Abstract
One-third of the world's population is infected with Mycobacterium tuberculosis (M.tb), which causes tuberculosis. Mycobacterium tuberculosis cell envelope components such as glycolipids, lipoglycans and polysaccharides play important roles in bacteria–host cell interactions that dictate the host immune response. However, little is known about the changes in the amounts and types of these cell envelope components as the bacillus divides during in vitro culture. To shed light on these phenomena, we examined growth-dependent changes over time in major cell envelope components of virulent M.tb by using sodium dodecyl sulfate–polyacrylamide gel electrophoresis, thin-layer chromatography, mass spectrometry, immunoblotting and flow cytometry. Our studies provide evidence that major mannosylated glycoconjugates on the M.tb cell envelope change as M.tb grows in vitro on the widely used Middlebrook 7H11 agar. In particular, our compositional analyses show that from Day 9 to 28 the amounts of mannose-containing molecules, such as mannose-capped lipoarabinomannan, lipomannan and phosphatidyl-myo-inositol mannosides, change continuously in both the cell envelope and outer cell surface. Along with these changes, mannan levels on the outer cell surface also increase significantly over time. The implications of these differences in terms of how M.tb is grown for studies performed in vitro and in vivo for assessing M.tb-host recognition and establishment of infection are discussed.
Keywords: cell envelope components, LAM, LM, Mycobacterium tuberculosis, PIMs
Introduction
According to the World Health Organization, 8.8 million people were newly infected with M.tb and 1.4 million died of tuberculosis (TB) in 2010 (WHO. Tuberculosis fact sheet). The M.tb cell envelope is rich in mannosylated components such as arabinomannan (AM), mannan, mannose-capped lipoarabinomannan (ManLAM), LM, phosphatidyl-myo-inositol mannosides (PIMs) and glycoproteins (Torrelles and Schlesinger 2010). Extensive studies have been conducted to understand the early interactions of M.tb with macrophages, its natural host cell niche. Mycobacterium tuberculosis cell envelope components such as glycolipids, lipoglycans and polysaccharides play important roles in bacteria–host interactions that dictate the host innate immune response (reviewed by Briken et al. 2004; Torrelles and Schlesinger 2010; Mishra et al. 2011).
Despite their importance, to our knowledge, there are few studies published on the quantity and types of these components within the M.tb cell envelope as the bacillus matures in vitro and in vivo. Furthermore, these studies have been largely conducted using mycobacteria grown in broth, where increasing amounts of extracellular material are released from the bacteria over time (Lemassu et al. 1996; Schwebach et al. 2001; Dhiman et al. 2011). Here, we hypothesized that virulent M.tb produces different amounts and types of major cell envelope components while M.tb matures on solid medium. We investigated the changes in ManLAM, LM and PIMs in whole cell lysates (WCL) and outer surface material (OM) obtained from M.tb grown on 7H11 agar plates for 9–28 days. Biochemical compositional analyses together with our immunoblotting and flow cytometry studies indicate that the levels of ManLAM, LM, PIMs and surface-exposed carbohydrates change significantly within the M.tb cell envelope over time.
Results
Changes in M.tb WCL mannosylated molecules over time
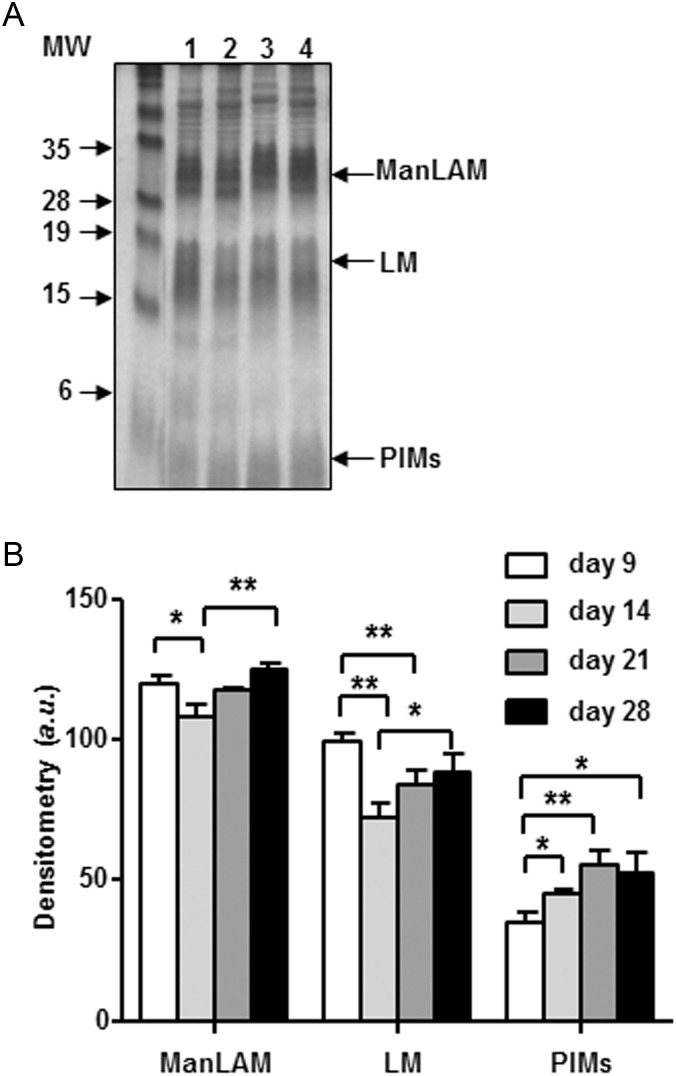
To evaluate the changes in lipoglycoconjugates (i.e., ManLAM, LM and PIMs) during the growth period of 9–28 days, WCLs, normalized by protein content (10 µg), were analyzed by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) followed by periodic acid silver nitrate staining. As seen in Figure 1A and B, the levels of ManLAM and LM changed in a similar fashion over time. It appeared that Day 14 was the turning point for ManLAM/LM production on the M.tb cell envelope, i.e., from Day 9 to 14, the levels of ManLAM/LM decreased but then increased afterwards (Figure 1B). In contrast, the levels of PIMs increased steadily from Day 9 to 21, after which time they remained at the same level until Day 28 (Figure 1B). There were no significant changes in the overall molecular sizes of ManLAM/LM as depicted by a similar migration profile over time.
Fig. 1.
Content of ManLAM, LM and PIMs in WCL. (A) A representative SDS–PAGE analysis of WCL lipoglycans. (B) Cumulative densitometry analysis of WCL (n = 3). Samples were normalized by protein content (10 µg protein per lane). Student's t-test was performed for the densitometry analysis. *P < 0.05 and **P < 0.01. Lanes 1–4 are Days 9, 14, 21 and 28 WCL, respectively.
Since arabinose (Ara), mannose (Man) and myo-inositol (myo-Inos) are constituents of ManLAM/LM/PIMs, the levels of these sugar residues were determined by gas chromatography (GC). Figure 2A shows that Ara, Man and myo-Inos in WCL increase over time. In particular, the level of Man increases significantly from Day 9 to 28. In order to assess the source of the increase in mannose, WCL was extracted with Triton X-114 to separate lipidated from nonlipidated mannosylated M.tb cell envelope components. Figure 2B shows that after removing lipidated components (i.e., ManLAM, LM, PIMs and mannosylated lipoproteins) the levels of the carbohydrates left in the aqueous phase had carbohydrate profiles similar to that seen in intact WCL. The absence of ManLAM/LM/PIMs was verified by SDS–PAGE of the aqueous phase followed by periodic silver staining (data not shown). This suggested that hydrophilic molecules such as mannan, AM and/or mannoproteins contained in the aqueous phase contribute greatly to the increase in Man observed over time in WCL. To determine whether mannoproteins were the source of this accumulation of mannose, we treated the aqueous phase with proteinase K followed by extensive dialysis. Our results (Figure 2C) show an increase in Man in the aqueous phase despite proteinase K treatment, indicating that mannosylated proteins are not directly responsible for the increase in Man observed over time during M.tb growth on agar medium. To discern whether the increase in Man is directly related to AM, mannan or both, we calculated the Man:Ara ratio in the aqueous phase. Our results presented in Figure 2D show that the Man:Ara ratio increases from Day 9 forward suggesting that the increase in Man observed in WCL is due mainly to an increase in mannan and not AM, since the latter has been reported to contain twice as much Ara as Man (Lemassu and Daffé 1994).
Fig. 2.
Compositional analysis of M.tb mannosylated components in the WCL. (A) Intact WCL before Triton X-114 extraction; (B) aqueous phase of WCL after Triton X-114 extraction before proteinase K digestion; (C) aqueous phase after proteinase K digestion and (D) Man:Ara ratio present in the aqueous phase after proteinase K digestion. Samples were normalized by protein content (100 µg protein). Student's t-test *P < 0.05; **P < 0.01 and ***P < 0.001. n = 3 for all graphs.
One of the unique features of ManLAM is its Man caps, which plays an important role in the interaction of M.tb with the host cell. The Man caps of ManLAM are positioned on the nonreducing end of its arabinan domain (Shi et al. 2008). To address whether ManLAM from bacteria grown over time changes its surface exposure of Man caps, we analyzed ManLAM in WCL by western blot and Far-western blot. As seen in Figure 3A, all WCL samples showed positive reactivity to the anti-LAM monoclonal antibody (mAb) CS-35, which recognizes preferentially the nonmannose-capped terminal Araf4, Araf5 and Araf6 motifs of ManLAM with a greater affinity for the latter two (Kaur et al. 2002; Rademacher et al. 2007). As the results show (Figure 3A and C), Araf4-, Araf5- and Araf6-containing ManLAM decreases from Day 9 to 28, although the total amount fluctuates during the same period of time (Figure 1A and B). This implies that the ManLAM nonreducing terminal structures change over time of growth and, in particular, that the extent of Man capping increases thereby reducing the ability of CS-35 to bind to its epitopes. To explore this possibility, Far-western blot (Figure 3B and D) analysis using the pulmonary collectin surfactant protein-D (SP-D), a protein that specifically recognizes the trimannoside caps of ManLAM and the single α(1 → 2)Manp branching residue of LM (Ferguson et al. 1999; Carlson et al. 2009), showed that the level of SP-D bound ManLAM increases steadily from Day 9 to 28. Thus, our overall results in Figure 3 suggest that there is an increase in Man capping of ManLAM as M.tb grows over time.
Fig. 3.
Immunoblot analysis of ManLAM in WCL obtained from M.tb harvest at different time points. (A) A representative experiment of a western blot using CS-35 anti-LAM Ab and (B) a Far-western blot using SP-D; (C) cumulative densitometry analysis of CS-35 western blots (n = 3) and (D) cumulative densitometry analysis of SP-D Far-western blots (n = 3). Bands in blots A and B correspond to ManLAM based on molecular weight, electrophoretic mobility and appearance of the bands. Samples were normalized by protein content (10 µg protein per lane). Student's t-test was performed for the densitometry analysis. *P < 0.05. Lanes 1–4 are Days 9, 14, 21 and 28 WCL, respectively.
Changes in mannosylated molecules in the M.tb OM over time
The M.tb OM was purified as previously described (Daffé and Lanéelle 2001) and analyzed in parallel with the WCL. The results show that from Day 9 to 21 the total amount of PIMs increases to a small but significant extent in the OM (Figure 4A and B), which is the same pattern as observed in WCL (Figure 1). In contrast, the changes in ManLAM and LM in the OM showed profiles different from those in the WCL, i.e., the amount of ManLAM in the OM fluctuated to a small degree from Day 9 to 28, while the level of LM decreased significantly from Day 9 to 28. Neutral sugar analysis of the OM obtained from M.tb grown at different time points also showed different or opposite profiles from those observed in the WCL. In particular, the amounts of Ara and myo-Inos decreased and Man kept steady over time in the OM (Figure 4C).
Fig. 4.
Content and compositional analyses of M.tb mannosylated components of the OM obtained at different time points of growth. (A) Representative gel image; (B) cumulative densitometry analysis of OM; (C) neutral sugar analysis of OM. *P < 0.05; **P < 0.01 and ***P < 0.001. n = 3.
SP-D binding to the M.tb surface over time
Given that the collective data obtained from WCL and OM above all pointed toward increased mannosylation on the outer surface of M.tb over time in culture, we next turned our attention to analyzing surface mannosylation in the context of intact bacteria. We used SP-D as a probe in flow cytometry experiments to evaluate changes in the degree of mannosylation on the M.tb surface over time. Our results in Figure 5 show that from Day 9 to 21 surface-bound SP-D increases significantly. Since the total amount of Man in the OM does not change over time, these results suggest rather that there is an increase in the exposure of SP-D reactive motifs [i.e. (1 → 2)Manp] on the M.tb cell surface over time.
Fig. 5.

Flow cytometry analysis of SP-D binding to the M.tb surface. (A) Histograms of M.tb grown for the indicated days and incubated in the absence of SP-D (negative control). (B) SP-D binding to the surface of M.tb grown for the indicated days. A negative control was used for M.tb grown for 9 days. (C) Fold change in SP-D binding to M.tb grown for the indicated days compared with the controls. Fold change = (MFIsample − MFIcontrol)/MFIcontrol, *P < 0.05 and **P < 0.01, n = 3.
Analysis of PIMs from M.tb grown over time
Figures 1 and 4 indicate that the total quantity of PIMs on the M.tb cell envelope (WCL and OM) changes from Day 9 to 28. In order to establish which PIM species is altered over time, we further analyzed PIM content by thin-layer chromatography (TLC). As shown in Figure 6A and B, glycolipids from both WCL and OM were separated into four spots by one-dimension TLC. The corresponding spots from WCL and OM have a similar migration profile (Supplementary data, Figure S1A). Matrix-assisted laser desorption-time of flight mass spectrometry (MALDI-TOF MS) analysis of purified spots from WCL and OM identified Spots 2, 3 and 4 as di- (PIM6), tri- (Ac1PIM6) and tetra-acylated PIM6 (Ac2PIM6), respectively (Figure 6C). Surprisingly, our results indicate that tri-acylated phosphatidyl-myo-inositol hexamannoside (AcPIM6) (Spot 3) decreases significantly in the M.tb cell envelope over time of growth. This effect on AcPIM6 was observed in both WCL (Figure 6A and D) and OM (Figure 6B and E), being more dramatic in the latter case. Conversely, a significant increase was observed for the most polar Spot 1. Although the identification of Spot 1 is still ongoing, our SDS–PAGE and compositional analyses revealed that Spot 1 migrates similar to PIMs by SDS–PAGE, and only contains Ara, Man and myo-Inos and fatty acids such 14:0, 16:0, 18:0 and tuberculosis stearic acid (TBST)] (Supplementary data, Figure S1B–D). Thus, these data suggest that the polar Spot 1 may relate to the family of higher-order PIMs.
Fig. 6.
Analysis of PIMs in the WCL and OM as M.tb ages in agar culture. (A) TLC analyses of WCL and (B) OM. (C) MALDI-TOF MS identification of Spots 2–4. Cumulative densitometry analysis of (D) WCL and (E) OM. Samples were normalized by protein content (100 µg protein per lane). Student's t-test was performed for the densitometry analysis *P < 0.05; **P < 0.01 and ***P < 0.001, n = 3.
Impact of the M.tb age of growth in association with human macrophages
Our results imply that as M.tb ages in culture, more lectin-binding Man motifs are exposed on its cell envelope surface. In order to evaluate the biological consequences of this observation further, we assessed the total association of M.tb grown for 9 or 28 days with human macrophages in monolayer culture, and the degree to which this association is mannose receptor (MR) dependent, i.e., inhibited by anti-MR Ab. Our results show that M.tb of these different ages associates with human macrophages to a similar extent (association in the presence of the immuoglobulin (IgG)-isotype control, Figure 7); however, the degree to which this association is due to mannan binding increases over time. This is indicated by a significant difference in the association of M.tb with the human macrophage MR, where there was a 56% decrease in association in the presence of anti-MR Ab in the case of 28-day-old M.tb versus a 22% decrease for 9-day-old M.tb (Figure 7).
Fig. 7.
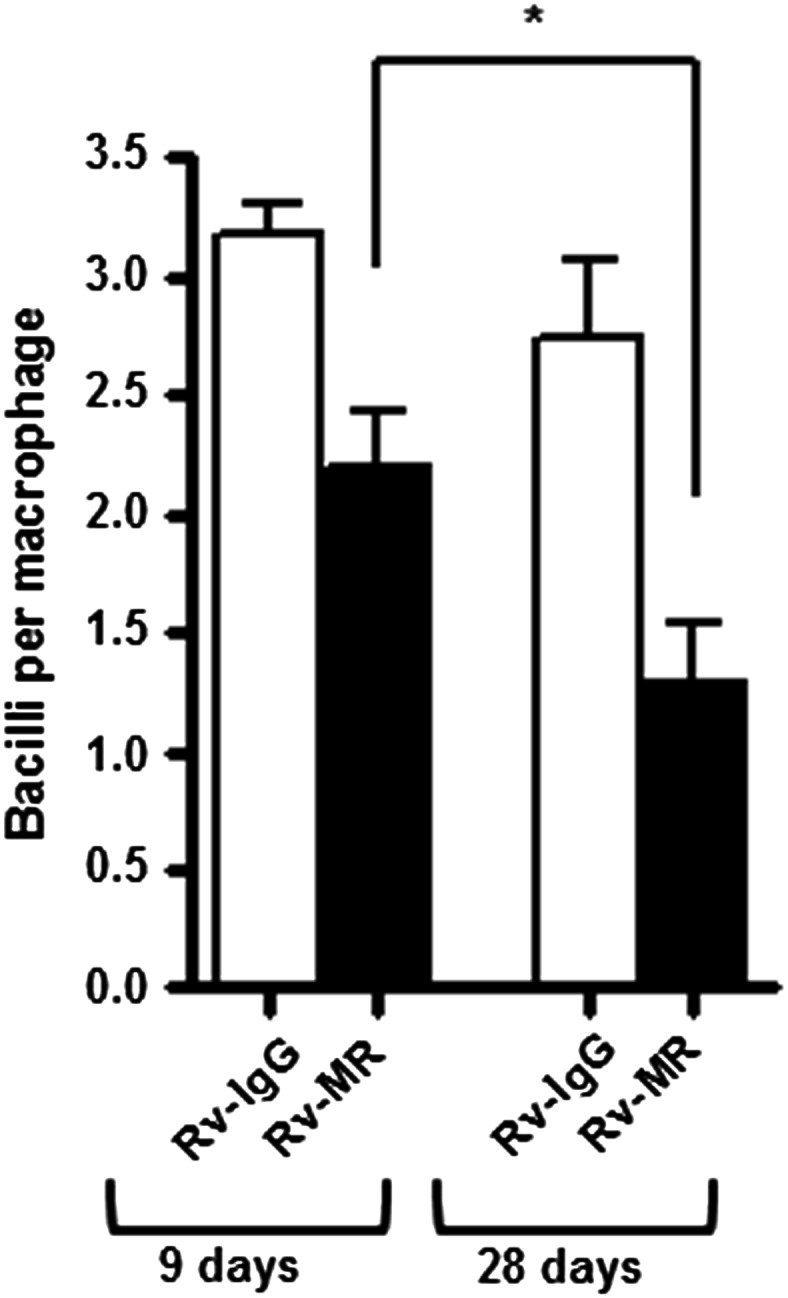
Human macrophage association with M.tb grown for either 9 days or 28 days on agar medium. MDM monolayers were preincubated with 10 μg/mL of IgG1 isotype (open bars) or anti-MR mAb (solid bars) before incubation with M.tb grown for the indicated days on agar medium (MOI 10:1). Shown are cumulative data (n = 3) and each experimental group was performed in triplicate. *P < 0.05; Student's t-test.
Discussion
In the laboratory setting, M.tb is usually cultured in either broth (using media such as Sauton's or Middlebrook 7H9) or on agar plates (such as Middlebrook 7H11 agar). It has been reported that as mycobacteria mature in broth culture, bacterial membrane vesicles or surface material mainly composed of exochelins, proteins, lipids and lipoglycans are shed (Lemassu et al. 1996; Prados-Rosales et al. 2011) and as a result the mycobacterial cell envelope composition at harvesting may be altered at different growth time points. However, little information is available regarding the nature of the cell envelope when M.tb is grown on solid agar over time. Furthermore, in the literature, there seems to be no consensus regarding the age of M.tb at harvesting for various research purposes. Previous studies, including ours, have used cultures in the range Days 9–14 from solid medium and 7 days to 6 weeks from broth culture with predicted viability in the range 50–90% (Schlesinger et al. 1994; Ortalo-Magné et al. 1995; Gobin and Horwitz 1996; Besra et al. 1997; Kaur et al. 2008; Arcos et al. 2011; Prados-Rosales et al. 2011; Torrelles et al. 2011). Here, we investigated the time-dependent changes in major M.tb cell envelope components (i.e., mannosylated glycoconjugates) implicated in pathogenesis for M.tb grown on solid medium. Based on our own experience, Day 9 is an early stage of M.tb growth on 7H11 agar. In Days 9–28, the colonies spread out and become progressively drier on the plates. Thus, we compared the WCL and OM constituents of M.tb on Days 9, 14, 21 and 28 of culture on solid agar.
The presence of Man on the M.tb surface is important in influencing M.tb pathogenesis (Ehlers and Daffé 1998; Vimr et al. 2004; Torrelles and Schlesinger 2010; Krishna et al. 2011). Our study shows that Man levels in the M.tb cell envelope change over time, and thus, the nature of surface-exposed Man varies directly depending on the time when M.tb is harvested from agar plates. This fact may have important consequences for M.tb interactions with the host cell and subsequent immune response to infection in models of M.tb pathogenesis. Lipoglycans on the M.tb cell envelope are heterogeneous in size and structure. This heterogeneity is observed between different mycobacterial species and also within strains of the same species (Torrelles and Schlesinger 2010). As a result of their structural differences, lipoglycans such as LAM and LM from different mycobacterial species trigger different immune responses [reviewed in Schlesinger et al. (2008); Rajaram et al. 2011]. Our data indicate that as M.tb grows on solid agar its major mannosylated cell envelope components vary in their biochemical nature, quantity and surface exposure. Growth on solid agar imparts an inherent heterogeneity whereby the harvested biomass contains older cells nearer the center of the colony which would presumably exist in a very different environment than cells at the periphery. Thus, the biochemical data presented reflect a population-wide analysis rather than single-cell analysis.
Our SP-D Far-western blot results suggest that as M.tb ages during in vitro growth, the amount of Man caps on ManLAM increases. The Man caps of ManLAM bind to the macrophage MR mediating M.tb phagocytosis and subsequent events by human macrophages, although this interaction varies for clinical isolates (Schlesinger 1993; Schlesinger et al. 1994, 1996; Torrelles et al. 2008). Similarly, both LM and PIMs mediate receptor recognition and regulate cytokine, oxidant and T cell responses (Barnes et al. 1992; Chan et al. 2001; Gilleron et al. 2001; De la Salle et al. 2005; Torrelles et al. 2006). We demonstrate that the levels of LM and PIMs change over time of culture in both WCL and OM. In particular, the level of LM and AcPIM6 decreases dramatically from Day 9 to 28 in the OM.
According to Ortalo-Magné et al. (1995), the outermost water-soluble cellular material of M.tb grown in broth culture is composed of 94–99% proteins and carbohydrates, where only a small fraction is of lipidic nature. In their studies of H37Rv M.tb grown on Sauton's medium, no ManLAM and LM were reported in the OM/capsular material (Ortalo-Magné et al. 1995) or in the exocellular material (culture filtrate material) (Lemassu and Daffé 1994). In contrast, our results with M.tb grown on solid medium show the presence of ManLAM, LM and PIMs in the OM and on the M.tb cell envelope surface. This discrepancy can be easily explained by the differences in growth conditions of the bacteria. Other published studies have been more akin to our results showing that the M.tb surface expression of mannose-capped AM during in vitro growth changes with culture age and is strain-dependent (Schwebach et al. 2001). In this study, the authors used antibodies reacting against AM arabinan domains to determine that exposure of AM on the M.tb surface is dynamic and increases over time.
Accordingly, our results provide evidence that the decrease in CS-35 recognition (less Ara on the surface) of older cultures of M.tb correlates with its increase in SP-D recognition (more Man on the surface). Thus, based on our results, a simple explanation for this observation is that: (i) there is more Man on the M.tb cell surface of older cultures, (ii) there is more Man capping on ManLAM and/or AM, and/or (iii) there is different spatial conformation, disposition and/or location of Man within the cell envelope as M.tb ages in culture. In this regard, our previous studies (Carlson et al. 2009) and current results of SP-D binding to whole M.tb indicate that the degree of surface-exposed Man (in the form of mannans) increases during time in culture. Since SP-D recognizes α(1 → 2)Man and α(1 → 3)Man linkages, our data cannot differentiate among the various surface-exposed constituents (e.g., ManLAM, LM, mannan, AM and mannoproteins); however, they indicate that there is an increase in Man as M.tb ages in culture which has implications for interactions with the host. Since studies using animal models have shown that surface-mannosylated OM components are produced during M.tb growth in vivo and that the amount of these components is dependent on the bacterial number per infected organ (Schwebach et al. 2001), it is imperative that we learn more about the nature and role of the M.tb cell envelope and OM components during the course of infection in humans.
Materials and methods
Reagents and antibodies
Phosphate buffered saline (PBS) containing CaCl2 and MgCl2 was from Invitrogen (Grand Island, NY). 7H11 agar and oleic acid-albumin-dextrose-catalase (OADC) enrichment were from Becton, Dickinson and company (Franklin Lakes, NJ). Glycerol, glass beads and bicinchoninic acid (BCA) kit were from Fisher Scientific (Pittsburgh, PA). CS-35 murine mAbs were kindly provided by the “TB Vaccine Testing and Research Materials” contract (NOI-AI-75320). Donkey anti-mouse antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). Of note, 0.22 µm filters and S-protein fluorescein isothiocyanate (FITC) conjugate were from EMD Millipore (Billerica, MA). S-protein horseradish peroxidase (HRP) conjugate was from Bio-Rad (Hercules, CA). Lysing matrix B tubes containing 0.1 mm silica spheres were from MP Biomedicals (Solon, OH). Endotoxin free water was from Baxter (Deerfield, IL). All the chemicals were of the highest grade from Sigma-Aldrich (Saint Louis, MO) unless otherwise specified.
Growth and preparation of M.tb
Mycobacterium tuberculosis strain H37Rv (ATCC 27294) was grown on 7H11 agar plates (composed of 7H11 agar, 5% OADC enrichment and 0.5% glycerol, following the manufacturer's instructions) for 9, 14, 21 and 28 days at 37°C, 5% CO2. To obtain WCL, M.tb was harvested to lysing matrix tubes containing 1 mL of endotoxin free water. Mycobacterium tuberculosis bacilli were lysed by FastPrep-24 (MP Biomedicals, Solon, OH) for 12 cycles (maximum speed 6.5 and 20 s/cycle). An established method (Daffé and Lanéelle 2001) was adapted to obtain OM. In brief, freshly harvested M.tb was subjected to gentle physical friction by 3 mm sterile glass beads followed by centrifugation at 4°C 250 × g for 10 min. The supernatant contained water-soluble OM. Both WCL and OM were sterile filtered through 0.22 µm filters and subjected to protein analysis by BCA protein assay. Aliquots were kept at −80°C.
Gel electrophoresis of WCL and OM and immunoblotting
Fifteen percent SDS–PAGE was prepared in house freshly using 40% acrylymide/bis-acrylymide (Bio-Rad Laboratories, Hercules, CA). WCL and OM (10 µg of protein content) were separated at a constant current of 10 mA for stacking gels and 15 mA for resolving gels. Gels were stained with periodic acid and silver nitrate as described (Tsai and Frasch 1982; Shi et al. 2008). For western blot, WCL separated by SDS–PAGE was transferred to a nitrocellulose membrane. After blocking, blots were incubated with the anti-LAM mAb CS-35 as the primary antibody and a donkey anti-mouse antibody as the secondary antibody followed by development with enhanced ChemiLuminescence western blot detection reagents (GE Healthcare, Buckinghamshire, UK). For Far-western blot, after transferring the samples to a nitrocellulose membrane, the blot was processed as described (Carlson et al. 2009). In brief, the blot was incubated with blocking buffer (20 mM Tris, 140 mM NaCl, 5 mM CaCl2, pH 7.4) containing 5% fatty acid free bovine serum albumin (BSA) for 3–5 h and 5 µg/mL SP-D in blocking buffer containing 1% fatty acid free BSA overnight. The blot was then labeled with S-protein HRP conjugate in blocking buffer containing 1% fatty acid free BSA followed by development and visualization as for western blot. Bands on the stained gel and immunoblots were analyzed by densitometry using the ImageJ program from the National Institute of Health (NIH) (n = 3). The densitometry approach used detects linear changes (Torrelles et al. 2004; and Supplementary data, Figure S2).
Carbohydrate and fatty acid analyses of WCL and OM by GC and gas chromatography-mass spectrometry
WCL and OM (10 µg of protein content) and purified Spot 1 (5 µg) were hydrolyzed with 2 M trifluoroacetic acid in water at 120°C for 2 h. Using scyllo-inositol as the internal standard, hydrolyzed samples were reduced with sodium borodeuteride and acylated by acetate anhydride at 120°C for 1 h (Shi et al. 2008). Resulting alditol acetates were analyzed using a gas chromatography-mass spectrometry (GC-MS) spectrometer (Thermo DSQII Trace coupled with GC Ultra) fitted with a Rtx-5MS column (30 m × 0.25 µm with 5 m of Intergra-guard, Restek, Bellefonte, PA) with an initial temperature of 150°C for 3 min, increased to 200°C at 2°C/min then to 250°C at 40°C/min and held for 4 min. Peak areas of the individually separated alditol acetate were used for relative quantification. Fatty acid methyl esters were obtained and analyzed as previously described (Torrelles et al. 2008).
PIM analysis by TLC and MALDI-TOF MS
WCL and OM (100 µg of protein content) were extracted sequentially by CHCl3/CH3OH (2:1 and 1:2, v/v) and CHCl3/CH3OH/H2O (10:10:3, v/v/v). The extracted lipid was separated on silica gel 60 aluminum-based TLC plates (Merck, Dardstadt, Germany) using CHCl3/CH3COOH/CH3OH/H2O (40:25:3:6). Total lipids were revealed by charring with 1% α-napthol/5% concentrated H2SO4 in CH3CH2OH at 120°C. To identify the glycolipids separated by TLC, WCL and OM (1 mg of protein content) were extracted and separated on preparative TLC plates as above. The separated PIMs were then scraped off and re-extracted with the organic solvent as above for Spots 2–4 and with water for Spot 1 due to its high hydrophilicity. Purified PIMs were analyzed by MALDI-TOF MS (Bruker Daltonic Reflex III, Bruker Dalltonic, Billerica, MA) at negative ion mode using 2,5-dihydroxybenzoic acid as matrix.
SP-D binding to M.tb surface
SP-D Neck + carbohydrate recognition domains (NCRD) was generated as described (Crouch et al. 2006, 2009) and kindly provided by Dr. Erika Crouch, Washington University. Bacterial single-cell suspensions (1.4 × 108 cells) were incubated in blocking buffer (3% fatty acid free BSA, 20 mM Tris, 140 mM NaCl, 5 mM CaCl2, pH 7.4) at room temperature for 1 h followed by the addition of SP-D NCRD to a final concentration of 5 µg/mL. To control samples, blocking buffer was added. After 1 h incubation, the bacteria were centrifuged at 4°C 14,000 × g for 10 min and washed twice with blocking buffer.
Bacteria were then re-suspended in 1 mL of blocking buffer with 0.1% S-protein FITC conjugate and incubated at room temperature for 1 h. After washing the cells twice with 2% fatty acid free BSA in PBS, the bacteria were fixed with 4% paraformadehyde for 10 min followed by re-suspension in PBS containing 2% BSA and detection on a BD FACS Canto II flow cytometer (BD Biosciences, San Jose, CA) at a wavelength of 488 nm.
Triton X-114 extraction and proteinase K digestion of WCL
Mannosylated lipoglycoconjugates from WCL (1 mg of protein content) were extracted with 8% Triton X-114 shaking at room temperature for 1 h followed by phase separation at 60°C for 30 min. The aqueous phase containing glycoproteins, arabinomannan (AM), mannan and glucans was subjected to carbohydrate analysis by GC-MS as described above. Proteinase K (Life Technologies, Grand Island, NY) was added to the aqueous phase to a concentration of 2 mg/mL and incubated at 37°C overnight. The resulting digest was heated at 80°C for 30 min to denature the excess proteinase K prior to dialysis against endotoxin free water with membrane MWCO 2000 for 24 h.
Isolation and preparation of human macrophages
Following an approved OSU IRB protocol, monocyte-derived macrophage (MDM) monolayers were prepared from healthy tuberculin-negative human volunteers as previously described (Schlesinger 1993). Monolayers (2 × 105 MDMs/well) for microscopy studies were obtained by adherence to acid-washed coverslips in 24-well tissue culture plates for 2 h at 37°C.
Association of M.tb with human MDMs
Mycobacterium tuberculosis association with MDMs in the presence or absence of anti-MR antibody or isotype control was performed as previously described (Torrelles et al. 2008). In brief, MDM monolayers were preincubated with anti-MR Ab or IgG isotype control (AbD Serotec, Raleigh, NC) at 10 µg/mL for 20 min at 37°C, 5% CO2 to block MR activity followed by M.tb infection (used single-cell suspensions at multiplicity of infection 10:1) for 2 h at 37°C. MDM monolayers were washed to remove nonassociated bacilli, fixed in 10% aqueous formalin and washed again several times. Associated M.tb was then stained with rhodamine/auramine. The mean ± SEM of associated M.tb per MDM was determined by counting ≥300 consecutive MDM per coverslip using phase contrast and fluorescence microscopy (Torrelles et al. 2008). Experiments were performed in triplicate (three independent experiments using different donors for each time point evaluated).
Statistics
For all statistical analysis, two-tailed t-tests were performed using GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA) to determine the significance of difference between test and control groups.
Supplementary data
Supplementary data for this article is available online at http://glycob.oxfordjournals.org/.
Funding
This work was supported in part by the National Institutes of Health (AI052458 and AI033004 to L.S.S.; NCR R000167948 to T.K.C.; and AI-073856 and the Parker B. Francis Fellowship to J.B.T.).
Conflict of interest
None declared.
Abbreviations
Ac2PIM6, tetra-acylated phosphatidyl-myo-inositol hexamannoside; AcPIM6, tri-acylated phosphatidyl-myo-inositol hexamannoside; AM, arabinomannan; Ara, arabinose; BCA, bicinchoninic acid; BSA, bovine serum albumin; GC, gas chromatography; GC-MS, gas chromatography-mass spectrometry; LM, lipomannan; M.tb, Mycobacterium tuberculosis (M.tb); mAb, monoclonal antibody; MALDI-TOF MS, matrix-assisted laser desorption-time of flight mass spectrometry; Man, mannose; ManLAM, mannose-capped lipoarabinomannan; MDM, monocyte-derived macrophage; MR, mannose receptor; myo-Inos, myo-inositol; NCRD, Neck + carbohydrate recognition domains; OADC, oleic acid-albumin-dextrose-catalase; OM, outer surface material; PIMs, phosphatidyl-myo-inositol mannosides; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; SP-D, surfactant protein; TB, tuberculosis; TLC, thin-layer chromatography; WCL, whole cell lysates.
Supplementary Material
Acknowledgements
We thank the Mass Spectrometry and Proteomics facility at the Campus Chemical Instrument Center at The Ohio State University for their technical assistance.
References
- Arcos J, Sasindran SJ, Fujiwara N, Turner J, Schlesinger LS, Torrelles JB. Human lung hydrolases delineate Mycobacterium tuberculosis-macrophage interactions and the capacity to control infection. J Immunol. 2011;187:372–381. doi: 10.4049/jimmunol.1100823. doi:10.4049/jimmunol.1100823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PF, Chatterjee D, Abrams JS, Lu S, Wang E, Yamamura M, Brennan PJ, Modlin RL. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- Besra GS, Morehouse CB, Rittner CM, Waechter CJ, Brennan PJ. Biosynthesis of mycobacterial lipoarabinomannan. J Biol Chem. 1997;272:18460–18466. doi: 10.1074/jbc.272.29.18460. doi:10.1074/jbc.272.29.18460. [DOI] [PubMed] [Google Scholar]
- Briken V, Porcelli SA, Besra GS, Kremer L. Mycobacterial lipoarabinomannan and related lipoglycans: From biogenesis to modulation of the immune response. Mol Biol. 2004;53:391–403. doi: 10.1111/j.1365-2958.2004.04183.x. [DOI] [PubMed] [Google Scholar]
- Carlson TK, Torrelles JB, Smith K, Horlacher T, Castelli R, Seeberger PH, Crouch EC, Schlesinger LS. Critical role of amino acid position 343 of surfactant protein-D in the selective binding of glycolipids from Mycobacterium tuberculosis. Glycobiology. 2009;19:1473–1484. doi: 10.1093/glycob/cwp122. doi:10.1093/glycob/cwp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan ED, Morris KR, Belisle JT, Hill P, Remigio LK, Brennan PJ, Riches DWH. Induction of inducible nitric oxide synthase-NO* by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-кB signaling pathways. Infect Immun. 2001;69:2001–2010. doi: 10.1128/IAI.69.4.2001-2010.2001. doi:10.1128/IAI.69.4.2001-2010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E, Hartshorn K, Horlacher T, McDonald B, Smith K, Cafarella T, Seaton B, Seeberger PH, Head J. Recognition of mannosylated ligands and influenza A virus by human surfactant protein D: Contributions of an extended site and residue 343. Biochemistry. 2009;48:3335–3345. doi: 10.1021/bi8022703. doi:10.1021/bi8022703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch EC, Smith K, McDonald B, Briner D, Linders B, McDonald J, Holmskov U, Head J, Hartshorn K. Species differences in the carbohydrate binding preferences of surfactant protein D. Am J Respir Cell Mol Biol. 2006;35:84–94. doi: 10.1165/rcmb.2005-0462OC. doi:10.1165/rcmb.2005-0462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daffé M, Lanéelle MA. Analysis of the capsule of Mycobacterium tuberculosis. In: Parish T, Stoker NG, editors. Mycobacterium tuberculosis Protocols, Methods in Molecular Medicine. Totowa (USA): Humana Press; 2001. pp. 217–227. Vol. 54. [DOI] [PubMed] [Google Scholar]
- De la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles LF, Malm D, Berg T, Paoletti S, Mitre B, Mourey L, et al. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–1324. doi: 10.1126/science.1115301. doi:10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- Dhiman RK, Dinadayala P, Ryan GJ, Lenaerts AJ, Schenkel AR, Crick DC. Lipoarabinomannan localization and abundance during growth of Mycobacterium smegmatis. J Bacteriol. 2011;193:5802–5809. doi: 10.1128/JB.05299-11. doi:10.1128/JB.05299-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MRW, Daffé M. Interactions between Mycobacterium tuberculosis and host cells: Are mycobacterial sugars the key? Trends Microbiol. 1998;6:328–335. doi: 10.1016/s0966-842x(98)01301-8. doi:10.1016/S0966-842X(98)01301-8. [DOI] [PubMed] [Google Scholar]
- Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol. 1999;163:312–321. [PubMed] [Google Scholar]
- Gilleron M, Ronet C, Mempel M, Monsarrat B, Gachelin G, Puzo G. Acylation state of the phosphatidylinositol mannosides from Mycobacterium bovis bacillus Calmette Guérin and ability to induce granuloma and recruit natural killer T cells. J Biol Chem. 2001;276:34896–34904. doi: 10.1074/jbc.M103908200. doi:10.1074/jbc.M103908200. [DOI] [PubMed] [Google Scholar]
- Gobin J, Horwitz MA. Exochelins of Mycobacterium tuberculosis remove iron from human iron-binding proteins and donate iron to mycobactins in the M. tuberculosis cell envelope. J Exp Med. 1996;183:1527–1532. doi: 10.1084/jem.183.4.1527. doi:10.1084/jem.183.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur D, Lowary TL, Vissa VD, Crick DC, Brennan PJ. Characterization of the epitope of anti-lipoarabinomannan antibodies as the terminal hexaarabinofuranosyl motif of mycobacterial arabinans. Microbiology. 2002;148:3049–3057. doi: 10.1099/00221287-148-10-3049. [DOI] [PubMed] [Google Scholar]
- Kaur D, Obregon-Henao A, Pham H, Chatterjee D, Brennan PJ, Jackson M. Lipoarabinomannan of Mycobacterium: Mannose capping by a multifunctional terminal mannosyltransferase. Proc Natl Acad Sci USA. 2008;105:17973–17977. doi: 10.1073/pnas.0807761105. doi:10.1073/pnas.0807761105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna S, Ray A, Dubey SK, Larrouly-Maumus G, Chalut C, Castanier R, Noguera A, Gilleron M, Puzo G, Vercellone A, et al. Lipoglycans contribute to innate immune detection of mycobacteria. PLoS One. 2011;6:e28476. doi: 10.1371/journal.pone.0028476. doi:10.1371/journal.pone.0028476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemassu A, Daffé M. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem J. 1994;297:351–357. doi: 10.1042/bj2970351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemassu A, Ortalo-Magne A, Bardou F, Silve G, Lanéelle MA, Daffé M. Extracellular and surface-exposed polysaccharids of non-tuberculous mycobacteria. Microbiology. 1996;142:1513–1520. doi: 10.1099/13500872-142-6-1513. doi:10.1099/13500872-142-6-1513. [DOI] [PubMed] [Google Scholar]
- Mishra AK, Driessen NN, Appelmelk BJ, Besra GS. Lipoarabinomannan and related glycoconjugates: Structure, biogenesis and role in Mycobacterium tuberculosis physiology and host-pathogen interaction. FEMS Microbiol Rev. 2011;35:1126–1157. doi: 10.1111/j.1574-6976.2011.00276.x. doi:10.1111/j.1574-6976.2011.00276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortalo-Magné A, Dupont MA, Lemassu A, Andersen AB, Goun P, Daffé M. Molecular composition of the outermost capsular material of the tubercle bacillus. Microbiology. 1995;141:1609–1620. doi: 10.1099/13500872-141-7-1609. doi:10.1099/13500872-141-7-1609. [DOI] [PubMed] [Google Scholar]
- Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Carmara C, Dosanchuk JD, Besra GS, Chen B, et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest. 2011;121:1471–1483. doi: 10.1172/JCI44261. doi:10.1172/JCI44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher C, Shoemaker GK, Kim HS, Zheng RB, Taha H, Liu C, Nacario RC, Schriemer DC, Klassen JS, Peters T, et al. Ligand specificity of CS-35, a monoclonal antibody that recognizes mycobacterial lipoarabinomannan: A model system for oligofuranoside-protein recognition. J Am Chem Soc. 2007;129:10489–10502. doi: 10.1021/ja0723380. doi:10.1021/ja0723380. [DOI] [PubMed] [Google Scholar]
- Rajaram MV, Ni B, Morris JD, Brooks MN, Carlson TK, Bakthavachalu B, Schoenberg DR, Torrelles JB, Schlesinger LS. Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrohphage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc Natl Acad Sci USA. 2011;108:17408–17413. doi: 10.1073/pnas.1112660108. doi:10.1073/pnas.1112660108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger LS. Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol. 1993;150:2920–2930. [PubMed] [Google Scholar]
- Schlesinger LS, Azad AK, Torrelles JB, Roberts E, Vergne I, Deretic V. Determinants of phagocytosis, phagosome biogenesis and autophagy for Mycobacterium tuberculosis. In: Haufmann SHE, Britton WJ, editors. Handbook of Tuberculosis: Immunology and Cell Biology. Weinheim, Germany: WILEY-CVH Verlag GmbH & Co. KGaA; 2008. pp. 1–22. [Google Scholar]
- Schlesinger LS, Hull SR, Kaufman TM. Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol. 1994;152:4070–4079. [PubMed] [Google Scholar]
- Schlesinger LS, Kaufman TM, Lyer S, Hull SR, Marciando LK. Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol. 1996;157:4568–4575. [PubMed] [Google Scholar]
- Schwebach JR, Casadevall A, Schneerson R, Dai Z, Wang X, Robbins JB, Glatman-Freedman A. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect Immun. 2001;69:5671–5678. doi: 10.1128/IAI.69.9.5671-5678.2001. doi:10.1128/IAI.69.9.5671-5678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Torrelles JB, Chatterjee D. Lipoglycans of Mycobacterium tuberculosis isolation, purification, and characterization. In: Parish T, Brown AC, editors. Mycobacteria Protocols. London (UK): Humana Press; 2008. pp. 23–45. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Azad AK, Schlesinger LS. Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol. 2006;177:1805–1816. doi: 10.4049/jimmunol.177.3.1805. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Khoo KH, Sieling PA, Modlin RL, Zhang N, Marques AM, Treumann A, Rithner CD, Brennan PJ, Chatterjee D. Truncated structural variants of lipoarabinomannan in Mycobacterium leprae and an ethambutol-resistant strain of Mycobacterium tuberculosis. J Biol Chem. 2004;279:41227–41239. doi: 10.1074/jbc.M405180200. doi:10.1074/jbc.M405180200. [DOI] [PubMed] [Google Scholar]
- Torrelles JB, Knaup R, Kolareth A, Slepushkina T, Kaufman TM, Kang P, Hill PJ, Brennan PJ, Chatterjee D, Belisle JT, et al. Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan. J Biol Chem. 2008;283:31417–31428. doi: 10.1074/jbc.M806350200. doi:10.1074/jbc.M806350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Schlesinger LS. Diversity in M. tuberculosis mannosylated cell envelope determinants impacts adaption to the host. Tuberculosis. 2010;90:84–93. doi: 10.1016/j.tube.2010.02.003. doi:10.1016/j.tube.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrelles JB, Sieling P, Arcos J, Knaup R, Bartling C, Rajaram MVS, Stenger S, Modlin RL, Schlesinger LS. Structural differences in lipomannans from pathogenic and nonpathogenic mycobacteria that impact CD1b-restricted T cell responses. J Biol Chem. 2011;286:35438–35446. doi: 10.1074/jbc.M111.232587. doi:10.1074/jbc.M111.232587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in polyacylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. doi:10.1016/0003-2697(82)90673-X. [DOI] [PubMed] [Google Scholar]
- Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev. 2004;68:132–153. doi: 10.1128/MMBR.68.1.132-153.2004. doi:10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO 2011. Tuberculosis fact sheet http://www.who.int/mediacentre/factsheets/fs104/en/index.html .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.