Abstract
Connexins form a family of transmembrane proteins that consists of 20 members in humans and 21 members in mice. Six connexins assemble into a connexon that can function as a hemichannel or connexon that can dock to a connexon expressed by a neighbouring cell, thereby forming a gap junction channel. Such intercellular channels synchronize responses in multicellular organisms through direct exchange of ions, small metabolites, and other second messenger molecules between the cytoplasms of adjacent cells. Multiple connexins are expressed in the cardiovascular system. These connexins not only experience the different biomechanical forces within this system, but may also act as effector proteins in co-ordinating responses within groups of cells towards these forces. This review discusses recent insights regarding regulation of cardiovascular connexins by mechanical forces and junctions. It specifically addresses effects of (i) shear stress on endothelial connexins, (ii) hypertension on vascular connexins, and (iii) changes in afterload and the composition of myocardial mechanical junctions on cardiac connexins.
Keywords: Gap junctions, Connexins, Cardiovascular system, Mechanotransduction
1. Connexins in the cardiovascular system
Connexins form a family of transmembrane proteins that consists of 20 members in humans and 21 members in mice. Oligomerization of six connexins results in the formation of a connexon. Such a connexon can dock to a connexon expressed by a neighbouring cell, thereby forming a gap junction channel.1 These channels are crucial for gap junctional intercellular communication (GJIC) as they allow passage of small molecules from the cytosol of one cell to another. Classically, ions and metabolites <1000 Da, e.g. ATP, Ca2+, cAMP, and IP3, are known to pass through gap junction channels. But larger molecules, such as peptides and small nucleotides, have also been shown to be able to diffuse through gap junctions.2–4 In addition, a connexon can also function as a hemichannel and allow diffusion of molecules between the cytoplasm and the extracellular space.5
The most abundantly expressed connexins in the cardiovascular system are connexin37 (Cx37), Cx40, Cx43, and Cx45. The expression of these proteins displays a large heterogeneity throughout the circulatory system (i.e. arteries, veins, lymphatic vessels, and the heart). In the vessels of healthy subjects, endothelial cells (ECs) of large conduit arteries express Cx37 and Cx40, whereas Cx43 and (to a much lower extent) Cx45 are mostly expressed by vascular smooth muscle cells (VSMCs).6,7 The level and pattern of connexin expression in conduit arteries changes with age and during atherogenesis,6,8 suggesting that connexins may be contributing to the latter process. In the apolipoprotein-E-deficient (ApoE−/−) mouse model for atherosclerosis, it has indeed been shown that Cx37 and Cx40 display atheroprotective properties,9,10 whereas Cx43 appeared pro-atherogenic.11 Recently, both Cx37 and Cx40 have been described to interact with endothelial nitric oxide synthase (eNOS) in aortic ECs.12,13 This suggests that both Cx37 and Cx40 may regulate release of eNOS-derived nitric oxide (NO). In agreement with these findings, it has been shown that Cx40-deficient (Cx40−/−) mice, which are also characterized by a reduced endothelial Cx37 expression, display diminished agonist-induced endothelium-dependent vasodilatation.12 In addition, in vitro evidence suggests that Cx37 inhibits eNOS-dependent NO production.13 However, arteries from Cx37-deficient mice (Cx37−/−) exhibit proper endothelium-dependent relaxation ex vivo.14 Compared with conduit arteries that fully depend on NO for endothelium-dependent relaxations, smaller blood vessels typically depend largely on endothelium-derived hyperpolarization for endothelium-mediated control of vascular tone.15 Interestingly, ECs and VSMCs of small muscular resistance arteries express Cx37, Cx40, and Cx43.16,17 In these arteries, connexins are involved in the spreading of endothelium-dependent vasodilatation and relaxation induced by endothelium-derived hyperpolarizing factor(s).18,19 ECs of large veins have been described to express Cx37, Cx40, and Cx43,20 although Cx40 and Cx37 expression levels seem lower than those of the aorta.21 Finally, lymphatic ECs express Cx37, Cx43, and Cx47,22,23 and recently Cx37 has been shown to be of crucial importance for the formation of lymphatic valves;22,23 i.e. Cx37−/− mice lack lymphatic valves and therefore display reduced lymph flow.22,23 More recently, Cx37 has been reported to be critically involved in the formation of venous valves as well.24
Classically, connexins expressed by the working myocardium contribute to synchronization of cardiac contractions by electrically coupling cardiomyocytes. However, cardiac connexins can also play a deleterious role, e.g. after periods of ischaemia by passing so-called ‘death signals’ from one cardiomyocyte to the next.25,26 Healthy ventricular myocardium mainly expresses Cx43 and, to a lesser extent, Cx45, and healthy atrial myocardium expresses high levels of both Cx40 and Cx43 in addition to small amounts of Cx45.25 Cells that form the electrical conduction system responsible for the rapid spread of electrical signals from the sinoatrial node towards the ventricles have been described to express a variety of connexins (i.e. Cx45, Cx43, Cx40, and Cx30). The particular set of connexins expressed by these cells is strongly dependent on their location.27 In summary, multiple connexins are expressed in the cardiovascular system that not only experience the different biomechanical forces within this system, but may also act as effector proteins in co-ordinating responses within groups of cells towards these forces.
2. Effect of shear stress in arteries
It is increasingly recognized that the endothelium lining the cardiovascular system is highly sensitive to haemodynamic shear stresses, which act at the vessel luminal surface in the direction of blood flow. Wall shear stress, the frictional force between blood and the endothelium, is more and more recognized as a key determinant of EC function, gene expression, and structure.28 In this respect, Krüppel-like factor 2 (KLF2) and nuclear factor-E2-related factor-2 (Nrf2) transcription factors have been identified as central regulators of physiological responses to arterial shear stress.29 In straight portions of arteries, KLF2 is induced in ECs exposed to prolonged high laminar shear stress (HLSS) where it regulates the expression of anti-inflammatory and anti-coagulant proteins, such as eNOS and thrombomodulin. HLSS also activates Nrf2, a transcription factor that regulates redox levels by induction of numerous anti-oxidant enzymes such as heme oxygenase-1. Physiological variations in shear stress regulate acute changes in blood vessel diameter and, when sustained, induce slow adaptive vascular wall remodelling. As a consequence, atherosclerotic lesions develop predominantly near side branches of arteries, i.e. where blood flow is oscillatory, or at the lesser curvature of bends of the arterial tree, i.e. where blood flow rates are relatively low.30 ECs in these areas of low or oscillatory shear stress (OSS) display activation of nuclear factor κB (NFκB) and activation protein 1 transcription factors, resulting in the expression of pro-inflammatory genes such as vascular cell adhesion molecule-1 (VCAM-1), E-selectin, and interleukin-8 (IL-8).31 In brief, the arterial endothelium is continuously exposed to regional differences in shear stress, which induces differential transcriptional activity in adjacent territories.
3. Connexins as effector proteins in co-ordinating shear stress responses?
Intercellular channels formed by connexins provide a simple method of synchronizing responses in multicellular organisms through direct exchange of ions, small metabolites, and other second messenger molecules between adjacent cells.32,33 This type of intercellular signalling is implicated in rapid co-ordinated activities such as contraction of the heart.34 In contrast, GJIC has to be restricted between adjacent territories with different function or activation state. Gap junctions in the arterial endothelium are known to consist mainly of Cx40 and Cx37.7,35 Cx43 is moderately expressed or absent in the quiescent arterial endothelium, but is induced under conditions associated with endothelial dysfunction. Gabriels and Paul36 observed that Cx43 was not detectable in most parts of the rat aorta but very abundant in small clusters of cells localized at the downstream edge of ostia of branching vessels or flow dividers. Moreover, when they created flow disturbances in vivo by surgical coarctation (by ligature) of the aorta leading to a 30% reduction in vessel diameter, increased Cx43 expression was observed in areas of pronounced oscillatory flow, whereas Cx40 expression levels were unchanged by this treatment. In contrast, Cx37 expression seemed down-regulated in these regions and very little co-expression of Cx37 and Cx43 was observed. Although aortic coarctation will induce significant flow disturbances, an effect of arterial compression on connexin expression cannot be completely ruled out using this model. It is, therefore, interesting that the relation between shear stress and connexin expression has also been investigated on upstream and downstream surfaces of rat cardiac valves that are naturally subjected to very different intensities of shear stress.37 The authors observed up to 200-fold greater expression of Cx43 in the ECs on the upstream compared with the downstream surfaces. In contrast, Cx37 expression was equal in both endothelia and Cx40 was not detected at all. Although ECs covering cardiac valves are also exposed to high pressures, it remains difficult to compare these cells with the arterial endothelium, given their different haemodynamic environment.
In addition to these early in vivo studies, several in vitro studies have been performed to investigate the correlation between Cx43 expression and different biomechanical forces on various types of ECs. In a first preliminary report,38 a four-fold increase in Cx43 mRNA levels in porcine aortic ECs was reported already after 1 h of 15 dynes/cm2 LSS, which remained elevated for the complete investigated period (24 h). This report was followed by a sophisticated and detailed study39 in which the spatial and temporal regulation of Cx43 mRNA and protein as well as intercellular communication was mapped in monolayers of bovine aortic ECs exposed to controlled flow conditions. At short term (5 h), endothelial Cx43 was increased under conditions of both laminar flow and in areas of flow separation and recirculation compared with no-flow controls. However, after 16 and 30 h of shear stress, Cx43 expression remained increased in cells subjected to disturbed flow, but Cx43 expression returned to normal levels in regions of undisturbed laminar flow. The differences in Cx43 expression were matched by an increased level of intercellular spread of Lucifer Yellow, a Cx43-permeable dye, in the region of disturbed flow. The effects of pulsed unidirectional and OSS in combination with different levels of hydrostatic pressure and circumferential stretch on Cx43 expression was investigated in PymT-transformed mouse ECs (bEnd.3), expressing all the three endothelial connexins, that were grown to confluence on the inside of elastic tubes.40 Interestingly, increased Cx43 expression was observed after 24 h of modest levels of both pulsatile LSS and OSS, although the rise in Cx43 expression reached higher levels (up to three-fold) under OSS. In addition, 4% (change of diameter) circumferential stretch caused a significant increase in Cx43 protein levels after 4 h that remained elevated after 24 h. In contrast, raising pressure from 100 to 150 mmHg for up to 24 h had no effect on Cx43 expression in ECs. Altogether, these studies provide clear evidence that endothelial Cx43 expression is sensitive to changes in the haemodynamic environment, in particular to OSS. Although NFκB and mitogen-activated protein kinase pathways are likely candidates,41,42 the mechanism by which OSS induces Cx43 expression in arterial ECs still remains to be established.
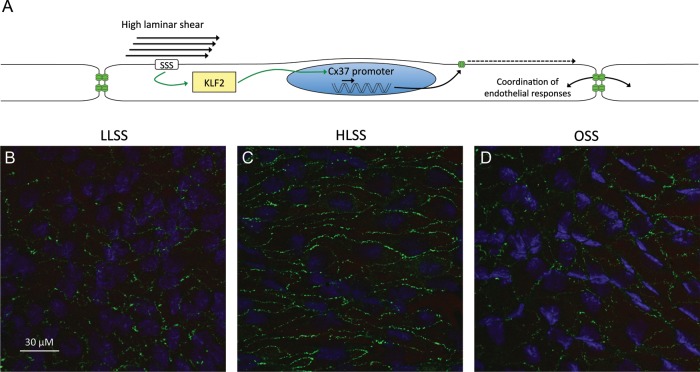
As mentioned before, most ECs in the aorta express high levels of Cx37, but this expression is lost in ECs at bifurcations and in ECs overlying atherosclerotic plaques.6 An increasing number of large microarray data sets have become available in which gene expression patterns in various shear stress regions have been compared with each other or to static culture conditions. These microarrays have provided rather inconsistent data regarding the regulation of endothelial Cx37 expression by shear stress.43–46 As described above, HLSS is known to be vaso-protective through the induction of KLF2. As the promoter region of the Cx37 gene contains many KLF consensus binding sites, we hypothesized that shear stress, through modulation of KLF2, may regulate Cx37 expression in the arterial endothelium. Using 16-week-old atherosusceptible ApoE−/− mice, we first confirmed that Cx37 is highly expressed in the straight region of the common carotid artery of these mice, whereas it is reduced at the carotid bifurcation.47 The differences in Cx37 expression were accompanied by a decreased level of intercellular spread of propidium iodide, a Cx37-permeable dye, in the bifurcation region. We then used shear stress-modifying vascular devices that impose specific flow patterns in mouse carotid arteries.48 We found that Cx37 expression was down-regulated in regions of altered flow (either LLSS or OSS), but was conserved in regions of HLSS (Figure 1).47
Figure 1.
(A) High laminar shear stress is atheroprotective partly through induction of Kruppel-like factor 2 that, in turn, up-regulates Cx37 expression and, therefore, ensures co-ordination of endothelial responses. Representative images of Cx37 (green) in the endothelium of carotid arteries exposed to low laminar (B), high laminar (C) or oscillatory shear stress (D) in vivo. HLSS, high laminar shear stress; KLF2, Kruppel-like factor 2; LLSS, low laminar shear stress; OSS, oscillatory shear stress; SSS, shear stress sensor.
To further study the mechanisms involved, bEnd.3 or human umbilical vein ECs (HUVECs) were exposed to shear stress in vitro. Cx37 and KLF2 expression were increased after 24 h of HLSS (25 dynes/cm2). Interestingly, Cx37 expression was reduced after silencing KLF2, suggesting that KLF2 indeed acts as a transcription factor for this gap junction protein. Moreover, binding of KLF2 to the promoter region of Cx37 was demonstrated by chromatin immunoprecipitation. We finally used a scrape-loading dye transfer approach and determined that gap junctional permeability to propidium iodide was severely impaired following KLF2 silencing in a monolayer of ECs. Altogether, this study demonstrated that shear stress, by modulating KLF2 expression, forms distinct communication compartments in arteries, with atherosusceptible regions expressing less Cx37 and displaying less intercellular diffusion of the cationic dye propidium iodide than the protected regions.47 This compartmentalization could, for example, be beneficial by limiting spread of specific pro-inflammatory mediators away from the region subjected to disturbed flow. On the other hand, shear stress-induced Cx37 may be involved in synchronizing endothelial responses to protective circulating factors.
Cx37 is also expressed in lymphatic ECs. However, in contrast to the arterial endothelium, its expression is mostly limited to regions of valve formation that are exposed to (low) OSS.23 Interestingly, recent studies demonstrated a severe impairment of lymphatic valve formation and function in Cx37−/− mice.22,23 Based on in vitro studies in which lymphatic ECs were exposed to physiological values of OSS, the authors proposed a mechanism for lymphatic valve formation in which Cx37 acts downstream of the lymphatic endothelial transcription factors Prox1/Foxc2 and shear stress, leading to a synchronized calcineurin/nuclear factor of activated T-cells (NFAT) activation in response to flow.23 Mechanistically, Cx37 gap junctions may mediate transfer of IP3, which triggers release of intracellular Ca2+ necessary for calcineurin/NFAT activation. However, involvement of other second messenger pathways cannot be excluded. Although the effector role of Cx37 in forming communication compartments in the lymphatic or arterial endothelium is similar, it remains very intriguing that the signalling pathways inducing the expression of the effector protein are dependent on a different, almost opposite, type of shear stress that up-regulates distinct transcription factors. Of note, Cx37−/− mice also lack venous valves.24 And although the mechanism of Cx37 expression in venous valves has not been investigated yet, one can imagine that similar molecular pathways control valve development in veins and lymphatic vessels, since they share a common embryonic origin.
Cx40 expression in the endothelium of large arteries seems to have little sensitivity to flow disturbances. Indeed, Cx40 expression is high in the straight portion of the mouse carotid artery and seems unaltered at the carotid bifurcation.47 Moreover, Cx40 expression levels in the mouse aortic ECs were not affected by surgical coarctation.36 Similar to KLF2, KLF4 transcription is induced in arteries by HLSS and this transcription factor subsequently regulates the expression of (vaso-protective) target genes. Interestingly, Cx40 mRNA expression in cultured ECs was moderately down-regulated by KLF4 siRNA, while it remained unaffected by KLF2 silencing.47 Whether arterial Cx40 expression is directly regulated by KLF4 or whether additional shear stress-responsive transcription factors are involved remains to be investigated.
More mechanistic details on the relation between shear stress and Cx40 are available from studies in the microvasculature, where shear stress causes a long-term induction of Cx40 protein expression. This Cx40 expression is greatly reduced by inhibiting PI3 kinase or Akt, with PI3K activity being required for basal Cx40 expression and Akt activity taking part in shear stress-dependent induction of Cx40.49 As endothelial Cx40 plays an important role in signal propagation along the wall of arterioles,35,50 this induction of Cx40 expression may have important functional consequences for the adaptation of microvascular networks.
Arterial pressure fluctuates as a result of the rhythmic activity of the heart. The effects of constant and pulsatile shear on the expression of arterial identity genes have been investigated by using a combination of approaches ranging from in vitro ECs cultures, the developing chicken embryo yolk sac vasculature, and mouse occlusion models. Recently, Buschmann et al.51 elegantly demonstrated that a combination of pulsatile shear and Cx40 is of critical importance for arterial identity and remodelling events during flow-driven arteriogenesis.
Altogether, the presently available studies point to a strong function for Cx40 in endothelial synchronization of mechanosensitive responses in the microvasculature, whereas Cx43 and Cx37 seem crucial for mechanosensitive compartmentalization in the arterial and lymphatic endothelium.
4. Hypertension and connexins
Hypertension is a chronic disease that can be subdivided in systemic hypertension and pulmonary hypertension. Systemic hypertension is estimated to affect up to one-third of the Western population and likely accounts for ∼16% of all deaths annually.52 The pathophysiology underlying the majority of cases remains poorly understood and therefore this disease is defined clinically. Systemic hypertension is defined as (i) a systolic blood pressure >140 mmHg or (ii) a diastolic blood pressure >90 mmHg or (iii) normal blood pressure but on antihypertensive therapy or (iv) being told at least twice by a health professional that one suffers from hypertension.52 Pulmonary hypertension has been defined as a mean pulmonary arterial pressure >25 mmHg at rest as assessed by right heart catheterization.53,54 Pulmonary hypertension affects 10–20% of the general population and is more prevalent in females compared with males.55–57
Connexins are widely expressed in organs involved in the pathogenesis of both systemic hypertension (e.g. ECs, VSMCs, and renin producing cells in the kidney) and pulmonary hypertension (e.g. pulmonary artery ECs and pulmonary fibroblasts). In addition, connexins are expressed in organs suffering from the consequences of both diseases (e.g. the left ventricle, which experiences increased afterload during systemic hypertension, and the right ventricle which is exposed to increased afterload during pulmonary hypertension). Despite the fact that many animal models have been used to study connexins in the contest of systemic hypertension, there is surprisingly little consensus regarding the effect of hypertension on connexin expression (for an overview, see Table 1). In addition, the molecular mechanisms by which hypertension affects connexins are largely unknown.
Table 1.
Effect of hypertension on connexin expression in the cardiovascular system
| Species | Model | Cx37 | Cx40 | Cx43 | Cx45 | Note | Ref. |
|---|---|---|---|---|---|---|---|
| Rat | SPSHR | ND | ND | →(mesenteric arterial cell culture) | ND | [112] | |
| Rat | DOCA-salt | ND | ND | ↑(aorta) | ND | [60] | |
| Rat | 2K1C | ND | ND | ↑(aorta), →(cardiomyocyte) | ND | [58,59] | |
| Rat | DOCA-salt | ND | ND | ↑(aorta), →(cardiomyocyte) | ND | [58,59] | |
| Rat | l-NAME | ND | ND | ↓(aorta),→(cardiomyocyte) | ND | [62] | |
| Rat | 2K1C | ND | ↑(both kidney) | ↑(unclipped kidney) | ND | [61] | |
| Rat | 3-week-old SHR | →(caudal ECs) | ↓(caudal ECs) | →(caudal ECs) | ND | [64] | |
| Rat | 12-week-old SHR | ↓(caudal ECs) | ↓(caudal ECs) | ↓(caudal ECs) | ND | [64] | |
| Rat | SHR | ↓(mesentric ECs) | ↓(mesenteric ECs) | ↑(mesenteric ECs) | ND | Candesartan increased expression of Cx37 and Cx40 but decreased expression of Cx43 | [66] |
| Rat | 3-week-old SHR | →(aortic ECs) | →(aortic ECs) | →(aortic ECs) | ND | [65] | |
| Rat | 12-week-old SHR | ↓(aortic ECs & VSMCs) | ↓(aortic ECs) | ↓(aortic VSMCs) | ND | Normalized endothelial connexin expression by ACE inhibition. | [65] |
| Rat | l-NAME | ↓(aortic ECs) | →(aortic ECs) | ↓(aortic ECs) | ND | Normalized Cx37 and Cx43 expression by carvedilol treatment | [63] |
| Mouse | Cx40−/− | →(RSC) | Undetectable | →(RSC) | ND | [68] | |
| Rat | SHR | ↓(renal arteriolar ECs) | → (renal arteriolar ECs) | → (renal arteriolar ECs) | ND | [67] | |
| Mouse | C57BL6 SK1C | ↑(aortic VSMCs) | ↑(aortic ECs) | ↑(aortic VSMCs) | ↑(aortic VSMCs) | Connexin expression partly normalized by candesartan | [41] |
| Mouse | C57BL6 l-NAME | ↑(aortic VSMCs) | ↑(aortic ECs) | → (whole aorta lysate) | ↑(aortic VSMCs) | [41] | |
| Mouse | Cx40−/− | ↑(aortic VSMCs), ↓(aortic ECs) | Undetectable | ↑(aortic VSMCs) | ↑(aortic VMCs) | Connexin expression in VSMCs reduced by candesartan | [41] |
ECs, endothelial cells; ND, not determined; RSC; renin-secreting cells; VSMCs, vascular smooth muscle cells.
5. Connexins in renin- and NO-dependent hypertension
In models of renin-dependent hypertension [e.g. the DOCA-salt and the 2-kidney, 1-clip Goldblatt (2K1C) models], the expression of both Cx40 and Cx43 increases albeit in a vessel- and time-dependent manner.41,58–60 Although the expression of Cx40 increases in both kidneys in the 2K1C model, the expression of Cx43 only increases in the unclipped kidney, suggesting that this increased Cx43 is a consequence of the rise in intravascular pressure rather than a more general stimulus.61 Moreover, Cx43 but not Cx40 increases in aortic VSMCs of DOCA-salt or 2K1C rats,61 whereas in mice these procedures induce an up-regulation of both Cx43 and Cx37 in aortic VSMCs.41 It has, therefore, been suggested that Cx43 is particularly sensitive to the haemodynamic changes that are associated with both models of hypertension rather than being sensitive to renin that displays opposite changes in these two animals models. This attractive suggestion has, however, been undermined by the fact that Cx43 expression appeared decreased in aortas from rats suffering from l-NAME-induced hypertension.62,63 Thus, haemodynamic changes per se do not necessarily lead to increased expression of Cx43 in aortic VSMCs. Importantly, decreased Cx43 expression in l-NAME-induced hypertension was fully reversed by a 7-day treatment with the non-selective beta-adrenergic blocker carvedilol, whereas treatment with the selective beta1-receptor antagonist atenolol only partially reversed the effects of hypertension on Cx43 expression (although the reduction in blood pressure was similar between the treatments), suggesting a blood pressure-independent role for beta2-receptor agonism on Cx43 expression in VSMCs. Of note, Cx40 expression was not altered by hypertension nor drug treatment in any of the groups.63
6. Connexins in rat and mouse models of genetic hypertension
In addition to studies focusing on changes in connexin expression in models that depend on pharmacological (e.g. l-NAME-induced hypertension) or surgical (e.g. the 2K1C model) interventions to induce hypertension, connexin expression has also been studied in spontaneously hypertensive rats (SHR). The endothelium lining caudal arteries isolated from 3-week-old (i.e. the pre-hypertensive period) SHR or Wistar Kyoto (WKY) control rats expresses similar amounts of Cx37 and Cx43, whereas the expression of Cx40 is significantly lower in SHR ECs.64 Moreover, the expression of Cx37, Cx40, and Cx43 was not affected by age in the WKY arteries. However, compared with arteries isolated from 3-week-old pre-hypertensive SHR rats, the expression of Cx37, Cx40, and Cx43 was significantly decreased in the arterial endothelium of 12-week-old SHR.64 A more recent study from the same group focused on the expression of connexins in the aortic wall of pre-hypertensive and hypertensive SHR compared with normotensive WKY.65 In this study, no differences were detected between SHR and WKY aortas in the pre-hypertensive period. However, aortas from hypertensive rats displayed reduced endothelial Cx37 and Cx40 but increased expression of Cx37 and Cx43 in the media.65 Mechanistically, treatment of SHR using an inhibitor of the angiotensin-converting enzyme (ACE) normalized blood pressure and connexin expression.65 However, since the study did not include a control group in which blood pressure was lowered using an aspecific vasorelaxant (e.g. hydralazine) it is unclear whether this effect of ACE inhibition was a direct effect on connexin expression or an indirect effect caused by a lower blood pressure. Indeed, an earlier study focusing on the molecular mechanism that regulates the expression of connexins in mesenteric arteries from SHR and WKY rats66 showed that endothelial Cx37 and Cx40 expression was significantly lower, whereas Cx43 was significantly higher in SHR compared with WKY mesenteric arteries. In this study, angiotensin receptor (AT) blockage reduced the blood pressure of SHR, increased the expression of both Cx37 and Cx40, and decreased the expression of Cx43. However, the combination of hydralazine and hydrochlorothiazide lowered the blood pressure to a similar extent, but did not correct the expression of either Cx37 or Cx40,66 thus ruling out a direct effect of lowered blood pressure on connexin expression. Finally, connexin abundance has also been studied in the renal resistance arteries of SHR and WKY rats.67 At this location, Cx40 and Cx43 expression was similar for both strains of rats. However, Cx37 was 2.4-fold decreased in SHR renal resistance arteries. Renal resistance arteries from normotensive Sprague–Dawley rats displayed equal Cx37 expression compared with SHR arteries suggesting that the decreased Cx37 expression in the SHR arteries was not due to the difference in blood pressure but was likely due to differences between the strains of rats. Finally, the effect of hypertension on connexin expression was studied in Cx40−/− mice.12,41,68,69 These mice are hypertensive and are characterized by an increased expression of Cx37, Cx43, and Cx45 in aortic VSMCs.41 Intriguingly, the expression of Cx37 in ECs from Cx40−/− mice is reduced,10,41,70 possibly pointing to a common pathway for Cx40 and Cx37 in the transport towards the plasma membrane. In contrast, the expression of Cx37 and Cx43 in renin-secreting cells does not seem to be affected by Cx40 deficiency.68
7. Role of Cx40 in hypertension
Despite all the above-mentioned data associating hypertension with changes in connexin expression, evidence that connexins are actually implicated in hypertensive disease was lacking for a long time and came only from studies of Cx40−/− mice.12,41,68,69 It appeared that Cx40 expression in renin-secreting cells in the kidney regulates their secretion of renin (for an excellent review, see Bosco et al.32). Indeed, mice ubiquitously deficient for Cx40 but also mice with specific deficiency of Cx40 in renin-secreting cells display increased renin plasma concentrations and are markedly hypertensive.68,71 Moreover, Cx40−/− mice treated with an AT1 receptor antagonist or with an ACE inhibitor show reduced blood pressure despite the fact that in these animals renin plasma levels are even further increased (compared with untreated Cx40−/− mice).68,71 Interestingly, five somatic mutations in GJA5, the human gene coding for Cx40, were reported in 2006.72 One of these mutations, coding for an Ala96→Ser substitution, results in the expression of a protein that localizes efficiently to the junctional plaque but is unable to form functional channels.72 Surprisingly, the expression of this Cx40 A96S mutation in C57BL6 mice caused renin-dependent hypertension characterized by similar blood pressure increases as observed in Cx40−/− animals.68,71,73 This suggests that actual GJIC between renin-producing cells may be required for Cx40-mediated control of renin release. However, a study from Kurtz et al.69 shows that the mechanism by which Cx40 deficiency leads to increased renin levels in mice lies in the structural changes that can be observed in the juxtaglomerular apparatus of Cx40−/− mice and not a reduced amount of GJIC communication.
GJA5 was cloned in 199474 and it was thereafter shown that mutations in the coding region of this gene are associated with various cardiac arrhythmias. Whether mutations in the coding region of GJA5 such as the Cx40 A96S are associated with hypertension in humans is currently unknown. Genetic polymorphisms in the human Cx40 gene promoter leading to reduced Cx40 expression have, however, been be associated with hypertension already in 2006.75 However, these results could not be confirmed in a recent study.76 Thus, although the exact molecular mechanism is not entirely clear and human data regarding the effect of mutations in the coding region of GJA5 are missing, studies of genetically altered mice have provided substantial evidence for a role of Cx40 in renin-dependent hypertension. Of note, several studies have shown that the increased blood pressure in Cx40−/− mice cannot be fully reversed by treating the animals with either an AT1 antagonist or an ACE inhibitor.68,71,77 Thus, additional Cx40-dependent pathways may be implicated in the regulation of blood pressure as well. In this regard, the fact that resistance arteries from Cx40−/− mice display impaired conducted dilatations and irregular vasomotion, possibly leading to increased peripheral vascular resistance, is of particular interest.77–79
8. Effect of afterload on the heart
In the beating heart, cardiomyocytes are subjected to a constant cycle of stretch and contraction. These mechanical forces regulate overall cell and organ function, both in health and in disease. The coupling of the contractile machinery of one cell with that of its neighbours is essential both for effective contraction, and for preservation of the elastic properties of the cardiac muscle. Molecular complexes (desmosomes, adherens junctions, and area composita) integrate at the site of cell contact (the intercalated disc) to provide a cell–cell mechanical continuum. Also at the intercalated disc, gap junctions allow for electrical synchrony between cells (Figure 2). When this electrical synchrony is disrupted, as seen in disease states causing a reduced and heterogeneous expression of Cx43, it predisposes to electrical re-entry and ultimately to the development of possibly fatal arrhythmias. While gap junctions and mechanical junctions were originally described as separate and independent entities, present data suggest that molecules classically defined as belonging to one group (e.g. desmosomes) can greatly affect the integrity of the other (e.g. gap junctions). The mechanisms responsible for this cross-talk are a subject of intense research in the field.
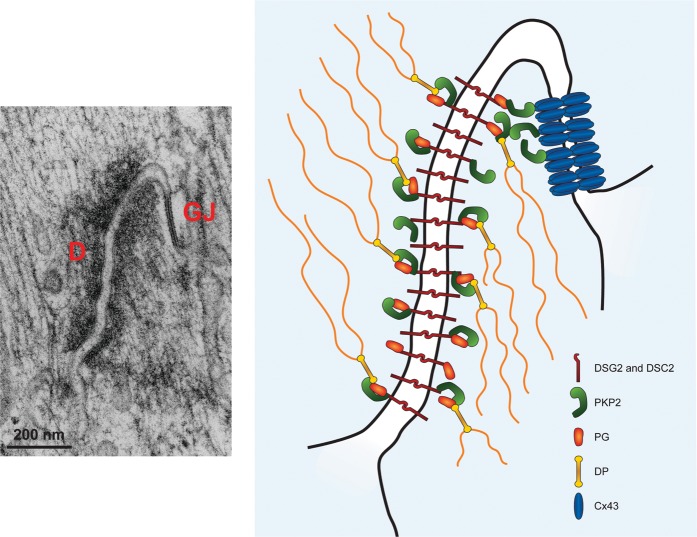
Figure 2.
Ultrastructure of the cardiac intercalated disc. Left: transmission electron microscopy image of adult murine heart showing the close juxtaposition of a desmosome to a gap junction. Right: schematic drawing of potential interaction between plakophilin-2 and Cx43 at the intercalated disc. D, desmosome; GJ, gap junction; DSG2, desmoglein-2; DSC2, desmocollin-2; PKP2, plakophilin-2; PG, plakoglobin; DP, desmoplakin. Electron microscopy image reproduced from Delmar and Liang111 with permission from Elsevier.
The response of cardiomyocytes to mechanical load has been extensively studied. Using monolayers of neonatal rat ventricular myocytes (NRVMs), Zhuang et al.80 showed that unidirectional pulsatile stretch up-regulates Cx43 expression. This connexin up-regulation was accompanied by an increase in conduction velocity. Separate studies showed an early and transient increase in Cx43 mRNA in cardiomyocytes subjected to pulsatile stretch.81,82 Thus, the regulation of Cx43 gene expression could underlie the observed induction of this protein by pulsatile stretch. However, a reduction of Cx43 protein degradation following stretch could also be involved. In fact, it seems that stretch is able to reduce contractile protein turnover in NRVMs under specific conditions of stretch degree and orientation.83 Whether a similar effect of stretch on Cx43 protein turnover also takes place remains to be demonstrated. Several pathways seem to be involved in the regulation of Cx43 expression by mechanical forces. NRVMs release autocrine–paracrine factors such as angiotensin II (Ang II), endothelin-1, vascular endothelial growth factor (VEGF), and transforming growth factor-β (TGF-β).82,84–87 Cx43 can be induced by exposure to VEGF or TGF-β and, more importantly, stretch-dependent induction of Cx43 expression can be abolished by antibodies directed against VEGF or TGF-β.88 In fact, early stretch-induced up-regulation of Cx43 seems to be partially mediated by VEGF, which acts downstream of TGF-β.88 Similarly, Ang II was shown to induce Cx43 expression in vitro, and stretch-dependent induction of Cx43 can be prevented by exposure to AT1 receptor antagonists such as losartan.82 It, thus, appears that several soluble factors that are released upon pulsatile stretch may promote GJIC between cardiomyocytes.
In addition to Cx43, short periods of pulsatile stretch were demonstrated to up-regulate mechanical junction proteins such as N-cadherin, plakoglobin, and desmoplakin at cell–cell junctions.80,89 Moreover, the degree of stretch and the micro-architecture of the cell–cell contact determine the formation of cell–cell adhesions. Indeed, pairs of NRVMs cultured on deformable substrate show variable expression of mechanical junction proteins depending on their shape (length-to-width ratio) and the stiffness of the substrate.90 Interestingly, studies of freshly dissociated adult rat ventricular myocytes that are allowed to reform cell–cell contact in culture showed that the formation of adherens junctions at the intercalated disc is a pre-requisite for Cx43 gap junction formation.89 In general, gap junction formation is seen as a process that occurs after mechanical coupling between cells (see also Meyer et al.91). It, thus, appears that, in vitro, mechanical stretch can also affect Cx43 expression by modifying the expression and localization of mechanical junction proteins.
The overall consensus of in vitro studies is that mechanical stretch favours Cx43 expression and GJIC in cardiomyocytes. Numerous studies of animal models and human patients have aimed to investigate the effect of stretch in vivo. Most studies involved increased left ventricular afterload, such as encountered with aortic stenosis in human or its animal model, transverse aortic constriction (TAC), or increased right ventricular afterload such as pulmonary hypertension. In an initial study, monocrotaline-induced pulmonary hypertension in rats resulted in the so-called remodelling of Cx43 expression, defined as a loss of gap junctions at the centre of intercalated discs and a redistribution of Cx43 to the lateral membrane of cardiomyocytes 4 weeks after induction.92 Interestingly, the remodelling of Cx43 was similar in rats subjected to TAC for 12 weeks.93 Thus, initial studies examining animals with severe pulmonary hypertension or TAC-induced left ventricular hypertrophy seemed to be in contradiction with in vitro studies. Subsequent studies that examined animals at different time-points after pressure overload found a transient increase in Cx43 expression up to 5 days after TAC, which was followed by a progressive decline in total expression.94 This biphasic response of Cx43 expression to increased mechanical stretch corroborates findings in patients suffering aortic stenosis; Kostin et al.95 showed that in patients with compensated aortic stenosis, Cx43 expression is increased compared with healthy subjects and the protein redistributes to the lateral membrane. However, in patients suffering from decompensated aortic stenosis, the total amount of Cx43 and the number of gap junctions are severely reduced, with a heterogeneous pattern of expression within the myocardium. One can thus postulate that the early increase and redistribution of Cx43 participates in the compensatory mechanism of the myocardium to overcome increased afterload. Once the compensatory mechanisms start to fail, Cx43 expression is reduced and becomes heterogeneous, which in turn can promote arrhythmias as seen in patients with decompensated aortic stenosis and, possibly, with pulmonary hypertension.96
The mechanisms leading to Cx43 remodelling are becoming increasingly understood. Dephosphorylation of Cx43 seems to be associated with the loss and lateralization of Cx43 in monocrotaline-induced pulmonary hypertension in rats as well as after TAC in mice.94,97 To determine whether Cx43 phosphorylation was responsible for gap junction remodelling, Remo et al.98 generated transgenic knock-in mice in which phosphomimetic glutamic acids replaced three serines in the C-terminal domain of Cx43. These serines are known to be phosphorylated by casein kinase 1δ and to play a role in gap junction assembly. Interestingly, these mice were resistant to gap junction remodelling after TAC and were less susceptible to the induction of ventricular arrhythmias, indicating that dephosphorylation of Cx43 is necessary for the remodelling of gap junctions after increased mechanical stretch.
The response to pharmacological blockers of the renin–angiotensin–aldosterone system also appears to be different between in vitro and in vivo studies. As mentioned above, Ang II increases Cx43 expression in vitro, and this effect is blocked by losartan. In contrast, losartan prevented Cx43 remodelling in rats after TAC,93 and spironolactone prevented or reversed dephosphorylation and lateralization of Cx43 in mice subjected to TAC.94 It is thus tempting to speculate that AT1 receptor antagonists and mineralocorticoid receptor antagonists may have a differential effect depending on the stage of the disease.
It is now increasingly recognized that Cx43 is part of a larger multimolecular complex involving several types of intercellular junction proteins. In fact, a recent study of adult sheep subjected to pulmonary hypertension demonstrated that in addition to Cx43, N-cadherin and desmosomal proteins (plakoglobin, desmocollin, desmoglein, desmoplakin, and plakophilin-2) were also remodelled to the lateral membrane of cardiomyocytes.99 The lateralization of Cx43 and mechanical junctions was associated with the microtubule-associated protein EB1 and the kinesin protein Kif5b, suggesting that the redistribution of these intercalated disc proteins is mediated by microtubule-dependent trafficking.
9. Effect of changes in the composition of myocardial mechanical junctions on connexins
Proper electrical and mechanical coupling between cardiomyocytes are crucial for a normal function of the heart. Alterations of both mechanical junction proteins and gap junction proteins are a prominent feature in many forms of heart disease such as ischaemia, hypertrophy, and various cardiomyopathies. There is growing evidence that the different junctional complexes at the intercalated disc behave in an integrated manner. Early in vitro studies of gap junction formation showed that preventing adherens junction formation in a tumour cell line through neutralizing antibodies impaired the assembly of gap junctions.91 As the formation of adherens junctions at the intercalated disc always precedes Cx43 gap junction formation in cardiomyocytes,89 it seems likely that any disease process involving adherens junctions would in turn disrupt Cx43 gap junctions. Similarly, disruption of desmosomes by silencing one of its components, PKP2, leads to a decrease and an internalization of Cx43 in NRVMs.100 In addition, pull-down experiments using tagged proteins showed that Cx43 and PKP2 could precipitate with each other. Recently, it was demonstrated that desmocollin-2 could bind to both Cx43 and plakoglobin, and could thus function as an adaptor protein between gap junction and desmosome.101 Therefore, it seems that gap junctions and desmosomes can interact in several ways, supporting the concept of a macromolecular complex of electrical and mechanical junctional proteins.
Several animal models have been used to investigate the consequences of altered mechanical junctions on connexins. First, a conditional heart-specific deletion of N-cadherin was shown to disrupt the intercalated disc, with the loss of adherens junctions and desmosomes. In this model, Cx43 (and Cx40 in the atria) was rapidly down-regulated in the myocardium after the loss of N-cadherin, which was associated with a reduction in ventricular conduction velocity.102,103 A whole class of proteins provides a mechanical link between the adherens junction or the desmosome and the cytoskeleton (actin filaments or intermediate filaments, respectively). Interestingly, alterations in these proteins also affect Cx43. For instance, deletion of the gene coding for αT-catenin, which binds β-catenin to actin, was shown to reduce Cx43 and PKP2 expression in cardiomyocytes.104 β-Catenin and plakoglobin, two other adaptor proteins, also affect Cx43. They both connect the adherens junction to actin filaments and seem to be able to substitute for each other. In addition, plakoglobin also binds to desmosomal cadherins. In that sense, mice deficient for β-catenin have a much milder cardiomyopathy than animals lacking plakoglobin, and mice deficient for both adaptor proteins suffer from much more severe disease. The expression of Cx43 seemed to parallel the severity of the phenotype, with a stepwise decrease in Cx43 from the control, to β-catenin-deficient, to plakoglobin-deficient, and finally to the double knock-out animals.105 Interestingly, this down-regulation of Cx43 preceded the onset of inducible arrhythmias, suggesting that additional mechanisms are necessary for the occurrence of arrhythmias.
Thus, in vitro, and in animal models, an intact mechanical junction between cardiomyocytes is necessary for a proper Cx43 expression and localization. The down-regulation of Cx43 caused by alterations in the mechanical junctions seems to be an early event in the progression of disease, and it predates overt arrhythmogenic events in animals. In humans, arrhythmogenic right ventricular cardiomyopathy (ARVC, also sometimes referred to as ARVD or AC) is considered a desmosomal disorder. Indeed, more than half of patients have a known mutation in one of the five genes coding for desmosomal proteins. Interestingly, a recent study investigating the expression of various junctional proteins in patients suffering from ARVC showed that both plakoglobin and Cx43 were down-regulated in all regions of the heart from the majority of ARVC patients, regardless of the underlying genetic disorder (11 out of 11 patients in Asimaki et al.106 and 14 out of 20 patients in Noorman et al.107). Other desmosomal proteins, such as desmoplakin and PKP2, were more variably expressed between patients. It, thus, appears that remodelling of Cx43 is a consistent feature in patients with ARVC, which could participate in the arrhythmogenic substrate of the disease. In fact, it is well known that remodelling of Cx43 occurs early in the progression of the disease, before overt structural damages.89,108,109 This is in contrast with other cardiac pathologies, such as ischaemic disease, hypertrophic cardiomyopathy, or dilated cardiomyopathy, where remodelling of Cx43 appears at later stages after considerable structural alterations.
Recent studies demonstrate that in addition to Cx43, the major α-subunit of the cardiac sodium channel, Nav1.5, is also remodelled in patients suffering from ARVC.107 In vitro studies showed that Nav1.5 is associated with PKP2 and that knockdown of PKP2 affects the properties of the sodium channel and reduces conduction velocity in cultured cardiomyocytes.110 Thus, in addition to inducing Cx43 remodelling, alterations in mechanical junction proteins could also affect the cardiac sodium current. This would constitute an additional mechanism underlying arrhythmias in ARVC.
In conclusion, physiological Cx43 expression in the myocardium is dependent on the proper expression and localization of mechanical junction proteins at the intercalated disc. Cx43 is consistently affected in arrhythmogenic diseases caused by mutations in mechanical junction proteins.
10. Summary
It has been well established that endothelial gap junctions are sensitive to shear stress, which changes their relative connexin composition. While Cx37 is up-regulated by HLSS and Cx43 by OSS, arterial Cx40 seems relatively insensitive to shear stress. These differences in connexin expression create endothelial communication compartments that may play a role in disease development, i.e. that may determine location of atherosclerotic lesions, but that may also participate in physiological processes such as valve formation. The effect of mechanical stretch as imposed by increased afterload on the heart is fairly established as well. Stretch seems to first induce a compensatory increase of Cx43 in cardiomyocytes, which is followed by a reduction and remodelling of Cx43 and associated mechanical junction proteins. Whether there is reciprocal regulation between mechanical and gap junctions in ECs and VSMCs is unclear at this moment. Moreover, with regard to hypertension, the effects on connexins seem to depend very much on the model studied, and data remain inconsistent even when the same model was used. Importantly, connexin expression is normalized by pharmacologically blocking the renin–angiotensin system in several models of systemic hypertension, making this an exciting new target for future drug development.
There is growing interest in the study of the inter-relation between mechanical forces, mechanical junctions, and connexin expression in the cardiovascular system. In the heart, the idea that the loss of Cx43 from the intercalated disc may increase the susceptibility to arrhythmias is quite straightforward. However, the consequences of an increase in Cx43 expression, as seen in the early stages after increased afterload, are less obvious. One can hypothesize that in addition to increased electrical coupling, the induction of Cx43 expression could also favour the transfer of larger molecules such as metabolites, siRNA,2 or miRNA3 between cardiomyocytes that are subjected to increased strain. This could lead to the homogeneous myocyte hypertrophy seen in compensated aortic stenosis. Interestingly, Cx43 is also up-regulated in VSMCs of arteries subjected to hypertension. One could imagine a similar compensatory mechanism in response to increased stretch, leading to increased resistance of the arteries to blood pressure. Regulation of connexins by shear stress seems to be a consistent feature, independently of the vascular bed. Whereas a function for Cx37 induction has been demonstrated in lymphatic valve development, the consequences of shear stress-dependent connexin regulation in blood vessels or in other regions of lymphatic vessels are only hypothetical so far. The main limitation resides in identifying the nature of the molecules diffusing through endothelial gap junctions. It is thought provoking that Cx43 and Cx37, which form gap junction channels with very different permeabilities, are inversely regulated by shear stress in ECs. It suggests that shear stress patterns, by modulating the ratio of Cx37 and Cx43, tightly regulate the permeability of endothelial gap junctions to distinct molecules. The precise identification of such molecules would greatly advance the understanding of the role of the synchronization induced by mechanotransduction.
Finally, long-standing questions remain as to the nature of the messages, the relevance of connexin diversity, and their importance on disease initiation or progression. Connexins have been seen as exciting pharmacological targets for years and yet, they have been hidden from the touch of selective drugs. Although the contribution of connexins to the maintenance of normal cellular functions is now relatively well understood, the specific contribution of connexins to disease is less clear. Whether it is in the blood vessels or in the heart, there is growing evidence that cross-talk of connexins with molecules involved in mechanical events is not coincidental or irrelevant, but rather the result of the fact that these molecules all live within molecular complexes where connexins are not only makers of gap junctions or, say, plakophilin-2 a maker of desmosomes. They are all part of a protein-interacting network that at the end regulates adhesion, cell–cell coupling, and excitability in the cardiovascular system.
Funding
This work was supported by grants from the Swiss National Science Foundation (310030_143343/1 and CRSII3_141811/1 to B.R.K.) and from the Fondation Leenaards (to B.R.K.). In addition, this work was supported by grants from the National Institutes of Health (O1-HL106632 and RO1-GM57691 to M.D.) and from the Leducq Foundation Transatlantic Network (to M.D.).
Conflict of interest: none declared.
References
- 1.Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev. 2003;83:1359–1400. doi: 10.1152/physrev.00007.2003. [DOI] [PubMed] [Google Scholar]
- 2.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, et al. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568(Pt 2):459–468. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lim PK, Bliss SA, Patel SA, Taborga M, Dave MA, Gregory LA, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–1560. doi: 10.1158/0008-5472.CAN-10-2372. [DOI] [PubMed] [Google Scholar]
- 4.Neijssen J, Herberts C, Drijfhout JW, Reits E, Janssen L, Neefjes J. Cross-presentation by intercellular peptide transfer through gap junctions. Nature. 2005;434:83–88. doi: 10.1038/nature03290. [DOI] [PubMed] [Google Scholar]
- 5.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 6.Kwak BR, Mulhaupt F, Veillard N, Gros DB, Mach F. Altered pattern of vascular connexin expression in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:225–230. doi: 10.1161/hq0102.104125. [DOI] [PubMed] [Google Scholar]
- 7.van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol. 1999;112:479–486. doi: 10.1007/s004180050432. [DOI] [PubMed] [Google Scholar]
- 8.Yeh HI, Chang HM, Lu WW, Lee YN, Ko YS, Severs NJ, et al. Age-related alteration of gap junction distribution and connexin expression in rat aortic endothelium. J Histochem Cytochem. 2000;48:1377–1389. doi: 10.1177/002215540004801008. [DOI] [PubMed] [Google Scholar]
- 9.Wong CW, Christen T, Roth I, Chadjichristos CE, Derouette JP, Foglia BF, et al. Connexin37 protects against atherosclerosis by regulating monocyte adhesion. Nat Med. 2006;12:950–954. doi: 10.1038/nm1441. [DOI] [PubMed] [Google Scholar]
- 10.Chadjichristos CE, Scheckenbach KE, van Veen TA, Richani Sarieddine MZ, de Wit C, Yang Z, et al. Endothelial-specific deletion of connexin40 promotes atherosclerosis by increasing CD73-dependent leukocyte adhesion. Circulation. 2010;121:123–131. doi: 10.1161/CIRCULATIONAHA.109.867176. [DOI] [PubMed] [Google Scholar]
- 11.Kwak BR, Veillard N, Pelli G, Mulhaupt F, James RW, Chanson M, et al. Reduced connexin43 expression inhibits atherosclerotic lesion formation in low-density lipoprotein receptor-deficient mice. Circulation. 2003;107:1033–1039. doi: 10.1161/01.cir.0000051364.70064.d1. [DOI] [PubMed] [Google Scholar]
- 12.Alonso F, Boittin FX, Beny JL, Heafliger JA. Loss of connexin40 is associated with decreased endothelium-dependent relaxations and eNOS levels in the mouse aorta. Am J Physiol Heart Circ Physiol. 2010;299:H1365–H1373. doi: 10.1152/ajpheart.00029.2010. [DOI] [PubMed] [Google Scholar]
- 13.Pfenniger A, Derouette JP, Verma V, Lin X, Foglia B, Coombs W, et al. Gap junction protein Cx37 interacts with endothelial nitric oxide synthase in endothelial cells. Arterioscler Thromb Vasc Biol. 2010;30:827–834. doi: 10.1161/ATVBAHA.109.200816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angelillo-Scherrer A, Fontana P, Burnier L, Roth I, Sugamele R, Brisset A, et al. Connexin 37 limits thrombus propensity by downregulating platelet reactivity. Circulation. 2011;124:930–939. doi: 10.1161/CIRCULATIONAHA.110.015479. [DOI] [PubMed] [Google Scholar]
- 15.Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Looft-Wilson RC, Billaud M, Johnstone SR, Straub AC, Isakson BE. Interaction between nitric oxide signaling and gap junctions: effects on vascular function. Biochim Biophys Acta. 2011;1818:1895–1902. doi: 10.1016/j.bbamem.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandow SL, Senadheera S, Bertrand PP, Murphy TV, Tare M. Myoendothelial contacts, gap junctions, and microdomains: anatomical links to function? Microcirculation. 2012;19:403–415. doi: 10.1111/j.1549-8719.2011.00146.x. [DOI] [PubMed] [Google Scholar]
- 18.Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- 19.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 20.Inai T, Shibata Y. Heterogeneous expression of endothelial connexin (Cx) 37, Cx40, and Cx43 in rat large veins. Anat Sci Int. 2009;84:237–245. doi: 10.1007/s12565-009-0029-y. [DOI] [PubMed] [Google Scholar]
- 21.Chang CJ, Wu LS, Hsu LA, Chang GJ, Chen CF, Yeh HI, et al. Differential endothelial gap junction expression in venous vessels exposed to different hemodynamics. J Histochem Cytochem. 2010;58:1083–1092. doi: 10.1369/jhc.2010.956425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanady JD, Dellinger MT, Munger SJ, Witte MH, Simon AM. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Dev Biol. 2011;354:253–266. doi: 10.1016/j.ydbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabine A, Agalarov Y, Maby-El Hajjami H, Jaquet M, Hagerling R, Pollmann C, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Dev Cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Munger SJ, Kanady JD, Simon AM. Absence of venous valves in mice lacking Connexin37. Dev Biol. 2013;373:338–348. doi: 10.1016/j.ydbio.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Severs NJ, Bruce AF, Dupont E, Rothery S. Remodelling of gap junctions and connexin expression in diseased myocardium. Cardiovasc Res. 2008;80:9–19. doi: 10.1093/cvr/cvn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Dorado D, Rodriguez-Sinovas A, Ruiz-Meana M. Gap junction-mediated spread of cell injury and death during myocardial ischemia-reperfusion. Cardiovasc Res. 2004;61:386–401. doi: 10.1016/j.cardiores.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 27.van Kempen MJ, ten Velde I, Wessels A, Oosthoek PW, Gros D, Jongsma HJ, et al. Differential connexin distribution accommodates cardiac function in different species. Microsc Res Tech. 1995;31:420–436. doi: 10.1002/jemt.1070310511. [DOI] [PubMed] [Google Scholar]
- 28.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boon RA, Horrevoets AJ. Key transcriptional regulators of the vasoprotective effects of shear stress. Hamostaseologie. 2009;29:39–40. 41–3. [PubMed] [Google Scholar]
- 30.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Heiden K, Cuhlmann S, Luong le A, Zakkar M, Evans PC. Role of nuclear factor kappaB in cardiovascular health and disease. Clin Sci (Lond) 2010;118:593–605. doi: 10.1042/CS20090557. [DOI] [PubMed] [Google Scholar]
- 32.Bosco D, Haefliger JA, Meda P. Connexins: key mediators of endocrine function. Physiol Rev. 2011;91:1393–1445. doi: 10.1152/physrev.00027.2010. [DOI] [PubMed] [Google Scholar]
- 33.Pfenniger A, Chanson M, Kwak BR. Connexins in atherosclerosis. Biochim Biophys Acta. 2013;1828:157–166. doi: 10.1016/j.bbamem.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Weidmann S. The electrical constants of Purkinje fibres. J Physiol. 1952;118:348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnstone S, Isakson B, Locke D. Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol. 2009;278:69–118. doi: 10.1016/S1937-6448(09)78002-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. [DOI] [PubMed] [Google Scholar]
- 37.Inai T, Mancuso MR, McDonald DM, Kobayashi J, Nakamura K, Shibata Y. Shear stress-induced upregulation of connexin 43 expression in endothelial cells on upstream surfaces of rat cardiac valves. Histochem Cell Biol. 2004;122:477–483. doi: 10.1007/s00418-004-0717-6. [DOI] [PubMed] [Google Scholar]
- 38.Cowan DB, Lye SJ, Langille BL. Regulation of vascular connexin43 gene expression by mechanical loads. Circ Res. 1998;82:786–793. doi: 10.1161/01.res.82.7.786. [DOI] [PubMed] [Google Scholar]
- 39.DePaola N, Davies PF, Pritchard WF, Jr., Florez L, Harbeck N, Polacek DC. Spatial and temporal regulation of gap junction connexin43 in vascular endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci USA. 1999;96:3154–3159. doi: 10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwak BR, Silacci P, Stergiopulos N, Hayoz D, Meda P. Shear stress and cyclic circumferential stretch, but not pressure, alter connexin43 expression in endothelial cells. Cell Commun Adhes. 2005;12:261–270. doi: 10.1080/15419060500514119. [DOI] [PubMed] [Google Scholar]
- 41.Alonso F, Krattinger N, Mazzolai L, Simon A, Waeber G, Meda P, et al. An angiotensin II- and NF-kappaB-dependent mechanism increases connexin 43 in murine arteries targeted by renin-dependent hypertension. Cardiovasc Res. 2010;87:166–176. doi: 10.1093/cvr/cvq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bao X, Clark CB, Frangos JA. Temporal gradient in shear-induced signaling pathway: involvement of MAP kinase, c-fos, and connexin43. Am J Physiol Heart Circ Physiol. 2000;278:H1598–H1605. doi: 10.1152/ajpheart.2000.278.5.H1598. [DOI] [PubMed] [Google Scholar]
- 43.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–H374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kefaloyianni E, Coetzee WA. Transcriptional remodeling of ion channel subunits by flow adaptation in human coronary artery endothelial cells. J Vasc Res. 2011;48:357–367. doi: 10.1159/000323475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.White SJ, Hayes EM, Lehoux S, Jeremy JY, Horrevoets AJ, Newby AC. Characterization of the differential response of endothelial cells exposed to normal and elevated laminar shear stress. J Cell Physiol. 2011;226:2841–2848. doi: 10.1002/jcp.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfenniger A, Wong C, Sutter E, Chulmann S, Dunoyer-Geindre S, Mach F, et al. Shear stress modulates the expression of the atheroprotective protein Cx37 in endothelial cells. J Mol Cell Cardiol. 2012;53:299–309. doi: 10.1016/j.yjmcc.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 48.Cheng C, van Haperen R, de Waard M, van Damme LC, Tempel D, Hanemaaijer L, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–3698. doi: 10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 49.Vorderwulbecke BJ, Maroski J, Fiedorowicz K, Da Silva-Azevedo L, Marki A, Pries AR, et al. Regulation of endothelial connexin40 expression by shear stress. Am J Physiol Heart Circ Physiol. 2012;302:H143–H152. doi: 10.1152/ajpheart.00634.2011. [DOI] [PubMed] [Google Scholar]
- 50.Brisset AC, Isakson BE, Kwak BR. Connexins in vascular physiology and pathology. Antioxid Redox Signal. 2009;11:267–282. doi: 10.1089/ars.2008.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Buschmann I, Pries A, Styp-Rekowska B, Hillmeister P, Loufrani L, Henrion D, et al. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development. 2010;137:2187–2196. doi: 10.1242/dev.045351. [DOI] [PubMed] [Google Scholar]
- 52.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. 2009;34:1219–1263. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 54.Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J. 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 55.Shah SJ. Pulmonary hypertension. JAMA. 2012;308:1366–1374. doi: 10.1001/jama.2012.12347. [DOI] [PubMed] [Google Scholar]
- 56.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 57.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173:1023–1030. doi: 10.1164/rccm.200510-1668OC. [DOI] [PubMed] [Google Scholar]
- 58.Haefliger JA, Castillo E, Waeber G, Aubert JF, Nicod P, Waeber B, et al. Hypertension differentially affects the expression of the gap junction protein connexin43 in cardiac myocytes and aortic smooth muscle cells. Adv Exp Med Biol. 1997;432:71–82. doi: 10.1007/978-1-4615-5385-4_8. [DOI] [PubMed] [Google Scholar]
- 59.Haefliger JA, Castillo E, Waeber G, Bergonzelli GE, Aubert JF, Sutter E, et al. Hypertension increases connexin43 in a tissue-specific manner. Circulation. 1997;95:1007–14. doi: 10.1161/01.cir.95.4.1007. [DOI] [PubMed] [Google Scholar]
- 60.Watts SW, Webb RC. Vascular gap junctional communication is increased in mineralocorticoid-salt hypertension. Hypertension. 1996;28:888–893. doi: 10.1161/01.hyp.28.5.888. [DOI] [PubMed] [Google Scholar]
- 61.Haefliger JA, Demotz S, Braissant O, Sutter E, Waeber B, Nicod P, et al. Connexins 40 and 43 are differentially regulated within the kidneys of rats with renovascular hypertension. Kidney Int. 2001;60:190–201. doi: 10.1046/j.1523-1755.2001.00786.x. [DOI] [PubMed] [Google Scholar]
- 62.Haefliger JA, Meda P, Formenton A, Wiesel P, Zanchi A, Brunner HR, et al. Aortic connexin43 is decreased during hypertension induced by inhibition of nitric oxide synthase. Arterioscler Thromb Vasc Biol. 1999;19:1615–1622. doi: 10.1161/01.atv.19.7.1615. [DOI] [PubMed] [Google Scholar]
- 63.Yeh HI, Lee PY, Su CH, Tian TY, Ko YS, Tsai CH. Reduced expression of endothelial connexins 43 and 37 in hypertensive rats is rectified after 7-day carvedilol treatment. Am J Hypertens. 2006;19:129–135. doi: 10.1016/j.amjhyper.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 64.Rummery NM, McKenzie KU, Whitworth JA, Hill CE. Decreased endothelial size and connexin expression in rat caudal arteries during hypertension. J Hypertens. 2002;20:247–253. doi: 10.1097/00004872-200202000-00014. [DOI] [PubMed] [Google Scholar]
- 65.Rummery NM, Grayson TH, Hill CE. Angiotensin-converting enzyme inhibition restores endothelial but not medial connexin expression in hypertensive rats. J Hypertens. 2005;23:317–328. doi: 10.1097/00004872-200502000-00014. [DOI] [PubMed] [Google Scholar]
- 66.Kansui Y, Fujii K, Nakamura K, Goto K, Oniki H, Abe I, et al. Angiotensin II receptor blockade corrects altered expression of gap junctions in vascular endothelial cells from hypertensive rats. Am J Physiol Heart Circ Physiol. 2004;287:H216–H224. doi: 10.1152/ajpheart.00915.2003. [DOI] [PubMed] [Google Scholar]
- 67.Braunstein TH, Sorensen CM, Holstein-Rathlou NH. Connexin abundance in resistance vessels from the renal microcirculation in normo- and hypertensive rats. APMIS. 2009;117:268–276. doi: 10.1111/j.1600-0463.2009.02432.x. [DOI] [PubMed] [Google Scholar]
- 68.Krattinger N, Capponi A, Mazzolai L, Aubert JF, Caille D, Nicod P, et al. Connexin40 regulates renin production and blood pressure. Kidney Int. 2007;72:814–822. doi: 10.1038/sj.ki.5002423. [DOI] [PubMed] [Google Scholar]
- 69.Kurtz L, Schweda F, de Wit C, Kriz W, Witzgall R, Warth R, et al. Lack of connexin 40 causes displacement of renin-producing cells from afferent arterioles to the extraglomerular mesangium. J Am Soc Nephrol. 2007;18:1103–1111. doi: 10.1681/ASN.2006090953. [DOI] [PubMed] [Google Scholar]
- 70.Jobs A, Schmidt K, Schmidt VJ, Lubkemeier I, van Veen TA, Kurtz A, et al. Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension. 2012;60:1422–1429. doi: 10.1161/HYPERTENSIONAHA.112.201194. [DOI] [PubMed] [Google Scholar]
- 71.Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- 72.Gollob MH, Jones DL, Krahn AD, Danis L, Gong XQ, Shao Q, et al. Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med. 2006;354:2677–2688. doi: 10.1056/NEJMoa052800. [DOI] [PubMed] [Google Scholar]
- 73.Lubkemeier I, Machura K, Kurtz L, Neubauer B, Dobrowolski R, Schweda F, et al. The connexin 40 A96S mutation causes renin-dependent hypertension. J Am Soc Nephrol. 2011;22:1031–1040. doi: 10.1681/ASN.2010101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kanter HL, Saffitz JE, Beyer EC. Molecular cloning of two human cardiac gap junction proteins, connexin40 and connexin45. J Mol Cell Cardiol. 1994;26:861–868. doi: 10.1006/jmcc.1994.1103. [DOI] [PubMed] [Google Scholar]
- 75.Firouzi M, Kok B, Spiering W, Busjahn A, Bezzina CR, Ruijter JM, et al. Polymorphisms in human connexin40 gene promoter are associated with increased risk of hypertension in men. J Hypertens. 2006;24:325–330. doi: 10.1097/01.hjh.0000200512.40818.47. [DOI] [PubMed] [Google Scholar]
- 76.Pfenniger A, van der Laan SW, Foglia B, Dunoyer-Geindre S, Haefliger JA, Winnik S, et al. Lack of association between connexin40 polymorphisms and coronary artery disease. Atherosclerosis. 2012;222:148–153. doi: 10.1016/j.atherosclerosis.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 77.de Wit C, Roos F, Bolz SS, Pohl U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol Genomics. 2003;13:169–177. doi: 10.1152/physiolgenomics.00169.2002. [DOI] [PubMed] [Google Scholar]
- 78.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, et al. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 79.Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, et al. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92:793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- 80.Zhuang J, Yamada KA, Saffitz JE, Kleber AG. Pulsatile stretch remodels cell-to-cell communication in cultured myocytes. Circ Res. 2000;87:316–322. doi: 10.1161/01.res.87.4.316. [DOI] [PubMed] [Google Scholar]
- 81.Wang TL, Tseng YZ, Chang H. Regulation of connexin 43 gene expression by cyclical mechanical stretch in neonatal rat cardiomyocytes. Biochem Biophys Res Commun. 2000;267:551–557. doi: 10.1006/bbrc.1999.1988. [DOI] [PubMed] [Google Scholar]
- 82.Shyu KG, Chen CC, Wang BW, Kuan P. Angiotensin II receptor antagonist blocks the expression of connexin43 induced by cyclical mechanical stretch in cultured neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2001;33:691–698. doi: 10.1006/jmcc.2000.1333. [DOI] [PubMed] [Google Scholar]
- 83.Simpson DG, Majeski M, Borg TK, Terracio L. Regulation of cardiac myocyte protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res. 1999;85:e59–e69. doi: 10.1161/01.res.85.10.e59. [DOI] [PubMed] [Google Scholar]
- 84.Sadoshima J, Xu Y, Slayter HS, Izumo S. Autocrine release of angiotensin II mediates stretch-induced hypertrophy of cardiac myocytes in vitro. Cell, 1993;75:977–984. doi: 10.1016/0092-8674(93)90541-w. [DOI] [PubMed] [Google Scholar]
- 85.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. EMBO J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seko Y, Takahashi N, Shibuya M, Yazaki Y. Pulsatile stretch stimulates vascular endothelial growth factor (VEGF) secretion by cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1999;254:462–465. doi: 10.1006/bbrc.1998.9969. [DOI] [PubMed] [Google Scholar]
- 87.Ruwhof C, van Wamel AE, Egas JM, van der Laarse Cyclic stretch induces the release of growth promoting factors from cultured neonatal cardiomyocytes and cardiac fibroblasts. Mol Cell Biochem. 2000;208:89–98. doi: 10.1023/a:1007046105745. [DOI] [PubMed] [Google Scholar]
- 88.Pimentel RC, Yamada KA, Kleber AG, Saffitz JE. Autocrine regulation of myocyte Cx43 expression by VEGF. Circ Res, 2002;90:671–677. doi: 10.1161/01.res.0000014823.75393.4d. [DOI] [PubMed] [Google Scholar]
- 89.Saffitz JE, Kleber AG. Effects of mechanical forces and mediators of hypertrophy on remodeling of gap junctions in the heart. Circ Res. 2004;94:585–591. doi: 10.1161/01.RES.0000121575.34653.50. [DOI] [PubMed] [Google Scholar]
- 90.McCain ML, Lee H, Aratyn-Schaus Y, Kleber AG, Parker KK. Cooperative coupling of cell-matrix and cell-cell adhesions in cardiac muscle. Proc Natl Acad Sci USA. 2012;109:9881–9886. doi: 10.1073/pnas.1203007109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meyer RA, Laird DW, Revel JP, Johnson RG. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J Cell Biol. 1992;119:179–189. doi: 10.1083/jcb.119.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uzzaman M, Honjo H, Takagishi Y, Emdad L, Magee AI, Severs NJ, et al. Remodeling of gap junctional coupling in hypertrophied right ventricles of rats with monocrotaline-induced pulmonary hypertension. Circ Res. 2000;86:871–878. doi: 10.1161/01.res.86.8.871. [DOI] [PubMed] [Google Scholar]
- 93.Emdad L, Uzzaman M, Takagishi Y, Honjo H, Uchida T, Severs NJ, et al. Gap junction remodeling in hypertrophied left ventricles of aortic-banded rats: prevention by angiotensin II type 1 receptor blockade. J Mol Cell Cardiol. 2001;33:219–231. doi: 10.1006/jmcc.2000.1293. [DOI] [PubMed] [Google Scholar]
- 94.Qu J, Volpicelli FM, Garcia LI, Sandeep N, Zhang J, Marquez-Rosado L, et al. Gap junction remodeling and spironolactone-dependent reverse remodeling in the hypertrophied heart. Circ Res. 2009;104:365–371. doi: 10.1161/CIRCRESAHA.108.184044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kostin S, Dammer S, Hein S, Klovekorn WP, Bauer EP, Schaper J. Connexin 43 expression and distribution in compensated and decompensated cardiac hypertrophy in patients with aortic stenosis. Cardiovasc Res. 2004;62:426–436. doi: 10.1016/j.cardiores.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 96.Benoist D, Stones R, Drinkhill MJ, Benson AP, Yang Z, Cassan C, et al. Cardiac arrhythmia mechanisms in rats with heart failure induced by pulmonary hypertension. American journal of physiology. Heart Circ Physiol, 2012;302:H2381–H2395. doi: 10.1152/ajpheart.01084.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sasano C, Honjo H, Takagishi Y, Uzzaman M, Emdad L, Shimizu A, et al. Internalization and dephosphorylation of connexin43 in hypertrophied right ventricles of rats with pulmonary hypertension. Circ J. 2007;71:382–389. doi: 10.1253/circj.71.382. [DOI] [PubMed] [Google Scholar]
- 98.Remo BF, Qu J, Volpicelli FM, Giovannone S, Shin D, Lader J, et al. Phosphatase-resistant gap junctions inhibit pathological remodeling and prevent arrhythmias. Circ Res. 2011;108:1459–1466. doi: 10.1161/CIRCRESAHA.111.244046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chkourko HS, Guerrero-Serna G, Lin X, Darwish N, Pohlmann JR, Cook KE, et al. Remodeling of mechanical junctions and of microtubule-associated proteins accompany cardiac connexin43 lateralization. Heart Rhythm. 2012;9:1133–1140 e6. doi: 10.1016/j.hrthm.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res. 2007;101:703–711. doi: 10.1161/CIRCRESAHA.107.154252. [DOI] [PubMed] [Google Scholar]
- 101.Gehmlich K, Lambiase PD, Asimaki A, Ciaccio EJ, Ehler E, Syrris P, et al. A novel desmocollin-2 mutation reveals insights into the molecular link between desmosomes and gap junctions. Heart Rhythm. 2011;8:711–718. doi: 10.1016/j.hrthm.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kostetskii I, Li J, Xiong Y, Zhou R, Ferrari VA, Patel VV, et al. Induced deletion of the N-cadherin gene in the heart leads to dissolution of the intercalated disc structure. Circ Res. 2005;96:346–354. doi: 10.1161/01.RES.0000156274.72390.2c. [DOI] [PubMed] [Google Scholar]
- 103.Li J, Patel VV, Kostetskii I, Xiong Y, Chu AF, Jacobson JT, et al. Cardiac-specific loss of N-cadherin leads to alteration in connexins with conduction slowing and arrhythmogenesis. Circ Res. 2005;97:474–481. doi: 10.1161/01.RES.0000181132.11393.18. [DOI] [PubMed] [Google Scholar]
- 104.Li J, Goossens S, van Hengel J, Gao E, Cheng L, Tyberghein K, et al. Loss of alphaT-catenin alters the hybrid adhering junctions in the heart and leads to dilated cardiomyopathy and ventricular arrhythmia following acute ischemia. J Cell Sci. 2012;125(Pt 4):1058–1067. doi: 10.1242/jcs.098640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Swope D, Cheng L, Gao E, Li J, Radice GL. Loss of cadherin-binding proteins beta-catenin and plakoglobin in the heart leads to gap junction remodeling and arrhythmogenesis. Mol Cell Biol. 2012;32:1056–1067. doi: 10.1128/MCB.06188-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, et al. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2009;360:1075–1084. doi: 10.1056/NEJMoa0808138. [DOI] [PubMed] [Google Scholar]
- 107.Noorman M, Hakim S, Kessler E, Groeneweg J, Cox MG, Asimaki A, et al. Remodeling of the cardiac sodium channel, connexin 43, and plakoglobin at the intercalated disk in patients with arrhythmogenic cardiomyopathy. Heart Rhythm. 2012 doi: 10.1016/j.hrthm.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaplan SR, Gard JJ, Protonotarios N, Tsatsopoulou A, Spiliopoulou C, Anastasakis A, et al. Remodeling of myocyte gap junctions in arrhythmogenic right ventricular cardiomyopathy due to a deletion in plakoglobin (Naxos disease) Heart Rhythm. 2004;1:3–11. doi: 10.1016/j.hrthm.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 109.Gomes J, Finlay M, Ahmed AK, Ciaccio EJ, Asimaki A, Saffitz JE, et al. Electrophysiological abnormalities precede overt structural changes in arrhythmogenic right ventricular cardiomyopathy due to mutations in desmoplakin-A combined murine and human study. Eur Heart J. 2012;33:1942–1953. doi: 10.1093/eurheartj/ehr472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sato PY, Musa H, Coombs W, Guerrero-Serna G, Patino GA, Taffet SM, et al. Loss of plakophilin-2 expression leads to decreased sodium current and slower conduction velocity in cultured cardiac myocytes. Circ Res. 2009;105:523–526. doi: 10.1161/CIRCRESAHA.109.201418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Delmar M, Liang FX. Connexin43 and the regulation of intercalated disc function. Heart Rhythm. 2012;9:835–838. doi: 10.1016/j.hrthm.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsai ML, Watts SW, Loch-Caruso R, Webb RC. The role of gap junctional communication in contractile oscillations in arteries from normotensive and hypertensive rats. J Hypertens. 1995;13:1123–1133. doi: 10.1097/00004872-199510000-00007. [DOI] [PubMed] [Google Scholar]