biomech. 2007;40:1594–1598. doi: 10.1016/j.jbiomech.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 53.Cheer AY, Dwyer HA, Barakat AI, Sy E, Bice M. Computational study of the effect of geometric and flow parameters on the steady flow field at the rabbit aorto-celiac bifurcation. Biorheology. 1998;35:415–435. doi: 10.1016/s0006-355x(99)80020-1. [DOI] [PubMed] [Google Scholar]
- 54.Vincent PE, Plata AM, Hunt AA, Weinberg PD, Sherwin SJ. Blood flow in the rabbit aortic arch and descending thoracic aorta. J R Soc Interface. 2011;8:1708–1719. doi: 10.1098/rsif.2011.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987;5:413–420. [PubMed] [Google Scholar]
- 56.Feldman CL, Coskun AU, Yeghiazarians Y, Kinlay S, Wahle A, Olszewski ME, et al. Remodeling characteristics of minimally diseased coronary arteries are consistent along the length of the artery. Am J Cardiol. 2006;97:13–16. doi: 10.1016/j.amjcard.2005.07.121. [DOI] [PubMed] [Google Scholar]
- 57.Anitschkow NN. Experimental atherosclerosis in animals. In: Cowdry EV, editor. Arteriosclerosis: A Survey of the Problem. New York: Macmillan; 1933. pp. 271–322. [Google Scholar]
- 58.Stalder AF, Russe MF, Frydrychowicz A, Bock J, Hennig J, Markl M. Quantitative 2D and 3D phase contrast MRI: optimized analysis of blood flow and vessel wall parameters. Magn Reson Med. 2008;60:1218–1231. doi: 10.1002/mrm.21778. [DOI] [PubMed] [Google Scholar]
- 59.Poelma C, van der Mijle RME, Mari JM, Tang M-X, Weinberg PD, Westerweel J. Ultrasound imaging velocimetry: towards reliable wall shear stress measurements. Eur J Mech-B/Fluids. 2012;35:70–75. [Google Scholar]
- 60.Cheng C, van Haperen R, de Waard M, van Damme LC, Tempel D, Hanemaaijer L, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–3698. doi: 10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 61.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 62.Chakraborty A, Chakraborty S, Jala VR, Haribabu B, Sharp MK, Berson RE. Effects of biaxial oscillatory shear stress on endothelial cell proliferation and morphology. Biotechnol Bioeng. 2012;109:695–707. doi: 10.1002/bit.24352. [DOI] [PubMed] [Google Scholar]
- 63.Ma P, Li X, Ku DN. Convective mass transfer at the carotid bifurcation. J Biomech. 1997;30:565–571. doi: 10.1016/s0021-9290(97)84506-x. [DOI] [PubMed] [Google Scholar]
- 64.Olgac U, Knight J, Poulikakos D, Saur SC, Alkadhi H, Desbiolles LM, et al. Computed high concentrations of low-density lipoprotein correlate with plaque locations in human coronary arteries. J Biomech. 2011;44:2466–2471. doi: 10.1016/j.jbiomech.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 65.Coppola G, Caro C. Oxygen mass transfer in a model three-dimensional artery. J R Soc Interface. 2008;5:1067–1075. doi: 10.1098/rsif.2007.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Doormaal MA, Ethier CR. Design optimization of a helical endothelial cell culture device. Biomech Model Mechanobiol. 2010;9:523–531. doi: 10.1007/s10237-010-0192-z. [DOI] [PubMed] [Google Scholar]
- 67.Peiffer V, Bharath AA, Sherwin SJ, Weinberg PD. A novel method for quantifying spatial correlations between patterns of atherosclerosis and haemodynamic factors. J Biomech Eng. 2013;135:021023. doi: 10.1115/1.4023381. [DOI] [PubMed] [Google Scholar]
- 68.Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weinberg PD. Disease patterns at arterial branches and their relation to flow. Biorheology. 2002;39:533–537. [PubMed] [Google Scholar]
- 70.McGillicuddy CJ, Carrier MJ, Weinberg PD. Distribution of lipid deposits around aortic branches of mice lacking LDL receptors and apolipoprotein E. Arterioscler Thromb Vasc Biol. 2001;21:1220–1225. doi: 10.1161/hq0701.091996. [DOI] [PubMed] [Google Scholar]
- 71.Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14:133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- 72.Cremers SG, Wolffram SJ, Weinberg PD. Atheroprotective effects of dietary L-arginine increase with age in cholesterol-fed rabbits. Br J Nutr. 2011;105:1439–1447. doi: 10.1017/S0007114510005234. [DOI] [PubMed] [Google Scholar]
- 73.Peiffer V, Rowland EM, Cremers SG, Weinberg PD, Sherwin SJ. Effect of aortic taper on patterns of blood flow and wall shear stress in rabbits: association with age. Atherosclerosis. 2012;223:114–121. doi: 10.1016/j.atherosclerosis.2012.04.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.