Abstract
We aimed to evaluate the lasting functional imprinting of exercise (EX) and catechin (CAT) on the vascular function of middle-age mice switched to a proatherogenic environment. C57BL/6J mice (n=10–15 in each group) fed a regular diet (RD) were exposed from the age of 1 to 9 months either to EX (voluntary running; 2.7± 0.2 km/day), to the polyphenol CAT (30 mg/kg/day in drinking water), or to physical inactivity (PI). At 9 months of age, EX and CAT were stopped and mice either remained on the RD or were fed a Western diet (WD) for an additional 3 months. At 12 months of age, mice from all groups fed a WD had similar body mass, systolic blood pressure, and plasma total cholesterol, glucose, insulin, and isoprostane. Compared to the RD, the WD induced an indomethacin-sensitive aortic endothelium-dependent and independent dysfunction in PI mice (p<0.05) that was prevented by both EX and CAT; this benefit was associated with a higher (p< 0.05) non-nitric oxide/non-prostacyclin endothelium-dependent relaxation. While EX, but not PI or CAT, prevented vascular dysfunction induced by the WD in cerebral arteries, it had no effect in femoral arteries. The profiles of activity of antioxidant enzymes and of proinflammatory gene expression in the aorta suggest a better adaptation of EX>CAT>PI mice to stress. In conclusion, our data suggest that a postnatal exposure to EX, but not to CAT, imprints an adaptive defense capacity in the vasculature against a deleterious change in lifestyle.
Keywords: Physical inactivity, Western diet, Endothelium, Antioxidant enzymes, Vascular smooth muscle cells
Introduction
Beyond genetic susceptibilities, the environment we live in is the main determinant of our health span [9]. It starts during the intrauterine environment that influences health outcome at adult age, as low birth weight is associated with an increased risk of diseases [2]. Epidemiological studies have clearly established that a lifelong healthy lifestyle, composed of moderate but regular physical exercise (EX) and a balanced diet, prolongs lifespan and reduces the odds of developing cardiovascular diseases (CVD) [10, 16, 45, 46, 59, 76]. The detrimental effects of the so-called Western diet (WD) and lifestyle on the cardiovascular system have, therefore, been established by numerous studies showing the contribution of physical inactivity (PI) and poor-quality/high-calorie intake on the development of obesity, hypertension, diabetes, and CVD [9, 37].
The primary target for stress induced by risk factors for CVD is the vascular endothelium. While not a medical condition, a chronic endothelial dysfunction is the initiating step of the atherosclerotic process [14, 73, 74]. Numerous preclinical studies have confirmed the cardiovascular benefits of EX on the endothelial function in aging mice [20], obese rats [7], and apoE−/− mice fed a high-fat diet [38]. In addition, the antioxidant polyphenol catechin (CAT) can prevent endothelial dysfunction in severely dyslipidemic mice if initiated early in life [19, 25]; we found, however, that chronic treatment with CAT was not effective and even deleterious if started in mice with established atherosclerosis [25], confirming the results of numerous clinical trials [66]. In contrast, the endothelial function of arteries from 1-year-old healthy C57BL/6 mice benefited more from CAT when initiated at the age of 9 months than when initiated at the age of 3 months [26]. Altogether, these data, in agreement with the clinical trials, suggest that (1) the timing of the treatment and (2) the environment (oxidative stress and inflammation) determine the endothelial protection given by antioxidants. It is unknown, however, if a healthy lifestyle, including EX, imprints the vascular endothelium, providing a better adaptive capacity to maintain its function against a deleterious and chronic change in environment.
In this study, we used two preventive interventions, voluntary EX and CAT, in young mice to test the hypothesis that these interventions will impose a unique molecular imprint to the endothelium, leading to a differential vascular reactivity after a switch in lifestyle at midlife. Our data demonstrate that lifelong PI combined or not with CAT limits the adaptive defense capacity of the vasculature compared to EX. They suggest, in agreement with our initial paradigm, that the magnitude of the age-dependent vascular dysfunction is directly related to its ability to limit the accumulation of damage.
Methods
The procedures and protocols in our study were approved by the Montreal Heart Institute Animal Ethics Committee and performed in accordance with the Guide for the Care and Use of Experimental Animals of the Canadian Council on Animal Care and the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Animals
C57BL/6J 1-month-old male mice (n=10–15 per group) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). Animals were kept under standard conditions (24 °C; 12:12 h light/dark cycle). At 1 month of age, mice were randomly selected to perform voluntary EX, receive CAT or neither (PI) until the age of 9 months (Supplementary Fig. S1). EX mice had free access to a running wheel (Lafayette Instrument, Lafayette, IN, USA) without reward [5] and ran an average of 2.7±0.2 km/day over the 8-month period of exposure. The polyphenol CAT was dissolved in the drinking water to deliver a daily dose of 30 mg/kg previously shown to lower oxidative stress [18, 25], prevent endothelial dysfunction and arterial wall remodeling [6, 19, 24, 25], and improve cognition [18]. During that period, all mice were fed a regular diet (RD, 2018; Harlan Teklad Laboratories, Madison, WI, USA). At 9 months of age, EX and CAT were stopped. Mice were either sacrificed for study or randomly assigned to receive a 3-month RD or WD (88137; Harlan Teklad Laboratories, Madison, WI, USA) until the age of 12 months (Fig. S1). During the 11 months of experimentation, blood pressure was recorded every other week by tail plethysmography (Kent Scientific Corporation, Torrington, CT, USA) as described before [6]. Values of systolic blood pressure (SBP) presented are a mean of the measures obtained during the last three sessions of recording prior to sacrifice. Food consumption was measured in each group 1 week before the age of 9 and 12 months. We observed that EX mice ate less (p<0.05) at 9 months than PI mice (PI=4.0±0.1 g/day, EX=3.4±0.2 g/day, CAT=3.8± 0.1 g/day, n=9–10). Following the WD, EX and CAT mice ate less (p<0.05) than PI mice (PI=3.9±0.1 g/day, EX=3.3± 0.1 g/day, CAT=3.4±0.2 g/day, n=7–10). At 9 months of age, EX mice had no access to the running wheel at least 48 h before euthanasia to avoid an acute effect of EX. Mice were fasted overnight before their morning terminal anesthesia (44 mg/kg ketamine and 2.2 mg/kg xylazine). Blood was collected and plasma was frozen at −80 °C. Thoracic and abdominal aortas were harvested and placed in ice-cold physiological salt solution (PSS, pH 7.4; in millimoles per liter: 119 NaCl, 4.7 KCl, 1.18 KH2PO4, 1.17 MgSO4, 24.9 NaHCO3, 1.6 CaCl2, 0.023 EDTA, and 10 glucose). Thoracic aortas were cut into 2-mm rings for vascular reactivity studies or embedded in optimal cutting temperature compound for immunostaining. Abdominal aortas were snap-frozen in liquid nitrogen and used for enzymatic activity and quantitative polymerase chain reaction (qPCR) studies. Femoral arteries were harvested and placed in ice-cold PSS to isolate a 2- to 3-mm-length arterial segments for endothelial function and compliance studies. Brain was removed from its cranial cavity and placed in ice-cold PSS to isolate posterior cerebral arteries (PCA) for endothelial function and compliance studies as previously reported [5].
Plasma parameters
Cholesterol and glucose levels were measured at the Montreal Heart Institute Clinical Biochemistry Laboratory (Montreal, QC, Canada). 8-Iso-prostaglandin F2α (isoprostane; Enzo Life Sciences, Farmingdale, NY, USA) and insulin (Alpco Diagnostics, Salem, NH, USA) were quantified using commercial kits and according to the manufacturers’ protocols.
Endothelial function in aortas
Rings of 2 mm isolated from the thoracic aorta were mounted on 20-μm tungsten wires in microvessel myographs (IMF, University of Vermont, Burlington, VT, USA) as previously described [70]. Vessels were stretched to obtain an optimal basal tension of 1.5 g as determined in preliminary studies; no differences in basal tension were observed between groups (data not included). The viability of the arterial rings was tested with a 40-mM KCl-PSS solution. After two washout periods, the vessels were equilibrated for 30–45 min alone, in the presence of Nω-nitro-L-arginine (L-NNA, 100 μM, a nitric oxide synthase [NOS] inhibitor), indomethacin (10 μM, a nonselective cyclooxygenase inhibitor), or with both L-NNA and indomethacin to study non-nitric oxide (NO)/non-prostanoid-dependent relaxations. First, a concentration–response curve with the thromboxane A2 analog 9,11-dideoxy-11α,9α-ep-oxy-methano-prostaglandin F2α (U46619, 0.1 nM to 10 μM) was obtained for each group (n=3–5, in duplicate) to measure the half-maximum effective concentration (EC50) (Table S1). In subsequent experiments (n = 3–9 per group), aorta segments were preconstricted at the EC50 and relaxation curves to acetylcholine (Ach, 0.1 nM to 30 μM) and sodium nitroprusside (SNP, 0.1 nM to 30 μM) were performed. Two consecutive concentration–response curves separated by 30–45 min washout periods were obtained from each aortic segment. Preliminary studies showed no effect of consecutive random concentration–response curves on relaxation (data not included). The maximal contraction was obtained at the end of the protocol by adding 127 mM KCl-PSS.
Reverse transcriptase quantitative polymerase chain reaction
Total RNA extraction was performed with the RNeasy Mini Kit (Qiagen Canada, Toronto, ON, Canada) following the manufacturer’s protocol. Reverse transcriptase reaction (100 ng total RNA) was performed as described previously [24] using the Moloney murine leukemia virus reverse transcriptase (200 U; Invitrogen, Carlsbad, CA, USA). qPCR were performed with platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA, USA). Primers were selected in two different exons spanning a large intronic sequence to avoid amplification of genomic DNA (Table S2). Annealing and elongation temperatures, cDNA template quantity, and primer concentrations were optimized for each pair of primers to generate a standard curve with an efficacy of 100±10 %. Cyclophilin A was used as a normalizer and relative gene expression was calculated by the ΔΔCT method [53].
Antioxidant enzyme activity in aortas
Abdominal aortas were disrupted with a mortar and pestle on dry ice and kept at −80 °C. The powder was manually homogenized in either 4-(2-hydroxyethyl)-1-piperazineetha-nesulfonic acid (HEPES) buffer (pH 7.2; in millimoles per liter: 20 HEPES, 1 ethylene glycol tetraacetic acid [EGTA], 210 mannitol, and 70 sucrose) for catalase and superoxide dismutase 2 (SOD2) assays or Tris–HCl buffer (pH 7.5; in millimoles per liter: 50 Tris–HCl, 5 EDTA, and 1 DTT) for glutathione peroxidase (GPx) assay. Samples homogenized in the HEPES buffer were split into two tubes and centrifuged 5 min at 1,500×g (4 °C) for SOD2 assay or 15 min at 10,000×g (4 °C) for catalase activity. Samples for GPx assay were centrifuged for 15 min at 10,000×g (4 °C). An aliquot of each sample was kept to measure protein concentration (Bradford assay). Further steps were performed as recommended by the manufacturer’s protocol (Cayman Chemical Company, Ann Arbor, MI, USA). SOD2 activity was determined in the presence of 1 mM of potassium cyanide to block copper–zinc SOD activities [62]. Activity values (in units) were adjusted with protein content.
Endothelial function in femoral arteries
Segments of the left or right gracilis artery were dissected in ice-cold PSS to remove surrounding fat and tissues [35]. A 2- to 3-mm-length arterial segment was isolated, cannulated at both ends, and pressurized at 80 mmHg in no flow condition in a pressure myograph. Internal pressure was maintained constant and real-time diameter changes were monitored using a pressure servo-control and a video dimension analyzer, respectively (Living System, Burlington, VT, USA). All experiments were conducted on segments with an internal diameter of 175–210 μM when pressurized at 80 mmHg. An equilibration time of 45 min was allowed before starting the experiment. A single cumulative concentration–response curve to Ach (1 nM to 30 μM) was obtained in vessel preconstricted with phenylephrine (1–30 μM). L-NNA, indomethacin, or a combination of both drugs was added during the equilibration period.
Endothelial function in cerebral arteries
PCAwere isolated, cannulated at both ends, and pressurized as previously described [17]. Flow-mediated dilations (FMD) were induced on phenylephrine (30 μM) preconstricted arteries [6]. Arteries were perfused with PSS and aerated with 12 % O2/5 % CO2/83 % N2. A single cumulative curve (from 0 to 20 dyn/cm2, with 2 dyn/cm2 steps between 0 and 10 dyn/cm2 followed by two 5 dyn/cm2 steps, at constant pressure of 60 mmHg) was performed on each segment [5]. The flow rate through the lumen (Q; in milliliters per second) required to match a given shear stress value (τ; in dynes per square centimeter) was calculated for each point on the curve according to Q=τπr3/4η, where η represents the viscosity (0.009 Poise) and r is the inside radius (in centimeters). The applied calculated shear stress was in the physiological range (≈0–25 dyn/cm2) [42, 50]. The data are presented as the percentage of maximal dilation for every shear stress value.
Compliance studies in femoral and cerebral arteries
Femoral arteries and PCA were used for the in vitro assessment of compliance, measured in passive conditions to reflect mechanical properties of the vascular wall [5, 6]. Passive pressure–diameter curves were conducted in a Ca2+-free PSS containing 1 mM of EGTA and 10 μM of SNP in order to abolish myogenic tone and to solely assess the mechanical properties of the arteries. Phenylephrine (10 μM) never induced a reduction in diameter in these conditions (data not shown). Lumen diameter and outer diameter changes were measured after each increment in the intraluminal pressure (from 10 to 120 mmHg, with a first 10-mmHg step followed by a 20-mmHg step) in order to calculate mechanical parameters of the arterial wall. The circumferential wall strain (in percent) was calculated according to [(D−D10 mmHg)/D10 mmHg], where D is the diameter at a given pressure and D10 mmHg is the initial diameter at the initial pressure. The circumferential wall stress (in dynes per square centimeter) was calculated according to [(P×1,334×D/2)×wall thickness], where P is the given intraluminal pressure (1 mmHg= 1,334 dyn/cm2) and wall thickness (in micromolars) is calculated with [(external diameter–lumen diameter)/2]. The stress–strain relationship was calculated by fitting the data to a nonlinear exponential curve (stress=stress0βstrain), where stress0 is the value when strain equals 0 and β;is a constant representing Young’s elastic modulus (YEM).
Statistical analyses
All groups were composed of 10 mice, except for CAT of 12 months fed RD or WD (n=15). The n refers to the number of animals used in each protocol. A study number of four animals per group of experiments has 90 % power to detect a 30 % absolute variation in vascular reactivity, the most variable parameter measured in this study assessed during preliminary studies. All protocols, including reactivity studies, were performed in duplicate and averaged before individual data inclusion for statistical analysis. Results are presented as the mean±standard error of the mean (SEM). EC50 and the pD2 value, the negative log of the EC50, were measured from individual concentration–response curves. Two-way analysis of variance (ANOVA) was used to test for interactions and main effects between initial treatments and age/diet of mice. When interactions were not statistically significant, marginal means were compared followed by the Bonferroni approach for multiple comparisons. When interactions were statistically significant, one-way ANOVA was used to compare cell means followed by the Bonferroni approach. For analysis of maximal relaxations (Emax) in aorta and femoral arteries, Dunnett’s approach was used instead of the Bonferroni approach. Usual assumptions such as normality and homoscedasticity of the variance were checked. When variances were unequal, usual transformations, such as logarithmic, square root, or inverse function, were used. If unequal variances remained after transformation, covariance pattern models were used. When normality was absent even after transformations, the Kruskal–Wallis test was used followed by the Mann–Whitney pairwise comparisons with adjusted p values. SPSS (IBM Corporation, Armonk, NY, USA) and SAS (SAS Institute, Cary, NC, USA) softwares were used for the statistical analyses. A value of p<0.05 was considered statistically significant.
Results
At the end of the preventive interventions, mice from the three groups had a similar body mass, SBP, as well as plasma glucose, insulin, and total cholesterol (TC) levels (Table 1). Glycemia was high at 9 months of age in all groups (Table 1) compared to 3-month-old mice [5]; this age-dependent increase in circulating glucose has been reported in rat [55], monkey [41], and human [3].
Table 1.
Body mass, plasma glucose, insulin, total cholesterol (TC), and SBP from PI, EX (voluntary wheel running), and CAT-treated (30 mg/kg/day in drinking water) mice from 1 to 9 months of age fed with a RD (9 months) and then maintained for 3 months in inactive conditions and fed either a RD (12 months) or a WD (12 months)
| Age/diets | 9 months RD
|
12 months RD
|
12 months WD
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatments | PI | EX | CAT | PI | EX | CAT | PI | EX | CAT | n |
| Body mass (g) | 30.4±1.3 | 32.4±1.2 | 31.1±1.2 | 33.4±1.6 | 35.7±1.9 | 31.0±0.8 | 44.9±1.5* | 43.9±1.6* | 45.5±0.9* | 9–15 |
| Glucose (mmol/L) | 17.5±1.1 | 15.9±1.7 | 19.2±0.8 | 16.4±0.9 | 20.0±1.9 | 15.7±0.8 | 22.3±0.8* | 19.5±2.6 | 20.5±1.9* | 4–7 |
| Insulin (ng/ml) | 0.17±0.04 | 0.27±0.06 | 0.19±0.06 | 0.29±0.09 | 0.23±0.08 | 0.21±0.05 | 0.49±0.12* | 0.57±0.18* | 0.44±0.11* | 8–10 |
| TC (mmol/L) | 2.4±0.1 | 2.8±0.1 | 2.8±0.1 | 2.6±0.1 | 2.6±0.3 | 2.9±0.1 | 6.9±1.1* | 7.2±1.1* | 5.4±0.6* | 4–7 |
| SBP (mmHg) | 141.0±6.2 | 142.3±2.9 | 148.1±2.8 | 143.3±5.5 | 140.6±3.7 | 149.0±1.8 | 134.1±8.1 | 145.5±4.3 | 138.8±3.2 | 4–14 |
Results are presented as the mean±SEM
p<0.05 compared to 12 months RD (treatment matching group)
Effect on endothelium-dependent and endothelium-independent function of EX, CAT, and PI at 9 months
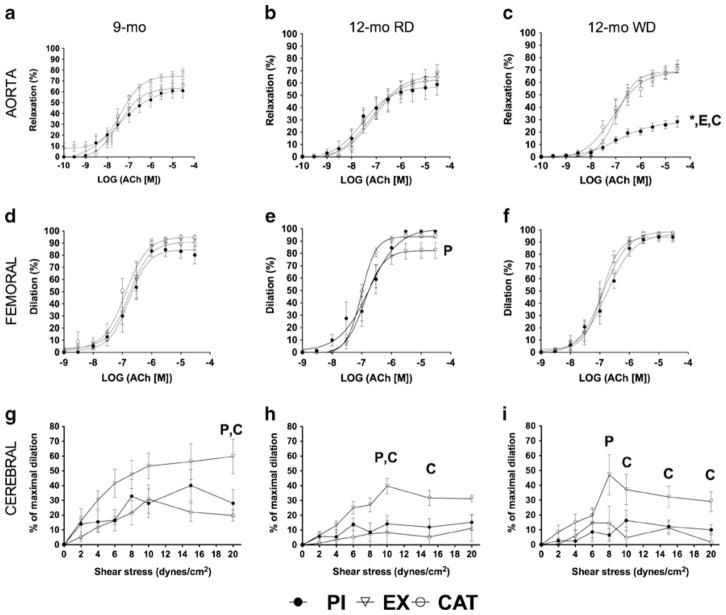
Maximal relaxations to Ach and SNP in aortas and to Ach in femoral arteries were similar in all groups (Fig. 1a, d; Tables S3 and S4), with no marked differences in sensitivity (data not included). In aortas isolated from PI and CAT mice, indomethacin (nonspecific COX1/2 inhibitor) significantly enhanced the maximal relaxation to Ach (Table S3). At 20 dyn/cm2, the highest shear stress imposed, FMD of cerebral arteries isolated from PI and CAT mice were lower than in arteries isolated from EX mice (Fig. 1g).
Fig. 1.
Main effects on vascular function of a switch from PI, EX (voluntary wheel running), or CAT (30 mg/kg/day in drinking water) with a RD from the age of 1 to 9 months to PI and a WD from the age of 9 to 12 months. Ach induced endothelium-dependent relaxation of the aorta (a, b, c; n=4–9) and femoral arteries (d, e, f; n=5–9) and flow-mediated endothelium-dependent dilation of cerebral arteries (g, h, i; n=6–10). Results are presented as the mean±SEM. *p<0.05 compared to 12 months of age with RD (treatment matching group), Pp<0.05 compared to PI, Ep<0.05 compared to EX, and Cp<0.05 compared to CAT
Antioxidant enzymatic activities at 9 months
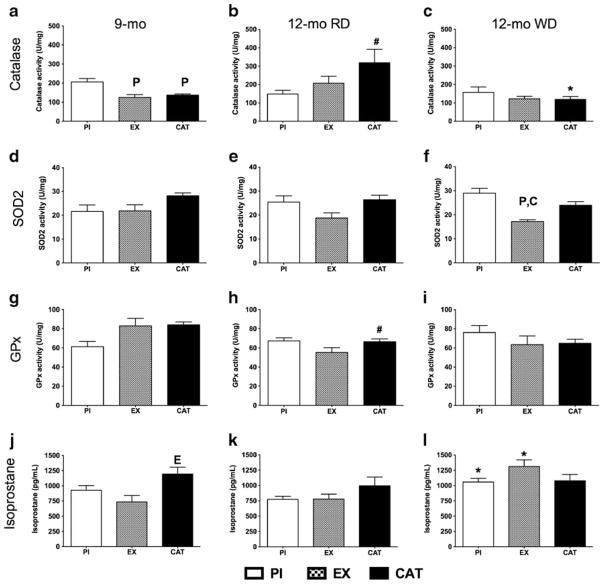
Catalase activity was higher in PI mice (Fig. 2a), without change in SOD2 and GPx activities (Fig. 2d, g). Systemic oxidative stress was evaluated by the levels of isoprostane in the plasma, a marker of lipid peroxidation [30]. Surprisingly, CAT-treated mice expressed higher levels of isoprostane in the blood than EX mice (Fig. 2j); aortic oxidative stress measured with the florescent probe dihydroethidium, however, was equivalent in all groups (data not included).
Fig. 2.
Effects on antioxidant activity of a switch from PI, EX (voluntary wheel running), or CAT (30 mg/kg/day in drinking water) with a RD from the age of 1 to 9 months to PI and a WD from the age of 9 to 12 months. Oxidative stress was evaluated by quantifying in the aorta the activity of catalase (a, b, c; n=4–5), SOD2 (d, e, f; n=4–5), and GPx (g, h, i; n=4–6) and by measuring the circulating levels of isoprostane (j, k, l; n=3–5). Results are presented as the mean± SEM. #p<0.05 compared to 9 months of age (treatment matching group), *p<0.05 compared to 12 months of age with RD (treatment matching group), Pp<0.05 compared to PI, Ep<0.05 compared to EX, and Cp<0.05 compared to CAT
Impact of the switch in lifestyle
At 12 months of age, mouse physiological and plasma parameters remained stable when switched to PI and a RD (Table 1). In all mice switched to a WD, however, body mass, glucose, insulin, and cholesterol increased (Table 1). No atherosclerotic plaques were visible in aortas.
Endothelium-dependent and endothelium-independent function 3 months after the switch of lifestyle
The maximal relaxant response induced by Ach in aortas isolated from PI mice was significantly reduced by the WD compared to the RD and abolished in the presence of L-NNA (Fig. 1b, c; Table S3); indomethacin, however, fully restored Ach-induced maximal relaxation compared to aorta segments isolated from the two other groups of mice (Table S3). These results, therefore, suggest (1) a reduction in NO production and (2) the presence of an antirelaxant constricting prostanoid, both limiting Ach-induced endothelium-dependent relaxation. In contrast to PI mice, the relaxation of aorta segments isolated from EX and CAT mice fed a WD was maintained at 12 months of age (Fig. 1c; Table S3). Indomethacin did not alter the relaxation induced by Ach, while L-NNA reduced it by ~60 % (Table S3). After combined NOS and COX1/2 inhibition, however, the relaxation induced by Ach was lower in PI mice compared to EX and CAT mice (Fig. S2a; Table S3), demonstrating that previous exposure to EX and CAT increased the contribution of a compensatory non-NO/non-prostacyclin (PGI2) relaxant factor while likely maintaining NO production. Similarly to Ach, the relaxation induced by SNP was lower in the aorta isolated from 12-month-old PI mice fed a WD compared to all other groups (Table S3), suggesting either an impairment of vascular smooth muscle cells (VSMC) to SNP-derived NO or, more likely, that the COX1/2 derivative limits as well the endothelium-independent SNP-induced VSMC relaxation.
The relaxation of the femoral arteries was not altered at 12 months in the three groups (Fig. 1e, f; Table S4). Nonetheless, differences in the contribution of NOS and COX1/2 activities were noted: individual inhibition of NOS and COX1/2 both reduced the relaxation induced by Ach in PI mice independently of the diet (Table S4). In contrast, the combination of L-NNA and indomethacin strongly reduced the relaxation induced by Ach in femoral arteries isolated from PI mice and from CAT mice fed a WD, but not an RD (Table S4). L-NNA and indomethacin, combined or not, did not significantly affect the relaxation of femoral arteries isolated from 12-month-old EX mice (Table S4).
Cerebral arteries isolated from EX mice and pressurized at 60 mmHg had a consistent greater FMD at 9 months (Fig. 1g) and at 12 months after 3 months of RD (Fig. 1h) or of WD (Fig. 1i). CAT neither prevented the age-related decline in FMD nor the deleterious impact of the WD on FMD (Fig. 1h, i). The WD, however, did not magnify the decline in endothelial function associated with a lifelong PI when fed an RD (Fig. 1h, i).
Antioxidant enzymatic activities and inflammatory genes expression 3 months after the switch of lifestyle
After 3 months of exposure to the WD, SOD2 activity was lower in EX mice only (Fig. 2f). Catalase and GPx activities did not increase with the WD compared to that measured at 9 months (Fig. 2c, i), but it increased in CAT mice after the RD (Fig. 2b). The isoprostane levels remained elevated in CAT-treated mice, while the WD increased isoprostane in PI and EX mice to become similar in all groups (Fig. 2l). No change in aortic oxidative stress was observed (data not included). Finally, the expression of the inflammation-related genes COX2 and angiopoietin-like 2 (angptl2) increased in CAT-treated mice only following the WD (Table S5).
Impact of the interventions on the femoral and cerebral artery biomechanics
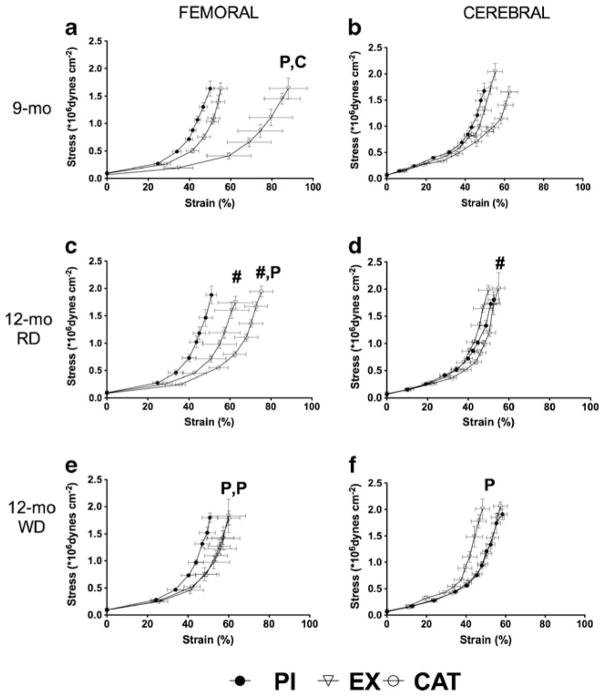
The biomechanical behavior of the wall of conductance and muscular arteries influences blood flow dynamics and can be modified by a change in endothelial function and EX training [5, 48]. At 9 months, femoral arteries isolated from EX mice were more compliant compared with PI and CAT mice, evidenced by a rightward shift of the passive stress–strain curve (Fig. 3a) and a lower YEM (Table S6). The compliance remained greater at 12 months of age in femoral arteries isolated from EX mice compared to those of PI mice, but nonetheless stiffened 3 months after preventing access to the EX wheel and exposure to either diets (Fig. 3c, e; Table S6). In contrast, removal of CAT at 9 months led to an increase in compliance at 12 months, leading to a similar level of compliance in femoral arteries isolated from CAT and EX mice with either diets (Fig. 3c, e; Table S6). PI, alone or combined with a WD, did not modify the compliance of the femoral artery (Fig. 3c, e; Table S6).
Fig. 3.
Major effects on the structure/function relationship (assessed by measuring compliance) of a switch from PI, EX (voluntary wheel running), or CAT (30 mg/kg/day in drinking water) with a RD from the age of 1 to 9 months to PI and a WD from the age of 9 to 12 months. Compliance was measured in the femoral (a, c, e; n=5–9) and the cerebral arteries (b, d, f; n=6–10). Results are presented as the mean±SEM. #p<0.05 compared to 9 months of age (treatment matching group), Pp<0.05 compared to PI, and Cp<0.05 compared to CAT
In contrast to the femoral artery, the compliance of mouse cerebral arteries is paradoxically increased by endothelial dysfunction [5, 6]. At 9 months of age, the compliance of the cerebral arteries isolated from CAT mice was greater compared to the two other groups (Fig. 3b), although the YEM was not changed significantly (Table S6), suggesting that CAT induced adverse remodeling of the cerebrovascular wall. At 12 months, the absence of CAT normalized the deleterious effect of CAT pretreatment on the compliance of the cerebral artery (Fig. 3d, f; Table S6). As expected, the WD shifted to the right the passive stress–strain curve in the cerebral arteries isolated from PI mice (Fig. 3f), together with a reduction of the YEM compared to EX mice (Table S6). These data demonstrate that the WD induced a weakening and a degradation of the biomechanical properties of the wall of the cerebral arteries in PI and CAT mice, while EX mice were protected.
Discussion
Vascular imprinting
The major finding of this study is that two interventions initiated at a very early age and known to be protective of the cardiovascular system imprint the vasculature so that it responds differentially in the face of a deleterious change of environment at middle age. In our experimental paradigm, early exposure to EX better protected the vasculature than CAT. This demonstrates that a healthy lifestyle made of voluntary EX and a balanced diet during development and maturation has a lasting protective impact on the vascular function. More importantly, our data reveal that the animals previously treated with CAT responded poorly to the WD with more evidence of inflammation and cerebrovascular adverse remodeling, making them at risk, although differently from PI animals, when transposed to a deleterious environment.
It is not well-known if the beneficial effects of a healthy lifestyle on the cardiovascular system would outlast a switch to a deleterious environment. One study reported that 3 weeks of EX was sufficient to prevent the development of obesity induced by a high-fat diet 10 weeks later [52]; yet, another reported that cessation of EX predisposed rats to hepatic steatosis [60]. By opposition, a short-term period of forced sedentary behavior (5 to 7 days) has been shown to be sufficient to alter vascular function [31, 43]. Our study is, therefore, unique because it reveals that voluntary EX during the first 9 months of life permitted the maintenance of the vascular (both endothelium-dependent and endothelium-independent) function, the optimal biomechanics of the cerebral and femoral arteries, together with a low SOD2 activity and low expression of inflammatory markers. These results suggest that EX mice were biologically equipped to face a metabolic stress, at least for 3 months.
EX facilitates adaptation
EX is known to stimulate endothelial NOS activity and the expression of antioxidant enzymes [27, 58]. Yet, EX is also known to transiently increase oxidative stress, and the beneficial adaptive changes can be prevented by a concomitant intake of antioxidants [29, 63]. Reactive oxygen species signaling is essential to regulate cellular function [28, 61], including to signal for adaptation to EX [23], and their inactivation at critical time points is deleterious [26, 29, 63]. This may explain why CAT alone, a potent antioxidant, neither fully preserved the vascular function nor the biomechanical properties compared to PI mice and even increased the expression of inflammatory markers and isoprostane. Hence, the impact of the two preventive approaches on the cardiovascular function diverged after 3 months of exposure to PI combined with a WD despite a similar increase in plasma TC, glucose, and insulin and in body mass (Table 1) that are associated with increased oxidative stress (Fig. 2l) and overall risk of CVD [39]. The lasting impact or memory of the biological molecular modeling induced by EX is, therefore, reflected by the overexpression of stress resistance pathways [40, 56–58] and might explain the better outcome in EX mice.
Adaptive mechanisms
We previously reported that dyslipidemia stimulated the expression of an adaptive NO-independent endothelial pathway in mouse femoral arteries [36] that had been identified as endothelium-derived hyperpolarizing factor in mesenteric arteries by others [11, 78]. The impairment of the NO pathway seen in inactive aging mice [38, 65] has been shown to be prevented by EX or an antioxidant treatment [20, 79]. Interestingly, EX permitted the maintenance of a better vascular function in cerebral arteries than CAT in all conditions, demonstrating that EX is more potent at protecting the cerebral circulation than a single antioxidant intervention. Our data are the first to demonstrate that only EX leads to a lasting protection of the vasculature against a change in environment.
Role of oxidative stress
Additional mechanisms are likely to contribute to the beneficial effects of EX. Aging is associated with an increase in oxidative damages associated with a decrease in antioxidant mechanisms [28, 32] accelerated by a WD [13]. The higher SOD2 activity in PI and CAT mice compared to EX mice following the WD suggests a higher production of superoxide anion in these two groups. Superoxide can originate from different vascular sources: NADPH oxidase, xanthine oxidase, COX1/2, and uncoupled NOS [8, 71]. Aging [14], PI [38], and dyslipidemia [19] can induce oxidative damage by the generation of superoxide anion that will impair NO and PGI2 relaxations [71]; EX can restore endothelial function and lower NADPH oxidase activity in the aorta of very old mice [20]. We reported that CAT reduces NADPH oxidase activity in cerebral arteries from 1-year-old atherosclerotic mice [19]. Excess of H2O2 can as well be deleterious for the vasculature and promote atherosclerosis [77]. At low levels of H2O2, GPx and peroxiredoxin prevent H2O2-related damage from normal metabolic activity; in cultured cells, however, an increase or excess of H2O2 inactivates GPx and peroxiredoxin, forcing catalase to compensate [44]. The higher level of catalase activity in PI mice at 9 months of age and in CAT mice fed a RD at 12 months of age suggests a greater load of H2O2 in these two groups. In CAT-treated mice, cessation of CAT at 9 months of age is likely responsible for the rise in catalase activity as an adaptive reaction to the reduction in antioxidant load. Surprisingly, however, the WD prevented this adaptive rise in catalase activity in CAT mice. The dominant, although likely necessary, role of antioxidant enzymes is, therefore, uncertain. Studies showed that EX prevented the decline in antioxidant enzymes function or expression [20, 51, 54], while others have reported no direct effect on SOD, catalase, or GPx activity or expression despite lower oxidative damage or increased NO bioavailability [4, 7, 22]. Similarly, antioxidant treatments are effective to prevent the decrease in anti-oxidant function in pro-inflammatory and pro-oxidant conditions, but cannot improve antioxidant enzyme profiles [1, 49]. In these healthy mice with no genetically augmented susceptibility to develop endothelial dysfunction and atherosclerosis, the WD neither produced a major global decline in antioxidant enzyme activity nor an increase in aortic oxidative stress: yet, PI induced a major reduction in vascular function in the aorta.
Inflammation
Low-grade inflammation is a hallmark of endothelial dysfunction [72]. The WD increased COX2 and angptl2 expression, two markers of inflammation, in CAT mice only. We previously reported that COX2 expression correlated with the level of damage in endothelial cells isolated from coronary artery disease patients [75], while others showed that oxidative stress stimulated COX2 expression [21]. In addition, we reported that 9 months of CAT magnified 50-folds the rise in COX2 expression associated with aging in the mouse aorta [26]. Angptl2, on the other hand, is associated with inflammation as its presence is increased in patients with rheumatoid arthritis and abdominal aortic aneurysm [47, 69], while its deletion can lower pro-inflammatory cytokines in rodents [67, 69]. Hence, our results suggest that early CAT treatment induces a pro-inflammatory environment upon the challenge of a WD: we propose that CAT limited the development of antioxidant defense mechanisms able to counteract the stress induced by the WD.
Endothelium-independent functions
Despite numerous studies showing no influence of cardiovascular risk factor on endothelium-independent relaxation [14, 20, 38, 51], the relaxation induced by SNP was markedly reduced in aortas isolated from PI mice fed a WD. Aging alters both the endothelium and the VSMC [34], and endothelium-independent response can be altered in presence of hyperinsulinemia in patients with metabolic syndrome [64] or by acute hyperhomocysteinemia in diabetic patients [12]. On the other hand, EX has been shown to increase the relaxant response to NO donors in humans and rabbits [15, 33], demonstrating that it can reverse and/or counteract the dysfunctional VSMC relaxant capacities. The mechanism involved in the impaired SNP response remains elusive; nonetheless, the equally reduced endothelium-dependent relaxation to Ach was prevented in the presence of indomethacin. Therefore, the default of relaxation is reversible and we can speculate that constricting COX1/2 derivatives may also counteract the VSMC relaxation to SNP. Altogether, our data suggest that an efficient vascular relaxation depends on a fine balance between relaxing and constricting factors highly sensitive to the environment.
Limitations
Our study has limitations. The statistical determination of the number of animals was set to only detect significant (>30 %) changes in functions between groups. This may have decreased the power to detect fine changes in functions that may be significant and could widen in later life. The design of the experiments is complex and the parameters are numerous; this study needs to be replicated to address specifically the modifications induced by an abrupt change in the environment, an approach that would increase the power to detect biological modifications, notably the role of COX1/2 derivatives on the vascular relaxation. We believe, however, that this design reflects a clinical reality associated to increasing prevalence in the migration of population with the consequent changes in lifestyles, a phenomenon restricted in the past to “Westernized” isolated societies [68].
In conclusion, we demonstrate that a healthy lifestyle at a young age including voluntary EX and a balanced diet, but not a single antioxidant, is protective at middle age when challenged by an environmental stress, likely by stimulating the expression of stress resistance pathways.
Acknowledgments
The authors would like to thank the staff of the animal facility of the Montreal Heart Institute. This study was supported by the Canadian Institutes for Health Research (E.T. MOP#14496). F. Leblond is supported by a Banting and Best Ph.D. scholarship from the Canadian Institutes for Health Research.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00424-012-1206-8) contains supplementary material, which is available to authorized users.
Ethical standard The experiments comply with the current laws of Canada in which they were performed.
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Abd El-Aziz TA, Mohamed RH, Pasha HF, Abdel-Aziz HR. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med. 2011 doi: 10.1007/s10238-011-0165-2. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ. The developmental origins of adult disease. J Am Coll Nutr. 2004;23(6 Suppl):588S–595S. doi: 10.1080/07315724.2004.10719428. [DOI] [PubMed] [Google Scholar]
- 3.Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, Vittone JL, Klee GG, Arora P, Jensen MD, Toffolo G, Cobelli C, Rizza RA. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003;52(7):1738–1748. doi: 10.2337/diabetes.52.7.1738. [DOI] [PubMed] [Google Scholar]
- 4.Bayod S, Del Valle J, Lalanza JF, Sanchez-Roige S, de Luxan-Delgado B, Coto-Montes A, Canudas AM, Camins A, Escorihuela RM, Pallas M. Long-term physical exercise induces changes in sirtuin 1 pathway and oxidative parameters in adult rat tissues. Exp Gerontol. 2012 doi: 10.1016/j.exger.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Bolduc V, Drouin A, Gillis MA, Duquette N, Thorin-Trescases N, Frayne-Robillard I, Des Rosiers C, Tardif JC, Thorin E. Heart rate-associated mechanical stress impairs carotid but not cerebral artery compliance in dyslipidemic atherosclerotic mice. Am J Physiol Heart Circ Physiol. 2011;301(5):H2081–H2092. doi: 10.1152/ajpheart.00706.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolduc V, Baraghis E, Duquette N, Thorin-Trescases N, Lambert J, Lesage F, Thorin E. Catechin prevents severe dyslipidemia-associated changes in wall biomechanics of cerebral arteries in LDLr−/−:hApoB+/+ mice and improves cerebral blood flow. Am J Physiol Heart Circ Physiol. 2012;302(6):H1330–H1339. doi: 10.1152/ajpheart.01044.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunker AK, Arce-Esquivel AA, Rector RS, Booth FW, Ibdah JA, Laughlin MH. Physical activity maintains aortic endothelium-dependent relaxation in the obese type 2 diabetic OLETF rat. Am J Physiol Heart Circ Physiol. 2010;298(6):H1889–H1901. doi: 10.1152/ajpheart.01252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87(10):840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 9.Carrera-Bastos P, Fontes-Villalba M, O’Keefe JH, Lindeberg S, Cordain L. The Western diet and lifestyle and diseases of civilization. Res Rep Clin Cardiol. 2011;2:15–35. [Google Scholar]
- 10.Chakravarty EF, Hubert HB, Lingala VB, Fries JF. Reduced disability and mortality among aging runners: a 21-year longitudinal study. Arch Intern Med. 2008;168(15):1638–1646. doi: 10.1001/archinte.168.15.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen SJ, Wu CC, Yen MH. Exercise training activates large-conductance calcium-activated K(+) channels and enhances nitric oxide production in rat mesenteric artery and thoracic aorta. J Biomed Sci. 2001;8(3):248–255. doi: 10.1007/BF02256598. [DOI] [PubMed] [Google Scholar]
- 12.Chousos I, Perrea D, Kyriaki D, Liatis S, Katsilambros N, Makrilakis K. Acute hyperhomocysteinaemia blunts endothelial dependent and endothelial independent vasodilatation in diabetic patients. Diabetes Vasc Dis Res: Off J Int Soc Diabetes Vascr Dis. 2010;7(3):186–194. doi: 10.1177/1479164110369687. [DOI] [PubMed] [Google Scholar]
- 13.Collins AR, Lyon CJ, Xia X, Liu JZ, Tangirala RK, Yin F, Boyadjian R, Bikineyeva A, Pratico D, Harrison DG, Hsueh WA. Age-accelerated atherosclerosis correlates with failure to upregulate antioxidant genes. Circ Res. 2009;104(6):e42–e54. doi: 10.1161/CIRCRESAHA.108.188771. [DOI] [PubMed] [Google Scholar]
- 14.Csiszar A, Ungvari Z, Edwards JG, Kaminski P, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90 (11):1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- 15.De Moraes R, Gioseffi G, Nobrega AC, Tibirica E. Effects of exercise training on the vascular reactivity of the whole kidney circulation in rabbits. J Appl Physiol. 2004;97(2):683–688. doi: 10.1152/japplphysiol.00923.2003. [DOI] [PubMed] [Google Scholar]
- 16.Dilis V, Katsoulis M, Lagiou P, Trichopoulos D, Naska A, Trichopoulou A. Mediterranean diet and CHD: the Greek European prospective investigation into cancer and nutrition cohort. Br J Nutr. 2012;108(4):699–709. doi: 10.1017/S0007114512001821. [DOI] [PubMed] [Google Scholar]
- 17.Drouin A, Thorin E. Flow-induced dilation is mediated by Akt-dependent activation of endothelial nitric oxide synthase-derived hydrogen peroxide in mouse cerebral arteries. Stroke. 2009;40(5):1827–1833. doi: 10.1161/STROKEAHA.108.536805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drouin A, Bolduc V, Thorin-Trescases N, Belanger E, Fernandes P, Baraghis E, Lesage F, Gillis MA, Villeneuve L, Hamel E, Ferland G, Thorin E. Catechin treatment improves cerebrovascular flow-mediated dilation and learning abilities in atherosclerotic mice. Am J Physiol Heart Circ Physiol. 2011;300(3):H1032–H1043. doi: 10.1152/ajpheart.00410.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drouin A, Farhat N, Bolduc V, Thorin-Trescases N, Gillis MA, Villeneuve L, Nguyen A, Thorin E. Up-regulation of thromboxane A(2) impairs cerebrovascular eNOS function in aging atherosclerotic mice. Pflugers Arch. 2011;462(3):371–383. doi: 10.1007/s00424-011-0973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587(Pt 13):3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng L, Xia Y, Garcia GE, Hwang D, Wilson CB. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-alpha, and lipopolysaccharide. J Clin Invest. 1995;95(4):1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filaire E, Rouveix M, Massart A, Gladine C, Davicco MJ, Durand D. Lipid peroxidation and antioxidant status in rat: effect of food restriction and wheel running. Eur J Appl Physiol. 2009;107(2):243–250. doi: 10.1007/s00421-009-1121-7. [DOI] [PubMed] [Google Scholar]
- 23.Fisher-Wellman K, Bloomer RJ. Acute exercise and oxidative stress: a 30 year history. Dyn Med. 2009;8:1. doi: 10.1186/1476-5918-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gendron ME, Thorin E. A change in the redox environment and thromboxane A2 production precede endothelial dysfunction in mice. Am J Physiol Heart Circ Physiol. 2007;293(4):H2508–H2515. doi: 10.1152/ajpheart.00352.2007. [DOI] [PubMed] [Google Scholar]
- 25.Gendron ME, Theoret JF, Mamarbachi AM, Drouin A, Nguyen A, Bolduc V, Thorin-Trescases N, Merhi Y, Thorin E. Late chronic catechin antioxidant treatment is deleterious to the endothelial function in aging mice with established atherosclerosis. Am J Physiol Heart Circ Physiol. 2010;298(6):H2062–H2070. doi: 10.1152/ajpheart.00532.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gendron ME, Thorin-Trescases N, Mamarbachi AM, Villeneuve L, Theoret JF, Mehri Y, Thorin E. Time-dependent beneficial effect of chronic polyphenol treatment with catechin on endothelial dysfunction in aging mice. Dose-Response. 2012;10(1):108–119. doi: 10.2203/dose-response.11-014.Thorin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gielen S, Schuler G, Adams V. Cardiovascular effects of exercise training: molecular mechanisms. Circulation. 2010;122(12):1221–1238. doi: 10.1161/CIRCULATIONAHA.110.939959. [DOI] [PubMed] [Google Scholar]
- 28.Giorgio M, Trinei M, Migliaccio E, Pelicci PG. Hydrogen peroxide: a metabolic by-product or a common mediator of ageing signals? Nat Rev Mol Cell Biol. 2007;8(9):722–728. doi: 10.1038/nrm2240. [DOI] [PubMed] [Google Scholar]
- 29.Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567(Pt 1):113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamburg NM, McMackin CJ, Huang AL, Shenouda SM, Widlansky ME, Schulz E, Gokce N, Ruderman NB, Keaney JF, Jr, Vita JA. Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27(12):2650–2656. doi: 10.1161/ATVBAHA.107.153288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 33.Haskell WL, Sims C, Myll J, Bortz WM, St Goar FG, Alderman EL. Coronary artery size and dilating capacity in ultradistance runners. Circulation. 1993;87(4):1076–1082. doi: 10.1161/01.cir.87.4.1076. [DOI] [PubMed] [Google Scholar]
- 34.Ishida S, Hamasaki S, Kamekou M, Yoshitama T, Nakano F, Yoshikawa A, Kataoka T, Saihara K, Minagoe S, Tei C. Advancing age is associated with diminished vascular remodeling and impaired vasodilation in resistance coronary arteries. Coron Artery Dis. 2003;14(6):443–449. doi: 10.1097/01.mca.0000087912.54460.bf. [DOI] [PubMed] [Google Scholar]
- 35.Krummen S, Falck JR, Thorin E. Two distinct pathways account for EDHF-dependent dilatation in the gracilis artery of dyslipidaemic hApoB+/+ mice. Br J Pharmacol. 2005;145(2):264–270. doi: 10.1038/sj.bjp.0706194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krummen S, Drouin A, Gendron ME, Falck JR, Thorin E. ROS-sensitive cytochrome P450 activity maintains endothelial dilatation in ageing but is transitory in dyslipidaemic mice. Br J Pharmacol. 2006;147(8):897–904. doi: 10.1038/sj.bjp.0706679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lairon D. Intervention studies on Mediterranean diet and cardiovascular risk. Mol Nutr & food Res. 2007;51(10):1209–1214. doi: 10.1002/mnfr.200700097. [DOI] [PubMed] [Google Scholar]
- 38.Laufs U, Wassmann S, Czech T, Munzel T, Eisenhauer M, Bohm M, Nickenig G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25(4):809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 39.Malik S, Wong ND, Franklin SS, Kamath TV, L’Italien GJ, Pio JR, Williams GR. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation. 2004;110(10):1245–1250. doi: 10.1161/01.CIR.0000140677.20606.0E. [DOI] [PubMed] [Google Scholar]
- 40.Mattson MP. Hormesis and disease resistance: activation of cellular stress response pathways. Hum Exp Toxicol. 2008;27(2):155–162. doi: 10.1177/0960327107083417. [DOI] [PubMed] [Google Scholar]
- 41.Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller JM, Davis MJ, Chilian WM. Integrated regulation of pressure and flow in the coronary microcirculation. Cardiovasc Res. 1996;32(4):668–678. [PubMed] [Google Scholar]
- 43.Navasiolava NM, Dignat-George F, Sabatier F, Larina IM, Demiot C, Fortrat JO, Gauquelin-Koch G, Kozlovskaya IB, Custaud MA. Enforced physical inactivity increases endothelial micro-particle levels in healthy volunteers. Am J Physiol Heart Circ Physiol. 2010;299(2):H248–H256. doi: 10.1152/ajpheart.00152.2010. [DOI] [PubMed] [Google Scholar]
- 44.Neumann CA, Cao J, Manevich Y. Peroxiredoxin 1 and its role in cell signaling. Cell Cycle. 2009;8(24):4072–4078. doi: 10.4161/cc.8.24.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nocon M, Hiemann T, Muller-Riemenschneider F, Thalau F, Roll S, Willich SN. Association of physical activity with all-cause and cardiovascular mortality: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. 2008;15(3):239–246. doi: 10.1097/HJR.0b013e3282f55e09. [DOI] [PubMed] [Google Scholar]
- 46.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. 2012;110(8):1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okada T, Tsukano H, Endo M, Tabata M, Miyata K, Kadomatsu T, Miyashita K, Semba K, Nakamura E, Tsukano M, Mizuta H, Oike Y. Synoviocyte-derived angiopoietin-like protein 2 contributes to synovial chronic inflammation in rheumatoid arthritis. Am J Pathol. 2010;176(5):2309–2319. doi: 10.2353/ajpath.2010.090865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Rourke MF, Hashimoto J. Arterial stiffness: a modifiable cardiovascular risk factor? J Cardpulm Rehabil Prev) 2008;28(4):225–237. doi: 10.1097/01.HCR.0000327179.21498.38. [DOI] [PubMed] [Google Scholar]
- 49.Palsamy P, Subramanian S. Resveratrol protects diabetic kidney by attenuating hyperglycemia-mediated oxidative stress and renal inflammatory cytokines via Nrf2-Keap1 signaling. Biochim Biophys Acta. 2011;1812(7):719–731. doi: 10.1016/j.bbadis.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Papaioannou TG, Karatzis EN, Vavuranakis M, Lekakis JP, Stefanadis C. Assessment of vascular wall shear stress and implications for atherosclerotic disease. Int J Cardiol. 2006;113(1):12–18. doi: 10.1016/j.ijcard.2006.03.035. [DOI] [PubMed] [Google Scholar]
- 51.Park Y, Booth FW, Lee S, Laye MJ, Zhang C. Physical activity opposes coronary vascular dysfunction induced during high fat feeding in mice. J Physiol. 2012;590(Pt 17):4255–4268. doi: 10.1113/jphysiol.2012.234856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):R290–R301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- 53.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging cell. 2011;10(6):1032–1037. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiang W, Weiqiang K, Qing Z, Pengju Z, Yi L. Aging impairs insulin-stimulated glucose uptake in rat skeletal muscle via suppressing AMPKalpha. Exp Mol Med. 2007;39(4):535–543. doi: 10.1038/emm.2007.59. [DOI] [PubMed] [Google Scholar]
- 56.Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6(1):71–75. doi: 10.1007/s10522-004-7386-7. [DOI] [PubMed] [Google Scholar]
- 57.Radak Z, Chung HY, Koltai E, Taylor AW, Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7(1):34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Radak Z, Zhao Z, Koltai E, Ohno H, Atalay M. Oxygen consumption and usage during physical exercise: the balance between oxidative stress and ROS-dependent adaptive signaling. Antioxid redox Signal. 2012 doi: 10.1089/ars.2011.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramsden CE, Faurot KR, Carrera-Bastos P, Cordain L, De Lorgeril M, Sperling LS. Dietary fat quality and coronary heart disease prevention: a unified theory based on evolutionary, historical, global, and modern perspectives. Curr Treat options in Cardiovasc Med. 2009;11(4):289–301. doi: 10.1007/s11936-009-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rector RS, Thyfault JP, Laye MJ, Morris RT, Borengasser SJ, Uptergrove GM, Chakravarthy MV, Booth FW, Ibdah JA. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. J Physiol. 2008;586(Pt 17):4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 62.Rippe C, Lesniewski L, Connell M, LaRocca T, Donato A, Seals D. Short-term calorie restriction reverses vascular endothelial dysfunction in old mice by increasing nitric oxide and reducing oxidative stress. Aging cell. 2010;9(3):304–312. doi: 10.1111/j.1474-9726.2010.00557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schinzari F, Tesauro M, Rovella V, Galli A, Mores N, Porzio O, Lauro D, Cardillo C. Generalized impairment of vasodilator reactivity during hyperinsulinemia in patients with obesity-related metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;299(6):E947–E952. doi: 10.1152/ajpendo.00426.2010. [DOI] [PubMed] [Google Scholar]
- 65.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol. 2009;587(Pt 23):5541–5549. doi: 10.1113/jphysiol.2009.178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steinberg D, Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105(17):2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 67.Tabata M, Kadomatsu T, Fukuhara S, Miyata K, Ito Y, Endo M, Urano T, Zhu HJ, Tsukano H, Tazume H, Kaikita K, Miyashita K, Iwawaki T, Shimabukuro M, Sakaguchi K, Ito T, Nakagata N, Yamada T, Katagiri H, Kasuga M, Ando Y, Ogawa H, Mochizuki N, Itoh H, Suda T, Oike Y. Angiopoietin-like protein 2 promotes chronic adipose tissue inflammation and obesity-related systemic insulin resistance. Cell Metab. 2009;10(3):178–188. doi: 10.1016/j.cmet.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Taylor R, Thoma K. Mortality patterns in the modernized Pacific Island nation of Nauru. Am J Public Health. 1985;75(2):149–155. doi: 10.2105/ajph.75.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tazume H, Miyata K, Tian Z, Endo M, Horiguchi H, Takahashi O, Horio E, Tsukano H, Kadomatsu T, Nakashima Y, Kunitomo R, Kaneko Y, Moriyama S, Sakaguchi H, Okamoto K, Hara M, Yoshinaga T, Yoshimura K, Aoki H, Araki K, Hao H, Kawasuji M, Oike Y. Macrophage-derived angiopoietin-like protein 2 accelerates development of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2012;32(6):1400–1409. doi: 10.1161/ATVBAHA.112.247866. [DOI] [PubMed] [Google Scholar]
- 70.Thorin E, Meerkin D, Bertrand OF, Paiement P, Joyal M, Bonan R. Influence of postangioplasty beta-irradiation on endothelial function in porcine coronary arteries. Circulation. 2000;101(12):1430–1435. doi: 10.1161/01.cir.101.12.1430. [DOI] [PubMed] [Google Scholar]
- 71.Ullrich V, Bachschmid M. Superoxide as a messenger of endothelial function. Biochem Biophys Res Commun. 2000;278(1):1–8. doi: 10.1006/bbrc.2000.3733. [DOI] [PubMed] [Google Scholar]
- 72.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: new perspectives. J Gerontol A Biol Sci Med Sci. 2010;65(10):1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanhoutte PM. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J: Off J Japan Circ Soc. 2009;73 (4):595–601. doi: 10.1253/circj.cj-08-1169. [DOI] [PubMed] [Google Scholar]
- 74.Versari D, Daghini E, Virdis A, Ghiadoni L, Taddei S. Endothelial dysfunction as a target for prevention of cardiovascular disease. Diabetes Care. 2009;32(Suppl 2):S314–S321. doi: 10.2337/dc09-S330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Voghel G, Thorin-Trescases N, Farhat N, Nguyen A, Villeneuve L, Mamarbachi AM, Fortier A, Perrault LP, Carrier M, Thorin E. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech Ageing Dev. 2007;128(11–12):662–671. doi: 10.1016/j.mad.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Wen CP, Wai JP, Tsai MK, Yang YC, Cheng TY, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378(9798):1244–1253. doi: 10.1016/S0140-6736(11)60749-6. [DOI] [PubMed] [Google Scholar]
- 77.Yang H, Roberts LJ, Shi MJ, Zhou LC, Ballard BR, Richardson A, Guo ZM. Retardation of atherosclerosis by overexpression of catalase or both Cu/Zn-superoxide dismutase and catalase in mice lacking apolipoprotein E. Circ Res. 2004;95(11):1075–1081. doi: 10.1161/01.RES.0000149564.49410.0d. [DOI] [PubMed] [Google Scholar]
- 78.Yen MH, Yang JH, Sheu JR, Lee YM, Ding YA. Chronic exercise enhances endothelium-mediated dilation in spontaneously hypertensive rats. Life Sci. 1995;57(24):2205–2213. doi: 10.1016/0024-3205(95)02127-5. [DOI] [PubMed] [Google Scholar]
- 79.Zou JG, Wang ZR, Huang YZ, Cao KJ, Wu JM. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int J Mol Med. 2003;11(3):317–320. [PubMed] [Google Scholar]