Abstract
Atherosclerosis initiates at predictable focal sites and develops to a spatially regional disease with limited distribution. There is compelling evidence that links haemodynamics to the localized origin of atherosclerotic lesions. Arterial flow in vivo is unsteady, dynamically complex, and regionally variable. Sites susceptible to atherosclerosis near arterial branches and curves are associated with regions of disturbed blood flow that contain repetitive phases of flow reversal resulting in steep multidirectional temporal and spatial gradients of wall shear stresses. Endothelium in atherosusceptible regions relative to protected sites shows activation of endoplasmic reticulum (ER) stress and the unfolded protein response (UPR), the altered expression of pro-inflammatory Nuclear Factor kappa B (NFκB) and oxidant/antioxidant pathways, and low expression of major protective factors, notably endothelial nitric oxide synthase and Kruppel-like Factors KLF2 and KLF4. At some atherosusceptible locations, reactive oxygen species levels are significantly elevated. Here we describe flow-related phenotypes identified in steady-state in vivo and outline some of the molecular mechanisms that may contribute to pre-lesional atherosusceptibility as deduced from complementary cell experiments in vitro. We conclude that disturbed flow is a significant local risk factor for atherosclerosis that induces a chronic low-level inflammatory state, an adaptive response to ensure continued function at the expense of increased susceptibility to atherogenesis. Surprisingly, when challenged by short-term hypercholesterolaemia in vivo, atherosusceptible endothelial phenotype was resistant to greater pro-inflammatory expression, suggesting that sustained hyperlipidaemia is required to overcome these protective characteristics.
Keywords: Endothelial phenotype, Haemodynamics, Atherosclerosis, Inflammation, Genomicsv
1. Introduction
In mammals, large quantities of blood are transported through the branching geometry of the arterial system under pulsatile pressure. The interactions of blood flow with the vessel geometry create complex haemodynamic characteristics including heterogeneous spatial and temporal mechanical stresses on the vessel wall. As the vascular interface with flow-mediated shear stresses, the arterial endothelium senses changes in local haemodynamic characteristics and responds by initiating acute changes in artery wall vasomotion and chronic structural remodelling.1 This important repertoire of regulatory physiological responses ensures acute adjustments of the vascular system and facilitates development and growth. However, localized regions of highly disturbed arterial flow are associated with metabolic stress in the endothelium that sensitizes the cells to local inflammatory changes that favour a pathological outcome, the initiation and development of atherosclerosis.
A biological definition of the characteristics of ‘susceptibility’ in an in vivo context remains challenging. Often treated as the absence or lowering of protective pathways, it also involves the active induction of a pathological stressed state. Stress arises from complex physical deformation forces that modify endothelial mechanotransduction. Furthermore, blood cells and undesirable metabolites [e.g. reactive oxygen species (ROS)] are retained within recirculating eddies with the potential for cell damage. The endothelium adapts by adjusting the equilibria of multiple pathways, but in doing so the cells may be locally more susceptible to systemic atherogenic risk factors.
Differential endothelial phenotypes identified at the actual sites where lesions will or will not develop can help identify the mechanisms involved. Here, we outline flow-related endothelial phenotypes in vivo, identified initially by transcriptomics, miRNA-omics, and traditional biochemistry, in susceptible and protected arterial sites in domestic swine and mouse models. Complete sequencing of the swine genome has recently provided access to a wealth of data for structural, genomic, epigenomic, translational, post-translational, and functional research in a species that is highly relevant to human physiology and pathology.
2. Disturbed blood flow predicts atherogenesis
In vivo, a tangential flow force component, shear stress, acts at the arterial luminal surface in the direction of flow to induce cyclic deformation of the endothelial surface throughout each cardiac cycle. As has been described for many years, arterial endothelial alignment in vivo and in vitro generally follows the shear stress direction.1–3 All flow in arteries is unsteady. In regions spared of atherosclerosis, the blood surges during the cardiac cycle at an increasing then decreasing velocity as the contraction subsides resulting in unsteady but unidirectional laminar flow that is atheroprotective.
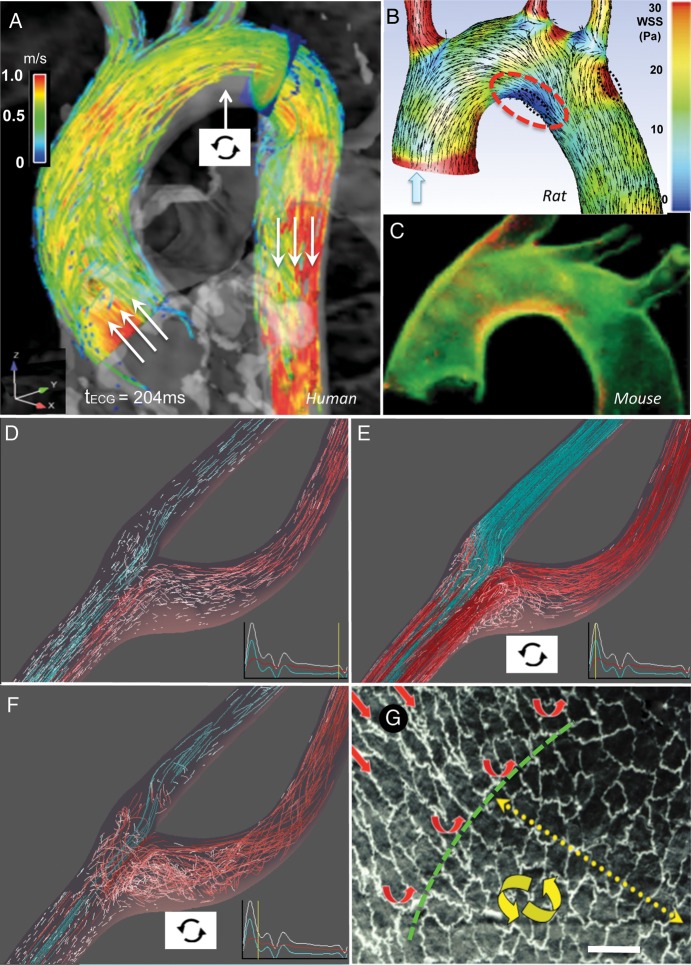
In contrast, atherosclerosis develops in spatially predictable regions (Figure 1 ) within large elastic and muscular distributing arteries near branches and bifurcations—where changes of vessel cross-sectional area occur over short distances—or as blood flow attempts to follow the tight inner curvature of the aortic arch. Within these regions, complicated flow patterns, collectively referred to as disturbed flow, originate from the separations of the mainstream to form recirculating eddies that contain non-uniform spatial and temporal gradients of shear stress. Disturbed flow typically includes periods of reciprocating flow reversal that create oscillating wall shear stresses, a characteristic notably absent in atheroprotected flow regions.
Figure 1.
(A–C) Flow disturbance at the inner curvature of the aortic arch. (A) Flow-velocity profile during systole with flow separation (arrow) in normal human. (B) Wall shear stress and velocity distributions (rat). (C) VCAM-1 immunostaining (red) in mouse aorta indicative of pro-inflammatory state. (D–F) Flow separation and disturbance in the normal human carotid sinus. (F) Endothelial cell morphology transitions from aligned (undisturbed flow) to polygonal (disturbed flow) adjacent to a branch artery in primate aorta. Bar 15 μm. From: (A) Markl M, Kilner PJ, Ebbers T. J Cardiovasc Magn Reson 2011;13:7 (Additional File 2).74 (A–C) Adapted from Bjorck et al. 2012.75 (B) Bjorck HM, Renner J, Maleki S, Nilsson SF, Kihlberg J, Folkersen L et al. PLoS One 2012;7:e52227.75 (D–F) Courtesy Professor D.A. Steinman, Biomedical Simulation Lab, University of Toronto. (G) Redrawn from Davies.1 (A, B, D–F) Computational fluid dynamic imaging from MRI.
The mean velocity of flow—and therefore the shear stress—is much lower in the separated flow sites; however, low shear stress is a gross oversimplification of the spatial and temporal complexity of the motion of fluid, blood cells, and molecules within the regions. Despite the complexity of disturbed flow, laminarity persists—fluid moving in parallel layers with frictional interactions between them—but with very different spatial and temporal relationships than exist outside of the regions. In extreme circumstances associated with congenital strictures, atherosclerotic stenoses, and cardiac valve dysfunction—where the flow path narrows and then expands—a chaotic state (turbulence) may occur during systole. Turbulence favours acute pro-coagulant risk and accelerated vessel-wall dysfunction leading to cardiovascular disease, e.g. downstream of aortic-valve stenosis.
3. Endothelial phenotypes in disturbed flow
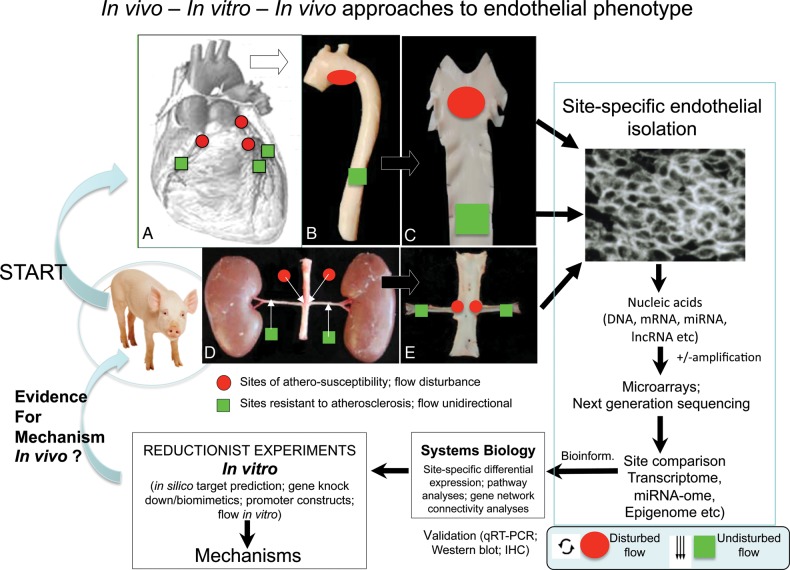
To generate an unbiased large-scale site-specific database in vivo, we performed ‘omic’ analyses (particularly transcriptomics and microRNA-omics) of the endothelium.4–7 The endothelium was isolated from disturbed and undisturbed flow regions (susceptible vs. protected) of pig arteries for nucleic acid extraction. Differential expression (mRNA; miRNA) databases were analysed for gene interactions, hierarchical structures, pathway associations, and other statistical analyses to identify site-specific endothelial interrelationships. The data were then used in the design of in vitro reductionist experiments under controlled conditions for insight into the mechanisms responsible. In the best circumstances, new mechanisms arising from the bench experiments were validated in vivo, thereby completing a circular strategy guided initially by the unbiased in vivo state (Figure 2). Such procedures have been used to identify characteristics of endothelial phenotypes in disturbed flow regions,4,5 to study the effects of risk factors such as hypercholesterolaemia on regional phenotypes,6,7 and to strengthen the in vivo relevance of the large body of literature reporting in vitro flow responses. Systems biology analyses have also been successfully applied to in vitro flow experiments;8 a point of discussion is whether the in vitro environment is adequately designed to capture sufficient characteristics (chemical as well as biomechanical) of the arterial environment.
Figure 2.
Outline of an in vivo ‘omics’ approach to endothelial phenotyping leading to in vitro experiments to investigate mechanisms which can then be probed in vivo.
4. Endothelial phenotype in atherosusceptible regions in vivo
We now outline several regulatory mechanisms in the endothelium for which current in vivo evidence is most robust in linking disturbed flow to an atherosusceptible phenotype.
4.1. The ‘atherosusceptible endothelium’
Long-known biological differences at disturbed flow arterial sites include increased endothelial permeability to plasma macromolecules,9 increased (but still very low) endothelial proliferation,10 and increased immuno-surveillance by monocytes that attach and migrate into the artery wall.11 These site-specific functional differences do not result in progression to significant inflammation unless additional systemic risk factors (e.g. hypercholesterolemia; hypertension; diabetes; smoking stress) are also present. The atherosusceptible phenotypes, therefore, may be considered to be in a sensitized—or ‘primed’12—pre-lesional state.
4.2. Systems biology approaches to localized endothelial phenotype in vivo
Prior to 2003, the association between endothelial phenotype and atherosusceptibility in vivo was inferred principally by extrapolation from in vitro flow experiments or by en face confocal imaging of immunostained candidate proteins within susceptible regions in situ. Notably, Cybulsky, Collins, and colleagues12 first reported increased in situ expression of several proteins associated with the pro-inflammatory nuclear factor κB (NFκB) pathway at such sites in mice. Lowered transcription of the vasculo-protective endothelial nitric oxide synthase (eNOS) were also noted.12,13 However, more comprehensive site-specific gene expression profiling in vivo in the mouse is limited by the small sample size available within haemodynamic regions of interest. The adult pig is an attractive model for comparative regional endothelial profiling. Linearly amplified RNA from freshly isolated endothelium of the inner aortic arch and descending thoracic aorta was hybridized to microarrays to profile differential gene expression reflecting the steady-state in vivo.4–8
4.3. Chronic low-level inflammation in atherosusceptible endothelium in swine
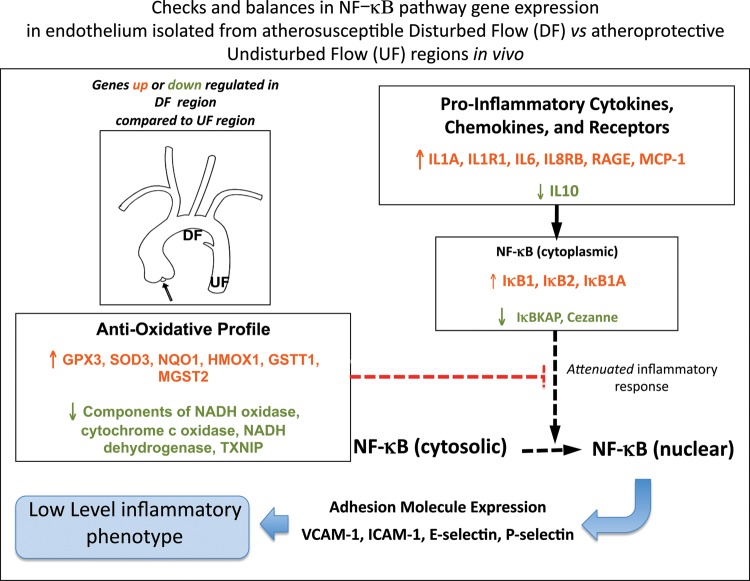
The lower expression of eNOS in disturbed flow regions reported in mice was confirmed in normal swine.8 Transcript profiles also showed the coexistence of enhanced pro- and anti-inflammatory gene expression patterns in susceptible endothelium.8 The differential expression of pro-inflammatory genes was primarily associated with NFκB pathway priming (Figure 3). In subsequent experiments, western blots showed a slightly elevated nuclear NFκB and modestly increased phosphorylation of IκBα,14 both suggestive of low-level inflammation and consistent with candidate gene and protein measurements in the mouse.12 Other prominent findings in the swine8 were the suppression of the protective endothelial transcription factors Krüppel-like factor (KLF) 2 and KLF4, and the differential expression of gap junctional gene connexin 43 that disrupted the signalling between cells in flow studies in vitro.15 On the protective side of the balance in the atherosusceptible cells were increased expression of glutathione peroxidase and other antioxidant genes. Increased procoagulant von Willebrand Factor expression was accompanied by protective enhancement of fibrinolysis-related gene expression.8 However, neither histological evidence of inflammation nor significant differences in the expression of vascular cell adhesion molecule-1 (VCAM-1), E-selectin, and intracellular cell adhesion molecule-1 (ICAM-1)—considered essential for transendothelial migration of monocytes into the arterial wall—were found in the disturbed flow regions in swine. Nor could measurable nuclear translocation of the NFκB p65 subunit, required to facilitate pro-inflammatory gene expression, be detected by en face immunocytochemistry,4 despite a modest increase measured by western blot. The coexistence of opposing mechanisms for pro/anti-inflammation in the same cells in vivo suggested that activation of inflammation is largely kept in check but with measurable biochemical evidence of low-level chronic NFκB activation.
Figure 3.
Differential gene expression in the atherosusceptible endothelium of normal swine. Pro-inflammatory NF-kB pathway activation coexisting with an enhanced antioxidative profile attenuates inflammation to a low level. Derived from Passerini et al.4
In vitro studies have corroborated and greatly extended the in vivo findings, principally through corollary experiments showing protection from inflammation by undisturbed laminar flow.16–18 In vivo, the local geometry of mouse carotid artery has been narrowed by cast or ligation to create flow disturbance resulting in ‘cause-effect’ inflammatory atherosclerosis19 and the identification of expected and new differential gene expression.20
4.4. Endoplasmic reticulum-stress and the unfolded protein response is a signature endothelial response to flow disturbance at multiple sites in vivo
The endothelial phenotype in vivo seems to be in dysequilibrium in areas of disturbed flow but without pathological consequences—suggesting successful adaptation to the prevailing haemodynamic conditions. Parallels with cell-adaptive responses to manage multiple kinds of cellular stress suggested to us that biosynthetic load may be increased during disturbed flow leading to endoplasmic reticulum (ER) stress.
4.4.1. ER stress induces unfolded protein response
ER stress occurs in response to excessive protein biosynthesis that interferes with normal peptide folding mechanisms in the ER lumen. If the rate of new synthesis exceeds its protein-folding capacity, unfolded protein response (UPR) activates a co-ordinated transcriptional up-regulation of ER chaperones and folding enzymes to promote the correct assembly of unfolded polypeptides and prevent incompletely folded proteins from aggregating.21
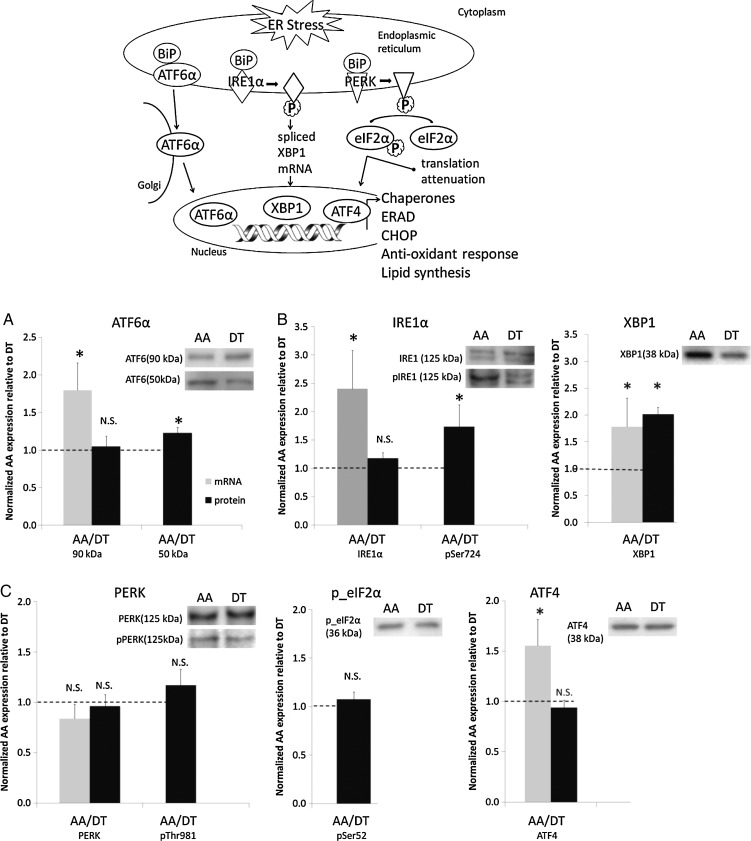
In the unstressed state, the ER chaperone-binding protein, BiP (also known as heat shock protein A5, HSPA5 and glucose-related protein 78, GRP78) binds to each of the three ER stress transducers. These are ER transmembrane proteins each having an ER-luminal domain for the sensing of unfolded proteins and a cytosolic domain for signalling. While bound, BiP maintains the inactive state of the transducers; however, when an imbalance occurs in the luminal flux of newly synthesized unfolded or misfolded peptides, UPR is activated (Figure 4). To bind unfolded/misfolded polypeptides in the ER lumen, BiP dissociates from the chaperones causing their phosphorylation. Activation of chaperones ATF6α (activating transcription factor 6α), IRE1α (inositol requiring kinase 1α), and PERK (protein kinase-like ER kinase) converge as transcriptional regulators in the nucleus to up-regulate ER chaperone and UPR transducer synthesis and to ubiquitinate unfolded/misfolded proteins for degradation through the proteosome. Both processes relieve ER stress accumulation of unfolded proteins and restore homeostasis. Failure to restore ER protein equilibrium to a normal range leads to apoptosis through transcriptional induction of CHOP (C/ERB homologous protein), inflammation through activation of NFκB, and the generation of ROS through excessive intracellular protein oxidation in the ER. ROS are also influential in initiating ER-stress since extracellular ROS itself promotes ER stress.
Figure 4.
Top panel: Schematic of ER-stress/UPR. (A–C) Activation of UPR chaperones ATF6α (A), IRE1α (B) but not of PERK (C) in atherosusceptible endothelium in vivo. From Civelek et al.23
4.4.2. Site-specific endothelial ER-stress/UPR in vivo is linked to atherosusceptibility
The association of ER stress with inflammatory genes in cultured endothelial cells following exposure to oxidized lipids was reported in 2006.22 Civelek et al.23 rediscovered the prominence of ER stress and the UPR in vivo during analyses of transcription profiles in a multi-site study in normal adult swine. Endothelium in the susceptible regions of the aortic arch, proximal brachiocephalic artery, aorto-renal branch region, and abdominal aorta were analysed relative to protected sites of the common carotid artery, descending thoracic aorta, and the distal renal artery. Each of the atherosusceptible regions is associated with complex disturbed blood flow. The most abundant common feature of the endothelium in atherosusceptibility regions was the up-regulation of genes associated with ER processing of proteins, ER stress, and the UPR. Differential gene-expression analysis identified 133 genes, 73% of which were involved in ER protein processing and folding and which form a highly connected and co-ordinated network up-regulated in the susceptible regions. Three independent and unbiased pathway mining approaches—Gene Ontology using the program DAVID, GSEA, and Ingenuity Pathway Analysis—identified ER stress and the UPR to be over-represented functional categories in atherosusceptible endothelium together with genes that function in protein folding, synthesis, and post-translational protein modification.23
To validate the genomics analyses, endothelial cell proteins were isolated from the aortic arch and descending thoracic aorta as well as from the atherosusceptible aorto-renal branch and the protected distal renal artery. At each atherosusceptible disturbed flow site, BiP transcript and/or protein expression was significantly up-regulated. Western blot demonstrated significantly elevated phospho-ATF6α (Figure 4A), phospho-IRE1α, and its target, spliced XBP-1 (Figure 4B). However, the third transducer pathway PERK was not activated (Figure 4C). A second study examined the disturbed and undisturbed flow regions of the coronary arteries, the endothelial origin of which is developmentally distinct from non-coronary arteries. Here, the role(s) of ER stress in the endothelium of coronary arteries may be particularly important. Civelek et al.5 used gene connectivity analyses to discriminate between coronary and non-coronary endothelial transcript profiles. Differential expression of 1300 endothelial genes were identified in the coronary artery endothelium with highest significance expressed in the gene modules enriched for biological functions related to ER stress and UPR, regulation of transcription and translation, and redox homeostasis. ROS load appeared to be heavier and antioxidant protection lower in coronary arteries. Overall, these studies, approached without pre-conceived expectations of differential expression of genes and proteins associated with ER stress/UPR, suggest that stresses associated with flow disturbance in vivo elicit partial activation of the UPR and that chronic ER stress is a signature for atherosusceptible endothelial phenotype in vivo.
Around the same time, connections between ER stress and the biomechanics of disturbed flow were reported from in vitro experiments.24,25
4.4.3. Flow characteristics in vitro induce BiP (GRP78; HSPA5) activation
Feaver et al. (2008)24 used in vitro flow to simulate human arterial shear stress waveforms. Atherosusceptible disturbed flow or atheroprotective laminar flow was applied to human endothelial cells. BiP (GRP78) was found to be significantly up-regulated in a sustained manner under atherosusceptible, but not atheroprotective flow up to 24 h. This response was dependent on both sustained activation of p38, as well as integrin α2β1. Increased BiP expression correlated with the activation of the ER stress sensing element promoter by atherosusceptible flow as a marker of the UPR. Shear stress regulation of BiP was through increased protein stability when compared with other flow-regulated proteins, such as connexin-43 and VCAM-1. Increased endothelial expression of BiP was also observed in atherosusceptible vs. atheroprotective regions of C57BL6 mice. These findings support a role for the haemodynamic environment in the preferential induction of ER stress and UPR in atherosusceptible regions.
4.4.4. Spliced XBP-1 chaperone pathway of UPR
Further in vitro evidence centres on spliced XBP-1 (sXBP-1), one of the three transduction arms of the UPR response. sXBP-1 encodes the XBP-1 transcription factor that translocates to the nucleus to activate selective pro-apoptotic target genes. Following the observation of endothelial expression of the XBP-1 pathway of UPR in the branching regions of ApoE−/− mice arteries and in atherosclerotic lesions that developed there, Zheng et al.25 reported that atherosusceptible flow waveforms induced XBP-1 splicing in cultured endothelial cells and that over-expression of (activated) sXBP-1 induced apoptosis. To extend the findings to an in vivo assay for atherogenesis, adenoviral-mediated over-expression of sXBP-1 was induced in an ApoE−/− murine aortic isograft model. In these animals, enhanced intimal hyperplasia and atherosclerosis developed in normally protected regions of the aorta, suggesting that when the XBP-1 UPR pathway is greatly over-stimulated, the adaptive protective function of UPR reverts to a pathological imbalance. While over-expression was not limited to the endothelium in the isograft model, the data are supportive of a prominent role for endothelial sXBP-1.
The role of ROS in arterial ER stress is further supported by the effects of oxidized and glycated lipoproteins which were recently shown to induce endothelial ER stress in vitro through a mechanism of sarcoplasmic/ER Ca2+ ATPase (SERCA) oxidation.26 This was inhibited by the activation of AMPK and evidence for such a mechanism in vivo was obtained by antioxidant administration resulting in the attenuation of impaired endothelial-mediated vasorelaxation. In ApoE−/− and ApoE/AMPK double knock-out mice fed a high-fat diet, antioxidants attenuated SERCA oxidation, ER stress, and atherosclerosis. A similar AMPK mechanism may influence ER stress in vascular smooth muscle,27 and it is clear that oxidation-related ER stress pathways are very important in macrophages for the ongoing development of atherosclerosis.28 These complementary approaches to endothelial ER stress provide compelling evidence for the existence of site-specific chronic adaptive UPR in atherosusceptible endothelium in vivo regulated through haemodynamics characteristics.
4.5. Regulation of eNOS function
Consideration of flow mechanisms responsible for the regulation of eNOS remains instructive despite most studies reporting responses to the transition from no-flow to flow in vitro.
4.5.1. Shear stress and eNOS phosphorylation
In contrast to Ca2+-dependent agonist stimulation of endothelial eNOS, the response to fluid shear stress does not require constant elevation in intracellular Ca2+. Rather the sensitivity of eNOS to intracellular Ca2+ is amplified by the phosphorylation of the enzyme. Of numerous eNOS phosphorylation sites, most is known about the functional consequences of phosphorylation of a serine residue (human eNOS sequence: Ser1177) in the reductase domain and a threonine residue (human eNOS sequence Thr495: bovine Thr497) within the calmodulin (CaM)-binding domain.
In cultured endothelial cells, Ser11 77 is rapidly phosphorylated after the application of fluid shear stress,29,30 VEGF,31,32 or bradykinin.33 The kinases involved vary with the stimuli applied and while shear stress elicits the phosphorylation of Ser1177 via protein kinase A,34,35 insulin, oestrogen, and VEGF mainly phosphorylate eNOS in the endothelial cells via Akt.31 The serine phosphorylation of eNOS alone is not sufficient to increase enzyme activity or NO production. This is because under basal conditions, the enzyme is constitutively phosphorylated on a threonine residue (human eNOS sequence: Thr495) within the CaM-binding domain and only when Thr495 is dephosphorylated can calmodulin bind to eNOS and NO production increase. Indeed most eNOS activating stimuli elicit the reciprocal regulation of Ser1177 and Thr495,32,33,36 although to different extents as receptor-dependent agonists elicit the almost complete dephosphorylation of this residue, while it remains largely phosphorylated in endothelial cells exposed to shear stress (in undisturbed flow). However, the status of this regulatory balance in endothelium subjected to disturbed flow has not been reported.
More recent studies suggest that the phosphorylation of an additional residue, i.e. Tyr 657 (human sequence) also acts like a brake to decrease NO generation.37 Indeed, this residue is phosphorylated in the endothelial cells exposed to shear stress in undisturbed flow but not to receptor-dependent agonists, and unlike the phosphorylation of Ser1177, the consequence of eNOS Tyr657 phosphorylation, is a complete loss of enzyme activity. A clue to why this particular tyrosine residue could have such dramatic effects can be found by considering the mechanisms known to regulate the activity of the neuronal NOS (nNOS), which was reported to be determined by preventing a large-scale swinging motion of the FMN domain to deliver electrons to the catalytic module in the holoenzyme essentially locking the FMN domain into its electron-accepting position, thus inhibiting enzyme activity.38 Since Tyr657 is the equivalent tyrosine residue in the human eNOS sequence, it is highly likely that its phosphorylation would be associated with a loss of NO production.
4.5.2. PYK2 and eNOS regulation
The kinase reported to phosphorylate eNOS Tyr657 is the focal adhesion protein tyrosine kinase, proline-rich tyrosine kinase 2 (PYK2), which is a rather unusual tyrosine kinase in that it contains no Src homology 2 (SH2) or SH3 domains. PYK2 is reported to mediate endothelial-cadherin-based cell–cell adhesion by tyrosine phosphorylation of β-catenin,39 has been implicated in regulating the organization of the actin cytoskeleton and can be activated by integrin stimulation (reviewed in Orr and Murphy-Ullrich40). Each of these mechanisms occurs in subcellular locations highly responsive in mechanotransduction.41 Whether or not Ca2+ influx alone, without concomitant integrin activation, increases PYK2 activity in endothelial cells remains controversial.42 PYK2 is also generally considered to be a redox-sensitive kinase that is activated following stimulation in situations associated with elevated oxidative stress,42,43 itself a hallmark of atherosusceptible sites and of most cardiovascular diseases and associated with impaired endothelial function. It is tempting to speculate that direct inactivation of eNOS via its tyrosine phosphorylation by PYK2 contributes to the phenomenon of endothelial dysfunction including sites of atherosusceptibility.
Circumstantial evidence to suggest that such a mechanism may exist includes thrombin-induced activation of PYK2 that leads to the activation of NFκB and its target genes, including VCAM-1 and MCP-1.44 Interestingly, PYK2 knock-down impaired the thrombin-induced activation of the IκB kinase and attenuated the release and the transcriptional capacity of RelA/p65.44 To what extent these effects can be explained by changes in NO production, which also inhibits NFκB activity,45 is unclear. Angiotensin-II-induced endothelial dysfunction is PYK2- and NADPH oxidase-dependent46 and the attenuated response to acetylcholine in arteries treated with a low concentration of H2O2 can be restored to control levels by the overexpression of the dominant negative PYK2 mutant.46 Moreover, aortic PYK2 was found to be activated as early as 7 days after starting a Western diet (i.e. before inflammatory cell activation) and PYK2 knockout mice bred onto an apolipoprotein E (ApoE)-deficient background demonstrated markedly decreased aortic lesions after 8 weeks of a Western diet.
Thus, it seems that shear stress elicits the phosphorylation of a tyrosine residue that negatively regulates eNOS activity and it is tempting to speculate that this event plays a key role in keeping NO output low and reducing the risk of co-factor, i.e. tetrahydrobiopterin depletion and eNOS uncoupling. Phosphorylation of the same residue in pathophysiological conditions may be responsible for decreasing NO generation. However, to what extent the latter mechanism competes with or complements the changes associated with eNOS uncoupling remain to be determined. Nevertheless, PYK2 may be an attractive therapeutic target as its inhibition would be expected to increase NO production.
4.6. Differential microRNA expression in atherosusceptible endothelium in vivo
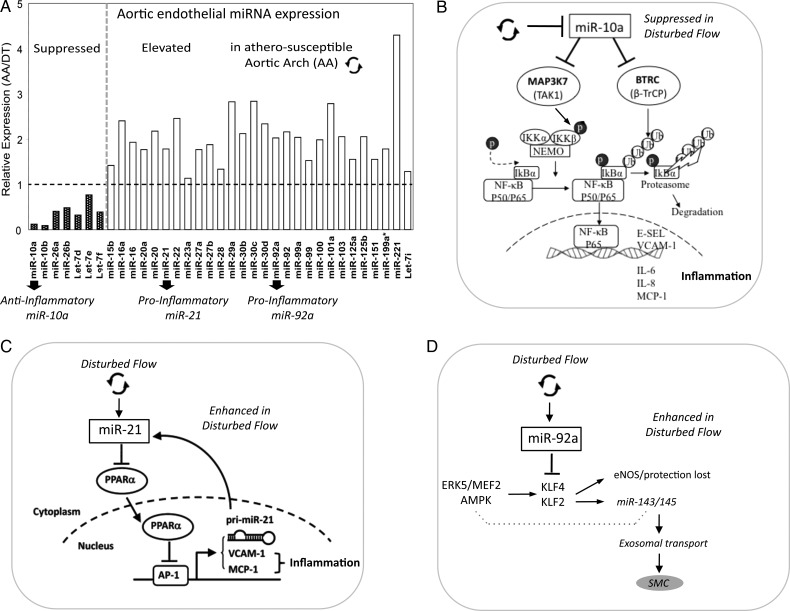
MicroRNAs (miRNAs) are highly-conserved, small non-coding RNAs of 19–26 nucleotides that post-transcriptionally regulate gene expression. Mammalian miRNAs usually bind to the 3′ untranslated region (3′UTR) of target mRNAs, promoting mRNA degradation and/or inhibiting translation of the protein-coding genes.47 Evolutionarily conserved Watson–Crick pairing between cognate mRNA 3′ UTR and miRNA 5′ regions centred on seed nucleotides (nt2–7) primarily determines miRNA target selection.48 Emerging evidence suggests that individual miRNAs fine-tune the synthesis of many genes and that miRNA-mediated proteomes are typically mirrored by transcriptomes.49,50 Given the widespread scope but modest repression of transcriptomes/proteomes by individual miRNAs, phenotypical consequences may arise by co-ordinated actions on multiple targets by single miRNAs or by an integrated regulatory effect on key pathways by multiple miRNAs.51 A subset of differentially expressed endothelial miRNAs was identified in atherosusceptible inner aortic arch compared with protected regions of thoracic aorta in normal swine,14,52 several of which show regulatory properties for NFκB pathway and the expression of transcription factors KLF2 and KLF4 (Figure 5A).
Figure 5.
(A) Differentially expressed miRNAs in atherosusceptible endothelium in vivo. (B–D) Three miRNAs promote inflammation, one by suppressed expression and two by enhanced expression. (B) miR-10a, (C) miR-21, (D) miR-92a. Modified from references (A and B)52 and Zhou et al. (C).56
4.6.1. miRNA-10a regulation of NFκB pathway
Endothelial miR-10a in swine aortic arch is suppressed by 70–80% compared with undisturbed flow regions of the thoracic aorta.52 A combination of genomic profiling, in silico analyses, miR manipulations, and molecular analyses in freshly isolated arterial endothelium and in cultured human endothelial cells (HAEC) demonstrated miR-10a as a novel negative regulator of NFκB signalling.52 Whole-genome transcriptome was profiled in cultured HAEC after the knock-down of endogenous miR-10a. Gene Set Enrichment Analysis (GSEA) identified IκB/NFκB-mediated inflammation as the top category of up-regulated biological processes. Phosphorylation of IκBα, a prerequisite for IκBα proteolysis and NFκB activation, was significantly up-regulated in miR-10a knock-down HAEC and was accompanied by increased nuclear translocation of NFκB p65. The inflammatory biomarkers monocyte chemoattractant protein-1 (MCP-1), interleukins IL-6, IL-8, VCAM-1, and E-selectin were elevated following miR-10a knock-down. Conversely, knock-in of miR-10a (a conservative 25-fold increase) inhibited the basal expression of VCAM-1 and E-selectin in HAEC. To investigate the molecular mechanisms underlying miR-10a suppression of pro-inflammatory molecules, genes in the canonical NFκB pathway were interrogated with miR-10a putative downstream targets predicted by TargetScan 5.1 that considers evolutionary conservation of miR seed sites.53 The in silico analyses identified two molecules: mitogen-activated protein kinase kinase kinase 7 (MAP3K7; also known as TAK1) gene, and beta-transducin repeat containing gene (BTRC; also known as β-TrCP). Both promote proteasomal degradation of IκBα and p65 nuclear translocation and contain evolutionarily conserved miR-10a binding sites in the 3′UTRs. MAP3K7 protein directly phosphorylates and activates IκB kinase β (IKKβ) stimulating phosphorylation of IκBs. BTRC recognizes phosphorylated IκBa and mediates phosphorylation-dependent ubiquitination leading to IκB proteolysis. Knock-down of endothelial miR-10a in HAEC significantly up-regulated MAP3K7 and BTRC expressions and the evidence of direct miR-10a binding to the 3′-UTR was demonstrated (Figure 5B).
The role of these newly identified molecules in vivo was investigated in the endothelium from atherosusceptible aortic arch and athero-protected descending thoracic aorta. MAP3K7, BTRC, phospho-IκBα, and nuclear p65 expression were all significantly up-regulated in atherosusceptible endothelium, where miR-10a expression is low. Collectively, these data demonstrate that differential expression of miR-10a contributes to the regulation of pro-inflammatory atherosusceptible endothelial phenotypes in vivo.
Regulation by miRs is multilayered; other mechanisms of control are emerging. Endothelial miR-126 has been shown to suppress VCAM-1 expression in vitro54 further implicating miRNAs in the control of endothelial responses to vascular perturbation; however, this has not been tested in vivo. Furthermore, flow-sensitive miR-663 and miR-21 have been demonstrated to provoke endothelial inflammation,55,56 the latter promoting activator protein 1 (AP-1) expression through the inhibition of PPARα (Figure 5C). eNOS has recently been reported as a direct target of miR-155.57 TNFα-induction of inflammation up-regulated miR-155 resulting in the reduced expression of eNOS, an effect that was reversed by simvastatin. However, differential expression of miR-155 in atherosusceptible endothelium has not been reported and this mechanism may require the development of atherosclerosis.
4.6.2. miRNA-92a regulation of transcription factors KLF2 and KLF4
We postulated that other differentially expressed miRs may target molecules known to be important in endothelial regions predisposed to atherosusceptibility or protection. The Kruppel-like family members KLF2 and KLF4 are endothelial transcription factors induced by (undisturbed, atheroprotective) laminar shear stress in vitro.58,59 KLF2 and KLF4 expression in vitro is suppressed by disturbed flow but this biomechanical regulation can be reversed by overexpression of KLF2 or KLF4, resulting in the induction of atheroprotective genes despite disturbed flow.60,61 KLF2 and KLF4 inhibit proinflammatory induction of VCAM-1 and E-selectin and attenuate leucocyte adhesion, induce thrombomodulin, and inhibit PAI-1 expression.20,60 Conversely, KLF4 deficiency enhanced the expression of cytokine-induced inflammatory VCAM-1, ICAM-1, and MCP-1 and suppressed eNOS,61 effects that were not mitigated by the simultaneous up-regulation of KLF2, suggesting some diversity in their regulatory mechanisms. Consistent with atherosusceptibility in vivo, KLF2 and KLF4 expression is low in endothelium within the regions of disturbed flow in swine and mouse.14,17
Both KLF2 and KLF4 induce eNOS, the up-regulation of which is itself anti-inflammatory and anti-coagulant.62 Transgenic experiments in mice demonstrated enhanced atherosclerosis in hemizygous KLF2- deficient mice.63 Recent endothelial-specific gain- and loss-of-function studies of human KLF4 in mice fed a high-fat diet demonstrated significant reduction in atherosclerosis lesion area in mice in which there was sustained expression of KLF4, while endothelial deletion of KLF4 notably augmented the atherosclerotic burden.61 KLF4 was also shown to interact with p300 transcriptional co-activator and does so in competition with other transcription factors (including NFκB) allowing differential transcriptional regulation through co-activator competition. This provides another layer of regulation that could result in the induction of some downstream KLF targets while inhibiting others.61
In contrast to miR-10a, miR-92a is expressed at a higher level in atherosusceptible disturbed flow regions in swine than in protected regions.14 In silico analyses predicted highly conserved binding sites in the 3′-untranslated region (3′UTR) of KLF4 for five miRs of the subset of site-specific differentially expressed miRs (miR-26a, -26b, -29a, -92a, and -103) and a single binding site for an miR-92a complex in the 3′UTR of KLF2. However, only miR-92a knock-down and knock-in resulted in responses of KLF4 and KLF2 expression in human arterial endothelial cells. Dual luciferase reporter assays demonstrated functional interactions of miR-92a with full-length 3′UTR sequences of both KLF4 and KLF2s and with the predicted binding elements. Two evolutionarily conserved miR-92a sites in KLF4 3′UTR and one site in KLF2 3′UTR were functionally validated.14 Knock-down of miR-92a in endothelial cells resulted in partial rescue from cytokine-induced inflammatory marker expression (MCP-1, VCAM-1, E-selectin, and eNOS), and the effects were attributable to enhanced KLF4 expression. Leucocyte-human arterial endothelial cell-adhesion experiments supported this conclusion.14 In atherosusceptible swine aortic arch endothelium, a site of elevated miR-92a expression, both KLFs were expressed at low levels consistent with miR-92a targeting (Figure 5D).
Wu et al.64 recently reported that an atheroprone flow waveform in vitro increased not only the level of endothelial miR-92a but also the association of miR-92a and KLF2 mRNA with both Ago1 and Ago2 proteins that are associated with the RNA-induced silencing complex. These findings are consistent with the above in vivo findings suggesting that KLF2 and KLF4 expression is regulated by the local haemodynamics through miR-92a (Figure 5D).
4.6.3. Flow-induced KLF2 regulates the expression of protective miR-143/145
In addition to direct miRNA regulation of KLF2 and KLF4 expression discussed earlier, miRNAs miR-143/145—downstream of KLF2—are themselves regulated by this transcription factor. miR-143/145 control the vascular smooth muscle phenotype (contractile/synthetic) during development;65 however, their expression is increased in endothelial cells exposed to shear stress in vitro.66 Furthermore, extracellular endothelium-derived microparticles (exosomes) secreted by miR-143/145-expressing endothelial cells were able to control target gene expression in co-cultured smooth-muscle cells (Figure 5D). Although the initial observation linked the endothelial expression of miR-143/145 with KLF2,66 a more recent study demonstrated that the shear stress-induced up-regulation of miR-143/145 can be attributed to the activation of the AMP-activated protein kinase (AMPK), the subsequent phosphorylation and activation of p53 and the post-transcriptional up-regulation of miR-143/145.18 These findings are significant inasmuch as metabolic diseases in general are associated with dysregulation of AMPK. Given that the AMPK is also activated by shear stress as well as an imbalance in the cellular AMP:ATP ratio, robust links are developing between haemodynamic stimuli, endothelial metabolism, post-transcriptional miRNA maturation, and the aspects of atherogenesis as a metabolic disease.
4.6.4. Interactions
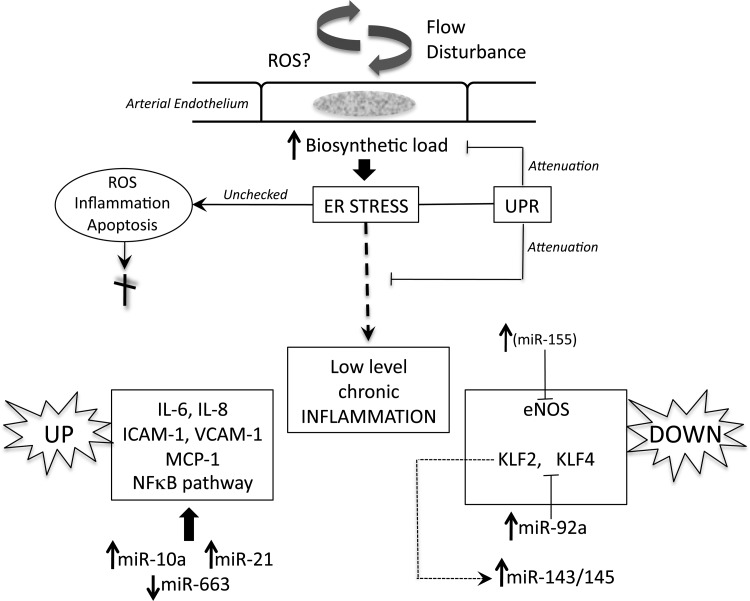
How the mechanisms discussed earlier may interact to influence susceptibility in vivo remains speculative. Figure 6 broadly outlines some connections. Despite the profiles being an average of a regional steady state, endothelial phenotype heterogeneity within a region of flow disturbance will likely result in a spectrum of individual cell susceptibilities.
Figure 6.
A schema of regulatory mechanisms of endothelial atherosusceptibility discussed in the text. Flow disturbance in regions susceptible to atherogenesis places a stress load on the biosynthetic capacity of the endothelial endoplasmic reticulum (ER) that leads to ER stress. Activation of the unfolded protein response (UPR) compensates to maintain normal cell function but a residual low-level chronic inflammatory state persists. Pro-inflammatory molecules are up-regulated (left) and protective molecules down-regulated (right). MicroRNAs (miRs) regulated up or down by disturbed flow regulate (at least in part) the pro-inflammatory state and are shown in a reciprocal transcriptional relationship to their targets. Notes: eNOS is a direct target of miR-155, inhibiting eNOS in cytokine-induced inflammation, however, differential miR-155 expression has not yet been demonstrated in disturbed flow. miR-143/145 is increased by flow-induced suppression of KLF2 and is shed in endothelial exosomes (Figure 5D). Not shown in this schema or discussed in the text (because of space limitations) are other regulatory mechanisms believed to contribute to endothelial pro-inflammatory phenotype through the MAPK pathway that mimics cytokine-induced mechanisms and through a low level of protective Nrf2 in atherosusceptible endothelium in mice.17
4.7. Transition of endothelial atherosusceptibility to inflammatory atherogenesis in vivo
The aforementioned outlines describe the regulation of some of the spatial differences of endothelial phenotype associated with atherosusceptibility. However, critical in vivo temporal information about endothelial phenotype change is lacking in longitudinal studies of the initiation of atherosclerosis. Studies of endothelial phenotype transition during progressive induction of atherosclerosis by hypercholesterolaemia are in an early stage and those conducted so far—involving short-duration high-fat diet in swine—revealed surprising resistance of the endothelium to the development of pathological phenotypes.6,7
4.7.1. Endothelial phenotype transition is resistant to short-duration hypercholesterolaemia
The effect of short duration (2 weeks) hypercholesterolaemia was tested in swine.6 Despite substantial elevation of circulating lipoprotein levels, endothelial ER stress/UPR status (vs. normocholesterolaemic controls) at comparative sites in vivo remained unchanged despite histological evidence of enhanced lipid permeability at the atherosusceptible sites. However, intimal macrophages were rarely observed despite subendothelial extracellular matrix-bound lipid. At face value, this result suggests that accumulation of a critical concentration of subendothelial lipoproteins may be necessary to trigger mechanisms that attract and facilitate the entry of monocyte-derived macrophages. Since monocyte transmigration requires the expression of adhesion molecules on the surface of endothelium, the rate-limiting step of site-specific transition to an overt inflammatory endothelial phenotype may be differential increase of endothelial permeability to circulating lipoproteins and monocyte-derived macrophages (which enter the arterial wall during normal immuno-surveillance at a higher rate in atherosusceptible sites11,67). Locally secreted macrophage cytokines may then act on the endothelium to develop the inflammatory phenotype signature, recruit more macrophages, and promote lesion development.
Is disturbed flow regulating endothelial permeability? Circumstantial evidence includes the enhanced turnover of the endothelial monolayer in turbulent flow that decreases junctional stability,68 the naturally increased immunosurveillance dynamics of monocytes in the regions of flow disturbance in vivo,11,67 the inhibitory effect of disturbed flow upon gap-junctional communication in vitro15 and in vivo,69 the KLF2-induced expression of connexin (Cx)37 in protective laminar flow in vitro and Cx37 suppression in disturbed flow in vivo,70 and perturbation of endothelial junctional molecule claudin-5 in disturbed flow regions in vivo71 and its regulation by SOX-18 in vitro.72
Limited investigation of the phenotypes of endothelial cells overlying clinical human atherosclerotic lesions obtained either as surgical by-products or as autopsy material73 suggests significant enhancement of inflammatory genes and pathways consistent with the general inflammatory profiles of the underlying lesions. Missing is knowledge of the phenotype-transition mechanisms (susceptible-to-inflammatory) and an understanding of the timing and dynamics involved. Nor should Nature's ‘checks and balances’ be ignored; in aortic-valve endothelium, significantly larger numbers of genes and pathways were changed following short-duration hypercholesterolaemia on the side of the valve leaflet susceptible to calcific valvular sclerosis.7 Most interestingly, however, the changes demonstrated the induction of protective rather than inflammatory pathways by hypercholesterolaemia despite enhanced lipid insudation. Unlike valve endothelium, significant site-specific protective networks were not induced in the arterial endothelium by hypercholesterolaemia. However, protective genes were enhanced in response to hypercholesterolaemia in all arterial endothelial sites studied irrespective of whether the sites were susceptible or protective;6 these included increased expression of reverse cholesterol transport regulators and antioxidant genes. Clarification of the timing and dynamics of inflammatory and protective transitions during hypercholesterolaemia are likely to emerge from longer periods of exposure to hypercholesterolaemia.
4.8. Future directions
Techniques to more thoroughly investigate cell phenotype than are reported in this review are already available through systems-targeted microarrays and whole genome sequencing in tandem with increasingly sophisticated statistical and bioinformatics analyses. Spatial and temporal accessibility to phenotype profiling is available over a range of scales down to the single cell embedded in vascular tissue. Measurement of the local mechanobiology of cells and extracellular matrices has rapidly advanced. Next-generation sequencing for analysis of entire systems (epigenetic, environmental, transcription factors, higher level chromatin organization, etc.), including the identification of genomic and epigenomic elements that regulate specific phenotypes, is now routinely accessible and can as readily be applied to vascular pathophysiology as to cell transformation, stem-cell differentiation, diabetes, and other metabolic diseases. Precise measurements of the complex arterial haemodynamics present in vivo, particularly, in the important coronary circulation, remain challenging. In addition to impacting mechanotransduction dynamics, these are also required to address the potential contributions of flow-dependent effects on the transport and local concentrations of potent signalling molecules, particularly, labile peptides and free radicals that undoubtedly play a significant role in arterial pathophysiology.
Funding
We gratefully acknowledge the US NIH National Heart Lung and Blood Institute (P01HL062250; T32HL07954, T32HL69766, K99 HL103789), the American Heart Association (0725385U; 11BGIA7080012; 0315286U), and the Deutsche Forschungsgemeinschaft (FL364/2-2, SFB834/A5 and Exzellenzcluster 147 ‘Cardio-Pulmonary Systems’).
Acknowledgements
We thank our collaborators Drs Elisabetta Manduchi and Chris Stoeckert of the Center for Bioinformatics at the University of Pennsylvania and Drs William Pritchard and John Karanian and their team at the Center for Devices and Radiological Health, US Food and Drug Administration, Laurel, MD, USA.
Conflict of interest: none declared.
References
- 1.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol. Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaherty JT, Pierce JE, Ferrans VJ, Patel DJ, Tucker WK, Fry DL. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ. Res. 1972;30:23–33. doi: 10.1161/01.res.30.1.23. doi:10.1161/01.RES.30.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Dewey CF, Gimbrone MA, Bussolari SR, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Engineer. 1981;103:177–185. doi: 10.1115/1.3138276. doi:10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- 4.Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, et al. Coexisting pro-inflammatory and anti-oxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. doi:10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Davies PF. Coronary artery endothelial transcriptome in vivo: identification of ER-stress and enhanced ROS by gene connectivity network analysis. Circ Cardiovasc Genet. 2011;4:243–252. doi: 10.1161/CIRCGENETICS.110.958926. doi:10.1161/CIRCGENETICS.110.958926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Civelek M, Grant GR, Irolla CR, Shi C, Riley R, Chiesa O, et al. Pre-lesional arterial endothelial responses in hypercholesterolemia: universal ATP-Binding Cassette A1 (ABCA1) upregulation contrasts with region-specific gene expression in vivo. Am J Physiol (Heart Circ) 2010;298:163–170. doi: 10.1152/ajpheart.00652.2009. doi:10.1152/ajpheart.00652.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerraty MA, Grant GR, Karanian JW, Chiesa OA, Pritchard WP, Davies PF. Hypercholesterolemia induces side-specific phenotype changes and PPARγ pathway activation in swine aortic valve endothelium. Arterioscler Thromb Vasc Biol. 2010;30:225–231. doi: 10.1161/ATVBAHA.109.198549. doi:10.1161/ATVBAHA.109.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA. Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. doi:10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell FP, Gallus AS, Schwartz CJ. Focal and regional patterns of uptake and the transmural distribution of 131-I-fibrinogen in the pig aorta in vivo. Exp Mol Pathol. 1974;20:281–292. doi: 10.1016/0014-4800(74)90060-4. doi:10.1016/0014-4800(74)90060-4. [DOI] [PubMed] [Google Scholar]
- 10.Wright HP. Mitosis patterns in aortic endothelium. Atherosclerosis. 1972;15:93–100. doi: 10.1016/0021-9150(72)90042-1. doi:10.1016/0021-9150(72)90042-1. [DOI] [PubMed] [Google Scholar]
- 11.Chien S. Molecular and mechanical bases of focal lipid accumulation in arterial wall. Prog Biophys Mol Biol. 2003;83:131–151. doi: 10.1016/s0079-6107(03)00053-1. doi:10.1016/S0079-6107(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 12.Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci USA. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. doi:10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Won D, Zhu SN, Chen M, Teichert AM, Fish JE, Matouk CC, et al. Relative reduction of endothelial nitric-oxide synthase expression and transcription in atherosclerosis-prone regions of the mouse aorta and in an in vitro model of disturbed flow. Am J Pathol. 2007;171:1691–1704. doi: 10.2353/ajpath.2007.060860. doi:10.2353/ajpath.2007.060860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang Y, Davies PF. Site-specific microRNA-92a regulation of Kruppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32:979–987. doi: 10.1161/ATVBAHA.111.244053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DePaola N, Davies PF, Pritchard WP, Polacek D. Spatial regulation of gap junction connexin43 in endothelial cells exposed to controlled disturbed flows in vitro. Proc Natl Acad Sci USA. 1999;96:3154–3159. doi: 10.1073/pnas.96.6.3154. doi:10.1073/pnas.96.6.3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gimbrone MA, Garcia-Cardena G. Vascular endothelium, hemodynamics, and the pathobiology of atherosclerosis. Cardiovasc Pathol. 2013;22:9–15. doi: 10.1016/j.carpath.2012.06.006. doi:10.1016/j.carpath.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warboys CM, Amini N, de Luca A, Evans PC. The role of blood flow in determining the sites of atherosclerotic plaques. F1000 Med Rep. 2011;3:5–12. doi: 10.3410/M3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kohlstedt K, Trouvain C, Boettger T, Shi L, Fisslthaler B, Fleming I. The AMP-activated protein kinase regulates endothelial cell angiotensin-converting enzyme expression via p53 and the post-transcriptional regulation of microRNA-143/145. Circ Res. 2013;112:1150–1158. doi: 10.1161/CIRCRESAHA.113.301282. [DOI] [PubMed] [Google Scholar]
- 19.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. doi:10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 20.Ni CW, Qiu H, Rezvan A, Kwon K, Nam D, Son DJ, et al. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood. 2010;116:e66–73. doi: 10.1182/blood-2010-04-278192. doi:10.1182/blood-2010-04-278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutkowski DT, Kaufman RJ. That which does not kill me makes me stronger: adapting to chronic ER stress. Trends Biochem Sci. 2007;32:469–476. doi: 10.1016/j.tibs.2007.09.003. doi:10.1016/j.tibs.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Gargalovic PS, Gharavi NM, Clark MJ, Pagnon J, Yang WP, He A, et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:2490–2496. doi: 10.1161/01.ATV.0000242903.41158.a1. doi:10.1161/01.ATV.0000242903.41158.a1. [DOI] [PubMed] [Google Scholar]
- 23.Civelek M, Manduchi E, Riley R, Stoeckert CJ, Davies PF. Chronic endoplasmic reticulum (ER)-stress activates unfolded protein response (UPR) in arterial endothelium in regions of susceptibility to atherosclerosis. Circ Res. 2009;105:453–461. doi: 10.1161/CIRCRESAHA.109.203711. doi:10.1161/CIRCRESAHA.109.203711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feaver RE, Hastings NE, Pryor A, Blackman BR. GRP78 upregulation by atheroprone shear stress via p38-, alpha2beta1-dependent mechanism in endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:1534–1541. doi: 10.1161/ATVBAHA.108.167999. doi:10.1161/ATVBAHA.108.167999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng L, Zampetaki A, Margariti A, Pepe AE, Alam S, Martin D, et al. Sustained activation of XBP1 splicing leads to endothelial apoptosis and atherosclerosis development in response to disturbed flow. Proc Natl Acad Sci USA. 2009;106:8326–833. doi: 10.1073/pnas.0903197106. doi:10.1073/pnas.0903197106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Zhang M, Wang S, Liang B, Zhao Z, Liu C, et al. Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes. 2010;59:1386–1396. doi: 10.2337/db09-1637. doi:10.2337/db09-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang B, Wang S, Wang Q, Zhang W, Viollet B, Zhu Y, et al. Aberrant ER stressing vascular smooth muscle increases vascular contractility and blood pressure in mice deficient of AMP-activated protein kinase α2 in vivo. Arterioscler Thromb Vasc Biol. 2013;33:595–604. doi: 10.1161/ATVBAHA.112.300606. doi:10.1161/ATVBAHA.112.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorp E, Iwawaki T, Miura M, Tabas I. A reporter for tracking the UPR in vivo reveals patterns of temporal and cellular stress during atherosclerotic progression. J Lipid Res. 2011;52:1033–1038. doi: 10.1194/jlr.D012492. doi:10.1194/jlr.D012492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. doi:10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 30.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, et al. Identification of flow-dependent endothelial nitric oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem. 1999;274:30101–30108. doi: 10.1074/jbc.274.42.30101. doi:10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 31.Fulton D, Gratton J-P, Mccabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. doi:10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. doi:10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 33.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:e68–e75. doi: 10.1161/hh1101.092677. doi:10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 34.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, et al. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. doi:10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 35.Dixit M, Loot AE, Mohamed A, Fisslthaler B, Boulanger CM, Ceacareanu B, et al. Gab1, SHP2, and protein kinase A are crucial for the activation of the endothelial NO synthase by fluid shear stress. Circ Res. 2005;97:1236–1244. doi: 10.1161/01.RES.0000195611.59811.ab. doi:10.1161/01.RES.0000195611.59811.ab. [DOI] [PubMed] [Google Scholar]
- 36.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, et al. Reciprocal phosphorylation and regulation of the endothelial nitric oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;19:16587–16591. doi: 10.1074/jbc.M100229200. doi:10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 37.Fisslthaler B, Loot AE, Mohamed A, Busse R, Fleming I. Inhibition of endothelial nitric oxide synthase activity by proline-rich tyrosine kinase 2 in response to fluid shear stress and insulin. Circ Res. 2008;102:1520–1528. doi: 10.1161/CIRCRESAHA.108.172072. doi:10.1161/CIRCRESAHA.108.172072. [DOI] [PubMed] [Google Scholar]
- 38.Garcin ED, Bruns CM, Lloyd SJ, Hosfield DJ, Tiso M, Gachhui R, et al. Structural basis for isozyme-specific regulation of electron transfer in nitric-oxide synthase. J Biol Chem. 2004;279:37918–37927. doi: 10.1074/jbc.M406204200. doi:10.1074/jbc.M406204200. [DOI] [PubMed] [Google Scholar]
- 39.van Buul JD, Anthony EC, Fernandez-Borja M, Burridge K, Hordijk PL. Proline-rich tyrosine kinase 2 (Pyk2) mediates vascular endothelial-cadherin-based cell-cell adhesion by regulating b-catenin tyrosine phosphorylation. J Biol Chem. 2005;280:21129–21136. doi: 10.1074/jbc.M500898200. doi:10.1074/jbc.M500898200. [DOI] [PubMed] [Google Scholar]
- 40.Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function by FAK and PYK2. Front Biosci. 2004;9:1254–1266. doi: 10.2741/1239. doi:10.2741/1239. [DOI] [PubMed] [Google Scholar]
- 41.Davies PF. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Clin Pract Cardiovasc Med. 2009;6:16–26. doi: 10.1038/ncpcardio1397. doi:10.1038/ncpcardio1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tai LK, Okuda M, Abe J, Yan C, Berk BC. Fluid shear stress activates proline-rich tyrosine kinase via reactive oxygen species-dependent pathway. Arterioscler Thromb Vasc Biol. 2002;22:1790–1796. doi: 10.1161/01.atv.0000034475.40227.40. doi:10.1161/01.ATV.0000034475.40227.40. [DOI] [PubMed] [Google Scholar]
- 43.Yin G, Yan C, Berk BC. Angiotensin II signaling pathways mediated by tyrosine kinases. Int J Biochem Cell Biol. 2003;35:780–783. doi: 10.1016/s1357-2725(02)00300-x. doi:10.1016/S1357-2725(02)00300-X. [DOI] [PubMed] [Google Scholar]
- 44.Bijli KM, Fazal F, Rahman A. Regulation of Rela/p65 and endothelial cell inflammation by proline-rich tyrosine kinase 2. Am J Respir Cell Mol Biol. 2012;47:660–668. doi: 10.1165/rcmb.2012-0047OC. doi:10.1165/rcmb.2012-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeiher AM, Fisslthaler B, Schray-Utz B, Busse R. Nitric oxide modulates the expression of monocyte chemoattractant protein 1 in cultured human endothelial cells. Circ Res. 1995;76:980–986. doi: 10.1161/01.res.76.6.980. doi:10.1161/01.RES.76.6.980. [DOI] [PubMed] [Google Scholar]
- 46.Loot AE, Schreiber JG, Fisslthaler B, Fleming I. Angiotensin II impairs endothelial function via tyrosine phosphorylation of the endothelial nitric oxide synthase. J Exp Med. 2009;206:2889–2896. doi: 10.1084/jem.20090449. doi:10.1084/jem.20090449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. doi:10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 48.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. doi:10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 49.Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature. 2008;455:64–71. doi: 10.1038/nature07242. doi:10.1038/nature07242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRNAs. Nature. 2008;455:58–63. doi: 10.1038/nature07228. doi:10.1038/nature07228. [DOI] [PubMed] [Google Scholar]
- 51.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. doi:10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fang Y, Shi C, Manduchi E, Civelek M, Davies PF. MicroRNA-10a regulation of proinflammatory phenotype in athero-susceptible endothelium in vivo and in vitro. Proc Natl Acad Sci USA. 2010;107:13450–13455. doi: 10.1073/pnas.1002120107. doi:10.1073/pnas.1002120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. doi:10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 54.Harris TA, Yamakuchi M, Ferlito M, Mendell JT, Lowenstein CJ. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA. 2008;105:1516–1521. doi: 10.1073/pnas.0707493105. doi:10.1073/pnas.0707493105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ni CW, Qiu H, Jo H. MicroRNA-663 upregulated by oscillatory shear stress plays a role in inflammatory response of endothelial cells. Am J Physiol Heart Circ Physiol. 2011;300:H1762–1769. doi: 10.1152/ajpheart.00829.2010. doi:10.1152/ajpheart.00829.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou J, Wang KC, Wu W, Subramaniam S, Shyy JY, Chiu JJ, et al. MicroRNA-21 targets peroxisome proliferators-activated receptor-alpha in an autoregulatory loop to modulate flow-induced endothelial inflammation. Proc Natl Acad Sci USA. 2011;108:10355–10360. doi: 10.1073/pnas.1107052108. doi:10.1073/pnas.1107052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun HX, Zeng DY, Li RT, Pang RP, Yang H, Hu YL, et al. Essential role of miR-155 in regulating endothelial-dependent vasorelaxation by targeting eNOS. Hypertension. 2012;60:1407–1414. doi: 10.1161/HYPERTENSIONAHA.112.197301. doi:10.1161/HYPERTENSIONAHA.112.197301. [DOI] [PubMed] [Google Scholar]
- 58.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. doi:10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 59.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. doi:10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou G, Hamik A, Nayak L, Tian H, Shi H, Lu Y, et al. Endothelial Kruppel-like factor 4 protects against atherothrombosis in mice. J Clin Invest. 2012;122:4727–4731. doi: 10.1172/JCI66056. doi:10.1172/JCI66056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamik A, Lin Z, Kumar A, Balcells M, Sinha S, Katz J, et al. Kruppel-like factor 4 regulates endothelial inflammation. J Biol Chem. 2007;282:13769–13779. doi: 10.1074/jbc.M700078200. doi:10.1074/jbc.M700078200. [DOI] [PubMed] [Google Scholar]
- 62.Atkins GB, Jain MK. Role of Kruppel-like transcription factors in endothelial biology. Circ Res. 2007;100:1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. doi:10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 63.Atkins GB, Wang Y, Mahabeleshwar GH, Shi H, Gao H, Kawanami D, et al. Hemizygous deficiency of Kruppel-like factor 2 augments experimental atherosclerosis. Circ Res. 2008;103:690–693. doi: 10.1161/CIRCRESAHA.108.184663. doi:10.1161/CIRCRESAHA.108.184663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu W, Xiao H, Laguna-Fernandez A, Villarreal G, Jr, Wang KC, Geary GG, et al. Flow-Dependent Regulation of Kruppel-Like Factor 2 Is Mediated by MicroRNA-92a. Circulation. 2011;124:633–641. doi: 10.1161/CIRCULATIONAHA.110.005108. doi:10.1161/CIRCULATIONAHA.110.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009;119:2634–2647. doi: 10.1172/JCI38864. doi:10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. doi:10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 67.Ogunrinade O, Kameya GT, Truskey GA. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann Biomed Eng. 2002;30:430–446. doi: 10.1114/1.1467924. doi:10.1114/1.1467924. [DOI] [PubMed] [Google Scholar]
- 68.Davies PF, Remuzzi A, Dewey CF, Gordon EJ, Gimbrone MA. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA. 1986;83:2114–2118. doi: 10.1073/pnas.83.7.2114. doi:10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabriels JE, Paul DL. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and 40 are more uniformly distributed. Circ Res. 1998;83:636–643. doi: 10.1161/01.res.83.6.636. doi:10.1161/01.RES.83.6.636. [DOI] [PubMed] [Google Scholar]
- 70.Pfenniger A, Wong C, Sutter E, Cuhlmann S, Dunoyer-Geindre S, Mach F, et al. Shear stress modulates the expression of the atheroprotective protein Cx37 in endothelial cells. J Mol Cell Cardiol. 2012;2:299–309. doi: 10.1016/j.yjmcc.2012.05.011. doi:10.1016/j.yjmcc.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 71.Karamanian A, Jimenez JM, Davies PF. Low expression of claudin-5, a flow-sensitive endothelial tight junction molecule, correlates with sites vulnerable to atherosclerosis in vivo. FASEB J. 2008;22:1120. Abstract. [Google Scholar]
- 72.Fontijn RD, Volger OL, Fledderus JO, Reijerkerk A, de Vries HE, Horrevoets AJ. SOX-18 controls endothelial-specific claudin-5 gene expression and barrier function. Am J Physiol (Heart Circ Physiol) 2008;294:H891–900. doi: 10.1152/ajpheart.01248.2007. doi:10.1152/ajpheart.01248.2007. [DOI] [PubMed] [Google Scholar]
- 73.Volger OL, Fledderus JO, Kisters N, Fontijn RD, Moerland PD, Kuiper J, et al. Distinctive expression of chemokines and transforming growth factor-beta signaling in human arterial endothelium during atherosclerosis. Am J Pathol. 2007;171:326–337. doi: 10.2353/ajpath.2007.061196. doi:10.2353/ajpath.2007.061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Markl M, Kilner PJ, Ebbers T. Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:7. doi: 10.1186/1532-429X-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bjorck HM, Renner J, Maleki S, Nilsson SF, Kihlberg J, Folkersen L, et al. Characterization of shear-sentive genes in the normal rat aorta identifies Hand2 as a major flow-responsive transcription factor. PLoS One. 2012;7:e52227. doi: 10.1371/journal.pone.0052227. [DOI] [PMC free article] [PubMed] [Google Scholar]