Abstract
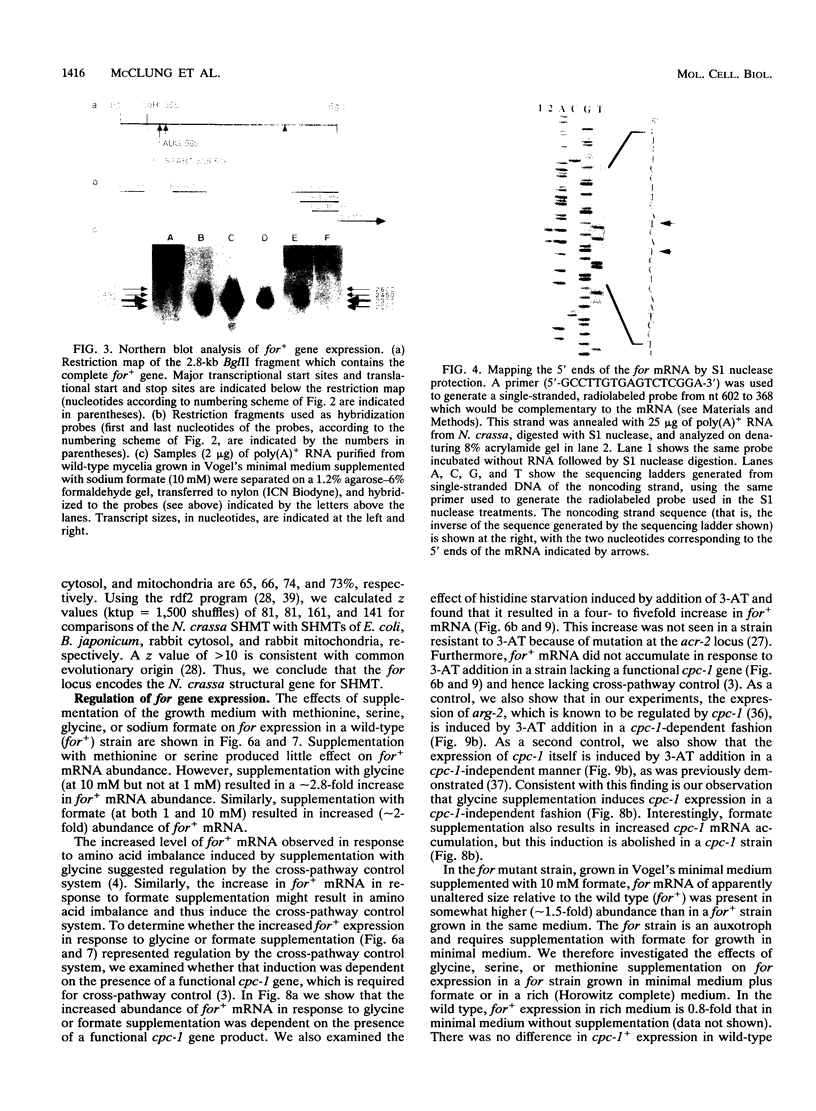
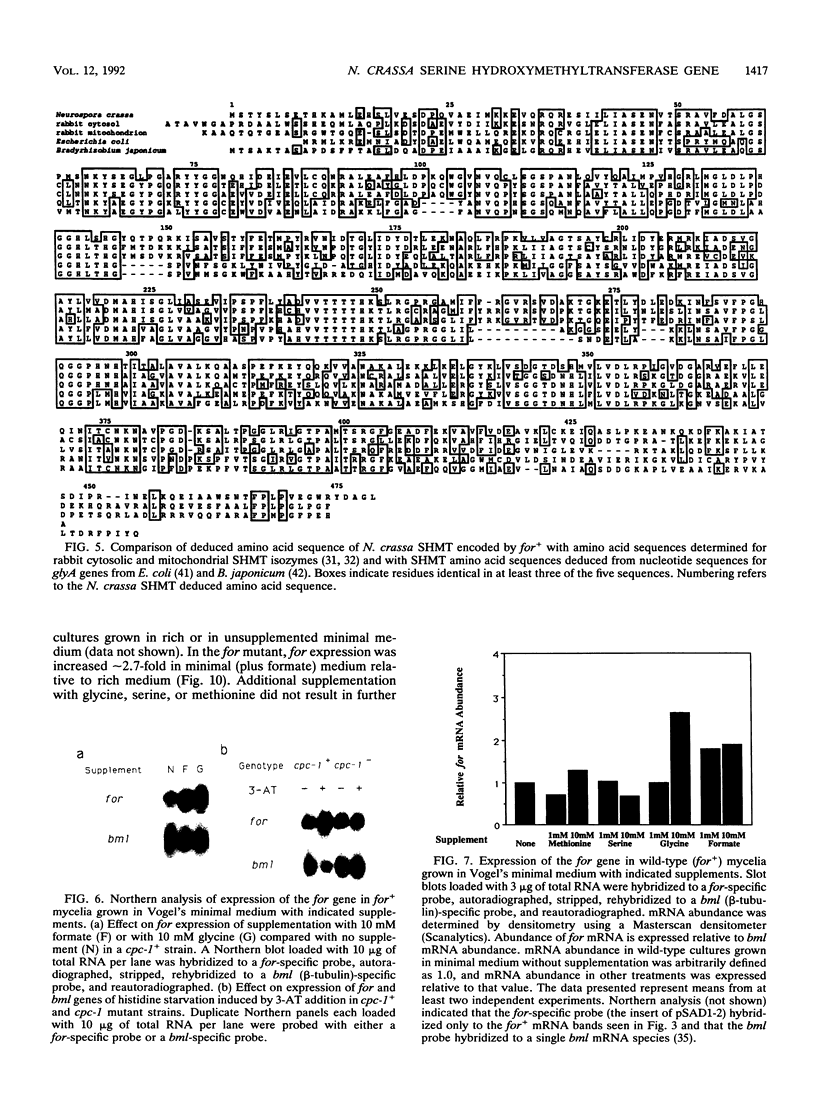
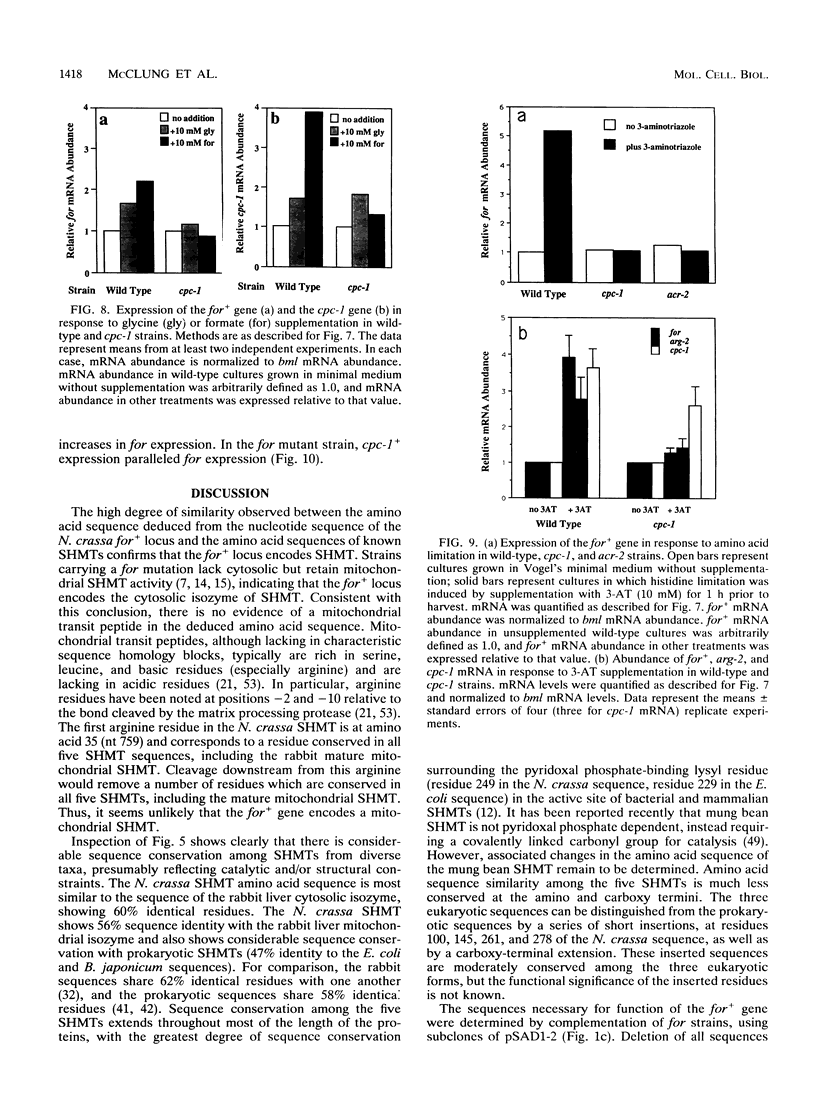
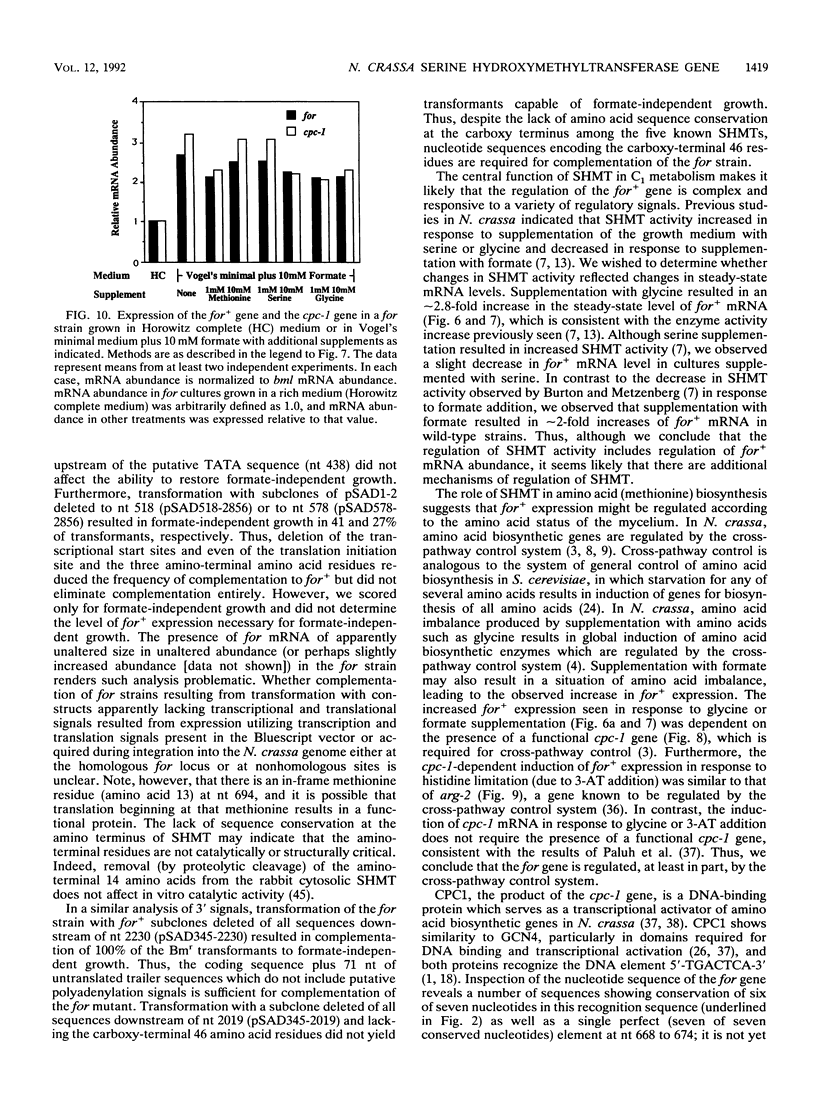
Serine hydroxymethyltransferase (SHMT) occupies a central position in one-carbon (C1) metabolism, catalyzing the reaction of serine and tetrahydrofolate to yield glycine and 5,10-methylenetetrahydrofolate. Methylenetetrahydrofolate serves as a donor of C1 units for the synthesis of numerous compounds, including purines, thymidylate, lipids, and methionine. We provide evidence that the formate (for) locus of Neurospora crassa encodes cytosolic SHMT. The for+ gene was localized to a 2.8-kb BglII fragment by complementation (restoration to formate-independent growth) of a strain carrying a recessive for allele, which confers a growth requirement for formate. The for+ gene encodes a polypeptide of 479 amino acids which shows significant similarity to amino acid sequences of SHMT from bacterial and mammalian sources (47 and 60% amino acid identity, respectively). The for+ mRNA has several different start and stop sites. The abundance of for+ mRNA increased in response to amino acid imbalance induced by glycine supplementation, suggesting regulation by the N. crassa cross-pathway control system, which is analogous to general amino acid control in Saccharomyces cerevisiae. This was confirmed by documenting that for+ expression increased in response to histidine limitation (induced by 3-amino-1,2,4-triazole) and that this response was dependent on the presence of a functional cross-pathway control-1 (cpc-1) gene, which encodes CPC1, a positively acting transcription factor. There are at least five potential CPC1 binding sites upstream of the for+ transcriptional start, as well as one that exactly matches the consensus CPC1 binding site in the first intron of the for+ gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt K., Fink G. R. GCN4 protein, a positive transcription factor in yeast, binds general control promoters at all 5' TGACTC 3' sequences. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8516–8520. doi: 10.1073/pnas.83.22.8516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthelmess I. B. Mutants affecting amino acid cross-pathway control in Neurospora crassa. Genet Res. 1982 Apr;39(2):169–185. doi: 10.1017/s0016672300020863. [DOI] [PubMed] [Google Scholar]
- Barthelmess I. B. Regulation of amino acid synthetic enzymes in Neurospora crassa in the presence of high concentrations of amino acids. Mol Gen Genet. 1986 Jun;203(3):533–537. doi: 10.1007/BF00422082. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Yanofsky C. Nucleotide sequence of the Neurospora crassa trp-3 gene encoding tryptophan synthetase and comparison of the trp-3 polypeptide with its homologs in Saccharomyces cerevisiae and Escherichia coli. J Biol Chem. 1989 Mar 5;264(7):3840–3848. [PubMed] [Google Scholar]
- Burton E. G., Metzenberg R. L. Regulation of methionine biosythesis in Neurospora crassa. Arch Biochem Biophys. 1975 May;168(1):219–229. doi: 10.1016/0003-9861(75)90244-1. [DOI] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F. Cross-pathway regulation: tryptophan-mediated control of histidine and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):889–892. doi: 10.1128/jb.119.3.889-892.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsiotis M., Jones R. F., Wesseling A. C. Cross-pathway regulation: histidine-mediated control of histidine, tryptophan, and arginine biosynthetic enzymes in Neurospora crassa. J Bacteriol. 1974 Sep;119(3):893–898. doi: 10.1128/jb.119.3.893-898.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C. M., Rajbhandary U. L. Regulation of the nuclear genes encoding the cytoplasmic and mitochondrial leucyl-tRNA synthetases of Neurospora crassa. Mol Cell Biol. 1989 Nov;9(11):4645–4652. doi: 10.1128/mcb.9.11.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins E. A., Chan P. Y., Combepine G. One-carbon metabolism in Neurospora crassa wild-type and in mutants partially deficient in serine hydroxymethyltransferase. Biochem J. 1976 Nov 15;160(2):305–314. doi: 10.1042/bj1600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossins E. A., Chan P. Y., Combepine G. Stimulation of folate metabolism by exogenous glycine in Neurospora crassa wild type. FEBS Lett. 1975 Jun 15;54(2):286–290. doi: 10.1016/0014-5793(75)80094-9. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbole D. J., Paluh J. L., Plamann M., Sachs M. S., Yanofsky C. cpc-1, the general regulatory gene for genes of amino acid biosynthesis in Neurospora crassa, is differentially expressed during the asexual life cycle. Mol Cell Biol. 1991 Feb;11(2):928–934. doi: 10.1128/mcb.11.2.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Melvold R. W., Nathenson S. G. Mitotic recombination in germ cells generated two major histocompatibility complex mutant genes shown to be identical by RNA sequence analysis: Kbm9 and Kbm6. Proc Natl Acad Sci U S A. 1986 May;83(10):3371–3375. doi: 10.1073/pnas.83.10.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARROLD C. E., FLING M. Two mutants of Neurospora crassa which utilize formate or formaldehyde for growth. J Biol Chem. 1952 Jan;194(1):399–406. [PubMed] [Google Scholar]
- Hendrick J. P., Hodges P. E., Rosenberg L. E. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4056–4060. doi: 10.1073/pnas.86.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III in DNA sequence analysis. Methods Enzymol. 1987;155:156–165. doi: 10.1016/0076-6879(87)55014-5. [DOI] [PubMed] [Google Scholar]
- Hilton J. L., Kearney P. C., Ames B. N. Mode of action of the herbicide, 3-amino-1,2,4-triazole(amitrole): inhibition of an enzyme of histidine biosynthesis. Arch Biochem Biophys. 1965 Dec;112(3):544–547. doi: 10.1016/0003-9861(65)90093-7. [DOI] [PubMed] [Google Scholar]
- Hinnebusch A. G. Mechanisms of gene regulation in the general control of amino acid biosynthesis in Saccharomyces cerevisiae. Microbiol Rev. 1988 Jun;52(2):248–273. doi: 10.1128/mr.52.2.248-273.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Loros J. J., Denome S. A., Dunlap J. C. Molecular cloning of genes under control of the circadian clock in Neurospora. Science. 1989 Jan 20;243(4889):385–388. doi: 10.1126/science.2563175. [DOI] [PubMed] [Google Scholar]
- Martini F., Angelaccio S., Pascarella S., Barra D., Bossa F., Schirch V. The primary structure of rabbit liver cytosolic serine hydroxymethyltransferase. J Biol Chem. 1987 Apr 25;262(12):5499–5509. [PubMed] [Google Scholar]
- Martini F., Maras B., Tanci P., Angelaccio S., Pascarella S., Barra D., Bossa F., Schirch V. The primary structure of rabbit liver mitochondrial serine hydroxymethyltransferase. J Biol Chem. 1989 May 25;264(15):8509–8519. [PubMed] [Google Scholar]
- McClung C. R., Fox B. A., Dunlap J. C. The Neurospora clock gene frequency shares a sequence element with the Drosophila clock gene period. Nature. 1989 Jun 15;339(6225):558–562. doi: 10.1038/339558a0. [DOI] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Orbach M. J., Porro E. B., Yanofsky C. Cloning and characterization of the gene for beta-tubulin from a benomyl-resistant mutant of Neurospora crassa and its use as a dominant selectable marker. Mol Cell Biol. 1986 Jul;6(7):2452–2461. doi: 10.1128/mcb.6.7.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orbach M. J., Sachs M. S., Yanofsky C. The Neurospora crassa arg-2 locus. Structure and expression of the gene encoding the small subunit of arginine-specific carbamoyl phosphate synthetase. J Biol Chem. 1990 Jul 5;265(19):10981–10987. [PubMed] [Google Scholar]
- Paluh J. L., Orbach M. J., Legerton T. L., Yanofsky C. The cross-pathway control gene of Neurospora crassa, cpc-1, encodes a protein similar to GCN4 of yeast and the DNA-binding domain of the oncogene v-jun-encoded protein. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3728–3732. doi: 10.1073/pnas.85.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh J. L., Yanofsky C. Characterization of Neurospora CPC1, a bZIP DNA-binding protein that does not require aligned heptad leucines for dimerization. Mol Cell Biol. 1991 Feb;11(2):935–944. doi: 10.1128/mcb.11.2.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D. D., Radford A., Newmeyer D., Björkman M. Chromosomal loci of Neurospora crassa. Microbiol Rev. 1982 Dec;46(4):426–570. doi: 10.1128/mr.46.4.426-570.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamann M. D., Stauffer L. T., Urbanowski M. L., Stauffer G. V. Complete nucleotide sequence of the E. coli glyA gene. Nucleic Acids Res. 1983 Apr 11;11(7):2065–2075. doi: 10.1093/nar/11.7.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossbach S., Hennecke H. Identification of glyA as a symbiotically essential gene in Bradyrhizobium japonicum. Mol Microbiol. 1991 Jan;5(1):39–47. doi: 10.1111/j.1365-2958.1991.tb01824.x. [DOI] [PubMed] [Google Scholar]
- Sachs M. S., Bertrand H., Metzenberg R. L., RajBhandary U. L. Cytochrome oxidase subunit V gene of Neurospora crassa: DNA sequences, chromosomal mapping, and evidence that the cya-4 locus specifies the structural gene for subunit V. Mol Cell Biol. 1989 Feb;9(2):566–577. doi: 10.1128/mcb.9.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirch V., Schirch D., Martini F., Bossa F. Serine hydroxymethyltransferase. Effect of proteases on the activity and structure of the cytosolic enzyme. Eur J Biochem. 1986 Nov 17;161(1):45–49. doi: 10.1111/j.1432-1033.1986.tb10122.x. [DOI] [PubMed] [Google Scholar]
- Snell K., Natsumeda Y., Eble J. N., Glover J. L., Weber G. Enzymic imbalance in serine metabolism in human colon carcinoma and rat sarcoma. Br J Cancer. 1988 Jan;57(1):87–90. doi: 10.1038/bjc.1988.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer G. V., Plamann M. D., Stauffer L. T. Construction and expression of hybrid plasmids containing the Escherichia coli glyA genes. Gene. 1981 Jun-Jul;14(1-2):63–72. doi: 10.1016/0378-1119(81)90148-7. [DOI] [PubMed] [Google Scholar]
- Steiert J. G., Rolfes R. J., Zalkin H., Stauffer G. V. Regulation of the Escherichia coli glyA gene by the purR gene product. J Bacteriol. 1990 Jul;172(7):3799–3803. doi: 10.1128/jb.172.7.3799-3803.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukanya N., Vijaya M., Savithri H. S., Radhakrishnan A. N., Rao N. A. Serine Hydroxymethyltransferase from Mung Bean (Vigna radiata) Is Not a Pyridoxal-5'-Phosphate-Dependent Enzyme. Plant Physiol. 1991 Feb;95(2):351–357. doi: 10.1104/pp.95.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowski M. L., Plamann M. D., Stauffer L. T., Stauffer G. V. Cloning and characterization of the gene for Salmonella typhimurium serine hydroxymethyltransferase. Gene. 1984 Jan;27(1):47–54. doi: 10.1016/0378-1119(84)90237-3. [DOI] [PubMed] [Google Scholar]
- Viebrock A., Perz A., Sebald W. The imported preprotein of the proteolipid subunit of the mitochondrial ATP synthase from Neurospora crassa. Molecular cloning and sequencing of the mRNA. EMBO J. 1982;1(5):565–571. doi: 10.1002/j.1460-2075.1982.tb01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer S. J., Yanofsky C. Efficient cloning of genes of Neurospora crassa. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4869–4873. doi: 10.1073/pnas.83.13.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waziri R., Baruah S., Hegwood T. S., Sherman A. D. Abnormal serine hydroxymethyl transferase activity in the temporal lobes of schizophrenics. Neurosci Lett. 1990 Dec 11;120(2):237–240. doi: 10.1016/0304-3940(90)90048-e. [DOI] [PubMed] [Google Scholar]
- Waziri R., Wilcox J., Sherman A. D., Mott J. Serine metabolism and psychosis. Psychiatry Res. 1984 Jun;12(2):121–136. doi: 10.1016/0165-1781(84)90012-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]