Summary
The urinary bladder is a common site of bacterial infection with a majority of cases attributed to uropathogenic Escherichia coli. Sequels of urinary tract infections (UTIs) include the loss of urothelial barrier function and subsequent clinical morbidity secondary to the permeation of urine potassium, urea and ammonia into the subepithelium. To date there has been limited research describing the mechanism by which this urothelial permeability defect develops. The present study models acute uropathogenic E. coli infection in vitro using intact canine bladder mucosa mounted in Ussing chambers to determine whether infection induces primarily a transcellular or paracellular permeability defect. The Ussing chamber sustains tissue viability while physically separating submucosal and lumen influences, so this model is ideal for quantitative measurement of transepithelial electrical resistance (TER) to assess alterations of urothelial barrier function. Using this model, changes in both tissue ultrastructure and TER indicated that uropathogenic E. coli infection promotes a paracellular permeability defect associated with the failure of umbrella cell tight junction formation and umbrella cell sloughing. In addition, bacterial interaction with the urothelium promoted secretion of cytokines from the urinary bladder with bioactivity capable of modulating epithelial barrier function including tumour necrosis factor-α, interleukin (IL)-6 and IL-15. IL-15 secretion by the infected bladder mucosa is a novel finding and, because IL-15 plays key roles in reconstitution of tight junction function in damaged intestine, this study points to a potential role for IL-15 in UTI-induced urothelial injury.
Keywords: urinary tract infection, urothelial permeability, tight junction, cytokine
Introduction
Urinary tract infections (UTIs) are one of the most common infections of the human body with Escherichia coli accounting for 70–90% of cases (Hooton and Stamm, 1997). Approximately 14% of dogs will be diagnosed with at least one UTI during their lifetime (Bartges, 2004) with 44–55% being caused by E. coli (Senior, 2007; Chew, 2011). The clinical morbidity associated with these infections may be attributed largely to failure of the urothelial barrier with permeation of urine potassium, urea and ammonia into the subepithelium (Parsons, 2007). A better understanding of how bacterial infection alters urothelial permeability may aid in the development of therapies that ameliorate the morbidity associated with UTI, particularly in dogs with resistant or recurrent infections.
In health, the urothelium forms a complex barrier that restricts the translocation of ions, solutes and bacteria into the body despite large changes in the volume, tonicity and composition of urine (Lewis, 2000). This barrier function is largely mediated by the most superficial umbrella cell layer of the urothelium. Transcellular permeability of solutes and water across membranes is restricted by the expression of uroplakins within the apical plasma membrane of the umbrella cells, while paracellular permeability of solutes and water between cells is controlled by tight junctions that adjoin the apical/lateral membranes of the umbrella cells (Lewis, 2000). In addition to providing a physical barrier, cytokine secretion is a major response of the urothelium to UTI (Agace et al., 1993; Funfstuck et al., 2001; Wood et al., 2011). These cytokines would be anticipated to play key roles in restoration or exacerbation of urothelial barrier defects (Capaldo and Nusrat, 2009).
To date there has been limited research describing the urothelial permeability defect mediated by either uropathogenic bacteria or urothelial cytokines secreted in response to urinary tract infection. Current knowledge and conventional approaches to studying the urothelial response to UTI are based largely on the use of cell culture or in-vivo models. While urothelial cell culture models are used to study single cell events, they are difficult to obtain as differentiated sheets of transitional epithelium and lack the presence of subepithelial influences on cellular behavior (Truschel et al., 1999; Lewis, 2000). By contrast, the complexity of in-vivo models and the confounding effect of recruited inflammatory cells often preclude a precise determination of urothelial-based acute disease mechanisms. An ideal model would hybridize the benefits of each approach by maintaining the cellular microenvironment while allowing for directed urothelial investigation.
The overall aim of this study was to characterize the acute effect of E. coli infection on barrier function and cytokine secretion by canine bladder mucosa using an in-vitro model system. The specific aims were to identify the mechanisms of barrier function loss and to identify cytokine targets that may be integral in the disruption or preservation of the urothelial barrier. These studies were undertaken using canine bladder mucosa mounted in Ussing chambers and infected with uropathogenic E. coli. This model system enabled the native architecture and biological responses of the urothelium and underlying lamina propria to be maintained for short term mechanistic studies in vitro. In the absence of a blood supply, the Ussing chamber system specifically isolates the actions of uropathogenic E. coli on the urothelium without the confounding effects of recruited inflammatory cells from the systemic circulation and underlying submucosal tissues. Furthermore, by providing physically separate saline reservoirs to warm, nourish and oxygenate the subepithelial and luminal sides of the mucosa independently, the approach enables quantitative measurements of epithelial barrier function and cytokine secretion (Clarke, 2009).
Materials and Methods
Animals
Intact urinary bladders were obtained from 15 beagle dogs (aged 6 months to 1 year; Covance Laboratories, Princeton, New Jersey, USA) at the completion of an unrelated terminal experiment that was limited to vascular studies in the caudal limbs. The dogs were humanely destroyed by intravenous injection of sodium pentobarbital. Urine sterility of each animal was confirmed by aerobic bacterial culture (10% blood agar for 14 days at 37°C) of urine aspirated from the bladder. Studies were approved by the North Carolina State University Institutional Animal Care and Use Committee.
Preparation of Bladder Mucosa
Urinary bladders were bathed in an oxygenated Ringer’s solution (154.1 mM Na+, 6.3 mM K+, 1.2 mM Ca2+, 0.7 mM Mg2+, 137.3 mM Cl−, 24 mM HCO3− and 1.65 mM HPO42−) and bisected longitudinally using sharp dissection. For aseptic experiments, the Ringer’s solution was sterilized by filtration (0.22 μm) and addition of 50 μg/ml of streptomycin and 50 IU/ml of penicillin (Mediatech, Manassas, Virginia, USA). Within the oxygenated Ringer’s solution, the seromuscular layers of the urinary bladder were removed by sharp dissection. The resulting mucosal sheets consisting of urothelium and lamina propria were divided into equal sections and mounted in 3.14 cm2 aperture Ussing chambers (World Precision Instruments, Sarasota, Florida, USA).
Ussing Chamber Studies
Within the Ussing chamber, the luminal and submucosal sides of the mucosa were bathed in 10 ml of Ringer’s solution with or without the previously described antibiotics. A final concentration of 10 mM of mannitol and 10 mM of glucose were added to the luminal and submucosal chamber reservoirs respectively. Both solutions were maintained at 37°C by water-jacketed reservoirs. The solutions were continuously oxygenated (95% O2, 5% CO2) and circulated by gas lift.
For measurement of transepithelial electrical resistance (TER), the spontaneous potential difference (PD) was measured using Ringer-agar bridges connected to calomel electrodes and the PD was short-circuited through Ag-AgCl electrodes using a voltage clamp that corrected for fluid resistance. Since the spontaneous PD for the urothelium falls between 1.0–1.0 mV, PD was determined by maintaining transepithelial current at 100 μA for 5 sec and then recording the PD. TER (Ω/cm2) was calculated from this current-clamped PD and applied current. Electrical readings were recorded at 30 min intervals over the course of each experiment after an initial 15 min tissue equilibration period.
Uropathogenic Escherichia coli
A uropathogenic strain of E. coli J96 (kindly provided by the laboratory of Dr. P. Orndorff, North Carolina State University) was cultured to log phase and added to the luminal reservoir of the Ussing chamber to achieve a final bacterial concentration of 1 × 108 colony forming units (cfu)/ml. Bacteria were added to the Ussing chamber immediately after the initial 15 min tissue equilibration period to allow for a total treatment contact time with the urothelium that ranged from 3–10 h.
Light and Transmission Electron Microscopy
At the conclusion of experiments, tissues were removed from the Ussing chamber and fixed in 10% neutral buffered formalin or Trump’s fixative (McDowell and Trump, 1976). For examination by light microscopy, formalin-fixed tissue was embedded in paraffin wax, sectioned (4 um), processed routinely and stained with haematoxylin and eosin (HE). For examination by transmission electron microscopy (TEM), Trump’s-fixed tissue was processed in accordance with standard techniques (Dykstra, 2003) and examined using a FEI/Philips EM 208S (FEI, Hillsboro, Oregon, USA) transmission electron microscope.
Immunofluorescence Microscopy
After removal from the Ussing chamber, mucosae were embedded in optimal temperature cutting medium and sectioned (4 μm) by use of a cryostat. Sections were fixed in 100% ethanol and blocked with 1% bovine serum albumin (v/w) and 2% goat serum (v/v) in phosphate buffered saline (PBS; with additional 0.12% of 1M CaCl2, pH 7.4) for 1 h at 4°C prior to incubation with primary antibodies. Primary antibodies (diluted 1 in 50 in blocking buffer) were applied for 1 h at room temperature and included polyclonal rabbit anti-human uroplakin-III (Abcam, Cambridge, Massachusetts, USA), polyclonal rabbit anti-canine zona occludens 1 (ZO-1) (Invitrogen, Eugene, Oregon, USA) and rabbit isotype control antibodies (Invitrogen). Fluorescence labelling was performed using strepavidin-conjugated Alexa Fluor 488 (diluted 1 in 100; Invitrogen), Cy3-labelled goat anti-rabbit (diluted 1 in 100; Jackson Immunoresearch, West Grove, Pennsylvania, USA), and FITC-labelled control mouse IgG1 (diluted 1 in 500; BD Pharmingen, Franklin Lakes, New Jersey, USA) for 30 min at room temperature. Sections were counterstained with DAPI and labelling was visualized using an epifluorescence microscope.
Cytokine/Chemokine Quantification
After 5 h of infection by E. coli or normal Ringer’s control, bathing fluid was removed from the submucosal reservoir of the Ussing chamber. Solutions (25 μl) were analyzed for concentrations of tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-4, IL-6, IL-10 and IL-15 using a canine-specific fluorescence multiplex assay as described by the manufacturer (MILLIPLEX MAP Canine Cytokine/Chemokine Panel; Millipore, Billerica, Massachusetts, USA). The lower limits of detection for these cytokines were: TNF-α, 0.4pg/ml; IFN-γ, 4.4pg/ml; IL-4, 28.8pg/ml; IL-10, 1.6pg/ml; IL-15, 14.8pg/ml; and IL-6, 12.1pg/ml.
Statistical Analysis
Statistical analyses were performed using commercially available software (SigmaStat software, Systat, San Jose, California, USA). Values are reported as means ± standard errors. For analysis of multiplex data, values that were lower than the limit of detection were assigned values of one half of the lower limit of detection (defined above) (Williams et al., 2002; Temperton et al., 2007; Li, 2011). Data were tested for normality and equal variance. Parametric data were analyzed using a student’s T-test. Non-parametric data were analyzed using a Mann-Whitney rank sum test and paired when appropriate.
Results
Microscopical Integrity of Canine Bladder Mucosa
To determine if the integrity of canine urothelium can be maintained in vitro, sheets of bladder mucosa were stripped from the adventitia and muscularis layers and placed in Ussing chambers for periods up to 10 h. At 0 h the acutely stretched urothelium consisted of three cell layers representing the basal cells, intermediate cells and large umbrella cells (Fig. 1a). The basal and intermediate cells retained associations with the intact basement membrane and each other by means of intact hemidesmosomes and desmosomes, respectively. The umbrella cells were characterized by numerous intracellular vesicles, abundant cytoplasm and a scalloped luminal surface consistent with an intact asymmetrical unit membrane (Fig. 1d). Notably absent from this acute preparation were well-developed tight junctions along the apical lateral interface of the umbrella cells (Fig. 1g).
Fig. 1.
Light and transmission electron microscopical appearance of canine bladder mucosa immediately after removal of the seromuscular layers (A, D and G) and after incubation in the Ussing chamber in the absence (B, E and H) or presence (C, F and I) of 1 × 108 cfu/ml E. coli J96 on the luminal side of the urothelium. Umbrella cells (UC), intermediate cells (IC) and basal cells (BC) can be identified ultrastructurally under each treatment condition (D, E and F). High magnification images of the umbrella cells immediately after removal of the seromuscular layers reveal small intercellular attachments (ia) without defined tight junctions between adjacent umbrella cells (G). After mounting in Ussing chambers, well-developed tight junctions (tj) between umbrella cells can be identified within a period of 5 h within tissue that obtained a TER >800 Ω/cm2 (H). After a 5 h exposure to E. coli J96 (1×108 cfu/ml), umbrella cells are observed to slough and those remaining lack defined intercellular junctions (ij)(I).
Functional Integrity of Canine Bladder Mucosa
TER is the most sensitive measure of epithelial barrier function since it reflects the ability of the epithelium to resist permeation by ions such as Na+. To assess the functional integrity of canine bladder urothelium over time, TER was calculated every 30 min for urothelium mounted within Ussing chambers. Beginning immediately after mounting the bladder mucosa in the Ussing chamber, urothelial resistance increased steadily, reaching a mean of 1384 ± 282 Ω/cm2 by 5 h (Fig. 2). TER was initially low, but following initial reduction the urothelium appeared to increase in functional integrity over the 5 h following placement in the Ussing chamber.
Fig. 2.
Maintenance of TER in response to normal Ringer’s solution (Rab) or lumen infection with uropathogenic E. coli J96 (1 × 108 cfu/ml). * P < 0.05, ** P < 0.01; n = 6 dogs for each treatment condition.
The increase in functional integrity of the bladder mucosa was hypothesized to be associated with changes in the urothelial structural integrity. To assess these changes, after 5 h within the Ussing chamber the tissue was examined ultrastructurally. The effect of tension (applied while mounting the mucosa within the Ussing chamber) was reflected by lateral movement of the basal and intermediate cells resulting in a more squamous appearing urothelium via light microscopy (Fig. 1b). Two cell layers were clearly visible with the third layer more difficult to discern consistently by TEM (Fig. 1e). These findings are consistent with the described electron microscopical appearance of distended urinary bladder (Richter and Moize, 1963). In all sections, an intact basement membrane interdigitated with, and connected to, the overlying urothelial cells by means of hemidesmosomes. Desmosomal connections, as well as interdigitating membrane between the cells of the urothelium, also remained. Umbrella cells retained the asymmetrical unit membrane; however, the apical undulations and scalloped appearance were less pronounced (Fig. 1e). In contrast to the acutely stretched urothelium, mucosa allowed to achieve TER >800 Ω/cm2 within the Ussing chamber demonstrated well-developed tight junctions along the apical lateral interface of umbrella cells (Fig. 1h).
Effects of Uropathogenic Esherichia coli on Urothelial Barrier Function
Exposing the luminal surface of canine urothelium to uropathogenic E. coli J96 for a period of 5 h resulted in a significant failure to recover urothelial barrier function culminating in a mean TER of 338 ± 146 Ω/cm2 (Fig. 2).
In order to determine the cause of reduced urothelial barrier function after infection with uropathogenic E. coli, the chambered urothelium was examined for changes in ultrastructural integrity. A minimal effect by E. coli on the urothelium was observed at a light microscopical level for up to 10 h (Fig. 1c). However, ultrastructurally, E. coli-infected tissue demonstrated periodic umbrella cell sloughing with exposure of the underlying intermediate cells by 5 h (Fig. 1f). Despite the retention of structural connections including hemidesmosomes and desmosomes, intact apical membrane tight junctions between the remaining umbrella cells were not identifiable (Fig. 1i).
Immunohistochemical Expression of ZO-1 and Uroplakin-III
In order to establish further whether E. coli impairs barrier function by a paracellular or transcellular mechanism, in-situ expression of the tight junction protein ZO-1 and the asymmetric unit membrane protein uroplakin-III were examined by immunofluorescence microscopy. In uninfected mucosa allowed to achieve a TER >800 Ω/cm2 within the Ussing chamber, ZO-1 was identified discretely at the intercellular junctions (Fig. 3a) of the umbrella cells consistent with tight junction formation. Uroplakin-III was expressed intensely, especially along the umbrella cell apical surface, as expected with an intact umbrella cell layer (Fig 3b).
Fig. 3.
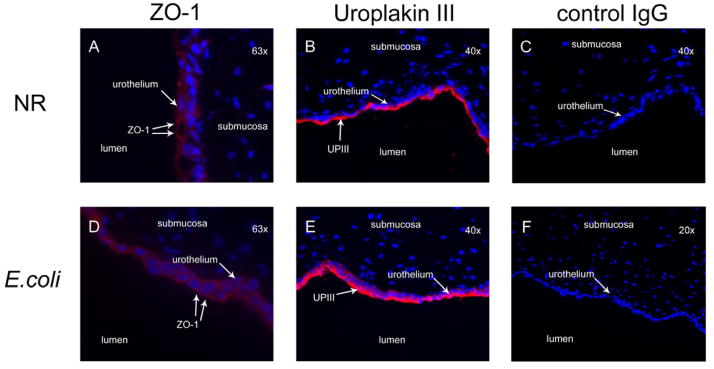
Immunofluorescence localization of ZO-1 and uroplakin-III in canine bladder mucosa treated with normal Ringer’s alone or infected with uropathogenic E. coli J96 (1 × 108 cfu/ml) for a period of 5 h in Ussing chambers. Localization of ZO-1 to the apical-lateral junctions of umbrella cells was well-defined in mucosa treated with normal Ringer’s (A) and absent in mucosa infected with E. coli (D). Uroplakin-III expression by the urothelium was localized primarily to the apical surface and umbrella cells in the normal Ringer’s treated tissue (B). In E. coli treated tissue the apical uroplakin-III remained present however an increase in uroplakin-III expression in all cellular layers of the urothelium was noted (E). FITC-labelled control mouse IgG1 (C) and rabbit antibody isotype control serum (F) served as the secondary and primary control antibodies respectively.
After 5h of E. coli infection, ZO-1 was conspicuously absent (Fig. 3d). In contrast, expression of uroplakin-III was intensified within all layers of the urothelium consistent with an urothelial response to insult (Lavelle et al., 2002) (Fig. 3e). There was no evidence of diminished uroplakin-III expression along the apical border, which would have been an expected sequela to generalized umbrella cell sloughing (Guiton et al., 2010).
Cytokine Secretion
In order to determine if bacterial infection in vitro mediates the acute secretion of cytokines having putative downstream roles in maintenance of epithelial barrier function, the concentrations of TNF-α, IFN-γ, IL-4, IL-10, IL-15 and IL-6 were measured by multiplex assay after treatment of Ussing chamber-mounted urothelial sheets with E. coli or normal Ringer’s solution for 5 h. Compared to Ringer’s solution, E. coli infection was associated with significant increases in concentrations of TNF-α, IL-6 and IL-15 (P ≤ 0.01) secreted into the subepithelial reservoir (Fig. 4). Two additional cytokines (IFN-γ and IL-4) were secreted by the urothelium after exposure to E. coli, but not in quantities different from Ringer’s solution alone. Of the six cytokines examined, only IL-10 was below the limit of detection of the multiplex assay for both the E. coli- and Ringer’s solution-treated tissue.
Fig. 4.
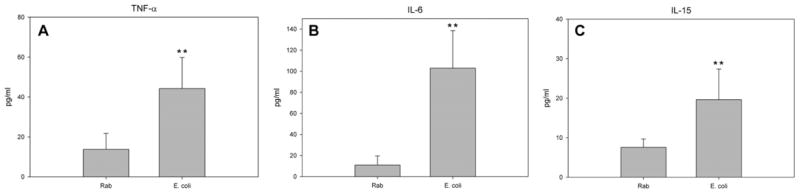
Secretion of cytokines by urothelium in response to normal Ringer’s (Rab) and E. coli J96 (1 × 108 cfu/ml). E. coli J96 induces the urothelium to secrete significant amounts of TNF-α, IL-6 and IL-15 into the subepithelial Ussing chamber 5 h after infection when compared with Rab treatment alone (A, B and C). ** P ≤ 0.01, n = 6.
Discussion
The results of the present study provide electrophysiological and anatomical evidence that E. coli damages urothelial barrier function by altering tight junction integrity and paracellular permeability. The act of mounting bladder mucosa within the Ussing chamber gave rise to an initially low TER that was rapidly restored to physiological levels in uninfected tissue commensurate with tight junction reformation between the umbrella cells. This rising TER is a characteristic response of urothelium within the Ussing chamber (Lewis and Hanrahan, 1990) and culminated in a peak TER consistent with those described in prior mammalian studies of bladder urothelium (Lavelle et al., 2000). In contrast, urothelium exposed to E. coli failed to recover this barrier function. This barrier defect was characterized by failure of the urothelium to re-form tight junctions between the umbrella cells and occasional umbrella cell sloughing as demonstrated both ultrastructurally and by means of immunofluorescence microscopy for expression of the tight junctional protein ZO-1. In contrast, integrity of the transcellular pathway components, including the asymmetric unit membrane ultrastructure and immunofluorescence-localized expression of uroplakin-III, appeared largely intact in E. coli-infected urothelium. Since the paracellular pathway is restricted by the tight junctions that adjoin the umbrella cells (Hicks et al., 1974; Lewis et al., 1976) and the transcellular pathway is largely restricted by the uroplakin-enriched asymmetric unit membrane (Lewis and Hanrahan, 1990; Negrete et al., 1996; Hu et al., 2002), these findings suggest that acute E. coli infection is associated with a paracellular permeability defect.
In the present study, the bacterial factor(s) responsible for failure of barrier function remain undetermined. Previous work demonstrated that soluble factors within an E. coli culture supernatant were sufficient to decrease the TER of bladder urothelium (Tay et al., 1996); however, in the present study all soluble factors were initially removed by washing the bacteria prior to infection of the mucosa. Barrier function between the normal Ringer’s- and E. coli-treated tissues was significantly different within the first 60 min after infection and persisted up to 5 h, suggesting that direct contact by bacteria may also play a role in altering urothelial permeability.
In other tissues such as the intestine, specific cytokines have been demonstrated to decrease (TNF-α, IFN-γ and IL-4), increase (IL-10 and IL-15) or have conflicting effects (IL-6) on epithelial barrier function (Al-Sadi et al., 2009). The effect of these cytokines on urothelial barrier function is unknown. Amongst these cytokines only IL-6 and TNF-α have been documented to be produced during acute UTI in people (Agace et al., 1993; Funfstuck et al., 2001; Rodhe et al., 2009). In the in-vitro model, acute E. coli infection of the canine bladder mucosa also produced significant increases in secretion of TNF-α and IL-6. Cytokine production by the urothelium is induced when virulence factors such as lipopolysaccharide and fimbriae expressed by uropathogenic E. coli interact with Toll-like receptor 4 and glycoconjugate receptors located on the urothelial surface, starting an intracellular signaling cascade (Svanborg et al., 1999; Samuelsson et al., 2004; Song et al., 2007). Given that in the present studies the effect of E. coli on barrier function occurred acutely within the first 60 min of urothelial exposure, a primary role of TNF-α or IL-6 in preventing urothelial tight junction reformation therefore seems unlikely. However, delayed effects of these cytokines on urothelial or inflammatory cells may contribute to a more sustained loss of barrier function. This possibility remains to be examined.
A unique finding in this study was the secretion of IL-15 in response to acute bacterial UTI in vitro. Throughout the body, IL-15 is recognized primarily for its role in activating or stimulating cells of the immune system including natural killer cells and neutrophils to initiate an innate immune response against invading pathogens (Shurin, 2003). However, its synthesis and biological actions are not limited to lymphoid tissue (Grabstein et al., 1994). In the intestinal mucosa, increased IL-15 secretion has been linked to both inflammatory cell recruitment (Di Sabatino et al., 2011) and tissue reparative responses including the formation and repair of tight junctions and epithelial barrier function (Nishiyama et al., 2001). Given that our model of E. coli infection identifies a paracellular defect coinciding with an increased secretion of IL-15, perhaps IL-15 may serve a similar role within the urothelium helping to recruit inflammatory cells while simultaneously initiating a protective response aimed at repairing damaged urothelial tight junctions.
The present studies have established a unique model of acute uropathogenic E. coli infection using canine bladder mucosa mounted in vitro in Ussing chambers. With this model, E. coli was demonstrated to mediate loss of urothelial barrier function and promote secretion by the bladder mucosa of cytokines with bioactivity reportedly capable of modulating epithelial barrier function. Loss of barrier function could be attributed to increased paracellular permeability of the urothelium as this was associated with failure of umbrella cell tight junction function and occasional umbrella cell sloughing, despite maintenance of expression of umbrella cell uroplakin-III. IL-15 has not been demonstrated previously to be secreted by the bladder mucosa and, because IL-15 plays key roles in reconstitution of tight junction function in damaged intestine, our study points to a new potential role for IL-15 in urothelial injury. Given that bladder permeability presumably increases in association with clinical signs of cystitis in naturally-occurring UTIs, the present study provides both new mechanistic insight into the cause of urothelial pathology and establishes a powerful model system with which to examine pharmacological approaches to ameliorate these effects.
Acknowledgments
M. Wood is supported by the Ruth L. Kirschstein National Research Service Award T32 RR024394 as part of North Carolina State University’s Comparative Medicine and Translational Research Training Program. The authors thank M. Stone, S. Stauffer and M. Suyemoto for their valuable technical assistance.
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agace W, Hedges S, Andersson U, Andersson J, Ceska M, et al. Selective cytokine production by epithelial cells following exposure to Escherichia coli. Infection and Immunity. 1993;61:602–609. doi: 10.1128/iai.61.2.602-609.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Frontiers in Bioscience. 2009;14:2765–2778. doi: 10.2741/3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartges JW. Diagnosis of urinary tract infections. Veterinary Clinics of North America: Small Animal Practice. 2004;34:923–987. doi: 10.1016/j.cvsm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochimica et Biophysica Acta: Biomembranes. 2009;1788:864–871. doi: 10.1016/j.bbamem.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew DJ, DiBartola SP, Scheneck PA. Canine and Feline Nephology and Urology. Elsevier; St Louis: 2011. pp. 240–271. [Google Scholar]
- Clarke LL. A guide to Ussing chamber studies of mouse intestine. American Journal of Physiology: Gastrointestinal and Liver Physiology. 2009;296:G1151–G1166. doi: 10.1152/ajpgi.90649.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabatino A, Calarota SA, Vidali F, MacDonald TT, Corazza GR. Role of IL-15 in immune-mediated and infectious diseases. Cytokine and Growth Factor Reviews. 2011;22:19–33. doi: 10.1016/j.cytogfr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Dykstra MJ. Biological Electron Microscopy: Theory, Techniques and Troubleshooting. Plenum Publishers; New York: 2003. pp. 5–78. [Google Scholar]
- Funfstuck R, Franke S, Hellberg M, Ott U, Knofel B, et al. Secretion of cytokines by uroepithelial cells stimulated by Escherichia coli and Citrobacter spp. International Journal of Antimicrobial Agents. 2001;17:253–258. doi: 10.1016/s0924-8579(01)00301-6. [DOI] [PubMed] [Google Scholar]
- Grabstein KH, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, et al. Cloning of a T-cell growth factor that interacts with the beta-chain of the interleukin-2 receptor. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- Guiton PS, Hung CS, Hancock LE, Caparon MG, Hultgren SJ. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infection and Immunity. 2010;78:4166–4175. doi: 10.1128/IAI.00711-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks RM, Ketterer B, Warren RC. Ultrastructure and chemistry of luminal plasma membrane of mammalian urinary bladder – structure with low permeability to water and ions. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1974;268:23–28. doi: 10.1098/rstb.1974.0013. [DOI] [PubMed] [Google Scholar]
- Hooton TM, Stamm WE. Diagnosis and treatment of uncomplicated urinary tract infection. Infectious Disease Clinics of North America. 1997;11:551–581. doi: 10.1016/s0891-5520(05)70373-1. [DOI] [PubMed] [Google Scholar]
- Hu P, Meyers S, Liang F-X, Deng F-M, Kachar B, et al. Role of membrane proteins in permeability barrier function: uroplakin ablation elevates urothelial permeability. American Journal of Physiology: Renal Physiology. 2002;283:F1200–F1207. doi: 10.1152/ajprenal.00043.2002. [DOI] [PubMed] [Google Scholar]
- Lavelle J, Meyers S, Ramage R, Bastacky S, Doty D, et al. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. American Journal of Physiology: Renal Physiology. 2002;283:F242–F253. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- Lavelle JP, Meyers SA, Ruiz WG, Buffington CA, Zeidel ML, et al. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. American Journal of Physiology: Renal Physiology. 2000;278:F540–F553. doi: 10.1152/ajprenal.2000.278.4.F540. [DOI] [PubMed] [Google Scholar]
- Lewis SA. Everything you wanted to know about the bladder epithelium, but were afraid to ask. American Journal of Physiology: Renal Physiology. 2000;278:F867–F874. doi: 10.1152/ajprenal.2000.278.6.F867. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Eaton DC, Diamond JM. The mechanism of Na+ transport by rabbit urinary bladder. Journal of Membrane Biology. 1976;28:41–70. doi: 10.1007/BF01869690. [DOI] [PubMed] [Google Scholar]
- Lewis SA, Hanrahan JW. Physiological approaches for studying mammalian urinary bladder epithelium. Methods in Enzymology. 1990;192:632–650. doi: 10.1016/0076-6879(90)92100-r. [DOI] [PubMed] [Google Scholar]
- Li J, Birkenheuer AJ, Marr HS, Levy MG, Yoder JA, et al. Expression and function of triggering receptor expressed on myeloid cells-1 (TREM-1) on canine neutrophils. Developmental and Comparative Immunology. 2011;35:872–880. doi: 10.1016/j.dci.2011.03.021. [DOI] [PubMed] [Google Scholar]
- McDowell EM, Trump BF. Histologic fixatives suitable for diagnostic light and electron microscopy. Archives of Pathology and Laboratory Medicine. 1976;100:405–414. [PubMed] [Google Scholar]
- Negrete HO, Lavelle JP, Berg J, Lewis SA, Zeidel ML. Permeability properties of the intact mammalian bladder epithelium. American Journal of Physiology: Renal Physiology. 1996;271:F886–F894. doi: 10.1152/ajprenal.1996.271.4.F886. [DOI] [PubMed] [Google Scholar]
- Nishiyama R, Sakaguchi T, Kinugasa T, Gu XB, MacDermott RP, et al. Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions. Journal of Biological Chemistry. 2001;276:35571–35580. doi: 10.1074/jbc.M106013200. [DOI] [PubMed] [Google Scholar]
- Parsons CL. The role of the urinary epithelium in the pathogenesis of interstitial cystitis/prostatitis/urethritis. Urology. 2007;69:9–16. doi: 10.1016/j.urology.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Richter WR, Moize SM. Electron microscopic observations on collapsed and distended mammalian urinary bladder (transitional epithelium) Journal of Ultrastructure Research. 1963;9:1–9. [PubMed] [Google Scholar]
- Rodhe N, Lofgren S, Strindhall J, Matussek A, Molstad S. Cytokines in urine in elderly subjects with acute cystitis and asymptomatic bacteriuria. Scandinavian Journal of Primary Health Care. 2009;27:74–79. doi: 10.1080/02813430902757634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson P, Hang L, Wullt B, Irjala H, Svanborg C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infection and Immunity. 2004;72:3179–3186. doi: 10.1128/IAI.72.6.3179-3186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior DF. Management of urinary tract infections. In: Elliott J, editor. BSAVA Manual of Canine and Feline Nephrology and Urology. British Small Animal Veterinary Association; Gloucester: 2007. pp. 282–289. [Google Scholar]
- Shurin MR, Tourkova IL, Hackstein H, Shurin GV. Interleukin-15 and 21. In: Thompson AW, Lotze MT, editors. The Cytokine Handbook. Vol. 1. Elsevier Science Ltd; Boston: 2003. pp. 431–453. [Google Scholar]
- Song J, Duncan MJ, Li G, Chan C, Grady R, et al. A novel TLR4-mediated signaling pathway leading to IL-6 responses in human bladder epithelial cells. PLoS Pathogenesis. 2007;3:e60. doi: 10.1371/journal.ppat.0030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg C, Godaly G, Hedlund M. Cytokine responses during mucosal infections: role in disease pathogenesis and host defence. Current Opinion in Microbiology. 1999;2:99–105. doi: 10.1016/s1369-5274(99)80017-4. [DOI] [PubMed] [Google Scholar]
- Tay H, Parsons CL, Stein PC. Electrophysiologic monitoring of the effects of soluble virulence factors produced by Escherichia coli infection in urine. Urology. 1996;48:389–392. doi: 10.1016/s0090-4295(96)00209-9. [DOI] [PubMed] [Google Scholar]
- Temperton NJ, Hoschler K, Major D, Nicolson C, Manvell R, et al. A sensitive retroviral pseudotype assay for influenza H5N1-neutralizing antibodies. Influenza and Other Respiratory Viruses. 2007;1:105–112. doi: 10.1111/j.1750-2659.2007.00016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truschel ST, Ruiz WG, Shulman T, Pilewski J, Sun TT, et al. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. Journal of Biological Chemistry. 1999;274:15020–15029. doi: 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- Williams SB, Flanigan TP, Cu-Uvin S, Mayer K, Williams P, et al. Human immunodeficiency virus (HIV) – specific antibody in cervicovaginal lavage specimens obtained from women infected with HIV. Clinical Infectious Diseases. 2002;35:611–617. doi: 10.1086/342201. [DOI] [PubMed] [Google Scholar]
- Wood MW, Breitschwerdt EB, Gookin JL. Autocrine effects of interleukin-6 mediate acute-phase proinflammatory and tissue-reparative transcriptional responses of canine bladder mucosa. Infection and Immunity. 2011;79:708–715. doi: 10.1128/IAI.01102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]




