Abstract
Peroxisome proliferator-activated receptor gamma (PPARγ) is emerging as a new pharmacotherapeutic target for chronic pain. When oral (3–30 mg/kg/day in chow for 7 wk) or twice-daily intraperitoneal (1–10 mg/kg/day for 2 wk) administration began before spared nerve injury (SNI), pioglitazone, a PPARγ agonist, dose-dependently prevented multiple behavioral signs of somatosensory hypersensitivity. The highest dose of intraperitoneal pioglitazone did not produce ataxia or reductions in transient mechanical and heat nociception, indicating that inhibitory effects on hypersensitivity were not secondary to adverse drug-induced behaviors or antinociception. Inhibitory effects on hypersensitivity persisted at least one week beyond cessation of pioglitazone administration, suggestive of long-lasting effects on gene expression. Blockade of PPARγ with GW9662, an irreversible and selective PPARγ antagonist, dose-dependently reduced the inhibitory effect of pioglitazone on hypersensitivity, indicating a PPARγ-dependent action. Remarkably, a single preemptive injection of pioglitazone 15 min before SNI attenuated hypersensitivity for at least 2 weeks; this was enhanced with a second injection delivered 12 hr after SNI. Pioglitazone injections beginning after SNI also reduced hypersensitivity, albeit to a lesser degree than preemptive treatment. Intraperitoneal pioglitazone significantly reduced the nerve injury-induced up-regulation of cd11b, GFAP, and p-p38 in the dorsal horn, indicating a mechanism of action involving spinal microglia and/or astrocyte activation. Oral pioglitazone significantly reduced touch stimulus-evoked phospho-extracellular signal-related kinase (p-ERK) in lamina I-II, indicating a mechanism of action involving inhibition of central sensitization. We conclude that pioglitazone reduces spinal glial and stimulus-evoked p-ERK activation and that PPARγ activation blocks the development of and reduces established neuropathic pain.
Keywords: Allodynia, Hyperalgesia, Nerve injury, Pioglitazone, extracellular signal-related kinase, Microglia, Astrocyte
1. INTRODUCTION
Neuropathic pain is defined as the pain caused by a lesion or disease of the somatosensory system (Jensen et al., 2011). Nerve injury produces numerous neurobiological events in the peripheral and central nervous system that contribute to chronic pain (Taylor, 2009). The mechanisms in the dorsal horn that underlie enhanced pain signaling include post-translational modifications (Woolf and Mannion, 1999) and microglia and/or astrocyte activation (Milligan and Watkins, 2009). For example, nerve injury induces activation of mitogen-activated protein kinases (MAPKs), as reflected by the phosphorylation of extracellular signal-regulated kinase (ERK) and p38 in spinal microglia (Ji et al., 2009; Jin et al., 2003; Zhuang et al., 2005). Such mechanisms provide multiple pharmacological targets for the treatment of chronic pain, but further research is required to improve neuropathic pain relief beyond currently available analgesic drugs, which yield only limited beneficial outcomes and produce serious side effects (Finnerup et al., 2010).
As insulin sensitizers, synthetic peroxisome proliferator-activated receptor gamma (PPARγ) agonists of the thiazolidinedione (TZD) class, such as rosiglitazone and pioglitazone, remain an important pharmacotherapeutic class for the treatment of type II diabetes (Evans et al., 2004; Martens et al., 2002). Beginning with our finding that intrathecal administration of rosiglitazone reduced behavioral signs of allodynia and hyperalgesia in the spared nerve injury (SNI) model of neuropathic pain (Churi et al., 2008), a growing number of studies report that systemic administration of TZDs reduce peripheral neuropathic pain (Jia et al., 2010; Maeda et al., 2008; Takahashi et al., 2011). PPARγ immunoreactivity is found in spinal cord and brain (Maeda et al., 2008; Moreno et al., 2004; Victor et al., 2006; Zhao et al., 2006). Thus, the translational potential of future TZD drugs for neuropathic pain is particularly exciting. Pioglitazone has a particular advantage as an experimental drug in that it readily crosses the blood brain barrier (Maeshiba et al., 1997) and thus has access to the PPARγ that is expressed in the dorsal horn of the spinal cord (Churi et al., 2008; Shibata et al., 2008). Furthermore, pioglitazone continues to be FDA-approved as a one-year therapy for diabetes. However, the existing literature provides only limited or conflicting information regarding the mechanism of anti-allodynic and anti-hyperalgesic action of TZDs such as pioglitazone (Jia et al., 2010; Maeda et al., 2008; Takahashi et al., 2011). Therefore, the present study evaluated timing, duration of action, and PPARγ-antagonist reversibility following oral and/or intraperitoneal administration. Most importantly, we tested the hypothesis that chronic administration of pioglitazone acts as a PPARγ agonist to inhibit the induction and/or maintenance phases of neuropathic pain.
To address this question, we used the intrathecal route to administer the selective PPARγ antagonist GW9662 before systemic pioglitazone. GW9662 has nanomolar affinity for PPARγ in binding experiments, with 10 and 600-fold less potency at PPARα and PPARδ, respectively (Leesnitzer et al., 2002). In addition, we evaluated the effect of pioglitazone on phosphorylation of extracellular signal-regulated kinase (p-ERK) in the superficial dorsal horn. P-ERK has emerged as a marker of increased neuronal activation in response to peripheral stimulation in inflammatory and neuropathic pain models (Gao and Ji, 2009; Ji et al., 1999; Zhuang et al., 2005).
Events that induce hyperalgesia, such as nerve injury, activate glia in the spinal cord (Ji and Suter, 2007; Milligan and Watkins, 2009; Scholz and Woolf, 2007; Svensson and Brodin, 2010). Spinal glia express a variety of receptors for neurotransmitters and neuromodulators, and produce and release numerous signaling molecules that could ultimately contribute to central sensitization and chronic pain (Milligan and Watkins, 2009). Because PPARγ agonists reduce glial activation in vitro (Bernardo et al., 2000; Jiang et al., 1998; Ricote et al., 1998) and in vivo in brain (Heneka et al., 2005; Petrova et al., 1999; Schintu et al., 2009) and spinal cord (Sauerbeck et al., 2011; Shibata et al., 2008), it is conceivable that they would have similar effects in nociceptive regions of the dorsal horn, ultimately leading to reductions in pain. Therefore, we administered pioglitazone and used antibodies against cd11b and glial fibrillary acidic protein (GFAP) to assess the spinal expression of microglia and astrocytes, respectively. We also measured p-p38 which is predominantly expressed in microglia (Ji and Suter, 2007).
We found that chronic pioglitazone reduces spinal glial and ERK activation, and operates at PPARγ to block the development of and reduce established neuropathic pain. The current results substantially extend our understanding of the pharmacodynamics and mechanism of the anti-allodynic and anti-hyperalgesic actions of TZDs, and, most importantly, establish spinal PPARγ systems as a promising pharmacotherapeutic target for the treatment of neuropathic pain using new PPARγ agonists.
2 METHODS
2.1 Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 200–300g at the time of surgery were housed 2–3 per bedded cage on a 12-hour light/dark cycle (7am/7pm) in a temperature (68–72° F) and humidity-controlled room with food and water provided ad libitum. All efforts were made to minimize animal suffering, to reduce the number of animals used, and to utilize alternatives to in vivo techniques, in accordance to the guidelines set forth by the National Institutes of Health (NIH Publications No.8023) and International Association for the Study of Pain regarding the proper treatment and use of laboratory animals. Tulane University and University of Kentucky Institutional Animal Care and Use Committees approved all experiments involving animals.
2.2 Spared nerve injury
Surgical anesthesia was achieved with isoflurane (5% induction, 1.5% maintenance in oxygen). As previously described (Decosterd and Woolf, 2000), an incision was made in the skin at the site of the trifurcation of the left sciatic nerve. The overlying muscles were retracted, exposing the common peroneal, tibial, and sural nerves. The common peroneal and tibial nerves were ligated with 6-0 silk (Ethicon, Somerville, NJ) and then the knot and adjacent nerve (2mm) were transected. Care was taken to avoid disturbing the sural nerve. In sham surgery, the overlying muscles were retracted to expose the nerves and then the muscle was sutured leaving all nerves intact. For SNI and sham surgeries, the muscle was sutured with 4-0 absorbable sutures (Ethicon) and the skin was closed with 9mm stainless steel wound clips. The day of SNI or sham surgery is referred to as day 0.
2.3 Behavioral tests of hyperalgesia and allodynia
Animals that received SNI were acclimated to a stainless steel grid within individual Plexiglas boxes for 60 min, and then first tested for mechanical allodynia, then cold allodynia, and then mechanical hyperalgesia. The observer (JM) was blinded to treatment by another experimenter.
2.3.1 Mechanical allodynia: von Frey
Mechanical allodynia was assessed using von Frey filaments (Stoelting, Inc, Wooddale, IL). The sural innervation of the lateral aspect of the plantar surface of each hind paw was mechanically stimulated with an incremental series of 8 monofilaments of logarithmic stiffness. Three areas of the sural receptive field were examined, in the following order: lateral heel, lateral mid-paw, and outermost digit. The 50% withdrawal threshold was determined using a modified up-down method of Dixon, as previously described (Chaplan et al., 1994). First, an intermediate von Frey monofilament (number 4.31, exerts 2.0g of force) was applied perpendicular to the glabrous skin, causing a slight bending. In the case of a positive response (immediate withdraw of the paw) in any of the three areas tested, a filament exerting less force was tested. In the case of a negative response to all three areas of stimulation, a filament exerting a larger force was tested.
2.3.2 Cold allodynia: acetone
A drop of acetone was applied to the plantar surface of the hindpaw using a syringe connected to PE-90 tubing, flared at the tip to a diameter of 3.5 mm. Surface tension maintained the volume of the drop at 10–12µl. The duration of time the animal would lift, shake or lick its paw was recorded. Animals were observed for 30 sec following each application of acetone. Three trials, with an interval of at least 3 min between each, were averaged.
2.3.3 Mechanical hyperalgesia: pin prick
Noxious pressure was applied to the lateral surface of the hindpaw using a safety pin to elicit a response. The duration of time the animal lifted, shook or licked its paw was recorded. Animals were observed for 30 sec following each pin application. Three trials, with an interval of at least 3 min between each, were averaged.
2.4 Behavioral tests of transient nociception and ataxia
2.4.1 Mechanical Nociception
Prior to baseline measurements, naïve rats were gently restrained with a towel and handled for 4 consecutive days, 5 min/day. On the day of testing, animals were gently wrapped in a towel, and increasing pressure was applied to the left and then right hind paw using the Paw Pressure Analgesia Instrument (Stoelting, Wood Dale, IL). Four observations were averaged.
2.4.2 Heat Nociception
Animals that received sham surgery were acclimated to an 8” × 8” clear Plexiglas box on a Plexiglas floor (Ugo Basile, Italy) for at least 1 hr. Absorbent paper towels, initially placed under the animal, were removed at least 30 min before testing. To facilitate acclimation and response reliability, fluctuations in room noise, vibrations and temperature were minimized. The thermal stimulus consisted of a radiant heat source (8V, 50W lamp, Ugo Basile, Italy) positioned under the glass floor directly beneath the hind paw. When triggered, a timer was activated, and light passed through a small aperture at the top of a movable case. One day before testing, voltage intensity was adjusted to standardize the average latency to paw withdrawal at 8±2 sec. At specified time-points, the stimulus was applied to the left paw (ipsilateral to injury) and the latency to paw withdraw was recorded. Three observations were averaged. If the rat did not respond within 20 s, the heat was discontinued to prevent tissue damage.
2.4.3 Ataxia
To test for possible effects of pioglitazone on motor coordination, naive rats were placed on an accelerating rotarod (Stoelting, Wood Dale, IL). The rotating bar was set to accelerate at a constant rate over 5 min, from an initial speed of 4 rpm to a final speed of 40 rpm. Rats quickly learn to walk on the rotarod, reaching a plateau within several acceleration trials. Therefore, training trials were repeated 5–10 times, until the average Pre-drug latency to fall was approximately 180 sec. One day later, we administered vehicle or pioglitazone. Each was administered i.p. twice daily at 9am and 4pm for 7 days. Animals were not exposed to the rotarod during this period. Post-drug testing was performed 2 hr after the 9am injection on day 7.
2.5 Drug administration, experimental timelines, and study design
2.5.1 Oral
Animals were randomly placed on research diets (Teklad 2018, Harlan Labs, Indianapolis, IN) that were factory customized to include Actos® (which contains 25% pioglitazone hydrochloride). The initial diet, begun 7 days before behavioral testing and surgery, was designed for rats weighing 250 ± 75g. Assuming a daily food consumption average of 20g, diets contained 0 (standard rat chow), 0.015, 0.15 or 1.5g of Actos® per kg of food to yield daily doses of 0, 0.3, 3.0 or 30.0mg/kg/day, respectively. After 4 weeks, pioglitazone concentration was increased to account for increased weight (375 ± 75g). Diets contained 0, 0.0225, 0.225 or 2.25g of Actos® per kg of food to continue the daily doses of 0, 0.3, 3.0 or 30.0mg/kg/day, respectively. On any given session, 1–2 animals from each group were tested in a randomized manner.
Six weeks after SNI, some animals were anesthetized with isoflurane (2%), and the lateral aspect of the hindpaw on the side ipsilateral to nerve injury was stroked from heel to toe with a cotton swab for 2 sec. This was repeated every five seconds for five minutes. After three additional minutes, isoflurane concentration was increased to 5% for two minutes, and the animals were perfused and spinal cords dissected and sliced for quantitative immunohistochemisty as described below.
2.5.2 Intraperitoneal
Pioglitazone potassium salt (Cayman Chemicals, Europe) and the PPARγ antagonist GW9662 (Sigma, St. Louis, MO) were dissolved in 0.9% sterile saline and 50% dimethyl sulfoxide in 0.1M PBS, respectively. Baseline behavioral measurements were taken, and animals were allocated into groups such that pre-drug baselines were similar. This matching was followed by injection of drug or vehicle (0.9% sterile saline). The first injection of pioglitazone (0.5–5mg/kg) or vehicle was administered 15 min before SNI, followed by an additional injection (0.5–5 mg/kg) at 4 pm, for a total daily dose of 1, 3 or 10 mg/kg. Subsequent injections continued at 9 am and 4 pm for an additional 7 days. GW9662 or vehicle (50% dimethyl sulfoxide) was administered 15 min prior to pioglitazone at 8:45am and 3:45pm, for a total daily dose of 0.1, 0.5 or 2 mg/kg. Fast anti-allodynic and anti-hyperalgesic actions of pioglitazone last up to 2.5 hr (Churi et al., 2008), so behavioral testing was initiated after 11:30 am (but before the 2nd injection at 4pm). On day 14, spinal cords were harvested for immunohistochemistry.
To evaluate the effect of timing of administration relative to SNI, six groups of rats (n=6 each) were tested concurrently and in randomized order using a balanced design with the experimenter blinded to treatment. Six animals, one from each group, were tested during each session in random order. The highest dose of pioglitazone (10mg/kg/day) was administered at one or more of the following times relative to SNI surgery: a single injection 15 min before SNI, a single injection 12 hr after SNI, or twice-daily injections begun 24 hr after SNI and lasting through day 7. Pioglitazone (P) or saline (S) was administered to the groups as follows: P at 15min, 12hr, and 24+ hr (PPP); P at 15min and 12hr, S at 24+ hr (PPS); S at 15min, P at 12hr and 24+ hr (SPP); P at 15min, S at 12 and 24+ hr (PSS); S at 15min and 12h, P at 24+ h (SSP); S at 15min, 12h, and 24+ h (SSS).
2.6 Immunohistochemistry
Animals were deeply anesthetized with pentobarbital (Fatal Plus, 200 mg/kg i.p., Med-Vet International, Mettawa, IL) or isoflurane (for p-ERK studies) and perfused transcardially with 200 ml of room temperature 0.1M phosphate buffered saline (PBS) with heparin (10,000 USP units/L) followed by 300 ml of ice-cold fixative (10% buffered formalin). The cord was removed and post-fixed for 4 hr in 10% buffered formalin and then cryoprotected (30% sucrose in 0.1M PBS for 36–96 hr). Transverse sections (40µm) were cut on a freezing microtome and collected in 0.1M PBS. The sections were washed three times in 0.1M PBS and then pretreated with 3% normal goat serum and 0.3% Triton X-100 to block non-specific binding. Sections were then incubated in a primary antibody, either GFAP (1:1000, ab7779, Abcam, Cambridge, MA), cd11b (1:2000, CBL1512, Chemicon, Billerica, MA), p-ERK (1:200, #4370, Cell Signaling Technology, Danvers, MA) or p-p38 (1:200, #4511, Cell Signaling Technology,) overnight at room temperature on a slow rocker. The tissue was then washed three times in 0.1M PBS, and incubated in goat anti-rabbit or goat anti-mouse secondary antibody for either enzyme (1:200 dilution) or fluorescent (1:700 dilution) labeling. For enzyme labeling, tissue was incubated in biotinylated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) for 2 hrs, washed in 0.1M PBS, exposed to avidin-biotin-peroxidase complex for 1hr, and the chromogen developed with 0.01% hydrogen peroxide and visualized with 0.05% diaminobenzidine. The tissue was then washed with 0.1M phosphate buffered distilled water (PB), mounted onto Superfrost Plus slides, air dried for at least 12 hours, dehydrated in a series of graded alcohols, cleared in xylene and cover-slipped. For fluorescent labeling, tissue was incubated in a secondary antibody conjugated to Alexa488 (1:700, Molecular Probes, Grand Island, NY) for 90 min, washed in 0.1M PBS followed by 0.01M PB, mounted onto Superfrost Plus slides, air dried, and cover-slipped with Prolong Gold with DAPI mounting medium (Molecular Probes).
2.7 Quantification of immunohistochemistry
All images were captured with a Nikon Eclipse TE2000-E microscope. Unless otherwise specified, regions of interest included lamina I-V of the dorsal horn at segments L4-L5.
GFAP- and cd11b-immunoreactivities were quantified with NIH ImageJ software. Integrated density was determined by thresholding the images using the default algorithm within ImageJ to reduce background and include positively stained cells in spinal cord dorsal horns from the L4-L5 lumbar region. Integrated density of the region of interest (ROI) is equal to the product of ROI area and mean gray value. The mean gray value represents the sum of the intensity values for all pixels above the threshold in the ROI divided by the number of pixels above threshold within the ROI. This method controls for differences in background between slices and subjects. For quantification of p-p38, an observer blinded to treatment manually counted punctate immunoreactive profiles in lamina I-V. Six animals per group and 4–6 slices per animal were quantified for cd11b, GFAP, and p-p38.
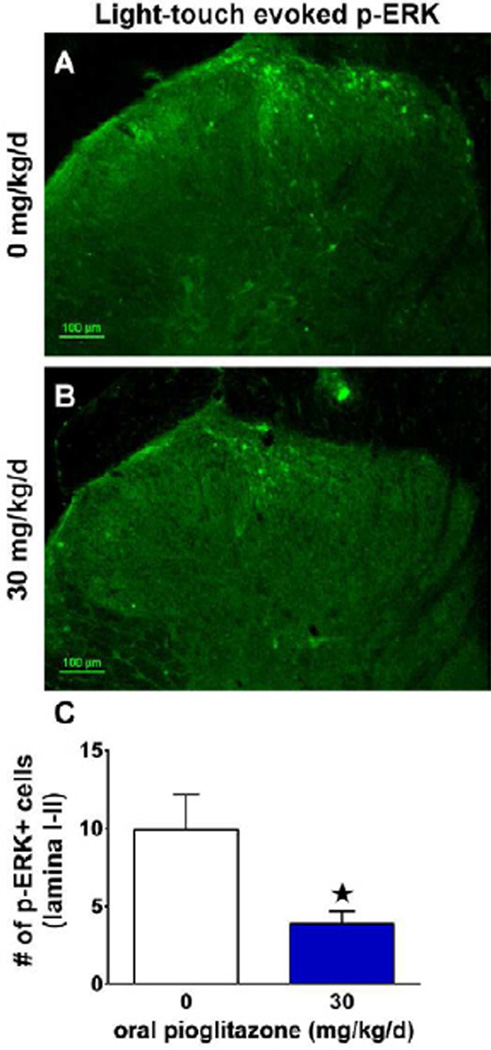
Phosphorylated Extracellular Signal-regulated Kinase (pERK) in Fig 7 was evoked by light-touch stimulation of the lateral hindpaw ipsilateral to spared nerve injury, followed by perfusion 10 min later. An observer blinded to treatment quantified the number of p-ERK positive profiles in lamina I-II of the L4-L5 dorsal horn ipsilateral to stimulation. The average number of p-ERK+ profiles per slice was calculated in 3–8 slices per animal, n=5–6 animals per group, and compared using an unpaired t-test with assumption of equal variance. The alpha value was pre-determined at p<0.05 for statistically significant differences between groups treated with 0 or 30 mg/kg/d pioglitazone.
Figure 7. Pioglitazone reduced light touch-evoked spinal ERK activation after SNI.
(A) Representative image 42 d after spared nerve injury showing that mechanical stimulation of the sural receptive field ipsilateral to injury (every 5 seconds for 5 minutes) resulted in a greater number of p-ERK positive profiles than the unstimulated contralateral dorsal horn (p<0.01; data not shown) in rats consuming standard rat chow (0 mg/kg/d pioglitazone). (B) Representative image showing a reduced number of p-ERK+ profiles in rats that were provided with 30 mg/kg/d pioglitazone in the rat chow. (C) Quantification of the number of p-ERK+ profiles in rats fed standard chow versus pioglitazone chow revealed a significant reduction in light touch-evoked spinal ERK activation (n = 6 per group). (★) denotes significant difference (p<0.05 via post-hoc t-tests). Scale bar = 100µm.
2.8 Data analysis and statistics
Differences between means were analyzed by two-way analysis of variance (ANOVA). For behavioral studies, Drug/Dose was a grouping factor and Time was the repeated measure. If a significant interaction was found (p<0.05), the ANOVA was followed by post-hoc Bonferroni tests. Area Under the Curve (AUC) was calculated using the trapezoidal method and effects of dose were analyzed using a one-way ANOVA followed by Dunnett multiple comparison test.
3. RESULTS
3.1 Pioglitazone reduces the development of nerve injury-induced hypersensitivity
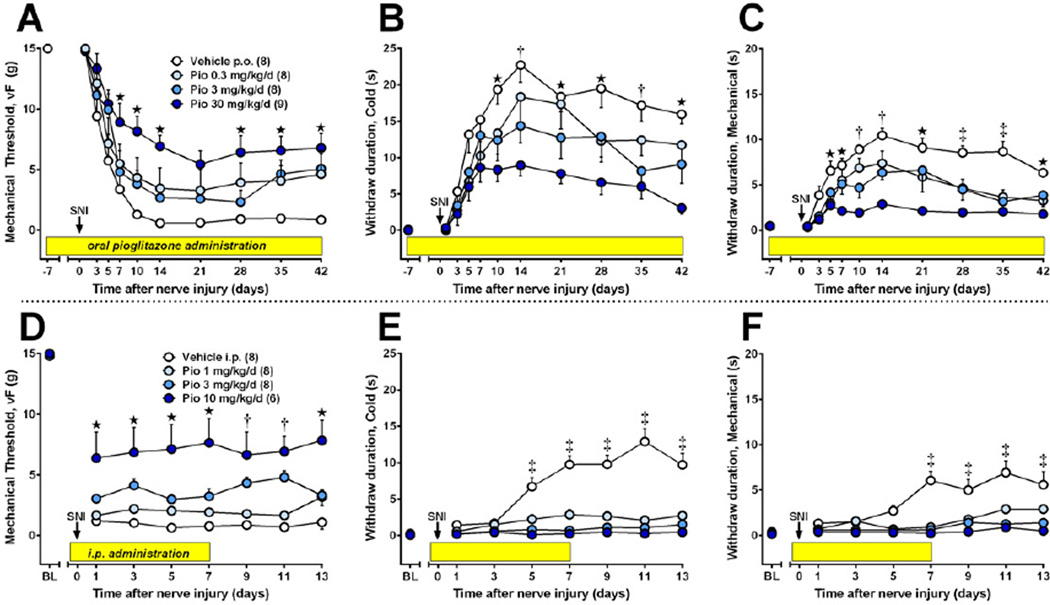
Previous studies indicate that oral gavage of pioglitazone reduced the heat hyperalgesia and/or mechanical allodynia associated with either L5 spinal nerve transection in rats or partial sciatic nerve ligation (PSNL) in mice (Jia et al., 2010; Maeda et al., 2008). However, there were several limitations inherent in these studies: Tests were limited to 1–2 week periods of drug administration; they did not evaluate two key modalities of neuropathic pain (hypersensitivity to cold temperature and noxious mechanical stimulation); they involved a stressful oral gavage procedure; and they did not rigorously evaluate drug-induced behavioral side effects such as ataxia. We addressed these limitations with two experiments. First, we determined whether the non-stressful administration of pioglitazone (via rat chow) for an extended period of time (7 weeks) would reduce cold allodynia and mechanical hyperalgesia. Fig 1A–C illustrates that oral pioglitazone dose-dependently reduced mechanical allodynia [F(3,290)=4.7, p<0.001], cold allodynia [F(3,290)=6.7, p<0.005] and mechanical hyperalgesia [F(3,290)=19.3, p<0.0001]. Second, we determined whether repeated i.p. injection of pioglitazone, beginning 15 min before SNI surgery, would reduce cold allodynia and mechanical hyperalgesia in the absence of ataxia. As illustrated in Fig 1D–F, pioglitazone dose-dependently reduced the development of mechanical allodynia [F(3,182)=17, p<0.0001] and reduced cold allodynia [F(3,182)=231, p<0.0001] and mechanical hyperalgesia [F(3,182)=93, p<0.0001].
Figure 1. The development of nerve injury-induced hypersensitivity is reduced by pioglitazone.
Pain-related responses to plantar application of von Frey hairs (A), acetone (B), or pin prick (C) were monitored from 7 d prior to 6 wk after spared nerve injury (SNI, t = 0 d, arrow). After baseline measurements, pioglitazone (mixed with rat chow in concentrations yielding daily doses of 0 to 30 mg/kg/day) was administered throughout the rest of the study (yellow bars). Oral pioglitazone dose-dependently reduced the development of hypersensitivity (n = 8–9). (D–F) Intraperitoneal injections began 15 min prior to SNI (arrow) and continued twice daily for 7 days (yellow bars). Pioglitazone reduced the development of hypersensitivity to vF, and prevented the development of hypersensitivity to cold and prick (n = 6–8). Values represent mean ± SEM. Statistical significance (p<0.05 by Bonferroni post-tests after repeated measures 2-way ANOVA) between vehicle and high (★), high + medium (†), or high + medium + low (‡) doses of pioglitazone.
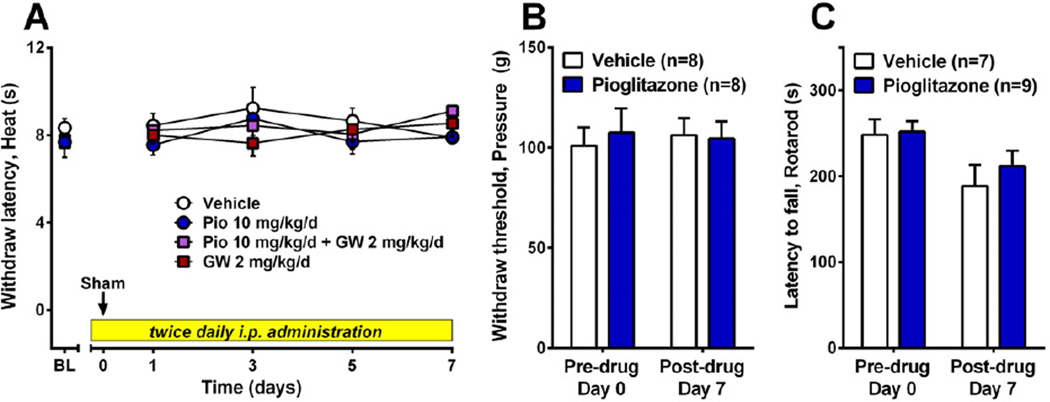
Fig 2A–C illustrates that twice-daily pioglitazone (10 mg/kg/d, i.p.) in uninjured animals did not change paw withdraw latency to heat or paw withdraw threshold to noxious pressure, and did not reduce performance in the accelerating rotarod test (p>0.05). The non-significant decrease in rotarod latency from Day 0 to Day 7 was likely due to lack of continual exposure to the rotarod, and thus loss of performance, during drug or vehicle administration. Importantly, control and treatment groups did not differ on testing days (p>0.05). Taken together, these results indicate that chronic pioglitazone inhibits behavioral signs of neuropathic pain, and is not confounded by the stress of drug administration, transient nociception or disruption of motor coordination.
Figure 2. Neither acute nociception nor motor coordination is disrupted by pioglitazone.
After baseline assessment of noxious heat, noxious pressure, or latency to fall off of a rotating bar (rotarod), the highest dose of pioglitazone (10 mg/kg/d, i.p.) was administered for 7 days, followed by behavioral reassessment. Pioglitazone did not change behavioral withdrawal responses to noxious heat (A) or pressure (B), nor did it affect motor coordination (C). n=7 for vehicle and n=9 for pioglitazone.
In a separate cohort of animals, we administered pioglitazone at 10mg/kg/d and continued behavioral testing for one month after the end of the 7 day administration protocol. As illustrated in Fig 3, von Frey, cold, and pin prick thresholds remained elevated for at least 6 days after the cessation of pioglitazone. Significant statistical interactions of pioglitazone with time indicate that pioglitazone delayed the development of mechanical allodynia [F(10,100)=2.0, p<0.05], cold allodynia [F(10,100)=9.9, p<0.0001] and mechanical hyperalgesia [F(10,100)=3.0, p<0.0001].
Figure 3. The anti-allodynic effects of chronic pioglitazone are long lasting.
Saline (Vehicle) or drug (Pio 10 mg/kg/d) administration (i.p.) was started 15 min prior to SNI surgery and continued twice daily for 7 days (yellow bars). Pioglitazone reduced hypersensitivity to vF (A), cold (B), and pin prick (C). These effects lasted well-beyond cessation of drug administration (n = 6 per group). Values represent mean ± SEM. (★) denotes significant difference (p<0.05 vs. vehicle by Bonferroni post-tests following repeated measures 2-way ANOVA).
3.2 PPARγ mediates the anti-hypersensitivity actions of pioglitazone
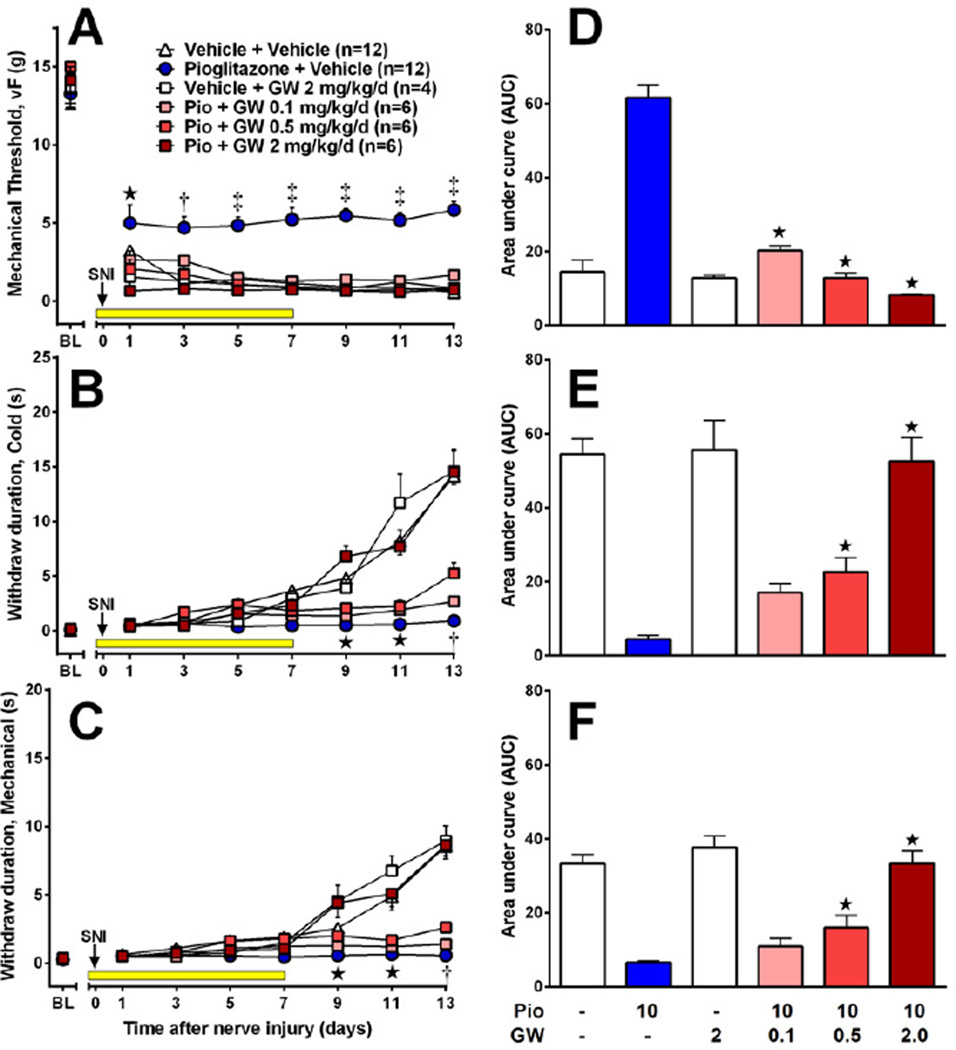
To determine the contribution of PPARγ to the anti-allodynic and anti-hyperalgesic actions of pioglitazone, we administered GW9662 (0.1, 0.5 or 2 mg/kg/d) or vehicle 15 min before pioglitazone (10mg/kg/d). As illustrated in Fig 4 A–C, GW9662 but not vehicle, dose-dependently reversed the inhibitory effect of chronic pioglitazone on mechanical allodynia [F(5,280)=29, p<0.0001], cold allodynia [F(5,280)=45, p<0.0001] and mechanical hyperalgesia [F(5,280)=58, p<0.0001]. In Fig 4 D–F, AUC bar graphs summarize the effect of pioglitazone (one-way ANOVA with subsequent Dunnett test versus pioglitazone+vehicle) on mechanical threshold [F(5, 40)=55, p<0.0001], cold withdraw duration [F(5, 40)=35, p<0.0001], and pin prick withdraw duration [F(5, 40)=33, p<0.0001] for days 1–13. When administered alone, GW9662 had no effect on the development of allodynia or hyperalgesia (p>0.05).
Figure 4. PPARγ mediates the inhibitory actions of pioglitazone on neuropathic pain.
The PPARγ antagonist GW9662 or its vehicle was administered 15 min prior to pioglitazone (10 mg/kg/d) or its vehicle. GW9662 reduced the anti-allodynic effects of pioglitazone on vF thresholds (A,D), cold allodynia (B,E), and pin prick hyperalgesia (C,F), but had no effect on its own (n = 4–12 per group). Values represent mean ± SEM. (A–C) Statistical significance (p<0.05 by Bonferroni post-tests after repeated measures 2-way ANOVA) between pioglitazone + vehicle and high (★), high + medium (†), or high + medium + low (‡) doses of GW9662 + pioglitazone denoted by symbols in parentheses. (D–F) Statistical significance (p<0.05 by Dunnett multiple comparison test after 1-way ANOVA) versus pioglitazone + vehicle group denoted by (★).
3.3 Pre-emptive and post-injury pioglitazone reduces neuropathic pain
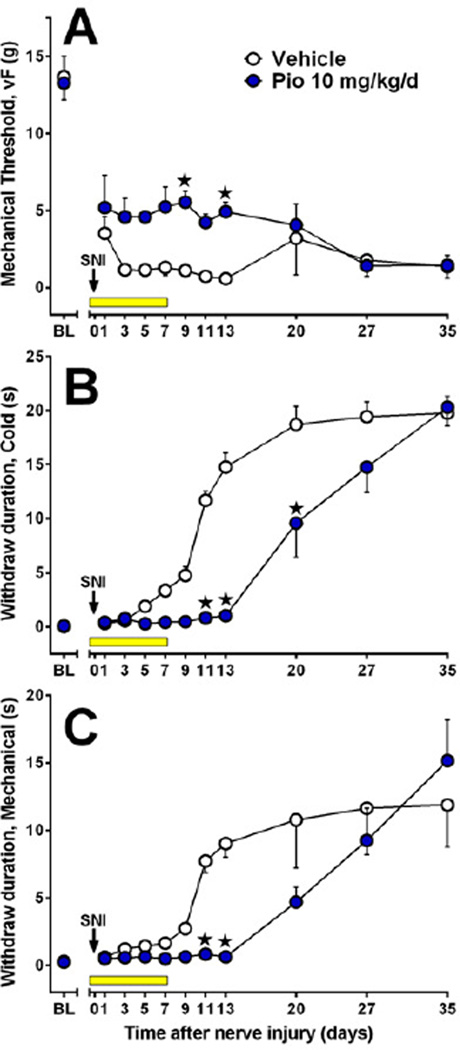
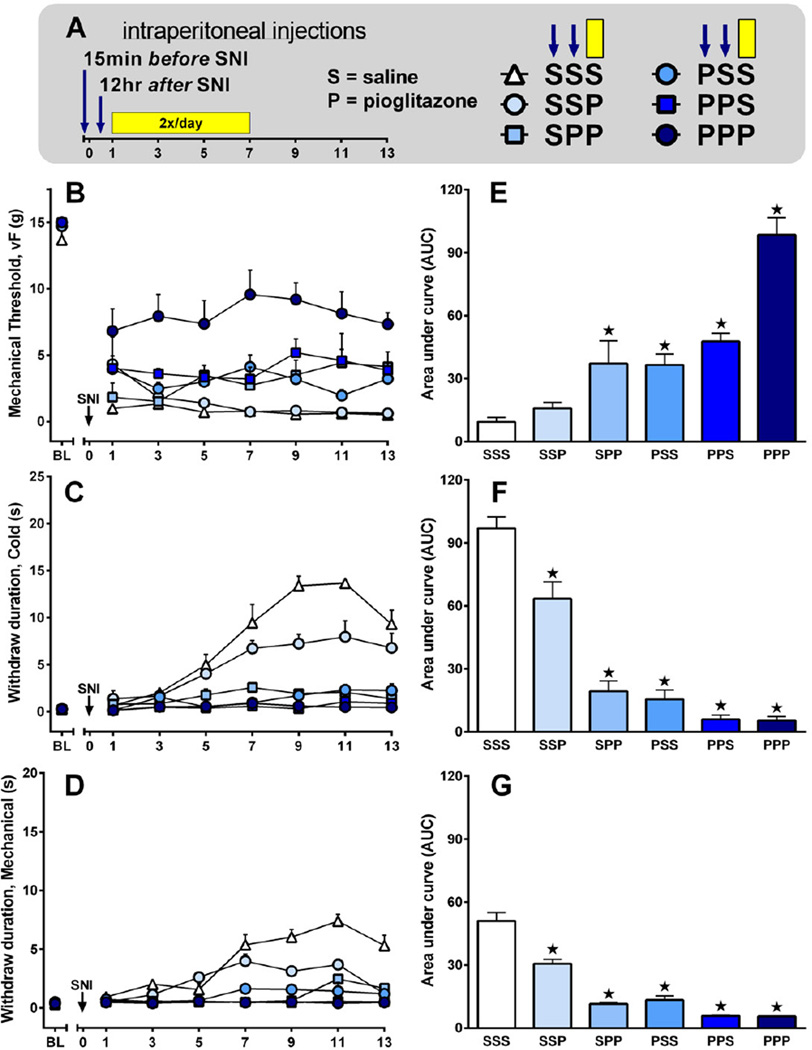
Previous studies indicate that chronic administration of pioglitazone, begun before and continued for several days to weeks after nerve injury, dramatically reduced both the development of and established hypersensitivity. To determine the importance of timing of drug administration to inhibition of hypersensitivity, and thus dissect the effects of pioglitazone on the development of vs. established chronic neuropathic pain, we administered pioglitazone 10 mg/kg/d at one or more of the following times relative to SNI surgery: a single injection 15 min before SNI; a single injection 12 hr after SNI; and/or twice-daily injections begun 24 hr after SNI and lasting through day 7. This experimental protocol is schematized in Fig 5A. Pioglitazone reduced mechanical hypersensitivity [F(5, 180)=23, p<0.0001] 5B, reduced cold allodynia [F(5, 180)=53, p<0.0001] 5C, and reduced mechanical hyperalgesia [F(5, 180)=70, p<0.0001] 5D. In Fig 5 E–G, data are presented as AUC bar graphs summarizing the effect of pioglitazone (one-way ANOVA with Dunnett test versus saline only) on mechanical threshold [F(5, 30)=23, p<0.0001], cold withdraw duration [F(5, 30)=57, p<0.0001], and pin prick withdraw duration [F(5, 30)=76, p<0.0001] for days 1–13.
Figure 5. Preemptive and post-surgery treatment with pioglitazone reduces neuropathic pain.
(A) Saline (S) or 10 mg/kg/day pioglitazone (P) was administered at one or more of the following times relative to SNI surgery in varying combinations: a single injection 15 min before SNI (first arrow: S or P); a single injection 12 hr after SNI (second arrow: S or P); or twice-daily injection begun 24 hr after SNI and lasting through day 7 (yellow bar: third S or P). For example, the first P in PPP reflects injection 1 (15 min prior to SNI), the second P in PPP represents injection 2 (12 hr after SNI), and the third P in PPP represents twice-daily injections (24 hr to 7 d after SNI) (n = 6 per group). (B–D) Line graphs illustrating behavioral responses to von Frey (B), acetone (C), or pin prick (D) stimuli applied to the ipsilateral plantar hindpaw. (E–G) AUC bar graphs summarizing the effect of pioglitazone (one-way ANOVA with Dunnett test versus SSS) on mechanical threshold [F(5, 35)=23, p<0.0001], cold withdraw duration [F(5, 35)=56, p<0.0001], and pin prick withdraw duration [F(5, 35)=67, p<0.001]. Values represent mean ± SEM. (★) denotes significant difference (p<0.05 vs. vehicle by Bonferroni post-tests following repeated measures 2-way ANOVA).
3.4 Pioglitazone inhibits the dorsal horn expression of cd11b, GFAP, and p-p38
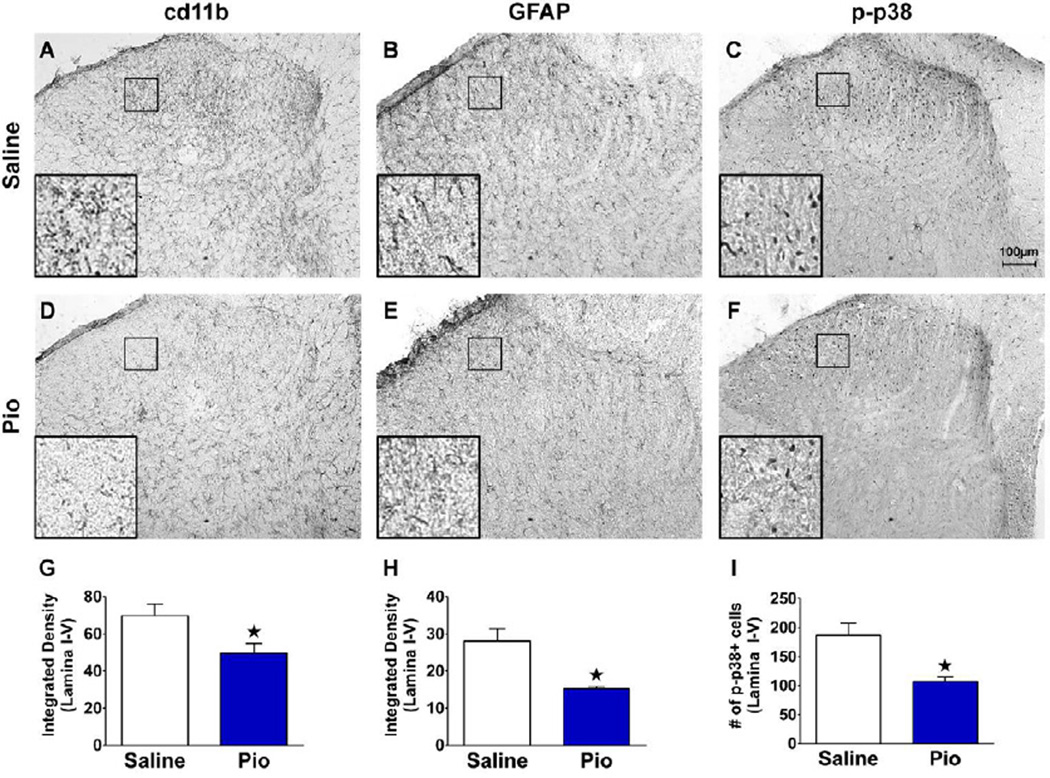
PPARγ agonists inhibit the activation of glial cells in the CNS (Carta et al., 2011; Sauerbeck et al., 2011). To test the hypothesis that repeated administration of pioglitazone inhibits nerve-injury induced activation of microglia and astrocytes in L4-L5 dorsal horn, we performed SNI and quantified the expression of cd11b and GFAP. We also evaluated phosphorylation of p38, another marker of the microglial contribution to neuropathic pain (Ji et al., 2009; Jin et al., 2003; Zhuang et al., 2005). As illustrated in Fig 6, compared to saline controls, systemic pioglitazone significantly reduced the expression of cd11b, GFAP, and p-p38 (all p<0.05).
Figure 6. Pioglitazone inhibits expression of cd11b, GFAP, and p-p38.
Intraperitoneal saline or drug administration was started 15 min prior to SNI surgery. Injections continued twice daily on days 1–7. Pioglitazone reduced the staining for cd11 b (A,D,G), GFAP (B,E,H) and phosphorylated p38 (C,F,I) (n = 6 per group). Insets in the lower left panels (A–F) represent 10× magnification of the 100 µm square portion of the sural innervation territory lamina II-III. Values represent mean ± SEM. (★) denotes significant difference (p<0.05 vs. vehicle by post-hoc t-tests). Scale bar = 100µm.
3.5 Pioglitazone inhibits the stimulus-induced expression of p-ERK
Non-noxious light touch produces activation (phosphorylation) of ERK in spinal cord neurons of nerve injured but not sham or naive animals(Gao and Ji, 2010a). Consistent with these reports, we found that light-touch stimulation of the spared sural receptive field increased p-ERK expression on the injured side of the lumbar dorsal horn (p<0.01; data not shown). We next tested the hypothesis that chronic oral administration of pioglitazone would reduce evoked p-ERK expression when tested 42 days after nerve injury. As illustrated in Fig 7, pioglitazone (30 mg/kg/d) reduced p-ERK expression to approximately 50% of vehicle animals (p<0.05).
4. DISCUSSION
A growing number of studies report that systemic administration of thiazolidinediones (TZDs) reduce peripheral neuropathic pain (Jia et al., 2010; Maeda et al., 2008; Takahashi et al., 2011). Here, we report that oral or twice-daily intraperitoneal pioglitazone, beginning before SNI and continuing for several weeks, dose-dependently prevented behavioral signs of mechanical and cold hypersensitivity.
4.1 Pioglitazone reduces the development of neuropathic pain
We found that the most effective treatment for preventing the development of allodynia was to start pioglitazone administration 15 min prior to SNI and continue delivery for 7 days. Remarkably, a single preemptive injection of pioglitazone 15 min before SNI substantially reduced hyperalgesia for at least 2 weeks, an effect that was enhanced even further with a second injection delivered 12 hr after SNI. Consistent with our results, Takahashi et al. found that three daily injections of rosiglitazone before partial sciatic nerve ligation (PSNL) attenuated the development of mechanical hypersensitivity(Takahashi et al., 2011). Also, Jia et al. found that 2 weeks of oral pioglitazone, beginning one hour before L5 spinal nerve transection, reduced the development of mechanical hypersensitivity (Jia et al., 2010). Finally, Maeda et al. reported administration of pioglitazone (25 mg/kg/d) starting one week prior to PSNL produced a sizeable (though not statistically significant) decrease in mechanical hypersensitivity when tested one week later (Maeda et al., 2008). Taken together with previous literature, our data indicate that chronic pioglitazone blocks the development of neuropathic pain.
4.2 Pioglitazone reduces the maintenance of neuropathic pain
Increased primary afferent discharge from injured nerves likely contributes to the development of behavioral signs of neuropathic pain (Taylor, 2001). However, while this ectopic activity largely subsides within several days (Liu et al., 2000), tactile and cold hypersensitivity remain constant for weeks. This suggests that other mechanisms are responsible for established neuropathic pain, such as spinal facilitation (Taylor, 2001) or descending facilitation from the rostral ventral medulla to the dorsal horn (Burgess et al., 2002). The current data indicates that when injections began after SNI, pioglitazone reduced established hypersensitivity, albeit to a lesser degree than preemptive treatment. Consistent with our results, Maeda et al. (2008) reported anti-allodynic effects of pioglitazone when administered 2 weeks after PSNL. Indeed, our studies indicate that 30–100 mg/kg doses of pioglitazone reverse mechanical allodynia and hyperalgesia and cold allodynia when first administered 2 weeks after SNI. We conclude chronic pioglitazone reduces established neuropathic pain.
4.3 Inhibition of neuropathic pain persists long after cessation of pioglitazone
PPARγ regulates gene transcription through several genomic mechanisms, such as ligand-dependent transactivation, ligand-dependent transrepression and ligand-independent transrepression (Ricote and Glass, 2007). In agreement with previous studies using 25 mg/kg/d pioglitazone for 6 days (Maeda et al., 2008), we found that pioglitazone reduced nerve injury-induced hypersensitivity not only during its administration, but also for an additional week after its termination. Since pioglitazone is metabolized with a half-life of 8–9 hours (Christensen et al., 2005), these effects are clearly mediated by long-term changes in gene expression associated with nuclear receptor activation and subsequent function as a transcription factor.
4.4 PPARγ mediates the inhibitory actions of pioglitazone on neuropathic pain
Our results indicate that blockade of PPARγ with GW9662 dose-dependently prevented the anti-allodynic and anti-hyperalgesic actions of pioglitazone. The robust effect of GW9662 speaks to the effective targeting of 10 mg/kg/d doses of pioglitazone at PPARγ; this dose is approximately 10-fold higher than the maximum daily dose (45 mg tablet) that is currently approved for use in humans. Although TZDs interact with non-PPARγ sites to exert numerous pharmacological effects(Feinstein et al., 2005; Sauerbeck et al., 2011), a strong body of evidence has emerged indicating that PPARγ mediates the reduction in pain-related behaviors elicited by TZDs after neuropathic injury. For example, GW9662 blocked the effect of pioglitazone and rosiglitazone in a rat model of spinal cord injury pain and a mouse model of peripheral neuropathic pain (Maeda et al., 2008; Park et al., 2007). Furthermore, Churi et al. (2008) demonstrated that the anti-allodynic effects of intrathecal rosiglitazone or 15d-PGJ2 (a putative endogenous PPARγ ligand) are blocked with intrathecal administration of the (less-selective) PPARγ antagonist, bisphenol A diglycidyl ether (Churi et al., 2008). Although we cannot rule out the possibility of non-PPARγ sites of action of pioglitazone, we conclude that PPARγ substantially contributes to the anti-hyperalgesic actions of pioglitazone.
4.5 Pioglitazone inhibits the stimulus-induced expression of p-ERK in SNI rats
In the absence of injury, non-noxious stimuli do not activate ERK in spinal cord neurons (Ji et al., 1999). By contrast, during cutaneous inflammation or after peripheral nerve injury (L5 spinal nerve ligation), light touch or movement elicits a robust expression of p-ERK in dorsal horn neurons, but not astrocytes nor microglia (Gao and Ji, 2010a; Wang et al., 2004). Thus, activation of p-ERK has emerged as a robust marker of sensitization of neurons to high-threshold sensory stimulation in the setting of nerve injury. We found pioglitazone reduced light touch-induced expression of p-ERK in the dorsal horn of SNI animals when neuropathic pain was already established. We conclude pioglitazone reduces stimulus-evoked ERK activation and suggest that PPARγ agonism reduces nerve injury-induced central sensitization of spinal cord neurons, possibly leading to its long-lasting anti-allodynic effects after peripheral nerve injury.
4.6 Pioglitazone inhibits microglial activation in lumbar dorsal horn
Microglia are intricately involved in the development of hyperalgesia (Abbadie et al., 2009; Ji and Suter, 2007; Milligan and Watkins, 2009; Scholz and Woolf, 2007). For example, Ji and colleagues found that L5 spinal ligation increased markers of spinal microglial activation, including Iba1 and p-p38 (Ji and Suter, 2007; Jin et al., 2003), and pharmacological inhibitors of p-p38 decrease behavioral signs of neuropathic pain (Ji et al., 2009; Lee et al., 2011; Yasuda et al., 2011). PPARγ immunoreactivity is expressed on microglia (Bernardo et al., 2005), and the current data indicate that pioglitazone decreased the expression of cd11b and p-p38. Our results are consistent with Park et al., who found that pioglitazone reduced microglia activation and the induction of proinflammatory genes including chemokines and cytokines after spinal cord injury (Park et al., 2007). We speculate that pioglitazone acts at PPARγ to inhibit the development of neuropathic pain by decreasing microglial activation in the dorsal horn. Future studies using PPARγ antagonists are needed to test this hypothesis. It is important to note the possibility that peripheral PPARγ binding sites might contribute to the anti-hyperalgesic effects of systemic pioglitazone (Hasegawa-Moriyama et al., 2012; Maeda et al., 2008; Takahashi et al., 2011). Further studies are needed to distinguish peripheral versus spinal influence of PPARγ on neuropathic pain.
4.7 Pioglitazone inhibits the nerve-injury induced activation of astrocytes
Astrocyte activation contributes to the maintenance of hyperalgesia (Gao and Ji, 2010b; Scholz and Woolf, 2007; Svensson and Brodin, 2010; Tsuda et al., 2011). PPARγ receptors are expressed on astrocytes in the CNS (Moreno et al., 2004) and spinal cord (Diab et al., 2002), and the current results indicate that pioglitazone significantly decreased GFAP immunoreactivity in the lumbar dorsal horn. We speculate that pioglitazone inhibits the maintenance of neuropathic pain by decreasing astrocyte activation in the dorsal horn.
4.8 Pioglitazone reduces multiple modalities of neuropathic hypersensitivity in the absence of tolerance, side effects, or stress
In addition to the data discussed above, our results differ from and extend the impact of previous studies in several important ways. First, previous studies of TZD-induced reductions in neuropathic pain-like behavior were limited to non-noxious mechanical and heat modalities (Jia et al., 2010; Maeda et al., 2008; Takahashi et al., 2011). However, clinical neuropathic pain is associated not only with mechanical allodynia and, to a lesser degree, heat hyperalgesia, but also mechanical hyperalgesia and cold allodynia (Taylor, 2001). Our current results indicate that pioglitazone reduced hypersensitivity to multiple stimulus modalities: von Frey fibers or noxious pin as non-noxious and noxious mechanical stimuli, respectively; and plantar acetone application as a cold stimulus. We conclude for the first time that pioglitazone reduces mechanical hyperalgesia and cold allodynia in addition to mechanical allodynia.
Second, while previous studies delivered TZDs for less than one month (Jia et al., 2010; Maeda et al., 2008; Takahashi et al., 2011), we found that oral pioglitazone reduced mechanical and cold hypersensitivity throughout a 6 wk period of administration after injury, without decrement over time. We conclude that analgesic tolerance does not develop with PPARγ agonists such as pioglitazone, an advantage over conventional pharmacotherapeutic approaches to chronic pain such as opioid narcotics.
Third, we show that the anti-allodynic and anti-hyperalgesic effects of a PPARγ agonist such as pioglitazone are not confounded by behavioral side effects. Previous nerve injury studies did not rigorously include these important controls (Jia et al., 2010; Maeda et al., 2008; Takahashi et al., 2011). We found that pioglitazone did not produce ataxia or reductions in transient mechanical and heat nociception, indicating that anti-allodynic and anti-hyperalgesic effects were not secondary to adverse drug-induced behaviors or analgesia. Our results in rats are consistent with the finding that pioglitazone (Actos®) does not produce a high incidence of psychotropic side effects in humans.
Fourth, the oral route of administration used here avoided confounds associated with the stress of injection or gavage (Jia et al., 2010; Maeda et al., 2008; Takahashi et al., 2011). Stress can activate antinociceptive systems, a phenomena termed stress-induced analgesia (SIA) (Carrive et al., 2011; Lewis et al., 1980). Even when vehicle controls are included, one cannot disregard the possibility of an interaction between the anti-allodynic and anti-hyperalgesic effects of TZDs and SIA. By including pioglitazone within the rat chow, we can conclude for the first time that pioglitazone reduces pain-like hypersensitivity in the absence of stress due to injection or gavage.
HIGHLIGHTS.
PPARγ activation blocks development of and established neuropathic pain.
Inhibition of neuropathic pain persists long after cessation of pioglitazone
Pioglitazone reduces spinal microglial and astrocyte activation
Pioglitazone reduces light touch stimulus-induced expression of p-ERK in dorsal horn
Anti-hyperalgesic effects extended to multiple stimulus modalities in the absence of side effects
Acknowledgements
Supported by 5R01NS62306 and 5K02DA19656 to BKT
List of Non-Standard Abbreviations
- GFAP
glial fibrillary acidic protein
- p-ERK
phosphorylated extracellular signal-regulated kinase
- PPARγ
peroxisome proliferator-activated receptor gamma
- TZD
thiazolidinedione
- PSNL
partial sciatic nerve ligation
- SNI
spared nerve injury
- SIA
stress-induced analgesia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbadie C, Bhangoo S, De Koninck Y, Malcangio M, Melik-Parsadaniantz S, White FA. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo A, Ajmone-Cat A, Gasparini L, Ongini E, Minghetti L. Nuclear receptor peroxisome-activated receptor-gamma is activated in rat microglial cells by the anti-inflammatory drug HCT 1026, a derivative of flurbiprofen. Journal of Neurochemistry. 2005;92:895–903. doi: 10.1111/j.1471-4159.2004.02932.x. [DOI] [PubMed] [Google Scholar]
- Bernardo A, Levi G, Minghetti L. Role of the peroxisome proliferator-activated receptor-gamma (PPAR-gamma) and its natural ligand 15-deoxy-Delta12, 14-prostaglandin J2 in the regulation of microglial functions. The European journal of neuroscience. 2000;12:2215–2223. doi: 10.1046/j.1460-9568.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- Burgess SE, Gardell LR, Ossipov MH, Malan TP, Jr., Vanderah TW, Lai J, Porreca F. Time-dependent descending facilitation from the rostral ventromedial medulla maintains, but does not initiate, neuropathic pain. J Neurosci. 2002;22:5129–5136. doi: 10.1523/JNEUROSCI.22-12-05129.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrive P, Churyukanov M, Le Bars D. A reassessment of stress-induced "analgesia" in the rat using an unbiased method. Pain. 2011;152:676–686. doi: 10.1016/j.pain.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Carta AR, Pisanu A, Carboni E. Do PPAR-Gamma Agonists Have a Future in Parkinson's Disease Therapy? Parkinsons Dis. 2011;2011:689181. doi: 10.4061/2011/689181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Christensen ML, Meibohm B, Capparelli EV, Velasquez-Mieyer P, Burghen GA, Tamborlane WV. Single- and multiple-dose pharmacokinetics of pioglitazone in adolescents with type 2 diabetes. J Clin Pharmacol. 2005;45:1137–1144. doi: 10.1177/0091270005279578. [DOI] [PubMed] [Google Scholar]
- Churi SB, Abdel-Aleem OS, Tumber KK, Scuderi-Porter H, Taylor BK. Intrathecal rosiglitazone acts at peroxisome proliferator-activated receptor-gamma to rapidly inhibit neuropathic pain in rats. J Pain. 2008;9:639–649. doi: 10.1016/j.jpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Diab A, Deng C, Smith JD, Hussain RZ, Phanavanh B, Lovett-Racke AE, Drew PD, Racke MK. Peroxisome proliferator-activated receptor-gamma agonist 15-deoxy-Delta(12,14)-prostaglandin J(2) ameliorates experimental autoimmune encephalomyelitis. J Immunol. 2002;168:2508–2515. doi: 10.4049/jimmunol.168.5.2508. [DOI] [PubMed] [Google Scholar]
- Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nature Medicine. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Spagnolo A, Akar C, Weinberg G, Murphy P, Gavrilyuk V, Dello Russo C. Receptor-independent actions of PPAR thiazolidinedione agonists: is mitochondrial function the key? Biochem Pharmacol. 2005;70:177–188. doi: 10.1016/j.bcp.2005.03.033. [DOI] [PubMed] [Google Scholar]
- Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? The open pain journal. 2009;2:11–17. doi: 10.2174/1876386300902010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia. J Neurochem. 2010a;115:505–514. doi: 10.1111/j.1471-4159.2010.06946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010b;7:482–493. doi: 10.1016/j.nurt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa-Moriyama M, Ohnou T, Godai K, Kurimoto T, Nakama M, Kanmura Y. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuates postincisional pain by regulating macrophage polarization. Biochem Biophys Res Commun. 2012;426:76–82. doi: 10.1016/j.bbrc.2012.08.039. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Hanke A, Dewachter I, Kuiperi C, O’Banion K, Klockgether T, Van Leuven F, Landreth GE. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain. 2005;128:1442–1453. doi: 10.1093/brain/awh452. [DOI] [PubMed] [Google Scholar]
- Jensen TS, Baron R, Haanpaa M, Kalso E, Loeser JD, Rice AS, Treede RD. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- Ji RR, Gereau RW, IV, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Research Reviews. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Suter MR. p38, microglial signaling, and neuropathic pain. Molecular Pain. 2007;1:3–33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Zhu S, Ji Q, Hui K, Duan M, Xu J, Li W. Repeated administration of pioglitazone attenuates development of hyperalgesia in a rat model of neuropathic pain. Experimental and clinical psychopharmacology. 2010;18:359–365. doi: 10.1037/a0020181. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 Mitogen-Activated Protein Kinase Is Activated after a Spinal Nerve Ligation in Spinal Cord Microglia and Dorsal Root Ganglion Neurons and Contributes to the Generation of Neuropathic Pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MK, Han SR, Park MK, Kim MJ, Bae YC, Kim SK, Park JS, Ahn DK. Behavioral evidence for the differential regulation of p-p38 MAPK and p-NF-kappaB in rats with trigeminal neuropathic pain. Mol Pain. 2011;7:57. doi: 10.1186/1744-8069-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, Plunket KD, Shenk JL, Stimmel JB, Therapontos C, Willson TM, Blanchard SG. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623–625. doi: 10.1126/science.7367889. [DOI] [PubMed] [Google Scholar]
- Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000;85:503–521. doi: 10.1016/S0304-3959(00)00251-7. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Kobayashi Y, Ozaki M, Kishioka S. Pioglitazone attenuates tactile allodynia and thermal hyperalgesia in mice subjected to peripheral nerve injury. J Pharmacol Sci. 2008;108:341–347. doi: 10.1254/jphs.08207fp. [DOI] [PubMed] [Google Scholar]
- Maeshiba Y, Kiyota Y, Yamashita K, Yoshimura Y, Motohashi M, Tanayama S. Disposition of the new antidiabetic agent pioglitazone in rats, dogs, and monkeys. Arzneimittelforschung. 1997;47:29–35. [PubMed] [Google Scholar]
- Martens FM, Visseren FL, Lemay J, de Koning EJ, Rabelink TJ. Metabolic and additional vascular effects of thiazolidinediones. Drugs. 2002;62:1463–1480. doi: 10.2165/00003495-200262100-00004. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Ceru MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Park SW, Yi JH, Miranpuri G, Satriotomo I, Bowen K, Resnick DK, Vemuganti R. Thiazolidinedione class of peroxisome proliferator-activated receptor gamma agonists prevents neuronal damage, motor dysfunction, myelin loss, neuropathic pain, and inflammation after spinal cord injury in adult rats. J Pharmacol Exp Ther. 2007;320:1002–1012. doi: 10.1124/jpet.106.113472. [DOI] [PubMed] [Google Scholar]
- Petrova TV, Akama KT, Van Eldik LJ. Cyclopentenone prostaglandins suppress activation of microglia: down- regulation of inducible nitric-oxide synthase by 15-deoxy-Delta12,14- prostaglandin J2. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4668–4673. doi: 10.1073/pnas.96.8.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Sauerbeck A, Gao J, Readnower R, Liu M, Pauly JR, Bing G, Sullivan PG. Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Experimental neurology. 2011;227:128–135. doi: 10.1016/j.expneurol.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson's disease. The European journal of neuroscience. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Shibata N, Kawaguchi-Niida M, Yamamoto T, Toi S, Hirano A, Kobayashi M. Effects of the PPARgamma activator pioglitazone on p38 MAP kinase and IkappaBalpha in the spinal cord of a transgenic mouse model of amyotrophic lateral sclerosis. Neuropathology : official journal of the Japanese Society of Neuropathology. 2008 doi: 10.1111/j.1440-1789.2008.00890.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Brodin E. Spinal astrocytes in pain processing: non-neuronal cells as therapeutic targets. Molecular Interventions. 2010;10:25–38. doi: 10.1124/mi.10.1.6. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Hasegawa-Moriyama M, Sakurai T, Inada E. The macrophage-mediated effects of the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone attenuate tactile allodynia in the early phase of neuropathic pain development. Anesthesia and analgesia. 2011;113:398–404. doi: 10.1213/ANE.0b013e31821b220c. [DOI] [PubMed] [Google Scholar]
- Taylor BK. Pathophysiologic mechanisms of neuropathic pain. Current pain and headache reports. 2001;5:151–161. doi: 10.1007/s11916-001-0083-1. [DOI] [PubMed] [Google Scholar]
- Taylor BK. Spinal inhibitory neurotransmission in neuropathic pain. Current pain and headache reports. 2009;13:208–214. doi: 10.1007/s11916-009-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Kohro Y, Yano T, Tsujikawa T, Kitano J, Tozaki-Saitoh H, Koyanagi S, Ohdo S, Ji RR, Salter MW, Inoue K. JAK-STAT3 pathway regulates spinal astrocyte proliferation and neuropathic pain maintenance in rats. Brain. 2011;134:1127–1139. doi: 10.1093/brain/awr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor NA, Wanderi EW, Gamboa J, Zhao X, Aronowski J, Deininger K, Lust WD, Landreth GE, Sundararajan S. Altered PPARgamma expression and activation after transient focal ischemia in rats. The European journal of neuroscience. 2006;24:1653–1663. doi: 10.1111/j.1460-9568.2006.05037.x. [DOI] [PubMed] [Google Scholar]
- Wang H, Dai Y, Fukuoka T, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Enhancement of stimulation-induced ERK activation in the spinal dorsal horn and gracile nucleus neurons in rats with peripheral nerve injury. The European journal of neuroscience. 2004;19:884–890. doi: 10.1111/j.0953-816x.2004.03203.x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Sugiura H, Tanaka H, Takigami S, Yamagata K. p38 MAP kinase inhibitors as potential therapeutic drugs for neural diseases. Cent Nerv Syst Agents Med Chem. 2011;11:45–59. doi: 10.2174/187152411794961040. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Patzer A, Herdegen T, Gohlke P, Culman J. Activation of cerebral peroxisome proliferator-activated receptors gamma promotes neuroprotection by attenuation of neuronal cyclooxygenase-2 overexpression after focal cerebral ischemia in rats. Faseb J. 2006;20:1162–1175. doi: 10.1096/fj.05-5007com. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Gerner P, Woolf CJ, Ji RR. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–159. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]