Abstract
PTPRJ/CD148 is a tyrosine phosphatase that has tumour suppressor-like activity. Quantitative PCR of various cells and tissues revealed that it is preferentially expressed in macrophage-enriched tissues. Within lymphoid tissues immunohistochemistry revealed that PTPRJ/CD148 co-localised with F4/80, indicating that macrophages most strongly express the protein. Macrophages express the highest basal level of ptprj, and this is elevated further by treatment with LPS and other Toll-like receptor ligands. In contrast, CSF-1 treatment reduced basal and stimulated Ptprj expression in human and mouse cells, and interferon also repressed Ptprj expression. We identified a 1006 nucleotide long noncoding RNA species, Ptprj-as1 that is transcribed antisense to Ptprj. Ptprj-as1 was highly expressed in macrophage-enriched tissue and was transiently induced by Toll-like receptor ligands with a similar time course to Ptprj. Finally, putative transcription factor binding sites in the promoter region of Ptprj were identified.
Introduction
Macrophages are key cells that recognise, ingest and destroy foreign microorganisms and their products as part of the innate immune system. Macrophages have an additional role as antigen-presenting cells, and so are central to the optimal functioning of both the innate and acquired immune response [1]. Pathogen-associated molecular patterns (PAMPs) such as bacterial endotoxin (LPS) and CpG DNA and other toll-like receptor (TLR) ligands, induce the release of proinflammatory products such as cytokines and chemokines, thereby enhancing pathogen clearance [2]. Ligation of surface receptors commonly activates protein phosphorylation cascades, which are mediated by the selective activation of protein tyrosine kinases (PTKs). Such responses are usually transient and can be negatively regulated by protein tyrosine phosphatases (PTPs) [3]–[5], and perturbation of the balance between PTK and PTP activity may result in a failure of inflammation to resolve or dysregulation of cell proliferation, which can lead to life-threatening chronic inflammatory diseases or malignancy [6]–[11]. An example of this is the constitutive tyrosine phosphorylation of the PI3 kinase/Akt pathway due to the reduction in the activity of SHP-1 in the allelic moth-eaten viable (Mev/Mev) mouse, resulting in severe autoimmunity [12].
PTPRJ (CD148, DEP-1, PTPη, Byp or PTPβ-like tyrosine phosphatase) is a type-III receptor PTP containing a single cytoplasmic phosphatase domain and an extracellular domain containing eight to twelve FNIII repeats, depending on species [13]. PTPRJ is found in a wide range of cell types [14], [15] and evidence for a tumour suppressor role has been indicated due to its reduced expression in some malignant tumours, its regulation by cell density, and the reversion of the transformed phenotype when PTPRJ function is restored [16]–[21]. PTPRJ has several substrates, including PDGF β-receptor [22], hepatocyte growth factor (HGF) receptor [23], vascular endothelial growth factor (VEGF) receptor-2 [24] and the p85 subunit of PI3-kinase [25].
Within human and mouse tissues, macrophages exhibit the highest expression of Ptprj [26]–[30] , and in this cell type Ptprj expression is up-regulated by LPS and down-regulated by CSF-1 [30]. Knockout of Ptprj indicates a positive role in monocyte activation as it dephosphorylates the negative regulatory tyrosine in src family kinases in a manner similar to CD45 [31], [32]. Little is known about the molecular mechanism underlying the regulation of Ptprj expression. Although there is little data on regulation of transcription, the 5′ end of the mRNA is thought to attenuate translation [33].
Recently, we characterized differential expression of Ptprj in normal and cancerous human breast tissue and in the developing mouse mammary gland [21]. Microarray analysis of the Ptprj gene locus identified seven probes that targeted long noncoding RNA species originating from the first intron [21], [34]. Although previously regarded as “junk”, it is now becoming increasingly understood that ncRNAs play a vital role in regulating and co-ordinating the developmental complexity of eukaryotic organisms [35]. The differential and developmental specificity of long ncRNA (lncRNA) expression, in combination with the widespread conservation of their promoters, splice sites and primary sequence [36], suggests that they are generally functional [37]–[39]. The molecular mechanisms of lncRNA functions are diverse and cannot be easily generalised, and unlike protein-coding genes, their function cannot currently be predicted from their primary sequence [40]. Previous functional studies of lncRNAs reveal that they can act by influencing target gene expression at specific genomic loci, either by directly interacting with chromatin regulatory proteins or by modulating the activity of their interacting partners [41]–[43]. While lncRNAs play important roles during normal cellular development and differentiation [44], lncRNAs are also associated with several diseases, including heart disease, Alzheimer’s disease, psoriasis, and cancer [45]. In addition to our observation of multiple lncRNAs originating from the Ptprj locus, the potential for RNA regulation of Ptprj was also recently highlighted by a report showing that PTPRJ is negatively regulated by the short RNA microRNA328 [46].
Here we investigate the expression of Ptprj and an antisense lncRNA originating from the Ptprj locus in macrophages. We show that Ptprj is highly expressed in macrophage-enriched tissues, that it is upregulated in response to various toll-like receptor ligands and downregulated by CSF-1. We further show that the expression of Ptprj-as-1, an lncRNA that is transcribed antisense to the coding sequence, is regulated by proinflammatory factors in a manner similar to the PTPRJ gene. Finally we characterise the promoter region of human and mouse Ptprj and identify putative transcription factor binding sites. An understanding of the biology of phosphatases such as Ptprj in macrophage-specific signalling cascades may enable the identification of key endogenous regulators of inflammation and therapeutic targets for inflammatory diseases.
Materials and Methods
Ethics Statement
All animal handing and treatment protocols were approved by the University of Queensland Animal Ethics Committee, certificate 570-08. Mice were monitored daily for any adverse reactions and euthanized by exposure to CO2.
Animal Handling
Bone marrow-derived macrophages (BMMs) were derived from cells obtained from the femurs of carbon dioxide euthanized C57BL/6 mice in accordance with University of Queensland ethics guidelines. Thioglycollate-elicited peritoneal macrophages (TEPMs) were obtained by injecting 6–8 week-old male mice with 1 mL of 10% w/v thioglycollate broth and recovering the peritoneal exudate by peritoneal cavity lavage with 10 mL of phosphate-buffered saline (PBS) 5 days post injection as described previously [47].
Cell Culture
RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% FCS (JRH Biosciences), 20 U/mL penicillin (Invitrogen Life Technologies), 20 µg/mL streptomycin (Invitrogen Life Technologies), and 2 mM L-glutamine (Invitrogen Life Technologies) (complete medium) was used for culture of RAW 264.7 cells and bone marrow-derived macrophages (BMMs). Briefly, bone marrow cells were cultured for 7 days in complete medium in the presence of 10,000 U/mL of recombinant human CSF-1 (Chiron Corporation, Emeryville, CA, USA) on bacteriological plastic plates. RAW 264.7 [48] cells were maintained on bacteriological plates (Sterilin, Staffordshire, UK) in complete media containing 5% heat-inactivated fetal bovine serum for a maximum of four weeks in culture. RAW 264.7 cells were passaged with an 18-gauge hypodermic needle and syringe. NIH3T3 and HEK 293 cell lines were maintained in D-MEM (Dulbecco/Vogt Modified Eagle’s Minimal Essential Medium, GIBCO) supplemented with 2 mM L-glutamine (Glutamax-1, Invitrogen), 10% heat-inactivated fetal bovine serum (JRH Biosciences, Lenexa, KS, USA), 20 U/mL penicillin and 20 µg/mL streptomycin (Invitrogen). The cells were cultured in T75 filter cap flasks (Nunc). All primary cells were maintained in a 37°C incubator containing 5% CO2. Lipopolysaccharide (from Salmonella Minnesota, Sigma Aldrich, St. Louis, MO, USA) was used at a final concentration of 10 ng/mL. Recombinant human colony stimulating factor-1 (a gift from Chiron, Emeryville, CA, USA) was used at a final concentration of 104 U/mL. Phorbol 12-myristate 13-acetate (PMA) (Sigma Chemical Co.) was used at a final concentration of 10−7 M. Where shown, cells were treated with 10 µg/mL of anti-CD148 antibody or hamster isotype control antibody (Serotec).
Quantitative Real Time PCR
RNA extraction from mammalian cells or tissues was performed using the RNeasy mini kit (Qiagen, Valencia, CA, USA) as per the manufacturer’s instructions. RNA was quantified by spectrophotometry at 260 and 280 wavelengths. RNA purity was ensured by an A260/A280 of at least 1.8. Genomic DNA contamination was removed from RNA preparations using DNase (Ambion, Austin, TX, USA) and cDNA was synthesised from 2–5 µg of total RNA using Superscript III (Invitrogen, Carlsbad, CA, USA), using oligo-dT primers (Geneworks, Adelaide, Australia). cDNA was quantitated using SYBR Green (Applied Biosystems, Foster City, CA, USA) in 20 µL reactions in a 96 well plate. Each cDNA was quantified in experimental triplicate. No amplification controls (a minus-reverse transcriptase control and a minus sample control) were included in each reaction plate to ensure the absence of contaminating genomic DNA. Data was collected and analysed using the ABI Prism software. Gene expression was determined relative to Hprt (hypoxanthine-guanine phosphoribosyl transferase) mRNA using the comparative threshold method as previously described [21]. Calculations were performed in Microsoft Excel following equations provided by Applied Biosystems. Unless otherwise stated, error bars in figures indicate the standard deviation of duplicate cDNA quantitation in the same thermal cycle run. Expression profiles were typically quantitated in at least two separate experiments (as stated in the figure legends) using completely independent preparations of cells and RNA extracts. Primers (f, forward; r, reverse) used were as follows: mouse csf1r f: CCAGAGCCCCCACAGATAA, r: AGCTTGCTGTCTCCACGTTTG; human csf1r f: CCTTCAGGAGCAGGCCCAAG, r: CCTTGCTCGCAGCAGGTCAG; mouse Ptprj f: CAGTACAGTGAATGGGAGCACTGAC, r: GTCCGTATTCTGCCACTCCAACT; human Ptprj f: AGTACACACGGCCCAGCAAT, r: GAGGCGTCATCAAAGTTCTGC; mouse Ptprj-as1 f: CCATCTCCCATT GTCCAAAC, r: TGATTGAAGGACAGCTGGAA mouse Hprt f: GCAGTACAGCCCCAAAATGG, r: AACAAAGTCTGGCCTGTATCCAA; human Hprt f: TCAGGCAGTATAATCCAAAGATGGT, r: AGTCTGGCTTATATCCAACACTTCG.
Antibodies
Monoclonal hamster anti-mouse CD148 antibody was generated as described [14] and affinity purified with Protein G sepharose. This antibody was a gift from Prof. Arthur Weiss (Howard Hughes Medical Institute, Rosalind Russell Medical Research Centre for Arthritis, University of California-San Francisco, San Francisco). Rat anti-mouse Mac-2 monoclonal antibody (un-purified culture supernatant from clone TIB-166 hybridoma, ATCC) was kindly provided by Dr Andrew Cook, University of Melbourne. Antibodies to the following proteins were purchased from commercial sources: F4/80 monoclonal antibody from Serotec, goat anti-hamster IgG biotin conjugated (as a secondary against hamster anti-mouse CD148 antibody) from Pierce and goat anti-rat IgG biotin conjugated (as a secondary against rat anti-mouse F4/80 and Mac-2 antibodies) from Serotec.
Immunoblotting
Total protein extracts were prepared using the SDS-boiling method [26]. Cells were washed twice with ice cold PBS and lysed on the culture plate with 500 µL boiling lysis buffer (66 mM Tris-HCl pH 7.4, 2% SDS) per 1×106 cells. Extracts were homogenised by repeated passage through a 26-gauge needle and further boiled for 5 minutes. Protein concentration of total lysates was determined using the BCA assay kit (Pierce, Rockford, IL, USA). Extracts were resolved by SDS-PAGE (4–12%), transferred to Imobilon-P (Millipore, North Ryde, NSW, Australia), blocked and probed with anti-CD148 antibody or antiphosphoprotein antibodies in the presence of phosphatase inhibitors. Blots were washed, probed with HRP-labelled secondary antibodies (Cell Signaling Technology) or Streptavidin-HRP-labelled (Pierce) tertiary antibody in case of CD148 blots, and detected using enhanced chemiluminescence (ECL) reagents (Amersham Pharmacia Biotech, Piscataway, NJ, USA). Membranes were stripped with 66 mM Tris-Cl (pH 6.7)/2% SDS/100 mM 2-mercapto-ethanol and reprobed with total Akt antibodies (Cell Signaling Technology).
Immunohistochemistry
Immunohistochemistry was performed using an immunoperoxidase technique with diaminobenzidine (DAB) as the chromogen as described previously [21], [49]. Expression of CD148, F4/80 and Mac-2 were examined in serial sections. Briefly, sections were deparaffinized and rehydrated followed by antigen retrieval. For CD148, microwave antigen retrieval was performed in citrate buffer pH 6.0 for 2 min and allowed to cool overnight. For F4/80 Carezyme Trypsin digestion kit (Biocare Medica) was used as per the manufacturer’s instructions. Sections were washed in Tris buffered saline (TBS) and endogenous peroxidase activity was blocked by incubating the sections in 3% H2O2 (diluted in TBS) for 30 min. Sections were incubated for 60 min in serum block [10% fetal calf serum (FCS) plus 10% normal serum (species of secondary antibody) in TBS] and then treated with the primary antibody for 60 min. Sections were subsequently incubated for 30 min with a biotinylated F(ab′)2 fragment of goat anti-rat or rabbit anti-hamster immunoglobulin (DakoCytomation), followed by horseradish peroxidase (HRP)-conjugated streptavidin (DakoCytomation) and developed with DAB chromogen (DakoCytomation) according to the manufacturer’s specifications. The specificity of the staining was confirmed by using matched isotype control antibodies. All sections were counterstained with Mayer’s haematoxylin. Slides were allowed to dry on the bench before mounting using permanent mounting media (Cytoseal, Stephens Scientific). All incubations were carried out at 25°C sections were washed between each step with TBS. Slides were examined and photographed using a transmitted light microscope (Olympus BX-51, with DP-70 camera).
Statistical Analysis
One-way analysis of variance (ANOVA) was used to determine any significance differences between the samples. In addition significance differences between individual time points for each group were calculated using a two-tailed independent Student’s t-test. Data sets yielding a p value greater than 0.05 were regarded as not statistically different.
Results
Ptprj is Preferentially Expressed in Macrophage-enriched Tissues and Cell Types
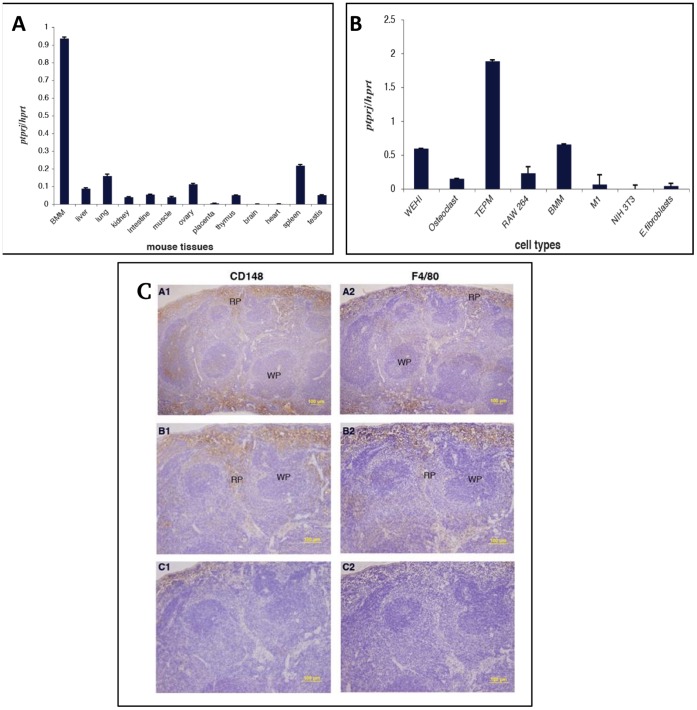
To characterize the expression of Ptprj in various mouse tissues, quantitative real-time PCR analysis was performed. Ptprj expression was highest in bone marrow derived macrophages (Figure 1A). Tissue distribution revealed that Ptprj was high in tissues with a significant macrophage content. In particular, expression of Ptprj was highest in the spleen. However, other macrophage-rich tissues such as lung, liver, kidney, intestine, thymus, ovary, muscle and testis showed detectable expression levels of Ptprj. Minimal expression of Ptprj was detected in brain, heart and placenta. The expression of Ptprj in a range of different cell types was also examined. Ptprj mRNA was expressed at relatively elevated levels in inflammatory (TEPMs) and primary macrophages (BMMs) and at lower levels in the macrophage-like cell line RAW 264.7, differentiated RAW/C4 osteoclast-like cells and myeloid precursor cell line M1 (Figure 1B). Ptprj mRNA levels were low in pre-B lymphoid cell line WEHI-231, embryonic fibroblasts and Ptprj mRNA was virtually undetectable in the fibroblast cell line NIH 3T3.
Figure 1. Ptprj mRNA expression in murine tissues.
(A). qPCR of Ptprj from RNA extracted from mouse tissues. (B) qPCR of Ptprj from RNA from pre-B lymphoid cell line WEHI-231, osteoclast-like cell line (RAW 264.7, C4), TEPM, macrophage-like cell line (RAW 264.7), BMM, myeloid cell line M1 and fibroblasts (NIH3T3 and mouse embryonic). (C). Immunohistochemistry of cell-specific expression of PTPRJ in mouse spleen sections. Sections were immunostained for CD148 (A1, B1) or F4/80 (A2, B2) and with CD148 (C1) and F4/80 (C2) isotype control antibodies. All sections were counterstained with haematoxylin. RP, red pulp; WP, white pulp. Original magnification: x100 (A), x200 (B, C). Bar, 100µm.
As Ptprj expression was highest in spleen compared to other tissues (Figure 1A), immunohistochemistry was performed to identify the cell types within spleen that express the protein product of Ptprj, CD148. CD148 expression pattern in the spleen was restricted to the red pulp region, and was indistinguishable from the expression pattern of F4/80, a mononuclear phagocyte marker (Figure 1C). This confirmed the macrophage lineage–specific expression of CD148 in the mouse spleen and was consistent with the quantitative real-time PCR data (Figure 1A,B).
Regulation of Ptprj mRNA and Protein in Response to Inflammatory Stimuli in Mouse Macrophages
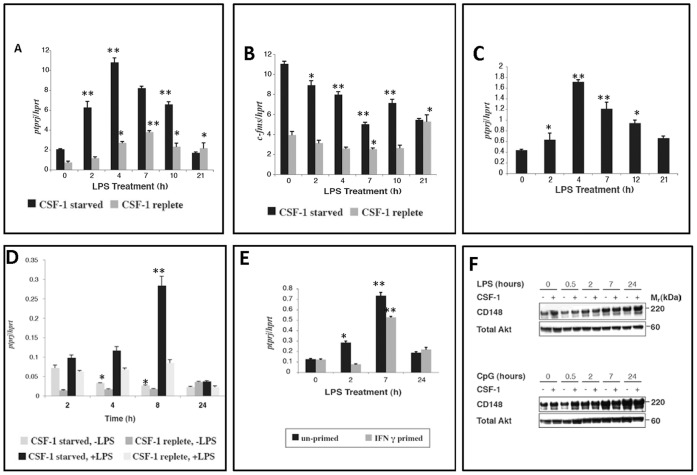
Quantitative real-time PCR analysis of BMMs stimulated with LPS showed regulated expression of Ptprj. In the absence of CSF-1, LPS induced Ptprj mRNA expression approximately six-fold peaking at 4 hours. However, the presence of CSF-1 caused a marked reduction in both the basal and LPS-induced expression levels of the Ptprj transcript (Figure 2A). As expected, the expression pattern of c-fms, a classical marker for mature, differentiated macrophages was suppressed by CSF-1 (Figure 2B). To investigate other murine macrophage populations, the expression profile of Ptprj was examined in the macrophage-like RAW 264.7 cell line and in thioglycollate-elicited peritoneal macrophages (TEPM). Although, the pattern of Ptprj induction in response to LPS in RAW 264.7 cells was identical to that of BMMs (Figure 2C), the fold induction was less compared to BMMs. Because RAW 264.7 cells are deficient in cell surface expression of CSF-1R [50], Ptprj mRNA expression in these cells was examined only in the absence of CSF-1. As with BMMs, in TEPM Ptprj was strongly induced by LPS and this was repressed by CSF-1 (Figure 2D). Pre-treatment of macrophages with IFNγ (priming) results in a more rapid and heightened response to LPS and other TLR agonists [48], [51]. Unlike LPS and CpG DNA, IFNγ alone had no effect on induction of Ptprj in BMMs (data not shown). Conversely, priming of BMMs with IFNγ repressed LPS-mediated induction of Ptprj mRNA expression at early time points (Figure 3E).
Figure 2. Regulation of Ptprj expression in mouse macrophages by proinflammatory stimuli.
A–C: Regulation of Ptprj expression by CSF-1 and LPS in mouse bone marrow-derived macrophages (BMMs). BMMs were maintained overnight in the presence or absence of CSF-1 (1×104 U/mL) before treatment with LPS (10 ng/mL) (A, B). RAW 264.7 cells were maintained overnight in the absence of CSF-1 before treatment with LPS (10 ng/mL) (C). Ptprj (A, C) and c-fms (B) expression profiles were assessed by quantitative real-time PCR. Profiles are representative of two independent experiments. D: Regulation of Ptprj expression by CSF-1 and LPS in mouse thioglycollate-elicited peritoneal macrophages (TEPMs). TEPMs were maintained overnight in the presence or absence of CSF-1 (1×104 U/mL) before treatment with LPS (10 ng/mL). Ptprj expression profile was assessed by quantitative real-time PCR. E: IFNγ treatment of bone marrow derived macrophages suppresses the LPS mediated induction of ptprj. BMMs were maintained overnight in the presence of CSF-1 (1×104 U/mL) and presence or absence of IFNγ (500 pg/mL) before treatment with LPS (10 ng/mL). RNA was extracted at each time point and used for the synthesis of cDNA. Ptprj expression profile was assessed by quantitative real-time PCR. Datapoints (+/− SD) represent the average of triplicate samples each from triplicate independent experiments. Significance values were determined by one-way analysis of variance (ANOVA). *denotes p<0.05; **denotes p<0.005; n = 3. F: Regulation of PTPRJ protein in response to LPS, CpG DNA and CSF-1. BMMs were maintained overnight in the presence or absence of CSF-1 (1×104 U/mL) before treatment with LPS (10 ng/mL) [top panel] or CpG DNA (0.1 µM) [bottom panel]. Protein lysates were separated by SDS-PAGE, transferred to PVDF membranes and immunoblotted for PTPRJ. The membrane was then stripped, and reprobed for total Akt as a loading control. Profiles are representative of two independent experiments.
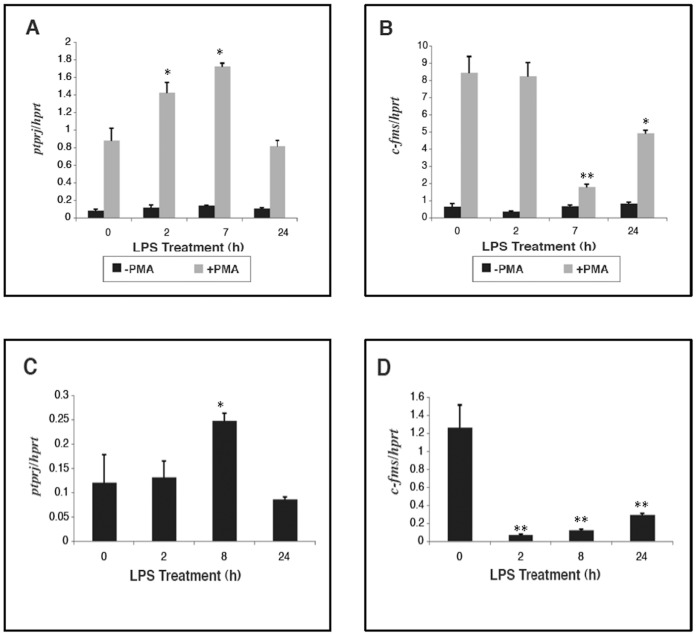
Figure 3. Ptprj expression in response to LPS in human mononuclear phagocytic cells.
THP-1 cells were maintained for 24 hours in the presence or absence of PMA (10−7 M) to induce differentiation, before treatment with LPS (10 ng/mL) (A, B). Human dendritic cells were treated with LPS (10 ng/mL) over a time course (C, D). Ptprj (A, C) and c-fms (B, D) expression profiles were assessed by quantitative real-time PCR. Datapoints (+/− SD) represent the average of triplicate samples. Significance values were determined by one-way analysis of variance (ANOVA). *denotes p<0.05; **denotes p<0.005; n = 3. *denotes p<0.05; **denotes p<0.005; n = 3.
To determine whether Ptprj was regulated at the protein level, western blot analyses of BMM cell lysates from LPS and CpG time courses were performed (Figure 2F). CSF-1 increased the basal level of CD148 protein (0 h lanes), however CD148 protein levels increased with time after treatment with LPS in both replete and CSF-1-starved cells. Although CpG DNA showed a similar basal pattern of induction of CD148 by CSF-1, an increased level of CD148 protein with time after stimulation was observed. Thus both qPCR (Figure 2D) and western blotting (Figure 2F) showed an increase in CD148 mRNA and protein respectively, albeit with a different timecourse.
Regulation of PTPRJ Gene Expression in Human Mononuclear Phagocytic Cells
The expression profile of PTPRJ in response to LPS was also examined in the human monocytic cell line THP-1 and in human dendritic cells. Unlike mature differentiated macrophages such as RAW 264.7 cells or BMMs, THP-1 cells are premonocytes that are committed to the monocytic lineage, but are non-adherent and lack many macrophage-specific cell surface markers. Therefore, for the induction of terminal differentiation to macrophage-like cells, THP-1 cells were cultured in the presence of PMA. PMA mimics some actions of CSF-1, but activates distinct signalling pathways [52]. In the absence of PMA, LPS stimulation had no effect on PTPRJ expression (Figure 3A). However, presence of PMA led to a dramatic induction of PTPRJ in response to LPS. Even in the absence of LPS, PMA induced basal PTPRJ expression approximately ten-fold, whereas in the presence of LPS, the induction was even stronger. PMA strongly induced c-fms, consistent with the view that PMA imparts a macrophage-like phenotype to these monocytic cells, however LPS treatment suppressed c-fms expression in PMA-differentiated cells (Figure 3B).
CD148 has been recognised as an accessory molecule present on the surface of peripheral blood dendritic cells [53]. Although overall fold induction was lower than in mouse macrophages, LPS induced PTPRJ expression in human peripheral blood dendritic cells (PBDCs) (Figure 3C). As with PMA-differentiated THP_ cells, c-fms was down-regulated by LPS in human dendritic cells (Figure 3D).
Expression of Long Noncoding RNA Ptprj-as1 during Macrophage Activation
Expression of Ptprj-as1 in murine tissues
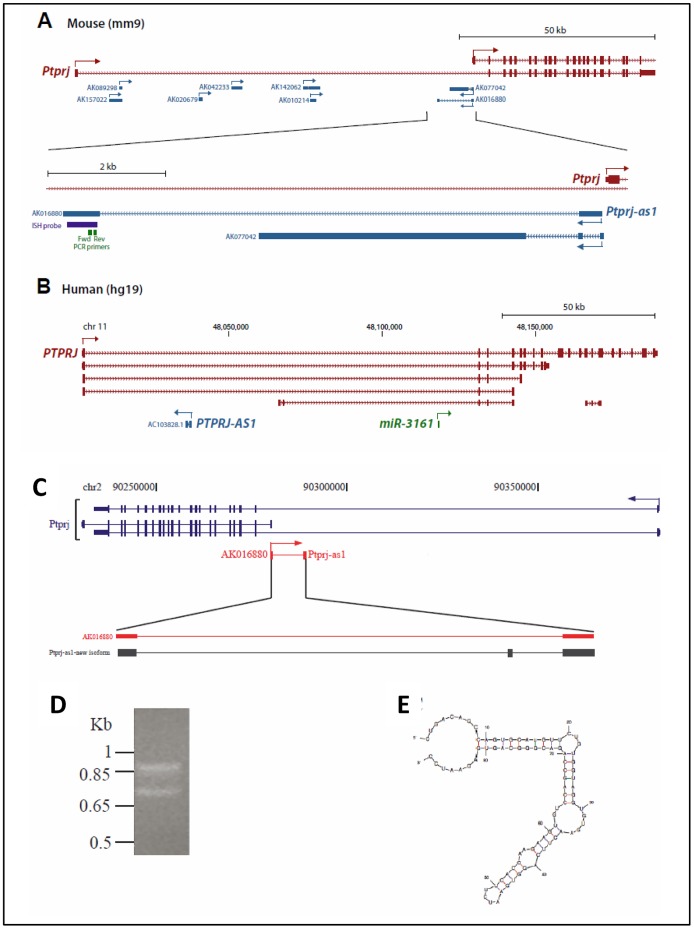
In a previous study, we examined microarray expression profiling data of mammary epithelial cells derived from pregnant, lactating and involuting mice [34]. The microarray that this dataset is based upon features probes targeting ∼29,550 mRNAs and 8,693 long ncRNAs. Within this dataset, we identified seven probes that targeted long ncRNAs that originated from the Ptprj locus [21]. All of these long ncRNAs occurred within the first intron of Ptprj; two were on the antisense strand and the remaining five were on the sense strand (Figure 4A). On the basis of its high expression in monocytic cells, cytoplasmic localization, and spliced character, we selected the lncRNA that we term Ptprj-as1 (GenBank Accession ID AK016880) for further examination. As the other lncRNAs were expressed at low levels or unexpressed in monocytic cells, and they were derived from unspliced transcripts, they were not pursued for further characterization as such low-expressed single-exon transcripts are challenging to study because they cannot be easily distinguished from genomic DNA in expression studies.
Figure 4. Characterisation of Ptprj-as1.
A–B: Ptprj-as1 maps to the reverse strand within the boundaries of the murine Ptprj gene. Comparison of the mouse Ptprj (A) and human PTPRJ (B) loci. Protein coding transcript isoforms of Ptprj/PTPRJ are shown in red and long noncoding transcripts are shown in blue. Arrows indicate the direction of transcription. The human microRNA miR-3161 is shown in green. Position of PCR primers used for qRT-PCR for mouse Ptprj-as1 are indicated. C-D: Mapping (C) and expression (D) of a splice variant of murine Ptprj-as1 in brain, kidney and testis. E: Predicted secondary structure of Ptprj-as1 splice variant.
Ptprj-as1 is a spliced 1,006 nt lncRNA that is transcribed antisense to Ptprj-as1 and is expressed at levels similar to Ptprj. Interestingly, Ptprj-as1 is antisense to the 5′UTR of a short isoform of Ptprj that lacks the canonical first exon, raising the possibility that Ptprj-as1 may be co-expressed with the short isoform of Ptprj-as1. Ptprj-as1 was identified as a long noncoding RNA of unknown function that is transcribed from the reverse strand of the Ptprj gene (Figure 4A). Note that the PTPRJ-AS1 in human is in a different location to Ptprj-as1 in mouse and should not be considered as an orthologue (Figure 4B). RT-PCR analysis of the murine transcript in different tissues confirmed the existence of tissue-specific splice variants (Figure 4C). Spleen, brain, and testis revealed two isoforms of Ptprjas-1, one; as reported previously [21] and a second that contains an additional 80 base exon (Figure 4D). The examination of the secondary structure of the additional exon showed a stable stem loop structure (Figure 4E).
Expression of Ptprj-as1 in murine macrophages
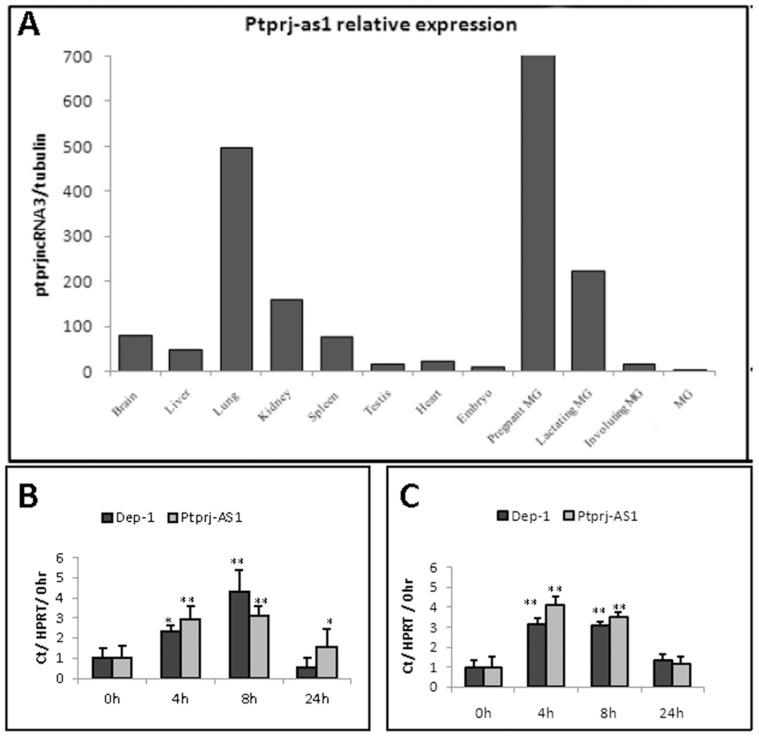
Ptprj-as1 was readily detectable by qRT-PCR (Figure 5A). Preliminary screens of murine tissues demonstrated that whilst Ptprj-as1 was expressed in many tissues, the transcript showed comparatively elevated levels in lung, brain, kidney and spleen; all tissues that have a sizeable macrophage component (Figure 5A). In unstimulated cells, LPS activation of murine bone marrow-derived macrophages (BMMs) induced a transient increase of Ptprj mRNA, peaking at a 4 fold increase 8 hours post addition of LPS. In parallel with Ptprj, Ptprj-as1 expression was also transiently induced by LPS activation of BMMs (Figure 5B). Furthermore, Pam3Cys induced a very similar expression pattern of Ptprj and Ptprj-as1 (Figure 5C). The comparable expression trends of Ptprj and Ptprj-as1 during mBMM activation is consistent with the developing hypothesis that the Ptprj and Ptprj-as1 are regulated by the activity of the same promoter.
Figure 5. Expression of Ptprj-as1 in murine tissues and in response to TRL ligands.
A: Expression of Ptprj-as1 in murine tissues. B, C: Ptprj and Ptprj-as1 mRNA expression in BMMs in response to LPS (B) or Pam3Cys (C). mRNA expression was quantified by qRT-PCR and expressed as fold change compared with untreated (0h). Plots represent mean fold change +/− SD; n = 3. Significance values were determined by one-way analysis of variance (ANOVA). *denotes p<0.05; **denotes p<0.005; n = 3. *denotes p<0.05; **denotes p<0.005; n = 3.
Bioinformatic Identification of Regulatory Sequences in the Ptprj Gene
To further investigate the potential mechanisms that direct the expression of Ptprj, bioinformatic analysis to identify putative promoter regions was performed.
Identification of Ptprj putative promoter by CAGE analysis
CAGE (cap analysis of gene expression) analysis identified the dominant start sites for transcription of the mouse and human Ptprj genes, which in turn allowed functional alignment of the promoters. Ptprj appears to have only one promoter, and both the mouse and human Ptprj promoters lack a TATA box and are rich in GC (data not shown). Whilst the majority of the macrophage-specific promoters also lack a TATA box [54]–[56] the Ptprj promoter differs from typical macrophage promoters such as c-fms [57], [58] in that these generally lack GC-rich elements. Analysis of the Ptprj-as1 promoter did not reveal the presence of either a TATA-box or GC-rich element, suggesting that it may be regulated independently to Ptprj.
Identification of Putative Transcription Factor Binding Sites
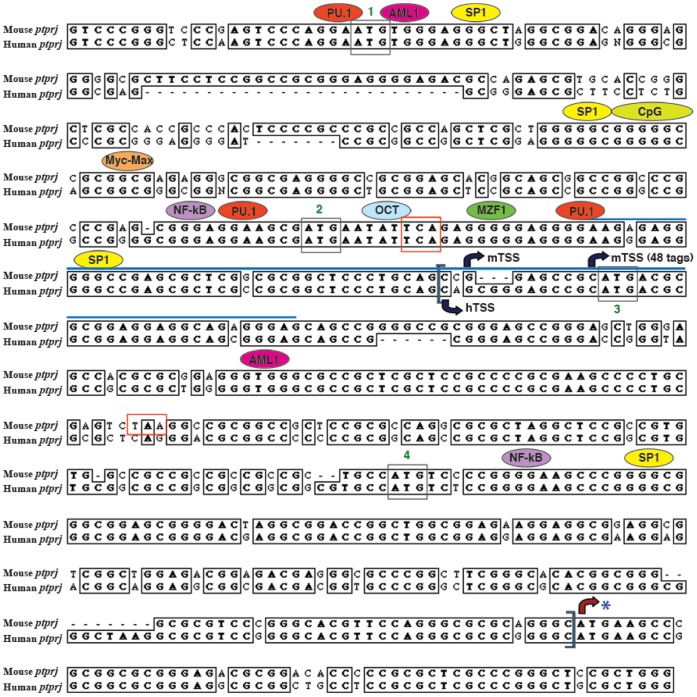
The mouse and human sequences corresponding to ECR1 were aligned using ClustalW alignment. This sequence was further examined for consensus transcription factor binding sites conserved in sequence and position between mice and humans using the RVista browser and TRANSFAC (Figure 6). This analysis revealed the presence of multiple transcription factor binding sites that are conserved in both sequence and position between the mouse and human putative promoters. These include many transcription factor binding sites that are commonly functional in myeloid promoters including PU.1, SP1, myeloid zinc finger protein (MZF1), nuclear factor kappa B (NF-κB) or p50, octamer factor binding sequences (OCT) and acute myelogenous leukemia (AML1). The presence of a CpG island upstream of the transcription start site might permit epigenetic control of transcriptional activity of the Ptprj promoter [58], [59]. One important observation of the assignment of TSS by CAGE is that the Ptprj mRNA is longer than previously appreciated. As a consequence, there may be up to four potential initiation codons or AUGs (uAUGs) in the 5′ untranslated region (UTR) upstream of the putative start site of translation of CD148.
Figure 6. Clustal W alignment of the mouse and human Ptprj putative promoters.
Consensus transcription factor binding sites conserved in sequence and position between mouse and human were predicted using RVista browser and TRANSFAC. Only the myeloid-specific transcription factors have been shown in this figure. Evolutionary conserved region (ECR1) lies within the blue brackets. Blue line marks the region of transcription start site (TSS) cluster represented in Figure 6. mTSS refers to the transcription start site for mouse ptprj and hTSS to the transcription start site for human Ptprj predicted by CAGE analysis. The four uAUG codons are highlighted within grey boxes and the stop codons within red boxes. Asterisk (*) represents the translation start site. The boxes represent the conserved sequences between mouse and human. N represents any nucleotide.
Discussion
PTPRJ has been widely accepted as an epithelial cell expressed gene and has been proposed to be a tumor suppressor in this cell type. However, we show here that Ptprj is highly expressed in macrophages and its expression is regulated in response to CSF-1 and proinflammatory stimuli. Ptprj expression was highest in spleen, lung, ovary, liver, kidney, intestine, thymus and testis; tissues that are rich in resident macrophage population. Indeed, approximately 18% of the cells in the spleen (the highest expressing tissue for Ptprj) are macrophages [60]. Others have shown CD148 is expressed in macrophages and related tissues of human origin [61]. The macrophage-enriched expression of CD148 was further confirmed by the strong correlation of the expression pattern of CD148 and F4/80 surface markers on the splenic macrophages in the red pulp. qRT_PCR showed a transient increase in CD148 mRNA after stimulation with proinflammatory factors such as LPS, and that both basal and stimulated expression is suppressed by CSF-1. Western blotting showed a general sustained increase in CD148 protein after LPS stimulation but no detectable suppression by CSF-1. Although the reason for this discrepancy is unknown, lack of correlation between RNA and protein may be related to the half-life of the protein within the cell. For example, if CD148 has a long half-life (as many membrane/cytoskeletal proteins do), then a short-term down-regulation in mRNA levels would not immediately translate into a down-regulation of protein. On the contrary, protein with a long half-life would continue to appear up-regulated due to the accumulation of the protein in the cell before degradation.
Whilst there have been a number of studies investigating the role of Ptprj in both epithelial and haematopoietic cells, its exact role in cell function has not been defined. Overall, Ptprj appears to be a negative regulator of cell proliferation in epithelial cells and fibroblasts, by down-regulating receptor tyrosine kinase activity [16], [17], [20]. In contrast, Ptprj plays a positive role in the activation of B cells and monocytes by dephosphorylating the negative regulatory carboxyterminal tyrosine of src family kinases (SFK), thereby activating these enzymes and potentiating a signalling cascade. Ptprj expression has been reported to be up-regulated in epithelial cells as they reach confluence and is down-regulated in some tumours [16], [17]. Although loss of heterogeity has been reported for colon, lung, breast and thyroid cancers [62], we have not detected any major down-regulation of PTPRJ protein or mRNA in a large cohort of breast cancers [21].
Clearly, the regulation of Ptprj is of profound importance in a number of cell types, however the regulation of PTPs in general and Ptprj in particular is poorly understood [11]. Ptprj translation has been shown to be regulated by the use of an alternative start site at the 5′ end of the gene [33]. There is growing evidence that microRNAs (miRNAs) can target the expression of both oncogenes and tumour suppressor genes and recently miRNA-328 has been reported to decrease Ptprj expression in epithelial cells [46]. In contrast to the relatively few miRNAs that have been identified, it appears that there are at least as many long noncoding RNAs (lncRNAs) as coding RNAs in the human genome [45], [63], and it is likely that many of these can regulate gene expression [64]. We have identified a lncRNA, Ptprj-as1, that is transcribed off the Ptprj gene locus and is co-regulated with Ptprj coding transcripts in murine monocytes. Although we have not investigated any direct roles of Ptprj-as1 in regulating Ptprj, its tissue-specific expression and co-incident location with Ptrpj in the genome, raise the possibility that it may have some role in the expression or splicing of Ptprj. Previous studies have revealed that antisense long ncRNAs can directly affect the expression or alternate splicing of nearby genes. For example, expression of a long ncRNAs antisense to the tumor-suppressor gene p15 results in silencing of the p15 gene by inducing heterochromatin formation [65]. In another example, differential expression of the lncRNA LUST, which originates antisense to an intronic region of RBM5, regulates the expression of RBM5 splice variants [66]. It also noteworthy that Ptpre, a related tyrosine phosphatase-encoding gene, also features a contextually analogous antisense noncoding RNA, Ptpreas [67]. Finally, although examination of the corresponding Ptprj loci in the human genome revealed a number of lncRNAs, because the human PTPRJ loci differs considerably in terms of both splice variants, exon number, primary sequence, and promoter initiation, it is not possible to identify orthologous lncRNAs. This in itself is not surprising, as lncRNAs seldom reveal identifiable primary sequence conservation, and as an intrinsic feature of their regulatory function as RNAs are thought generally to show great plasticity in their sequence constraints compared to protein-coding genes [37].
CAGE analyses revealed the presence of clustered transcription start sites in both mouse and human Ptprj promoters, including conserved multiple upstream AUG (uAUG) codons in the 5′ UTR (data not shown). These uAUGs are common features of mRNAs that encode regulatory proteins. These upstream open reading frames (uORFs) encode for upstream peptides (uPEPs), which are highly evolutionarily conserved and are important for regulating translation of the main CDS [68], [69]. It is conceivable that one of these AUGs is the preferred start codon, instead of the one (marked with asterisk in Figure 6) recognised by the publicly available sequence databases. As shown in Figure 6, there is a conserved stop codon in the 5′ UTR and another in the mouse putative promoter. In addition, translational analysis initiating from the fourth uAUG encodes for a 52 amino acid long peptide in case of mouse Ptprj.
As there was a similar pattern of Ptprj gene regulation across a range of mouse and human macrophages, a combination of bioinformatic data mining and functional analysis was used to dissect the potential mechanisms underlying the transcriptional regulation of Ptprj. NF-κB binding sites were identified in the Ptprj promoter (Figure 6). NF-κB has a key role in inflammation as it regulates apoptosis, cell-cycle progression, proliferation and cell differentiation [70], [71]. The responsiveness of both mouse and human Ptprj genes to LPS and CpG DNA could therefore be attributed to the presence of an NF-κB binding site in the Ptprj putative promoter.
The Ptprj promoter is a GC-rich TATA-less promoter, which is the major promoter class in mammals and is a more broad and evolvable category of promoters compared to the TATA box-containing promoters [59]. A number of multiple transcription factor binding sites that are conserved in both sequence and position between the mouse and human putative promoters were identified. The presence of PU.1, SP1, MZF1, NF-κB, AML1 and OCT binding sites in the Ptprj promoter could explain the macrophage-specific expression of Ptprj. Many myeloid promoters have a functional PU.1 binding site upstream of the transcription start site. PU.1 binds to and recruits TATA binding protein (TBP), the primary component of the basal transcription factor TFIID in promoters lacking a TATA box [55], [72]. The expression of PU.1 has been shown to be important for macrophage differentiation and the expression of a number of molecules that mediate some of the immunological actions of macrophages [73]. Amongst other transcription factors that are involved in myeloid gene regulation and can interact with TBP are SP1 and OCT [55]. MZF1 expression is restricted to myeloid cell lines and is implicated in the development of cells of the myeloid lineage. The presence of a conserved MZF1 binding site in the Ptprj promoter is consistent with its expression in myeloid cells. The RNA binding zinc finger proteins EWS and FUS/TLS bind to the consensus binding sites for MZF1, implicating their significance in the assembly of the basal transcriptional complex [74]. Taken together, the bioinformatic analyses of the Ptprj putative promoter revealed that the Ptprj gene is highly regulated in macrophages and the Ptprj promoter resembles a macrophage-specific promoter.
IFNγ augments the immune response to LPS and other TLR agonists, thereby orchestrating distinct cellular signalling pathways through transcriptional control over a large number of genes [50]. In contrast, IFNγ priming of BMMs repressed Ptprj induction in response to LPS at early time points and had no effect at later time points (Figure 2E). Thus, Ptprj differs from many other macrophage-specific genes, which are regulated by both LPS and IFNγ. Many LPS-inducible genes including Mpeg-1, Itm2b and Ramp2, are repressed by CSF-1, but LPS-inducible in the presence of CSF-1 [75]. However, LPS-induction of Ptprj expression was more pronounced in the absence of CSF-1. In this respect, Ptprj regulation is similar to that of Tlr9, as absence of CSF-1 causes an elevation in Tlr9 mRNA expression, but differs in response to IFNγ. In both mouse macrophages and human monocytes or dendritic cells, LPS induced CD148 expression whereas it repressed c-fms expression. Thus the increases in Ptprj gene expression are unlikely to be dependent on CSF-1 signalling, as LPS treatment not only suppresses expression of the CSF-1 receptor (C-fms) but also induces cleavage of the surface receptor making cells refractive to CSF-1 [75]. Dendritic cells are a heterogeneous population of cells with a number of different lineage relationships. C-fms is downregulated upon differentiation of monocytes into myeloid-derived dendritic cells and is also expressed at lower levels in other debdritic cell polulations [76]. Whilst expression is low in these cells, clearly, c-fms is functional since absence of CSF-1 results in >50% reduction in dendritic cell numbers [76], and CSF-1 can also regulate the number of tissue and blood elicited dendritic cells [77]–[79]. Thus ligation of CSF-1 to its receptor c-fms, provides both survival and differentiation signals for dendritic cells [80].
CD148 expression is up-regulated in chronic inflammatory diseases, such as Crohn’s disease and Cogan’s syndrome [61], [81]. The possible function of CD148 in inflammation could be similar to that of CD45, which plays an important role in lymphocyte development and function and has a crucial role in inflammation and cancer [82]. CD45 enhances T-cell and BCR signalling by dephosphorylating the auto-inhibitory phospho-tyrosine residues on Src family tyrosine kinases (SFKs) [83]. Indeed, recent studies of double knockout mice have revealed that CD45 and CD148 have overlapping functions, and can activate src family kinases in monocytes [84]; therefore activation or enhancement of CD148 expression could lead to modulation of the inflammatory response. The current results indicate that Ptprj is highly regulated by inflammatory stimuli in vitro and in vivo, however, the precise mechanism involved in the regulation of inflammation by Ptprj remains to be elucidated.
Acknowledgments
We thank Prof. Arthur Weiss for providing the anti-CD148 antibody.
Funding Statement
Part of this work was funded by the CRC for Chronic Inflammatory Diseases. RKD was in receipt of an NHMRC Dora Lush scholarship. MED is in receipt of an NHMRC Career Development Award. The authors wish to acknowledge the ARC Special Research Centre for Functional and Applied Genomics. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stoy N (2001) Macrophage biology and pathobiology in the evolution of immune responses: A functional analysis. Pathobiology 69: 179–211. [DOI] [PubMed] [Google Scholar]
- 2. Gordon S (2012) Innate Immune Functions of Macrophages in Different Tissue Environments. Journal of Innate Immunity 4: 409–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shanley TP (2002) Phosphatases: Counterregulatory role in inflammatory cell signaling. Crit Care Med 30: S80–S88. [PubMed] [Google Scholar]
- 4. Weiss A (2009) Kinases and phosphatases of the immune system. Immunological Reviews 228: 5–8. [DOI] [PubMed] [Google Scholar]
- 5. Hermiston ML, Zikherman J, Zhu JW (2009) CD45, CD148, and Lyp/Pep: critical phosphatases regulating Src family kinase signaling networks in immune cells (vol 228, pg 288, 2009). Immunological Reviews 229: 387–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vang T, Miletic AV, Arimura Y, Tautz L, Rickert RC, et al. (2008) Protein tyrosine phosphatases in autoimmunity. Annu Rev Immunol 26: 29–55. [DOI] [PubMed] [Google Scholar]
- 7. Vang T, Miletic AV, Bottini N, Mustelin T (2007) Protein tyrosine phosphatase PTPN22 in human autoimmunity. Autoimmunity 40: 453–461. [DOI] [PubMed] [Google Scholar]
- 8. Mustelin T, Vang T, Bottini N (2005) Protein tyrosine phosphatases and the immune response. Nat Rev Immunol 5: 43–57. [DOI] [PubMed] [Google Scholar]
- 9. Lin G, Aranda V, Muthuswamy SK, Tonks NK (2011) Identification of PTPN23 as a novel regulator of cell invasion in mammary epithelial cells from a loss-of-function screen of the ‘PTP-ome’. Genes Dev 25: 1412–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boivin B, Yang M, Tonks NK (2010) Targeting the reversibly oxidized protein tyrosine phosphatase superfamily. Sci Signal 3: pl2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tonks NK (2012) Protein Tyrosine Phosphatases: From Housekeeping Enzymes to Master-Regulators of Signal Transduction. FEBS J. [DOI] [PMC free article] [PubMed]
- 12. Shultz LD, Rajan TV, Greiner DL (1997) Severe defects in immunity and hematopoiesis caused by SHP-1 protein-tyrosine-phosphatase deficiency. Trends in Biotechnology 15: 302–307. [DOI] [PubMed] [Google Scholar]
- 13. Ostman A, Yang Q, Tonks NK (1994) EXPRESSION OF DEP-1, A RECEPTOR-LIKE PROTEIN-TYROSINE-PHOSPHATASE, IS ENHANCED WITH INCREASING CELL-DENSITY. Proceedings of the National Academy of Sciences of the United States of America 91: 9680–9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin J, Zhu JW, Baker JE, Weiss A (2004) Regulated expression of the receptor-like tyrosine phosphatase CD148 on hemopoietic cells. Journal of Immunology 173: 2324–2330. [DOI] [PubMed] [Google Scholar]
- 15.Gaya A, Pirotto F, Palou E, Autschbach F, Del Pozo V, et al.. (1999) CD148, a new membrane tyrosine phosphatase involved in leukocyte function. Leukemia & Lymphoma 35: 237–+. [DOI] [PubMed]
- 16. Iuliano R, Trapasso F, Le Pera I, Schepis F, Sama I, et al. (2003) An adenovirus carrying the rat protein tyrosine phosphatase eta suppresses the growth of human thyroid carcinoma cell lines in vitro and in vivo. Cancer Res 63: 882–886. [PubMed] [Google Scholar]
- 17. Keane MM, Lowrey GA, Ettenberg SA, Dayton MA, Lipkowitz S (1996) The protein tyrosine phosphatase DEP-1 is induced during differentiation and inhibits growth of breast cancer cells. Cancer Research 56: 4236–4243. [PubMed] [Google Scholar]
- 18. Pera IL, Iuliano R, Florio T, Susini C, Trapasso F, et al. (2005) The rat tyrosine phosphatase eta increases cell adhesion by activating c-Src through dephosphorylation of its inhibitory phosphotyrosine residue. Oncogene 24: 3187–3195. [DOI] [PubMed] [Google Scholar]
- 19. Ruivenkamp CAL, van Wezel T, Zanon C, Stassen APM, Vlcek C, et al. (2002) Ptprj is a candidate for the mouse colon-cancer susceptibility locus Scc1 and is frequently deleted in human cancers. Nature Genetics 31: 295–300. [DOI] [PubMed] [Google Scholar]
- 20. Kellie S, Craggs G, Bird IN, Jones GE (2004) The tyrosine phosphatase DEP-1 induces cytoskeletal rearrangements, aberrant cell-substratum interactions and a reduction in cell proliferation. Journal of Cell Science 117: 609–618. [DOI] [PubMed] [Google Scholar]
- 21.Smart CE, Amiri MEA, Wronski A, Dinger ME, Crawford J, et al.. (2012) Expression and Function of the Protein Tyrosine Phosphatase Receptor J (PTPRJ) in Normal Mammary Epithelial Cells and Breast Tumors. Plos One 7. [DOI] [PMC free article] [PubMed]
- 22. Kovalenko M, Denner K, Sandstrom J, Persson C, Gross S, et al. (2000) Site-selective dephosphorylation of the platelet-derived growth factor beta-receptor by the receptor-like protein-tyrosine phosphatase DEP-1. Journal of Biological Chemistry 275: 16219–16226. [DOI] [PubMed] [Google Scholar]
- 23. Palka HL, Park M, Tonks NK (2003) Hepatocyte growth factor receptor tyrosine kinase met is a substrate of the receptor protein-tyrosine phosphatase DEP-1. Journal of Biological Chemistry 278: 5728–5735. [DOI] [PubMed] [Google Scholar]
- 24. Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E (2006) Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. Journal of Cell Biology 174: 593–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tsuboi N, Utsunomiya T, Roberts RL, Ito H, Takahashi K, et al. (2008) The tyrosine phosphatase CD148 interacts with the p85 regulatory subunit of phosphoinositide 3-kinase. Biochemical Journal 413: 193–200. [DOI] [PubMed] [Google Scholar]
- 26. Dave RK, Hume DA, Elsegood C, Kellie S (2009) CD148/DEP-1 association with areas of cytoskeletal organisation in macrophages. Experimental Cell Research 315: 1734–1744. [DOI] [PubMed] [Google Scholar]
- 27. Tangye SG, Aversa G, Lanier LL, de Vries JE, Phillips JH (1998) CD148: A cell-surface phosphatase involved in T cell activation. Faseb Journal 12: A941–A941. [PubMed] [Google Scholar]
- 28. Matozaki T, Murata Y, Mori M, Kotani T, Okazawa H, et al. (2010) Expression, localization, and biological function of the R3 subtype of receptor-type protein tyrosine phosphatases in mammals. Cell Signal 22: 1811–1817. [DOI] [PubMed] [Google Scholar]
- 29.Arimura Y, Yagi J (2010) Comprehensive Expression Profiles of Genes for Protein Tyrosine Phosphatases in Immune Cells. Science Signaling 3. [DOI] [PubMed]
- 30. Osborne JM, den Elzen N, Lichanska AM, Costelloe EO, Yamada T, et al. (1998) Murine DEP-1, a receptor protein tyrosine phosphatase, is expressed in macrophages and is regulated by CSF-1 and LPS. Journal of Leukocyte Biology 64: 692–701. [DOI] [PubMed] [Google Scholar]
- 31. Zhu JW, Brdicka T, Katsumoto TR, Lin J, Weiss A (2008) Structurally distinct Phosphatases CD45 and CD148 both regulate B cell and macrophage immunoreceptor signaling. Immunity 28: 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stepanek O, Kalina T, Draber P, Skopcova T, Svojgr K, et al. (2011) Regulation of Src Family Kinases Involved in T Cell Receptor Signaling by Protein-tyrosine Phosphatase CD148. Journal of Biological Chemistry 286: 22101–22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Karagyozov L, Godfrey R, Boehmer S-A, Petermann A, Hoelters S, et al. (2008) The structure of the 5′-end of the protein-tyrosine phosphatase PTPRJ mRNA reveals a novel mechanism for translation attenuation. Nucleic Acids Research 36: 4443–4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Askarian-Amiri ME, Crawford J, French JD, Smart CE, Smith MA, et al. (2011) SNORD-host RNA Zfas1 is a regulator of mammary development and a potential marker for breast cancer. RNA 17: 878–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amaral PP, Mattick JS (2008) Noncoding RNA in development. Mamm Genome 19: 454–492. [DOI] [PubMed] [Google Scholar]
- 36. Ponjavic J, Ponting CP, Lunter G (2007) Functionality or transcriptional noise? Evidence for selection within long noncoding RNAs. Genome Res 17: 556–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dinger ME, Amaral PP, Mercer TR, Mattick JS (2009) Pervasive transcription of the eukaryotic genome: functional indices and conceptual implications. Brief Funct Genomic Proteomic 8: 407–423. [DOI] [PubMed] [Google Scholar]
- 38. Dinger ME, Pang KC, Mercer TR, Crowe ML, Grimmond SM, et al. (2009) NRED: a database of long noncoding RNA expression. Nucleic Acids Res 37: D122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mercer TR, Dinger ME, Mattick JS (2009) Long non-coding RNAs: insights into functions. Nat Rev Genet 10: 155–159. [DOI] [PubMed] [Google Scholar]
- 40. Rinn JL, Chang HY (2012) Genome regulation by long noncoding RNAs. Annual review of biochemistry 81: 145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, et al. (2007) Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 129: 1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Khalil AM, Guttman M, Huarte M, Garber M, Raj A, et al. (2009) Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci U S A 106: 11667–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, et al. (2010) Long noncoding RNA as modular scaffold of histone modification complexes. Science 329: 689–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dinger ME, Amaral PP, Mercer TR, Pang KC, Bruce SJ, et al. (2008) Long noncoding RNAs in mouse embryonic stem cell pluripotency and differentiation. Genome Res 18: 1433–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS (2010) Non-coding RNAs: regulators of disease. J Pathol 220: 126–139. [DOI] [PubMed] [Google Scholar]
- 46.Paduano F, Dattilo V, Narciso D, Bilotta A, Gaudio E, et al.. (2012) Protein tyrosine phosphatase PTPRJ is negatively regulated by microRNA-328. FEBS J. [DOI] [PubMed]
- 47.Irvine KM, Burns CJ, Wilks AF, Su S, Hume DA, et al.. (2006) A CSF-1 receptor kinase inhibitor targets effector functions and inhibits pro-inflammatory cytokine production from murine macrophage populations. Faseb Journal 20: 1921–+. [DOI] [PubMed]
- 48. Sweet MJ, Stacey KJ, Kakuda DK, Markovich D, Hume DA (1998) IFN-gamma primes macrophage responses to bacterial DNA. Journal of Interferon and Cytokine Research 18: 263–271. [DOI] [PubMed] [Google Scholar]
- 49. Pettit AR, Walsh NC, Manning C, Goldring SR, Gravallese EM (2006) RANKL protein is expressed at the pannus-bone interface at sites of articular bone erosion in rheumatoid arthritis. Rheumatology 45: 1068–1076. [DOI] [PubMed] [Google Scholar]
- 50. Fowles LF, Stacey KJ, Marks D, Hamilton JA, Hume DA (2000) Regulation of urokinase plasminogen activator gene transcription in the RAW264 murine macrophage cell line by macrophage colony-stimulating factor (CSF-1) is dependent upon the level of cell-surface receptor. Biochemical Journal 347: 313–320. [PMC free article] [PubMed] [Google Scholar]
- 51. Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-gamma: an overview of signals, mechanisms and functions. Journal of Leukocyte Biology 75: 163–189. [DOI] [PubMed] [Google Scholar]
- 52.Stacey KJ, Fowles LF, Coleman MS, Ostrowski M, Hume DA (1993) REGULATION OF THE UROKINASE-TYPE PLASMINOGEN-ACTIVATOR GENE BY CSF-1 AND PHORBOL ESTER. Journal of Leukocyte Biology: 63–63.
- 53. Woodhead VE, Stonehouse TJ, Binks MH, Speidel K, Fox DA, et al. (2000) Novel molecular mechanisms of dendritic cell-induced T cell activation. International Immunology 12: 1051–1061. [DOI] [PubMed] [Google Scholar]
- 54. Schroder K, Irvine KM, Taylor MS, Bokil NJ, Cao K-AL, et al. (2012) Conservation and divergence in Toll-like receptor 4-regulated gene expression in primary human versus mouse macrophages. Proceedings of the National Academy of Sciences of the United States of America 109: E944–E953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tenen DG, Hromas R, Licht JD, Zhang DE (1997) Transcription factors, normal myeloid development, and leukemia. Blood 90: 489–519. [PubMed] [Google Scholar]
- 56. Rehli M, Den Elzen N, Cassady AI, Ostrowski MC, Hume DA (1999) Cloning and characterization of the murine genes for bHLH-ZIP transcription factors TFEC and TFEB reveal a common gene organization for all MiT subfamily members. Genomics 56: 111–120. [DOI] [PubMed] [Google Scholar]
- 57. Ross IL, Yue X, Ostrowski MC, Hume DA (1998) Interaction between PU.1 and another Ets family transcription factor promotes macrophage-specific basal transcription initiation. Journal of Biological Chemistry 273: 6662–6669. [DOI] [PubMed] [Google Scholar]
- 58. Caiafa P, Zampieri M (2005) DNA methylation and chromatin structure: the puzzling CpG islands. J Cell Biochem 94: 257–265. [DOI] [PubMed] [Google Scholar]
- 59. Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, et al. (2005) The transcriptional landscape of the mammalian genome. Science 309: 1559–1563. [DOI] [PubMed] [Google Scholar]
- 60. Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, et al. (2003) A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101: 1155–1163. [DOI] [PubMed] [Google Scholar]
- 61. Autschbach F, Palou E, Mechtersheimer G, Rohr C, Pirotto F, et al. (1999) Expression of the membrane protein tyrosine phosphatase CD148 in human tissues. Tissue Antigens 54: 485–498. [DOI] [PubMed] [Google Scholar]
- 62. Ruivenkamp C, Hermsen M, Postma C, Klous A, Baak J, et al. (2003) LOH of PTPRJ occurs early in colorectal cancer and is associated with chromosomal loss of 18q12–21. Oncogene 22: 3472–3474. [DOI] [PubMed] [Google Scholar]
- 63. Dinger ME (2011) lncRNAs: finding the forest among the trees? Mol Ther 19: 2109–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, et al. (2011) lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 477: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yu W, Gius D, Onyango P, Muldoon-Jacobs K, Karp J, et al. (2008) Epigenetic silencing of tumour suppressor gene p15 by its antisense RNA. Nature 451: 202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rintala-Maki ND, Sutherland LC (2009) Identification and characterisation of a novel antisense non-coding RNA from the RBM5 gene locus. Gene 445: 7–16. [DOI] [PubMed] [Google Scholar]
- 67. Pang KC, Dinger ME, Mercer TR, Malquori L, Grimmond SM, et al. (2009) Genome-wide identification of long noncoding RNAs in CD8+ T cells. J Immunol 182: 7738–7748. [DOI] [PubMed] [Google Scholar]
- 68. Crowe ML, Wang XQ, Rothnagel JA (2006) Evidence for conservation and selection of upstream open reading frames suggests probable encoding of bioactive peptides. BMC Genomics 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang XQ, Rothnagel JA (2004) 5′-untranslated regions with multiple upstream AUG codons can support low-level translation via leaky scanning and reinitiation. Nucleic Acids Res 32: 1382–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schottelius AJ, Dinter H (2006) Cytokines, NF-kappaB, microenvironment, intestinal inflammation and cancer. Cancer Treat Res 130: 67–87. [DOI] [PubMed] [Google Scholar]
- 71. Hanada T, Yoshimura A (2002) Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev 13: 413–421. [DOI] [PubMed] [Google Scholar]
- 72. Eichbaum QG, Iyer R, Raveh DP, Mathieu C, Ezekowitz RA (1994) Restriction of interferon gamma responsiveness and basal expression of the myeloid human Fc gamma R1b gene is mediated by a functional PU.1 site and a transcription initiator consensus. J Exp Med 179: 1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nagamura-Inoue T, Tamura T, Ozato K (2001) Transcription factors that regulate growth and differentiation of myeloid cells. Int Rev Immunol 20: 83–105. [DOI] [PubMed] [Google Scholar]
- 74. Hume DA, Sasmono T, Himes SR, Sharma SM, Bronisz A, et al. (2008) The Ewing sarcoma protein (EWS) binds directly to the proximal elements of the macrophage-specific promoter of the CSF-1 receptor (csf1r) gene. Journal of Immunology 180: 6733–6742. [DOI] [PubMed] [Google Scholar]
- 75. Sester DP, Trieu A, Brion K, Schroder K, Ravasi T, et al. (2005) LPS regulates a set of genes in primary murine macrophages by antagonising CSF-1 action. Immunobiology 210: 97–107. [DOI] [PubMed] [Google Scholar]
- 76. MacDonald KP, Rowe V, Bofinger HM, Thomas R, Sasmono T, et al. (2005) The colony-stimulating factor 1 receptor is expressed on dendritic cells during differentiation and regulates their expansion. J Immunol 175: 1399–1405. [DOI] [PubMed] [Google Scholar]
- 77. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, et al. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327: 656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tagliani E, Shi C, Nancy P, Tay CS, Pamer EG, et al. (2011) Coordinate regulation of tissue macrophage and dendritic cell population dynamics by CSF-1. J Exp Med 208: 1901–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Merad M, Manz MG (2009) Dendritic cell homeostasis. Blood 113: 3418–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Fancke B, Suter M, Hochrein H, O’Keeffe M (2008) M-CSF: a novel plasmacytoid and conventional dendritic cell poietin. Blood 111: 150–159. [DOI] [PubMed] [Google Scholar]
- 81. Lunardi C, Bason C, Leandri M, Navone R, Lestani M, et al. (2002) Autoantibodies to inner ear and endothelial antigens in Cogan’s syndrome. Lancet 360: 915–921. [DOI] [PubMed] [Google Scholar]
- 82. Frame MC (2002) Src in cancer: deregulation and consequences for cell behaviour. Biochimica Et Biophysica Acta-Reviews on Cancer 1602: 114–130. [DOI] [PubMed] [Google Scholar]
- 83. Hermiston ML, Xu Z, Weiss A (2003) CD45: A critical regulator of signaling thresholds in immune cells. Annual Review of Immunology 21: 107–137. [DOI] [PubMed] [Google Scholar]
- 84. Zhu JW, Doan K, Park J, Chau AH, Zhang H, et al. (2011) Receptor-like Tyrosine Phosphatases CD45 and CD148 Have Distinct Functions in Chemoattractant-Mediated Neutrophil Migration and Response to S. aureus. Immunity 35: 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]