Abstract
Background
Anopheles sinensis, Anopheles anthropophagus, Anopheles minimus and Anopheles dirus are the major vectors of malaria transmission in China. Anopheles sinensis is considered a secondary vector due to its relatively low malaria-transmission ability. However, in 2005, an outbreak of over 40,000 Plasmodium vivax malaria cases was reported in areas where Anopheles sinensis was the only major vector. Therefore, it is necessary to reassess the malaria transmission ability of this vector species in China.
Methods
Laboratory colonies of An. sinensis and An. anthropophagus, and first-generation progeny (F1) of An. sinensis that had been collected in central China, were infected by direct membrane feeding assay with mono-vivax gametocyte-containing blood collected from vivax-infected patients. The mosquitoes were kept for 7 to 14 days post-blood feeding to allow parasites to develop into oocysts and sporozoites. Infectivity was measured by dissecting midguts and salivary glands. The presence of oocysts and sporozoites was determined by microscopy at 7 and 14 days post-blood feeding, and the numbers of gametocytes and asexual parasites, as well as mosquito parasite infections, were determined.
Results
The positive oocyst and sporozoite feed rates of the 142 pairs of lab-colony An. sinensis and An. anthropophagus were not significantly different, and the same results were found with the 10 pairs of laboratory and F1 An. sinensis. An. sinensis had more oocysts/midgut at 7 days post-feeding than An. anthropophagus, but the gametocytemia, asexual parasitemia, and ratio of macrogametocytes to microgametocytes, did not correlate with either oocyst or sporozoite infection. However, in the oocyst-positive mosquitoes, there was a correlation between gametocytemia and the average oocyst number/midgut.
Conclusions
The susceptibility of An. sinensis (both laboratory and F1) to P. vivax-infected blood is similar to Anopheles anthropophagus, when evaluated by membrane feeding assay under laboratory conditions. In recent years, in central China, the vivax malaria transmission ability of An. sinensis has probably been underestimated. Further studies of this species in other regions are needed. An. sinensis could also be a good candidate vector for evaluating candidate malaria transmission-blocking vaccines (TBV).
Keywords: Membrane blood feeding, Plasmodium vivax, Anopheles sinensis, Anopheles anthropophagus
Background
Anopheles sinensis, An. anthropophagus, An. minimus, and An. dirus are the major vectors of malaria transmission in China [1], and species in the An. maculatus complex may be major transmission vectors in Tibet Autonomous Region [2]. Among these species, An. minimus and An. dirus are mostly distributed in southern China (Yunnan and Hainan provinces), where the geographical environment is markedly different from the central region. An. sinensis and An. anthropophagus are relatively more widely distributed in China. According to the updated distribution records of the An. hyrcanus species group (Diptera: Culicidae), An. sinensis is found in over 29 provinces and regions in China [3]. It is noteworthy that An. sinensis has become the only major vector in central China, where Plasmodium vivax is the only prevalent, locally transmitted, malaria parasite; however, a few imported falciparum malaria cases have been reported among travellers [4].
An. sinensis and An. anthropophagus are both members of the An. hyrcanus complex, sharing similar morphological characteristics, and a ribosomal DNA-internal transcribed spacer 2 (rDNA-ITS2) -based method is required to distinguish the two species [5,6]. However, in addition to the distinct distributions of An. sinensis and anthropophagus, the species differ strongly in host preference, resting habitat, and other features involved in malaria transmission. First, An. anthropophagus prefers to bite humans rather than animals, whereas An. sinensis is a more zoophilic mosquito and demonstrates a marked preference for cattle and other warm-blooded animals. Second, An. anthropophagus prefers indoor resting after blood feeding; residual insecticide spraying in areas of central China where An. anthropophagus predominated as the major vector for falciparum effectively reduced malaria mortality and morbidity, from 1980 to 1990. As a result, falciparum malaria has been eliminated in central China. However, An. sinensis tends toward outdoor resting after indoor blood feeding, which has made vector control of this species more difficult. Third, An. anthropophagus is much more susceptible to vivax malaria parasites [7]. The regions in China containing both An. anthropophagus and An. sinensis have suffered more serious malaria epidemics than those areas where An. sinensis is the only vector [8].
Taking the above-mentioned factors into consideration, one possible conclusion is that An. sinensis plays a less important role in malaria transmission than other species in central China. Unexpectedly, frequent outbreaks of vivax malaria started appearing in areas where An. sinensis was the main vector, with over 40,000 reported vivax cases in 2005, accounting for 67% of all cases in China [9]; this suggests that the susceptibility and other features of An. sinensis that affect its interaction with vivax parasites have changed. Thus, the comparative malaria-transmission ability of An. sinensis with other major vectors should be reassessed. In this study, we assessed the susceptibility of An. sinensis to P. vivax in central China by membrane-feeding assay and compared the results with An. anthropophagus and a field strain of An. sinensis. This study will help to explain the vivax epidemic situation in central China better, and improve the current elimination programmes of this species in China.
Methods
Study site and patients
The study was conducted in Bengbu, Anhui Province, central China (Figure 1). P. vivax is the only malaria parasite in this region. In 2004, the total number of malaria cases in Anhui reached 8,909, which was 22.9% of all cases in China (Figure 2). Patients aged 18 years of age or more, who sought clinical treatment for malaria, were included in this study. Thick and thin blood smears were prepared from each individual and stained with 10% Giemsa by experienced microscopists to exclude mixed infection with P. falciparum. In addition, gametocyte and asexual parasite densities were determined for all P. vivax-positive patients by counting the number of parasites per 500 leukocytes in a thick blood smear under oil immersion microscopy. The raw counts were converted into parasites/microliter by assuming a count of 8,000 leukocytes/μL. If gametocytes were present, the patient was asked to enrol in the study. After the patients were briefed on the project and completed consent forms, approximately 5 mL of blood was collected by venepuncture and used for membrane blood feeding (detailed below) of starved mosquitoes. After mosquito feeding, the volunteers were released from the study and received antimalarial treatment.
Figure 1.
Study area for vivax malaria patient recruitment from 2005 to 2007.
Figure 2.
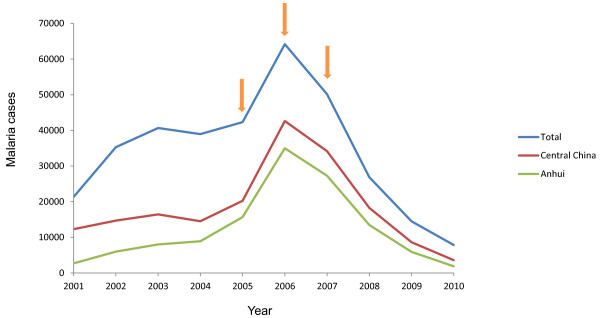
Malaria transmission in China from 2003 to 2010.
Mosquitoes
An. sinensis and An. anthropophagus have been maintained in the insectary of the Key Technical Laboratory for Prevention and Control of Parasitic Diseases of the Ministry of Health (MOH) in the Jiangsu Institute of Parasitic Diseases (JIPD), Wuxi, China, for over 30 years. The mosquitoes were reared at 27 ± 1°C with a relative humidity of 70–80% and provided with 10% (w/v) glucose in water. One hundred mosquitoes in each carrying cage were transported from JIPD to the field sites in a cooler box. Next, 6- to 8-day-old mosquitoes were provided only water for 12 hours prior to blood feeding. In this study, engorged female anopheline mosquitoes from Bengbu, Anhui, were also collected, and their progeny (F1) were identified via both morphological characteristics and an rDNA ITS2-based method [5] to confirm species. The mosquitoes were maintained as described above, prior to blood feeding.
Membrane feeding
Five hundred microliters (500 μL) of whole blood from each patient were centrifuged at 5,000 g for 5 minutes. The serum was then removed and replaced with approximately 300 μL of AB serum from a P. vivax-naive donor. The packed red blood cells and donor sera were carefully mixed and added to the membrane feeder. A constant-temperature (37°C) circulating-water system was used to prevent exflagellation of microgametocytes [10]. The blood feeding lasted for 30 minutes, after which the glass membrane feeder was removed from the top of the carton and all the unengorged mosquitoes were removed and freeze-killed. After feeding, all engorged mosquitoes were transported back to JIPD’s insectary in Wuxi, where they were provided with a 12-h light/dark cycle and daily sugar solution before dissection.
Mosquito dissection
On day 7 post-blood feeding, the mosquitoes were aspirated into glass tubes and immobilized by placing the tube on ice. At least 10 midguts of each species were dissected in a drop of mercurochrome in phosphate-buffered saline, and the number of oocysts per midgut was counted under 10× or 40× microscopic examination. On day 14 post-feeding, if the mosquitoes were oocyst-positive, another 10 mosquitoes of each species were dissected, and the number of oocysts per midgut was first counted, as above. Then, the infecting sporozoite level was recorded after direct determination under phase contrast microscopy (Leica DM2500, US) without staining. The level was recorded as follows: “+”, 1–10 sporozoites; “++”, 11–100 sporozoites; “+++”, 101–500 sporozoites and “++++”, >500 sporozoites.
Statistical analysis
The chi-square test was used to compare the proportion of mosquitoes infected with oocysts, the proportion of mosquitoes infected with sporozoites, and the proportion of infected mosquitoes per positive feeding, between paired lab-colony An. anthropophagus and An. sinensis, and between paired F1 and lab-colony An. sinensis. Paired T tests were used to compare oocyst loads (mean oocyst number per infected midgut) between the feeding groups. A regression test was used to detect any linear correlation between parasite load and infection rate.
Ethical approval
All human-subject research conducted in this study was reviewed and approved by the Institutional Ethics Committee of the National Institute for Parasitic Diseases (NIPD), Chinese Center for Disease Control and Prevention. All the patients involved in this study read and signed the informed consent forms.
Results
Patient data
From 2005 to 2007, over 200 symptomatic malaria patients came to the clinic in Bengbu, Anhui. In total, 142 volunteers were finally enrolled in this study after excluding subjects aged less than 18 years and those with mixed infections with falciparum malaria, or zero gametocytes by thick blood-smear count. Patient age and parasite density data are shown in Table 1.
Table 1.
Age and parasite density of the study patients (n = 142)
| Minimum | Maximum | Average | |
|---|---|---|---|
| Age of patients (years) |
18 |
71 |
34.6 ± 3.1 |
| No. of asexual parasites (/μL) |
0 |
21696 |
4332.8 ± 371.2 |
| No. of macrogametocytes (/μL) |
32 |
6560 |
1057.6 ± 88 |
| No. of microgametocytes (/μL) | 64 | 4800 | 486.4 ± 51.2 |
Membrane feeding
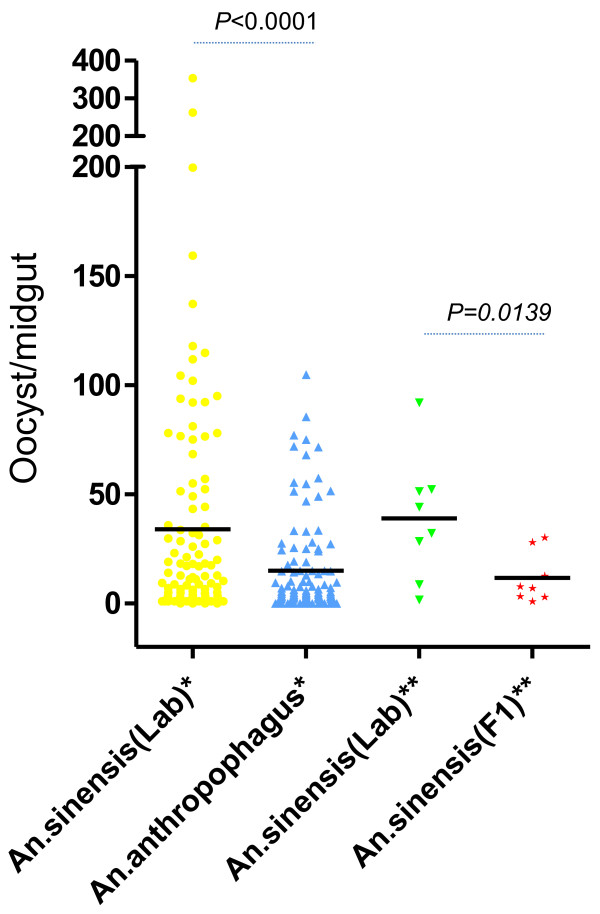
In total, the blood of 142 vivax patients was fed via membrane feeding to the laboratory colonies of An. sinensis and An. anthropophagus. Among these 142 patients, blood from 10 patients was also fed to lab-colony and F1 An. sinensis mosquitoes. The engorged feeding percentages of the paired laboratory strain An. sinensis and An. anthropophagus were 64.86% (9210/14200) and 62.86% (8926/14200), respectively. The F1 An. sinensis had the lowest engorged feeding rate, at 16.5% (165/1000) (χ2 = 934.05, p < 0.01). Oocyst number and sporozoite index were counted and recorded using a normal and phase-contrast microscope, respectively (Figure 3). The positive oocyst feed rate (positive feeds/total feeds) and positive mosquito infection rate (positive mosquitoes/total mosquitoes) of the lab-colony An. sinensis and An. anthropophagus did not differ at day 7 post-blood feeding (χ2 = 0.82, P > 0.05, χ2 = 3.22, P > 0.05, respectively). Likewise, the positive sporozoite feed rate and positive mosquito infection rate at day 14 post-blood feeding did not differ (χ2 = 0.09, P > 0.05, χ2 = 0.21, P > 0.05, respectively) (Table 2). In 10 paired cases, both the F1 and laboratory strain had the same positive oocyst feed (80%) and sporozoite feed (30%) rates. In the 10 paired membrane feeding tests, the lab-strain An. sinensis had a higher oocyst infection rate at day 7 than F1 (Figure 4), as did the laboratory strain An. sinensis in the 142 paired feedings with laboratory strain An. anthropophagus (Figure 4). However, interestingly, the two laboratory strains had similar sporozoite levels at day 14 post-feeding (z = 0.866, p = 0.38, Table 3).
Figure 3.
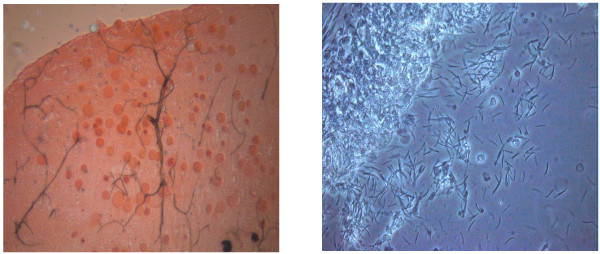
Oocyst and sporozoite infection in midgut and salivary glands. (A) Oocysts in the midgut were counted using a normal microscope at 10× objective magnification with mercurochrome staining. (B) Sporozoites in the salivary glands were assessed using 40× objective magnification and a phase-contrast microscope without staining.
Table 2.
Comparison of blood feeding, and oocyst and sporozoite infection, forAn. sinensis and An. anthropophagus
|
Species |
% of feeds infecting mosquitoes |
% of mosquitoes that fed on all infectious patients that developed parasite infection |
Mean number of oocysts per positive mosquito |
||
|---|---|---|---|---|---|
| (Positive feeds/total feeds) | (Positive mosquitoes/total mosquitoes) | ||||
| Days post-feeding |
7 |
14 |
7 |
14 |
7 |
|
An. sinensis (Lab) |
72.5 (103/142) |
28.9 (41/142) |
45.7 (15536/340) |
11.1 (135/1216) |
45.7 (15536/340) |
|
An. sinensis (F1) |
80.0 (8/10) |
30.0 (3/10) |
13.4 (281/21) |
20.0 (9/45) |
13.4 (281/21) |
| An. anthropophagus (Lab) | 67.6 (96/142) | 26.8 (38/142) | 21.0 (6437/306) | 11.84 (96/811) | 21.0 (6437/306) |
Figure 4.
Scatter plots of the results of the species comparisons. The median oocyst load of Anopheles is shown by a horizontal black line; *: range of oocysts/midgut between the 142 pairs of lab-colony An. sinensis (lab) and An. anthropophagus, **: range of oocysts/midgut between the 10 pairs of lab-colony (Lab) and first-generation (F1) An. sinensis.
Table 3.
Sporozoite infection of An. sinensis and An. anthropophagus 14 days’ post-feeding
|
Sporozoite infective level |
No. of sporozoites |
|
|---|---|---|
| An. sinensis(Lab) | An. anthropophagus | |
| + |
12 |
10 |
| ++ |
42 |
24 |
| +++ |
31 |
19 |
| ++++ |
50 |
43 |
| Total | 135 | 96 |
*: “+”, 1–10 sporozoites; “++”, 11–100 sporozoites; “+++”, 101–500 sporozoites and “++++”, >500 sporozoites.
Correlation of parasitemia and infection
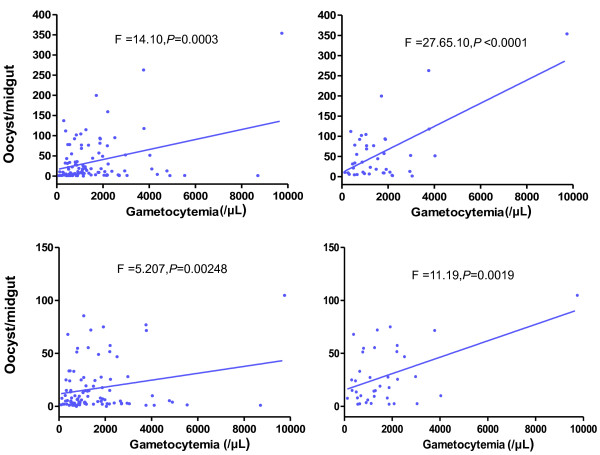
In total, 41/103 and 38/96 infected lab-colony An. sinensis and An. anthropophagus, respectively, developed sporozoites in the salivary glands at 14 days’ post-blood feeding. The other 62 and 58 respective cases only had oocysts in the midgut at day 7 post-feeding, and 32 of 142 cases were negative for both oocyst and sporozoite infection in both An. sinensis and An. anthropophagus (Table 4). The effects of parasite density (macrogametocyte, microgametocyte, asexual-stage parasite) and ratio of macrogametocytes to microgametocytes, in the five groups referred to above, were evaluated. The parasite density or the ratio of macrogametocyte to microgametocyte had no effect on parasite infection as the data showed that there was no significant difference between the negative and positive cases. However, in cases of positive infection, regression analysis revealed a significant linear correlation between blood gametocyte density and midgut parasite infection load in both An. sinensis and An. anthropophagus. The cases with more oocysts or sporozoites had higher gametocytemia levels, particularly in the sporozoite-positive cases (Figure 5).
Table 4.
Parasitemia and oocyst and sporozoite infection in An. sinensis and An. anthropophagus
|
Species |
An. sinensis |
An. anthropophagus |
An. sinensis/An. anthropophagus |
||
|---|---|---|---|---|---|
| Oocyst(+)/ Sporozoite(−) | Oocyst(+)/ Sporozoite(+) | Oocyst(+)/ Sporozoite(−) | Oocyst(+)/ Sporozoite(+) | Oocyst(−)/ Sporozoite(−) | |
| Cases |
62 |
41 |
58 |
38 |
32 |
| Mean oocysts/midgut |
20.81 |
58.03 |
18.36 |
53.68 |
0 |
| Mean gametocyte density (/μL) |
1579.6 ± 154.8 |
1682.3 ± 255.4 |
1618.2 ± 163.4 |
1652.2 ± 267.9 |
1386.5 ± 254.0 |
| Mean asexual parasite density (/μL) |
4308.2 ± 444.4 |
5061.8 ± 709.6 |
4325.0 ± 456.6 |
4329.7 ± 663.2 |
3781.5 ± 714.2 |
| Female gametocytes/male gametocytes | 3.24 ± 0.25 | 3.41 ± 0.46 | 3.31 ± 0.26 | 3.07 ± 0.35 | 3.98 ± 0.92 |
Figure 5.
Correlation between number of Plasmodium vivax gametocytes per microliter of blood and number of oocysts per midgut. (A) Lab-colony An. sinensis (103 oocyst-positive cases), (B) Lab-colony An. sinensis (41 sporozoite-positive cases), (C) Lab-colony An. anthropophagus (96 oocyst-positive cases) and (D) Lab-colony An. anthropophagus (38 sporozoite-positive cases).
Discussion
This is the first study to evaluate the susceptibility of An. sinensis to vivax parasites in central China by membrane feeding, after the re-emergence of malaria in central China. In this study, An. sinensis (both laboratory colony and F1) were equally susceptible to vivax malaria parasites as An. anthropophagus, which was believed for many years to be the major vector in central China. Despite that belief, the laboratory colony of An. sinensis had a higher oocyst infection rate. Although the same was not found in the F1 mosquitoes, their low observed vivax susceptibility could have been due to their low engorged feeding rate, caused by the switch of emergent environment from field to laboratory. In addition, the difficulty of maintaining the engorged mosquitoes under laboratory conditions cannot be ignored [11], as this definitely reduced the quantity of mosquitoes dissected at day 7 post-feeding. Furthermore, in the 10 paired cases, both laboratory and field An. sinensis mosquitoes presented the same infection rate and 100% concordance with the positive case selection (both positive oocyst and sporozoite feedings), suggesting the lab-colony An. sinensis in this study could well represent actual current vector susceptibility to parasites in the field. In this case, An. sinensis was more able to carry P. vivax in the midgut stage than An. anthropophagus, given an equal opportunity to feed on malaria patients. This contrasts with previous results in central China [12]. Indeed, only mosquitoes with sporozoites in the salivary glands can infect humans, although both An. sinensis and An. anthropophagus had similarly low sporozoite infection rates in this study, which should raise some suspicion [13]. However, it is reasonable to note that malaria parasites also reduce mosquito survival rates [14]. In addition, our objective was to evaluate the susceptibility of An. sinensis compared with An. anthropophagus and not to count sporozoite quantities long-term. Both mosquito species had more sporozoites after 14 days’ post-feeding than at 14 days. Thus, oocyst infection in the midgut stage could reflect potential transmission capacity.
A well-known preference for human biting, a tendency to rest indoors, and great susceptibility to parasites with sufficient longevity, are essential criteria for evaluating vectors for malaria transmission capacity. In China, although An. sinensis is the most widely distributed, with a large population in most mainland regions, the species had for decades been judged not to be the predominant vector for malaria due to its exophilic and exophagic features, and relatively low susceptibility to parasites compared with other vectors. Nevertheless, the vivax malaria outbreak in 2005 in central China, in which An. sinensis served as the main vector, suggested an updated evaluation of the vector capacity and transmission role of this species was necessary [15]. Along with agricultural and industrial progress in China, frequently moving populations have become an important group at risk of carrying parasites from malaria-epidemic areas to malaria-free or low-transmission regions. During the malaria transmission season, from June to September, farmers and construction workers habitually sleep in the open without net protection, which increases the chance for An. sinensis, which in this study had a strong propensity to develop vivax malaria parasites following blood-feeding from infected humans, to bite several different people. Due to the exophilic nature of An. sinensis and continuously increasing insecticide resistance [16-21], the regular insecticide residual spray (IRS) methods used in malaria-transmission regions do not kill all mosquitoes [22]. Another possible reason for the malaria outbreak in central China is climatic and environmental change [10]. If An. sinensis mosquitoes are unable to find their usual animal blood feeding targets, because of the construction of buildings or other such changes, they may resort to biting humans [23].
Only macrogametocytes and microgametocytes can develop in the mosquito midgut; all other asexual parasites are digested after blood feeding. This study supports the previous finding that although the average asexual parasitemia counts in the negative feed groups were lower than the positive feed groups, no significant differences were found [24]. This was also supported by some positive feeds with zero asexual parasites somehow achieving a high oocyst infection number; however, the oocysts were absent in the midgut in some cases with high asexual parasite counts. Although the same phenomenon was noted with the relationship of gametocytemia to midgut infection in the negative and positive groups, oocyst load (oocysts per positive mosquito) increased with gametocyte density in all infection-positive groups, suggesting that the blood of patients with high levels of gametocytemia had a greater potential to induce mosquitoes, post-feeding, to develop oocysts in the midgut and thereby be at higher risk of transferring parasites to other humans. Although the results from this study confirm the previous finding that oocysts developed well in the mosquito midgut, with a ratio of macrogametocytes to microgametocytes of less than 4 [25], the number of negative-infection cases producing oocysts in this study demonstrated that the presence of oocysts in the midgut following feeding is not a good indicator of infectivity, a conclusion supported by a study in west Thailand [26]. The malaria parasite is under intense attack from the mosquito’s innate immune system during its development in the midgut and salivary glands [27]. Several mosquito immune genes play important roles in the parasite evasion stage by influencing parasite-mosquito interactions [28-30]. In other words, the susceptibility of mosquitoes to malaria infection could be related to an enhanced or weakened immune response of mosquitoes to parasite infection [31]. Additionally, the genotypes of the invading parasites play an important role, i.e., parasites with VK210 and VK247, two main genotypes of circumsporozoite protein (CSP), have an obvious preference for infecting mosquitoes [32]. Therefore, further study of the susceptibility of An. sinensis to parasites from various geographic areas in China is necessary.
Although the direct-feeding method more accurately reflects epidemiologic reality [33], most volunteers prefer to provide blood by venepuncture rather than allowing mosquitoes to bite their skin directly [34]. In the membrane-feeding assay used in this study, patient sera were replaced by naive malaria-free human AB serum, to avoid interference from varying antibody levels in patient blood samples [35]. Furthermore, the constant-temperature cycling system allowed unlimited maintenance of parasite activity and equalised blood-feeding conditions among the mosquito groups [36]. The membrane feeding assay is a valuable tool for the evaluation and validation of candidate markers of transmission-blocking vaccine (TBV) following the modification of target genes [37,38]. Because An. sinensis is the largest of the four major vectors in China, as well being relatively easier to maintain under laboratory conditions, and with high susceptibility to vivax parasites, it could be used as a valuable candidate species to evaluate TBV, in particular.
Compared to the previous studies [39,40] the An sinensis from this study showed higher susceptibility rates to P. vivax isolates in Central China, although it is known that the An. sinensis strain from Korea, China and Japan was compatible genetically and/or nearly identical to that from Thailand, based on the crossing experiments and comparative sequence analyses of the ribosomal DNA (rDNA) internal transcribed spacer 2 (ITS2) [41]. The genetic diversity of the parasites and their compatibility to the vectors in each location may contribute to the difference in vector susceptibility. In this study we did not analyse the genetic diversity of the parasites as the study aimed to compare the susceptibility of An. sinensis and An. anthopophagus in Central China to the same parasite isolates collected in this region.
Conclusions
To our knowledge, this is the first report of the susceptibility of the widely distributed malaria vector An. sinensis to P. vivax following artificial membrane feeding after the re-emergence of malaria in central China. The An. sinensis mosquitoes in the laboratory maintained a similar capacity to become infected with vivax parasites as the field mosquitoes, and their parasite-carrying ability was also similar to that of An. anthropophagus. The vector capacity of An. sinensis for malaria transmission during the vivax re-emergence period, particularly in central China, has probably been underestimated. Due to its morphological characteristics and high susceptibility to parasites, An. sinensis could be a good vector candidate for vivax malaria TBV evaluation.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
GD, QG, and JS contributed equally to the study design and data analysis. HX, HY, JL, FL, YB and JC managed the parasites and mosquitoes in the field. GD, HY, JL and YB contributed to the dissection of mosquitoes. GD drafted the manuscript. QG provided scientific supervision. JS revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Guoding Zhu, Email: jipdzhu@hotmail.com.
Hui Xia, Email: xiahui912@hotmail.com.
Huayun Zhou, Email: zhou_huayun@hotmail.com.
Julin Li, Email: lijulin301@hotmail.com.
Feng Lu, Email: lufeng981@hotmail.com.
Yaobao Liu, Email: boao0721@hotmail.com.
Jun Cao, Email: caojuncn@hotmail.com.
Qi Gao, Email: gaoqi54@hotmail.com.
Jetsumon Sattabongkot, Email: jetsumon@hotmail.com.
Acknowledgements
We acknowledge the outstanding technical support of the entomology teams of the Armed Forces Research Institute of Medical Sciences (AFRIMS, Bangkok, Thailand). We are grateful to Qiang Fang, Zhiyong Tao and Kaiming Hu from Bengbu Medical College for their assistance in field work and are grateful to Ming-chieh Lee from the Program in Public Health, University of California at Irvine, USA, for drafting the map. This research was supported by the US Military Infectious Diseases Research Program, Jiangsu Province’s Construction project (BM2009902) and Jiangsu Province’s Medical High Tech Platform (ZX201108).
References
- Chen B, Harbach RE, Butlin RK. Molecular and morphological studies on the Anopheles minimus group of mosquitoes in southern China: taxonomic review, distribution and malaria vector status. Med Vet Entomol. 2002;16:253–265. doi: 10.1046/j.1365-2915.2002.00373.x. [DOI] [PubMed] [Google Scholar]
- Wu S, Pan JY, Wang XZ, Zhou SS, Zhang GQ, Liu Q, Tang LH. Anopheles pseudowillmori is the predominant malaria vector in Motuo County. Tibet Autonomous Region. Malaria J. 2009;8:46. doi: 10.1186/1475-2875-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda LM, Zhao TY, Ma YJ, Gao Q, Zhu GD, Khuntirat B, Sattabongkot J, Wilkerson RC. Updated distribution records of the Anopheles (Anopheles) hyrcanus species-group (Diptera: Culicidae) in China. Zootaxa. 2007;1407:43–55. [Google Scholar]
- Zhou SS, Wang Y, Fang W, Tang LH. [Malaria situation in the People's Republic Of China in 2007] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2008;26(6):401–403. [PubMed] [Google Scholar]
- Gao Q, Beebe NW, Cooper RD. Molecular identification of the malaria vectors Anopheles anthropophagus and Anopheles sinensis (Diptera: Culicidae) in central China using polymerase chain reaction and appraisal of their position within the Hyrcanus group. J Med Entomol. 2004;41(1):5–11. doi: 10.1603/0022-2585-41.1.5. [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhou HY, Cooper RD, Li FH, Su YP, Zhu GD, Cao J, Beebe NW. A simple technique for the genetic identification of different Anopheline mosquitoes among the Anopheles hyrcanus complex. Chin J Zoonoses. 2005;21(3):193–196. [Google Scholar]
- Li JL, Zhou HY, Shen BX. The susceptibility investigation of Anopheles sinensis and Anopheles anthropophagus to Plasmodium vivax. Chin J Schisto Control. 1996;8(4):1. [Google Scholar]
- Liu CF, Qian HL, GU ZC, Pan JY, Zhen X. The current comparsion of malaria transmission effect ofAnopheles sinensisandAnopheles anthropophagusin China. J Med Res. 1991;20(5):2. [Google Scholar]
- Zhou SS, Wang Y, Tang LH. [Malaria situation in the People's Republic of China in 2006] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2007;25(6):439–441. [PubMed] [Google Scholar]
- Billker O, Shaw MK, Margos G, Sinden RE. The roles of temperature, pH and mosquito factors as triggers of male and female gametogenesis of Plasmodium berghei in vitro. Parasitol. 1997;115(Pt 1):1–7. doi: 10.1017/s0031182097008895. [DOI] [PubMed] [Google Scholar]
- Bangs MJ, Soelarto T, Barodji, Wicaksana BP, Boewono DT. Colonization of Anopheles maculatus from Central Java, Indonesia. J Am Mosq Control Assoc. 2002;18(4):359–363. [PubMed] [Google Scholar]
- Rong YQ, Fang HY, Yang ZY, Zhou HY, Mei HG. The susceptibility study of Anopheles sinensis and Anopheles anthropophagus to Plasmodium vivax and Plasmodium falciparum. JiangSu Ji Fang. 1986;3:4. [Google Scholar]
- Solarte Y, Manzano MR, Rocha L, Hurtado H, James MA, Arevalo-Herrera M, Herrera S. Plasmodium vivax sporozoite production in Anopheles albimanus mosquitoes for vaccine clinical trials. Am J Trop Med Hyg. 2011;84(2 Suppl):28–34. doi: 10.4269/ajtmh.2011.09-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heather M, Ferguson AFR. Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol. 2002;18(6):256–261. doi: 10.1016/S1471-4922(02)02281-X. [DOI] [PubMed] [Google Scholar]
- Zhou SS, Zhang SS, Wang JJ, Zheng X, Huang F, Li WD, Xu X, Zhang HW. Spatial correlation between malaria cases and water-bodies in Anopheles sinensis dominated areas of Huang-Huai plain, China. Parasit Vectors. 2012;5:106. doi: 10.1186/1756-3305-5-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann T, Hume JC, Licht M, Burns CS, Wollenberg K, Simard F, Ribeiro JM. Molecular evolution of immune genes in the malaria mosquito Anopheles gambiae. PLoS One. 2009;4(2):e4549. doi: 10.1371/journal.pone.0004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan WL, Wang ZM, Li CX, Chu HL, Xu Y, Dong YD, Wang ZC, Chen DY, Liu H, Liu DP. et al. First report on co-occurrence knockdown resistance mutations and susceptibility to beta-cypermethrin in Anopheles sinensis from Jiangsu Province. China PLoS One. 2012;7(1):e29242. doi: 10.1371/journal.pone.0029242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen K, Van Bortel W, Trung HD, Sochantha T, Keokenchanh K, Coosemans M. Knockdown resistance in Anopheles vagus, An. sinensis, An. paraliae and An. peditaeniatus populations of the Mekong region. Parasit Vectors. 2010;3(1):59. doi: 10.1186/1756-3305-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui F, Raymond M, Qiao CL. Insecticide resistance in vector mosquitoes in China. Pest Manag Sci. 2006;62(11):1013–1022. doi: 10.1002/ps.1288. [DOI] [PubMed] [Google Scholar]
- Wang J. Resistance and response to selection to deltamethrin in Anopheles sinensis from Zhejiang, China. J Am Mosq Control Assoc. 2000;16(1):9–12. [PubMed] [Google Scholar]
- Zhang Z, Yang C. Application of deltamethrin-impregnated bednets for mosquito and malaria control in Yunnan, China. Southeast Asian J Trop Med Public Health. 1996;27(2):367–371. [PubMed] [Google Scholar]
- The malERA Consultative Group on Vector Control. A research agenda for malaria eradication: vector control. PLoS Med. 2011;8(1):e1000401. doi: 10.1371/journal.pmed.1000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadei WP, Dutary Thatcher B. Malaria vectors in the Brazilian amazon: Anopheles of the subgenus Nyssorhynchus. Rev Inst Med Trop São Paulo. 2000;42(2):87–94. doi: 10.1590/S0036-46652000000200005. [DOI] [PubMed] [Google Scholar]
- Zollner GE, Ponsa N, Garman GW, Poudel S, Bell JA, Sattabongkot J, Coleman RE, Vaughan JA. Population dynamics of sporogony for Plasmodium vivax parasites from western Thailand developing within three species of colonized Anopheles mosquitoes. Malaria J. 2006;5:68. doi: 10.1186/1475-2875-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Luo SH, Shu HP, Fu RD. Research on the factors influencing the sporogonic multiplication of Plasmodium vivax in the mosquito vector. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing ZaZhi. 1995;13:21–24. [PubMed] [Google Scholar]
- Nacher M, Silachamroon U, Singhasivanon P, Wilairatana P, Phumratanaprapin W, Fontanet A, Looareesuwan S. Risk factors for Plasmodium vivax gametocyte carriage in Thailand. Am J Trop Med Hyg. 2004;71(6):693–695. [PubMed] [Google Scholar]
- Cirimotich CM, Dong Y, Garver LS, Sim S, Dimopoulos G. Mosquito immune defenses against Plasmodium infection. Dev Comp Immunol. 2010;34(4):387–395. doi: 10.1016/j.dci.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 2011;7(5):e1002043. doi: 10.1371/journal.ppat.1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmakelis A, Moustaka M, Poulakakis N, Louis C, Slotman MA, Marshall JC, Awono-Ambene PH, Antonio-Nkondjio C, Simard F, Caccone A. et al. Anopheles immune genes and amino acid sites evolving under the effect of positive selection. PLoS One. 2010;5(1):e8885. doi: 10.1371/journal.pone.0008885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford JE, Guelbeogo WM, Sanou A, Traore A, Vernick KD, Sagnon N, Lazzaro BP. De novo transcriptome sequencing in Anopheles funestus using Illumina RNA-seq technology. PLoS One. 2010;5(12):e14202. doi: 10.1371/journal.pone.0014202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahia AC, Kubota MS, Tempone AJ, Pinheiro WD, Tadei WP, Secundino NF, Traub-Cseko YM, Pimenta PF. Anopheles aquasalis Infected by Plasmodium vivax displays unique gene expression profiles when compared to other malaria vectors and plasmodia. PLoS One. 2010;5(3):e9795. doi: 10.1371/journal.pone.0009795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Ceron L, Rodriguez MH, Nettel JC, Villarreal C, Kain KC, Hernandez JE. Differential susceptibilities of Anopheles albimanus and Anopheles pseudopunctipennis to infections with coindigenous Plasmodium vivax variants VK210 and VK247 in southern Mexico. Infect Immun. 1999;67(1):410–412. doi: 10.1128/iai.67.1.410-412.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M-T RC. The malarial infectivity of an African village population to mosquitoes (Anopheles gambiae) Am J Trop Med Hyg. 1957;6:971–979. doi: 10.4269/ajtmh.1957.6.971. [DOI] [PubMed] [Google Scholar]
- Awono-Ambene HP, Diawara L, Robert V. Comparison of direct and membrane feeding methods to infect Anopheles arabiensis with Plasmodium falciparum. Am J Trop Med Hyg. 2001;64(1–2):32–34. doi: 10.4269/ajtmh.2001.64.32. [DOI] [PubMed] [Google Scholar]
- Sattabongkot J, Tsuboi T, Hisaeda H, Tachibana M, Suwanabun N, Rungruang T, Cao YM, Stowers AW, Sirichaisinthop J, Coleman RE. et al. Blocking of transmission to mosquitoes by antibody to Plasmodium vivax malaria vaccine candidates Pvs25 and Pvs28 despite antigenic polymorphism in field isolates. Am J Trop Med Hyg. 2003;69(5):536–541. [PubMed] [Google Scholar]
- Sattabongkot J, Maneechai N, Phunkitchar V, Eikarat N, Khuntirat B, Sirichaisinthop J, Burge R, Coleman RE. Comparison of artificial membrane feeding with direct skin feeding to estimate the infectiousness of Plasmodium vivax gametocyte carriers to mosquitoes. Am J Trop Med Hyg. 2003;69(5):529–535. [PubMed] [Google Scholar]
- Tachibana M, Sato C, Otsuki H, Sattabongkot J, Kaneko O, Torii M, Tsuboi T. Plasmodium vivax gametocyte protein Pvs230 is a transmission-blocking vaccine candidate. Vaccine. 2012;30(10):1807–1812. doi: 10.1016/j.vaccine.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Tonwong N, Sattabongkot J, Tsuboi T, Iriko H, Takeo S, Sirichaisinthop J, Udomsangpetch R. Natural infection of Plasmodium falciparum induces inhibitory antibodies against gametocyte development in human hosts. Jpn J Infect Dis. 2012;65(2):152–156. [PubMed] [Google Scholar]
- Joshi D, Choochote W, Park MH, Kim JY, Kim TS, Suwonkerd W, Min GS. The susceptibility of Anopheles lesteri to infection with Korean strain of Plasmodium vivax. Malaria J. 2009;8(12):42. doi: 10.1186/1475-2875-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi D, Kim JY, Choochote W, Park MH, Min GS. Preliminary vivax malaria vector competence for three members of the Anopheles hyrcanus group in the Republic of Korea. J Am Mosq Control Assoc. 2011;27(3):312–314. doi: 10.2987/10-6086.1. [DOI] [PubMed] [Google Scholar]
- Park MH, Choochote W, Kim SJ, Somboon P, Saeung A, Tuetan B, Tsuda Y, Takagi M, Joshi D, Ma Y, Min GS. Nonreproductive isolation among four allopatric strains of Anopheles sinensis in Asia. J Am Mosq Control Assoc. 2008;24(4):489–495. doi: 10.2987/08-5753.1. [DOI] [PubMed] [Google Scholar]