Abstract
Embryoid body (EB) formation is a traditional method of inducing differentiation of pluripotent stem cells (PSCs). It is a routine in vitro test of pluripotency as well as the first stage in many differentiation protocols targeted toward the production of a specific lineage or cellular population, as in neural differentiation (see Chapters 29 and 30). The induction of differentiation via EB formation is fairly straight-forward. However, depending on the specific PSC culture conditions – substrate, feeders, medium, and eventual cell type of interest – various methods are applied in order to most routinely obtain healthy EB cultures.
Keywords: embryoid body, differentiation, aggrewell, ROCK inhibitor
1. Introduction
In this chapter, we present several protocols for EB formation. These are provided as general examples that have worked well in our hands and that can be used for both feeder-free and feeder-based culture systems. We also present methods for controlling the size of EBs through the use of Aggrewell dishes (1).
2. Materials
2.1. Reagents and Supplies
Matrigel™, reduced growth factor (BD Biosciences #354231).
Dulbecco’s phosphate-buffered saline, without Mg2 and Ca2 (DPBS).
StemPro™ SFM kit (includes 50× supplement, DMEM-F12 with GlutaMax, and 25% BSA Solution, Invitrogen # A1000701).
2-Mercaptoethanol (Invitrogen#21985-023).
Accutase (Millipore# SCR005).
Human bFGF/FGF2 (Stemgent #03-0002).
Y27632 ROCK Inhibitor (Stemgent # 04–0012). Prepare a 10 mM (1,000×) solution: dilute 2 mg of ROCK inhibitor in 643 μL of DMSO. Aliquot and store at −20°C.
Six-well vacuum gas plasma-treated tissue culture dishes (BD Falcon#353046).
Corning Costar Ultra-Low-Attachment six-well Plates (Corning# 3471).
9-in. glass Pasteur pipettes (unplugged).
AggreWell™ 400 plates (Stem Cell Technologies #27845).
Centrifuge, swinging bucket style, high speed capable of 2,000 × g, with adapters to spin cell culture plates.
3. Methods
Although these protocols describe how to make EBs from cells that are grown in a six-well format, they can be scaled for other culture formats as well.
3.1. EB Formation via Cell Clusters
Exchange the medium in the well(s) to be used for differentiated with freshly prepared PSC medium.
Using a dissection microscope stationed within a laminar flow hood, mechanically dissociate healthy, undifferentiated colonies into medium-sized pieces using a needle, pipette tip, or fire- drawn glass Pasteur pipette as described in detail in Chapter 8 under mechanical subculturing (see Note 1).
After the desired colonies have been mechanically dissociated, collect in a tube and transfer, 1:1, to a low-attachment dish (see Note 2).
Place dish in the incubator.
The next day, EBs should be present. They are characterized by the pieces of colonies taking on a round appearance, with smooth borders. Irregularly shaped but smooth-bordered EBs may in fact be several EBs that have agglomerated.
-
Begin feeding on the second day and feed every other day thereafter.
To feed these suspension cultures, collect and pool the contents of the wells in a 15-mL conical tube.
Allow the EBs to settle to the bottom, for about 5 min in the tissue culture hood at room temperature or in the incubator or water bath at 37°C.
Carefully aspirate or pipette off the medium without disturbing the EBs, which have settled in the bottom of the tube.
Add the same volume of fresh, warm medium to the tube and GENTLY resuspend the EBs – excessive trituration will break them apart.
Dispense the EBs suspended in fresh medium into the ultra-low-attachment dish, and return to the incubator.
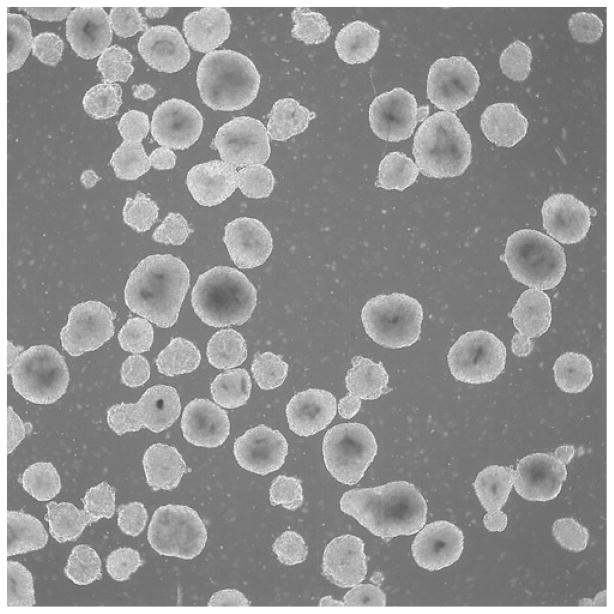
After 4–7 days, the EBs will be ready for plating onto slides for staining, or for further differentiation using a specific protocol (Fig. 1).
Fig. 1.
Phase-contrast image with 4× objective showing EBs after 1 week.
3.2. EB Formation via Dissociation to Single Cells
During EB formation from single cells, the cells are dissociated and resuspended in medium containing ROCK inhibitor to improve cell survival (2). The cells may be allowed to agglomerate and freely form EBs of varying size within an ultra-low-attachment dish or they may be seeded onto an Aggrewell™ dish, where their size can be more easily controlled.
Both variations of EB formation via single cells in suspension begin with the following:
Aspirate medium from the well(s) to be lifted, and gently rinse each well with 1 mL DPBS. Aspirate the DPBS.
Add 1 mL warm Accutase, place at 37°C for about 30 s, and then observe under a microscope to monitor cell dissociation (see Chapter 10).
When the cells appear to be phase-bright, or are noticeably lifting, immediately dilute the cells and Accutase using 9 mL of DPBS, and transfer the suspension to a 15 mL conical tube.
Rinse the well with 5 mL of DPBS to remove any remaining cells, pool with the contents of the 15 mL conical tube, and spin at 100 g for 5 min.
At this point, both protocols call for the introduction of ROCK inhibitor (Y27632), which is a potent inhibitor of apoptosis (see note 3).
3.2.1. Free-Form EB Formation on Low-Attachment Surfaces
Resuspend the pellet in an appropriate volume of ROCK inhibitor-containing culture medium.
Add this suspension to a low-attachment dish. As a simple assay to determine EB-forming ability, plating the cells 1:1 from their original surface area usually produces satisfactory results. Increasing or decreasing the ratio will roughly alter the resulting sphere size, but not in a controlled, quantitative fashion.
3.2.2. Aggrewell™ Plating Method
Aggrewell™ plates make it easier to control the size of the EBs and reduce variability among EBs by controlling the initial starting size of each EB. The plates are similar to a standard 24-well dish, except that the center eight wells are textured to create hundreds of tiny, triangular microwells. When a cell suspension with a known concentration of cells is added to this plate, and the plate is then spun in an adapted centrifuge, each microwell collects a small, defined number of cells, which form an embryoid body. The EBs are removed after a period of 24–48 h for further culture. (The manufacturer’s protocol and full technical data are available at http://www.stemcell.com.)
Resuspend the pellet in 2 mL of culture medium containing 10 μM ROCK inhibitor.
Count the cells using 10 μL of cell suspension, 10 μL of trypan blue, and a hemacytometer.
Refer to the chart in Table 1 (excerpted with permission from SCT) for detail on which seeding density should be used to obtain a particular EB size. Calculate the necessary volume of media to obtain the desired density of cells, while taking into account that each well holds 1 mL of medium. Divide this volume in half, as half the volume will be used to remove air bubbles from the Aggrewell™ plate (see step 6 below). For example, to create eight wells of EBs that are approximately 500 cells each, would require 4.8 × 106 cells. Suspend 4.8 × 106 cells in 4 mL of medium, and proceed.
Fill each of the wells to be seeded with 500 μL of ROCK inhibitor-containing medium.
Place the Aggrewell™ plate (which does not yet contain cells) in a centrifuge with a swinging bucket rotor and the necessary adapters for spinning culture plates (Fig. 2). Spin at 2,000 × g for 5 min. This step is crucial, as it removes bubbles that can become trapped in the microwells. The cells can be placed in an incubator to keep them warm during this time.
After the spin is complete, seed the cell suspension at the appropriate density.
Re-spin the plate at a lower speed of 150 g for 5 min.
Place the plate in the incubator overnight.
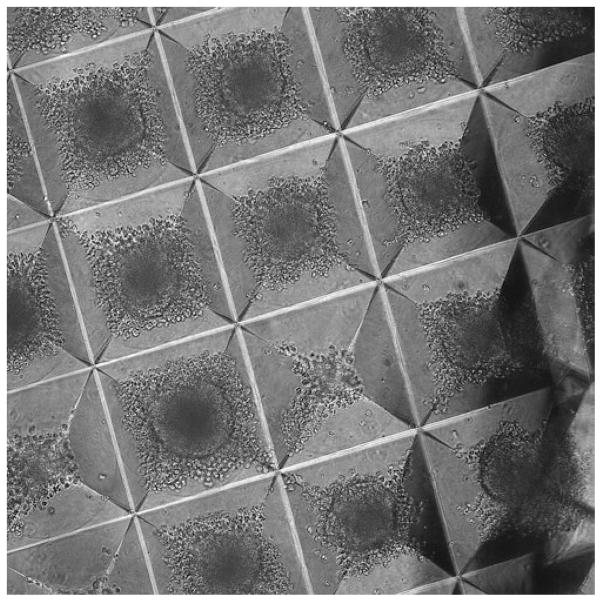
The next day, the cells should have formed EBs (Fig. 3). If they do not appear to have done so, another 2–3 days may be necessary. They should not need to be fed during this time.
When EBs have formed, they can be removed from the Aggrewell™ microwells using gentle trituration and shaking of the plate and further cultured in a low-attachment plate (see Notes 4 and 5, Fig. 4).
Table 1.
Table from stem cell technologies giving seeding densities for generating EBs of various sizes
| Desired number of hESCs/hiPSCs per EB | AggreWell™ 400 plate eight wells, each with approximately 1,200 microwells per well |
|---|---|
| Required number of hESCs/hiPSCs per well | |
| 50 | 6 × 104 cells |
| 100 | 1.2 × 105 cells |
| 200 | 2.4 × 105 cells |
| 500 | 6 × 105 cells |
| 1,000 | 1.2 × 106 cells |
| 2,000 | 2.4 × 106 cells |
| 3,000 | 3.6 × 106 cells |
| 4,000 | 4.8 × 106 cells |
Fig. 2.

Example of a low-speed centrifuge with adapter for spinning Aggrewell plates.
Fig. 3.
EBs forming in an Aggrewell plate.
Fig. 4.
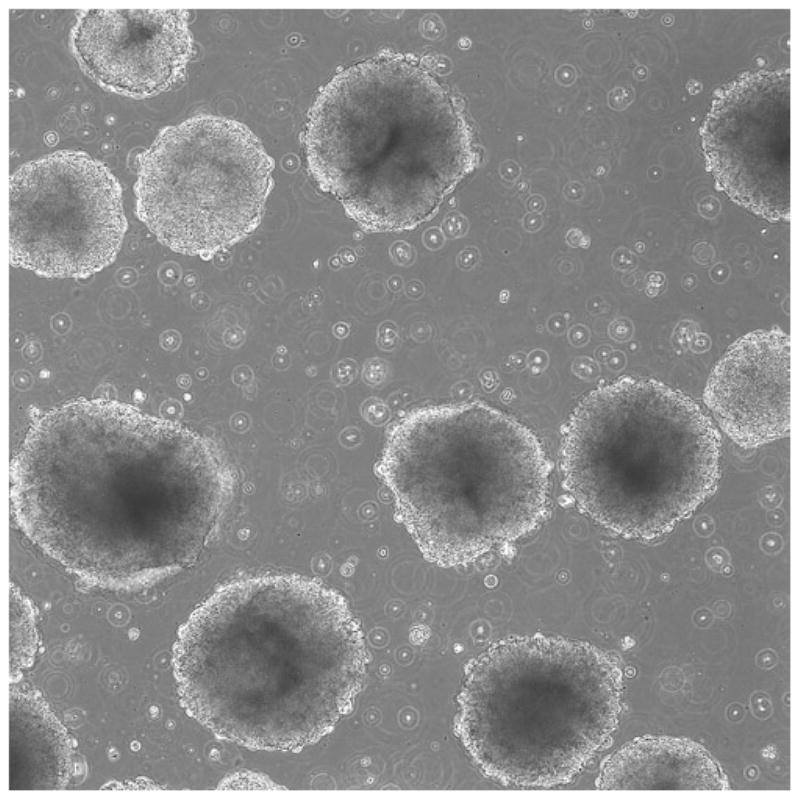
EBs harvested from an Aggrewell plate (10× objective).
Acknowledgments
This work has been funded by the National Institutes of Health (T15HL074286, R21MH087925, R01HD059967). The NCI Preclinical Repository supplied FGF-2. We also recognize Richard Pepple for proof-reading this chapter.
Footnotes
For EB formation, medium-large pieces tend to have the best survival. Extremely small colony chunks of only a few cells in size tend to have very low survival, and make very poor EBs.
If you have passaged a full six-well dish, transfer everything to a low-attachment six-well dish.
Without ROCK inhibitor, there is a strong likelihood that cells will die as a result of the shock of being put into suspension as single cells. We have noticed that some PSC cell lines that have been in culture for a long time are able to survive and form EBs without the use of ROCK inhibitor. However, this has only been at high seeding densities. For younger cell lines seeded at low densities, ROCK inhibitor is crucial.
We have found that the smaller the embryoid body size, the more difficult it is to remove them. Forceful trituration is effective but this often breaks the EBs apart. If small EBs are desired, they may not be easily or safely removed until they have grown to a larger size while still in the microwell. At this time, the effects of extended culture in the microwells are unknown.
Regardless of the method used to create them, EBs have a tendency to agglomerate once they are pooled into a low attachment dish. This adds a further complication to controlling sphere size. If the conjoined EBs become too large (greater than 1 mm across), the cells on the inside of the mass will have only restricted access to oxygen and nutrients, and viability will suffer. In some variations of differentiation protocols, limited conjoining of EBs is desired. For example, in our lab we have found that conjoined masses of 5–10 EBs tend to form rosettes well when seeded back onto a substrate. For other protocols, this may not be the case, however, and steps may be taken to prevent this. Other researchers have reported seeding EBs at an extremely low density, as well as routine gentle trituration to discourage aggregation.
References
- 1.Ungrin MD, Joshi C, Nica A, Bauwens C, Zandstra PW. Reproducible, Ultra-High-Throughput Formation of Multicellular Organization from Single Cell Suspension-Derived Human Embryonic Stem Cell Aggregates. PLoS One. 2008;3(2):e1565. doi: 10.1371/journal.pone.0001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]