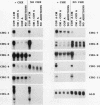
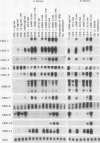
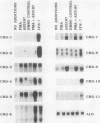
Abstract
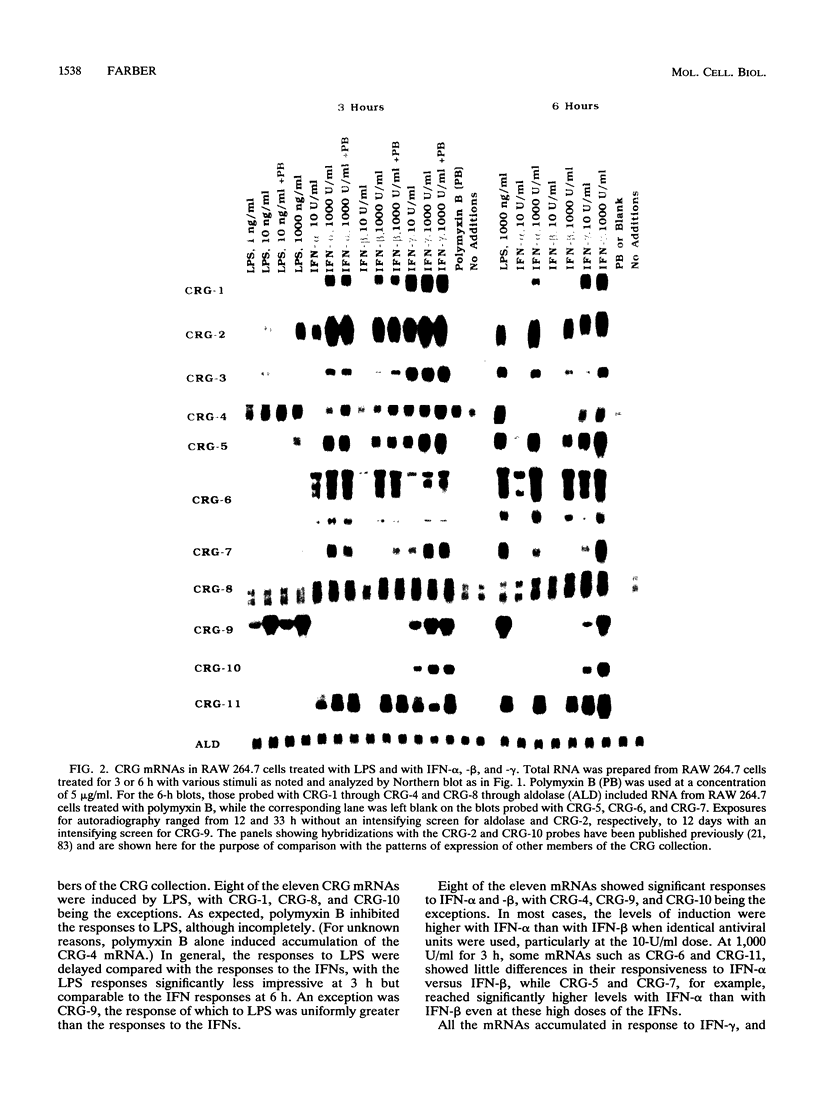
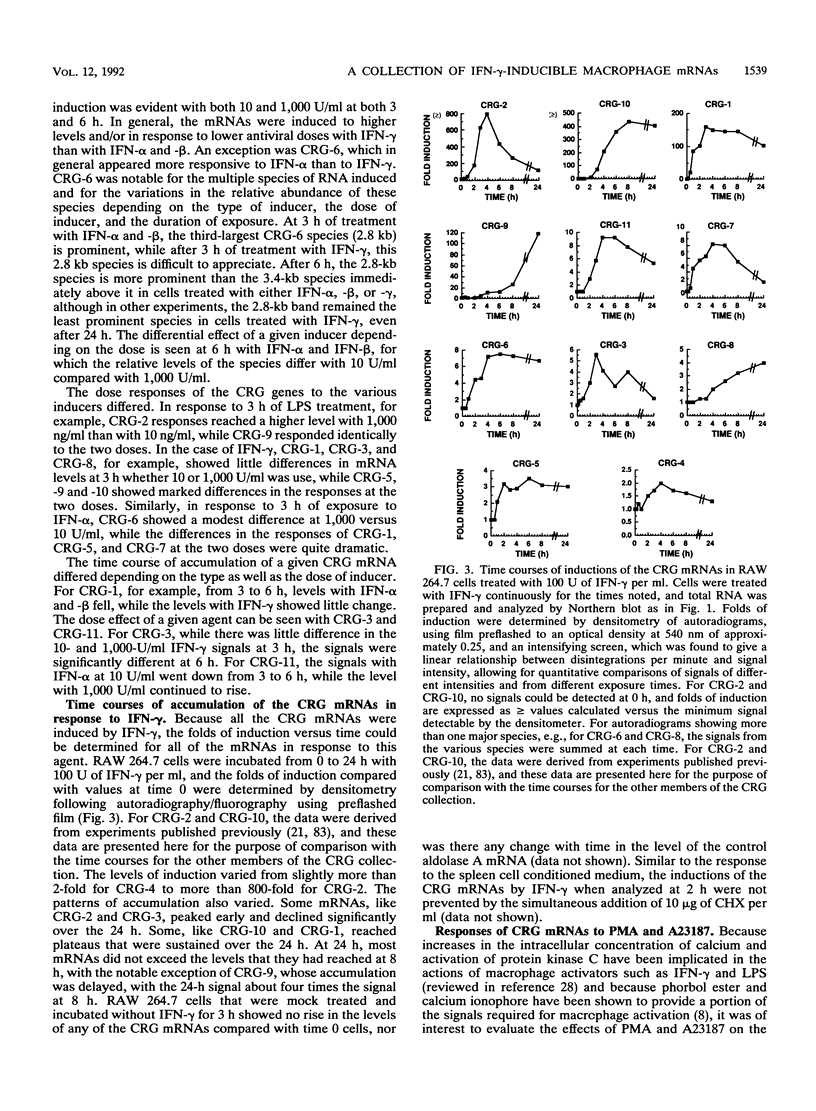
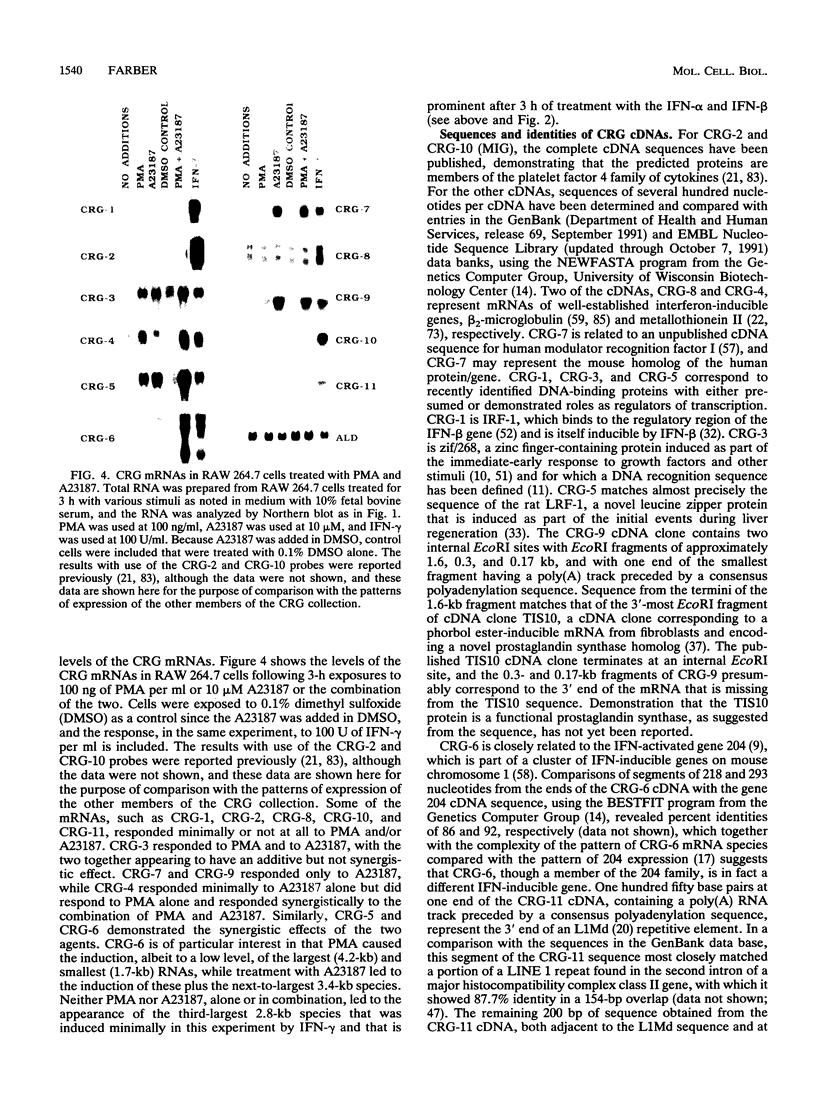
To identify genes induced during macrophage activation, a cDNA library was prepared from cultures of the RAW 264.7 mouse macrophage cell line that had been treated with conditioned medium from mitogen-stimulated spleen cells, and the cDNA library was screened by differential plaque hybridization. Eleven cDNA clones, designated CRG-1 through CRG-11, corresponding to mRNA species inducible in RAW 264.7 cells by the spleen cell conditioned medium, were isolated. Inductions were not blocked by cycloheximide. All of the mRNAs were inducible by gamma interferon, and some were also inducible by alpha and beta interferons, by lipopolysaccharide, by phorbol 12-myristate 13-acetate, and by the calcium ionophore A23187. Sequencing of the cDNAs revealed that CRG-1, CRG-3, and CRG-5 are cDNAs of recently identified transcription factors IRF-1, zif/268, and LRF-1 respectively. As previously reported, CRG-2 and CRG-10 (MIG) encode new members of the platelet factor 4 family of cytokines. CRG-6 corresponds to a new member of a family of interferon-inducible genes clustered on mouse chromosome 1, CRG-9 corresponds to a prostaglandin synthase homolog, CRG-8 corresponds to beta 2-microglobulin, and CRG-4 corresponds to metallothionein II. CRG-11 contains sequences of a truncated L1Md repetitive element as well as nonrepetitive sequences. The nonrepetitive sequence of CRG-11 as well as the sequences of CRG-7 are not closely related to published sequences. The CRG genes and proteins are of interest because of their involvement in macrophage activation, because of their roles as mediators of the effects of gamma interferon and other pleiotropic agents, and because of their usefulness as tools for studying the signal pathways through which gamma interferon and other inducers exert their effects on gene and protein expression.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Aguet M., Dembić Z., Merlin G. Molecular cloning and expression of the human interferon-gamma receptor. Cell. 1988 Oct 21;55(2):273–280. doi: 10.1016/0092-8674(88)90050-5. [DOI] [PubMed] [Google Scholar]
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- Arenander A. T., Lim R. W., Varnum B. C., Cole R., de Vellis J., Herschman H. R. TIS gene expression in cultured rat astrocytes: induction by mitogens and stellation agents. J Neurosci Res. 1989 Jul;23(3):247–256. doi: 10.1002/jnr.490230302. [DOI] [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Censini S., Bartalini M., Tagliabue A. Regulation of arachidonic acid metabolism in macrophages by immune and nonimmune interferons. J Immunol. 1985 Jul;135(1):502–505. [PubMed] [Google Scholar]
- Celada A., Schreiber R. D. Role of protein kinase C and intracellular calcium mobilization in the induction of macrophage tumoricidal activity by interferon-gamma. J Immunol. 1986 Oct 1;137(7):2373–2379. [PubMed] [Google Scholar]
- Choubey D., Snoddy J., Chaturvedi V., Toniato E., Opdenakker G., Thakur A., Samanta H., Engel D. A., Lengyel P. Interferons as gene activators. Indications for repeated gene duplication during the evolution of a cluster of interferon-activatable genes on murine chromosome 1. J Biol Chem. 1989 Oct 15;264(29):17182–17189. [PubMed] [Google Scholar]
- Christy B. A., Lau L. F., Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with "zinc finger" sequences. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy B., Nathans D. DNA binding site of the growth factor-inducible protein Zif268. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8737–8741. doi: 10.1073/pnas.86.22.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Pang R. H. Induction of unique mRNAs by human interferons. J Biol Chem. 1982 Aug 25;257(16):9234–9237. [PubMed] [Google Scholar]
- Dale T. C., Imam A. M., Kerr I. M., Stark G. R. Rapid activation by interferon alpha of a latent DNA-binding protein present in the cytoplasm of untreated cells. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1203–1207. doi: 10.1073/pnas.86.4.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley J. P. Discrete high molecular weight RNA transcribed from the long interspersed repetitive element L1Md. Nucleic Acids Res. 1987 Mar 25;15(6):2581–2592. doi: 10.1093/nar/15.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel D. A., Snoddy J., Toniato E., Lengyel P. Interferons as gene activators: close linkage of two interferon-activatable murine genes. Virology. 1988 Sep;166(1):24–29. doi: 10.1016/0042-6822(88)90142-0. [DOI] [PubMed] [Google Scholar]
- Faltynek C. R., Princler G. L., Gusella G. L., Varesio L., Radzioch D. A functional protein kinase C is required for induction of 2-5A synthetase by recombinant interferon-alpha A in Daudi cells. J Biol Chem. 1989 Aug 25;264(24):14305–14311. [PubMed] [Google Scholar]
- Fan X. D., Stark G. R., Bloom B. R. Molecular cloning of a gene selectively induced by gamma interferon from human macrophage cell line U937. Mol Cell Biol. 1989 May;9(5):1922–1928. doi: 10.1128/mcb.9.5.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G. Size and structure of the highly repetitive BAM HI element in mice. Nucleic Acids Res. 1983 Aug 11;11(15):5073–5091. doi: 10.1093/nar/11.15.5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M. A macrophage mRNA selectively induced by gamma-interferon encodes a member of the platelet factor 4 family of cytokines. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5238–5242. doi: 10.1073/pnas.87.14.5238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabstein K. H., Urdal D. L., Tushinski R. J., Mochizuki D. Y., Price V. L., Cantrell M. A., Gillis S., Conlon P. J. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986 Apr 25;232(4749):506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- Gupta M. P., Gupta M., Zak R., Sukhatme V. P. Egr-1, a serum-inducible zinc finger protein, regulates transcription of the rat cardiac alpha-myosin heavy chain gene. J Biol Chem. 1991 Jul 15;266(20):12813–12816. [PubMed] [Google Scholar]
- Hamilton T. A., Becton D. L., Somers S. D., Gray P. W., Adams D. O. Interferon-gamma modulates protein kinase C activity in murine peritoneal macrophages. J Biol Chem. 1985 Feb 10;260(3):1378–1381. [PubMed] [Google Scholar]
- Hamilton T. A., Bredon N., Ohmori Y., Tannenbaum C. S. IFN-gamma and IFN-beta independently stimulate the expression of lipopolysaccharide-inducible genes in murine peritoneal macrophages. J Immunol. 1989 Apr 1;142(7):2325–2331. [PubMed] [Google Scholar]
- Hamilton T. A., Somers S. D., Becton D. L., Celada A., Schreiber R. D., Adams D. O. Analysis of deficiencies in IFN-gamma-mediated priming for tumor cytotoxicity in peritoneal macrophages from A/J mice. J Immunol. 1986 Nov 15;137(10):3367–3371. [PubMed] [Google Scholar]
- Harada H., Fujita T., Miyamoto M., Kimura Y., Maruyama M., Furia A., Miyata T., Taniguchi T. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell. 1989 Aug 25;58(4):729–739. doi: 10.1016/0092-8674(89)90107-4. [DOI] [PubMed] [Google Scholar]
- Hsu J. C., Laz T., Mohn K. L., Taub R. Identification of LRF-1, a leucine-zipper protein that is rapidly and highly induced in regenerating liver. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3511–3515. doi: 10.1073/pnas.88.9.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler D. S., Veals S. A., Fu X. Y., Levy D. E. Interferon-alpha regulates nuclear translocation and DNA-binding affinity of ISGF3, a multimeric transcriptional activator. Genes Dev. 1990 Oct;4(10):1753–1765. doi: 10.1101/gad.4.10.1753. [DOI] [PubMed] [Google Scholar]
- Klein J. B., Schepers T. M., Dean W. L., Sonnenfeld G., McLeish K. R. Role of intracellular calcium concentration and protein kinase C activation in IFN-gamma stimulation of U937 cells. J Immunol. 1990 Jun 1;144(11):4305–4311. [PubMed] [Google Scholar]
- Kole L. B., Haynes S. R., Jelinek W. R. Discrete and heterogeneous high molecular weight RNAs complementary to a long dispersed repeat family (a possible transposon) of human DNA. J Mol Biol. 1983 Apr 5;165(2):257–286. doi: 10.1016/s0022-2836(83)80257-5. [DOI] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Kusari J., Tiwari R. K., Kumar R., Sen G. C. Expression of interferon-inducible genes in RD-114 cells. J Virol. 1987 May;61(5):1524–1531. doi: 10.1128/jvi.61.5.1524-1531.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner A. C., Chaudhuri A., Darnell J. E., Jr Transcriptional induction by interferon. New protein(s) determine the extent and length of the induction. J Biol Chem. 1986 Jan 5;261(1):453–459. [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Reich N., Darnell J. E., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988 Apr;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Levy D., Darnell J. E., Jr Interferon-dependent transcriptional activation: signal transduction without second messenger involvement? New Biol. 1990 Oct;2(10):923–928. [PubMed] [Google Scholar]
- Lew D. J., Decker T., Darnell J. E., Jr Alpha interferon and gamma interferon stimulate transcription of a single gene through different signal transduction pathways. Mol Cell Biol. 1989 Dec;9(12):5404–5411. doi: 10.1128/mcb.9.12.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R. W., Varnum B. C., Herschman H. R. Cloning of tetradecanoyl phorbol ester-induced 'primary response' sequences and their expression in density-arrested Swiss 3T3 cells and a TPA non-proliferative variant. Oncogene. 1987;1(3):263–270. [PubMed] [Google Scholar]
- Lin A. H., Bienkowski M. J., Gorman R. R. Regulation of prostaglandin H synthase mRNA levels and prostaglandin biosynthesis by platelet-derived growth factor. J Biol Chem. 1989 Oct 15;264(29):17379–17383. [PubMed] [Google Scholar]
- Luster A. D., Unkeless J. C., Ravetch J. V. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins. Nature. 1985 Jun 20;315(6021):672–676. doi: 10.1038/315672a0. [DOI] [PubMed] [Google Scholar]
- MacKay R. J., Russell S. W. Protein changes associated with stages of activation of mouse macrophages for tumor cell killing. J Immunol. 1986 Aug 15;137(4):1392–1398. [PubMed] [Google Scholar]
- Marcucci F., Klein B., Kirchner H., Zawatzky R. Production of high titers of interferon-gamma by prestimulated murine spleen cells. Eur J Immunol. 1982 Sep;12(9):787–790. doi: 10.1002/eji.1830120916. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Miyamoto M., Fujita T., Kimura Y., Maruyama M., Harada H., Sudo Y., Miyata T., Taniguchi T. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell. 1988 Sep 9;54(6):903–913. doi: 10.1016/s0092-8674(88)91307-4. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Manser T., Pearson G. D., Daley M. J., Gefter M. L. Effect of IFN-gamma on the immune response in vivo and on gene expression in vitro. 1984 Jan 26-Feb 1Nature. 307(5949):381–382. doi: 10.1038/307381a0. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols F. C., Garrison S. W. Interferon-gamma potentiation of lipopolysaccharide-induced eicosanoid release from human monocytes. J Interferon Res. 1987 Feb;7(1):121–129. doi: 10.1089/jir.1987.7.121. [DOI] [PubMed] [Google Scholar]
- Opdenakker G., Snoddy J., Choubey D., Toniato E., Pravtcheva D. D., Seldin M. F., Ruddle F. H., Lengyel P. Interferons as gene activators: a cluster of six interferon-activatable genes is linked to the erythroid alpha-spectrin locus on murine chromosome 1. Virology. 1989 Aug;171(2):568–578. doi: 10.1016/0042-6822(89)90626-0. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Prpic V., Yu S. F., Figueiredo F., Hollenbach P. W., Gawdi G., Herman B., Uhing R. J., Adams D. O. Role of Na+/H+ exchange by interferon-gamma in enhanced expression of JE and I-A beta genes. Science. 1989 Apr 28;244(4903):469–471. doi: 10.1126/science.2541500. [DOI] [PubMed] [Google Scholar]
- Raschke W. C., Baird S., Ralph P., Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978 Sep;15(1):261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Pfeffer L. M. Evidence for involvement of protein kinase C in the cellular response to interferon alpha. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8761–8765. doi: 10.1073/pnas.87.22.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa F., Hatat D., Abadie A., Wallach D., Revel M., Fellous M. Differential regulation of HLA-DR mRNAs and cell surface antigens by interferon. EMBO J. 1983;2(9):1585–1589. doi: 10.1002/j.1460-2075.1983.tb01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Gupta S. L. Differential efficacies of human type I and type II interferons as antiviral and antiproliferative agents. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5928–5932. doi: 10.1073/pnas.77.10.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders K. M., Littman B. H. Lymphokine stimulation of human macrophage C2 production is partially due to interferon-gamma. J Immunol. 1986 Aug 1;137(3):876–879. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneck J., Rager-Zisman B., Rosen O. M., Bloom B. R. Genetic analysis of the role of cAMP in mediating effects of interferon. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1879–1883. doi: 10.1073/pnas.79.6.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz R. M., Kleinschmidt W. J. Functional identity between murine gamma interferon and macrophage activating factor. Nature. 1983 Sep 15;305(5931):239–240. doi: 10.1038/305239a0. [DOI] [PubMed] [Google Scholar]
- Schumann R. R., Leong S. R., Flaggs G. W., Gray P. W., Wright S. D., Mathison J. C., Tobias P. S., Ulevitch R. J. Structure and function of lipopolysaccharide binding protein. Science. 1990 Sep 21;249(4975):1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- Searle P. F., Davison B. L., Stuart G. W., Wilkie T. M., Norstedt G., Palmiter R. D. Regulation, linkage, and sequence of mouse metallothionein I and II genes. Mol Cell Biol. 1984 Jul;4(7):1221–1230. doi: 10.1128/mcb.4.7.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Skowronski J., Singer M. F. Expression of a cytoplasmic LINE-1 transcript is regulated in a human teratocarcinoma cell line. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6050–6054. doi: 10.1073/pnas.82.18.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckle M. Y., Barker K. A. Two burgeoning families of platelet factor 4-related proteins: mediators of the inflammatory response. New Biol. 1990 Apr;2(4):313–323. [PubMed] [Google Scholar]
- Sukhatme V. P., Kartha S., Toback F. G., Taub R., Hoover R. G., Tsai-Morris C. H. A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Res. 1987 Sep-Oct;1(4):343–355. [PubMed] [Google Scholar]
- Tannenbaum C. S., Koerner T. J., Jansen M. M., Hamilton T. A. Characterization of lipopolysaccharide-induced macrophage gene expression. J Immunol. 1988 May 15;140(10):3640–3645. [PubMed] [Google Scholar]
- Thorens B., Mermod J. J., Vassalli P. Phagocytosis and inflammatory stimuli induce GM-CSF mRNA in macrophages through posttranscriptional regulation. Cell. 1987 Feb 27;48(4):671–679. doi: 10.1016/0092-8674(87)90245-5. [DOI] [PubMed] [Google Scholar]
- Ulker N., Samuel C. E. Mechanism of interferon action: inhibition of vesicular stomatitis virus replication in human amnion U cells by cloned human gamma-interferon. II. Effect on viral macromolecular synthesis. J Biol Chem. 1985 Apr 10;260(7):4324–4330. [PubMed] [Google Scholar]
- Uzé G., Lutfalla G., Gresser I. Genetic transfer of a functional human interferon alpha receptor into mouse cells: cloning and expression of its cDNA. Cell. 1990 Jan 26;60(2):225–234. doi: 10.1016/0092-8674(90)90738-z. [DOI] [PubMed] [Google Scholar]
- Vanguri P., Farber J. M. Identification of CRG-2. An interferon-inducible mRNA predicted to encode a murine monokine. J Biol Chem. 1990 Sep 5;265(25):15049–15057. [PubMed] [Google Scholar]
- Wahl S. M., Wahl L. M., McCarthy J. B., Chedid L., Mergenhagen S. E. Macrophage activation by mycobacterial water soluble compounds and synthetic muramyl dipeptide. J Immunol. 1979 Jun;122(6):2226–2231. [PubMed] [Google Scholar]
- Wallach D., Fellous M., Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982 Oct 28;299(5886):833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]
- Weiel J. E., Hamilton T. A., Adams D. O. LPS induces altered phosphate labeling of proteins in murine peritoneal macrophages. J Immunol. 1986 Apr 15;136(8):3012–3018. [PubMed] [Google Scholar]
- Weil J., Epstein C. J., Epstein L. B., Sedmak J. J., Sabran J. L., Grossberg S. E. A unique set of polypeptides is induced by gamma interferon in addition to those induced in common with alpha and beta interferons. Nature. 1983 Feb 3;301(5899):437–439. doi: 10.1038/301437a0. [DOI] [PubMed] [Google Scholar]
- Weinshank R. L., Luster A. D., Ravetch J. V. Function and regulation of a murine macrophage-specific IgG Fc receptor, Fc gamma R-alpha. J Exp Med. 1988 Jun 1;167(6):1909–1925. doi: 10.1084/jem.167.6.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Wright T. M., Farber J. M. 5' regulatory region of a novel cytokine gene mediates selective activation by interferon gamma. J Exp Med. 1991 Feb 1;173(2):417–422. doi: 10.1084/jem.173.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C., Sehgal P. B., Tamm I. Signal transduction pathways in the induction of 2',5'-oligoadenylate synthetase gene expression by interferon alpha/beta. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2243–2247. doi: 10.1073/pnas.86.7.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap W. H., Teo T. S., Tan Y. H. An early event in the interferon-induced transmembrane signaling process. Science. 1986 Oct 17;234(4774):355–358. doi: 10.1126/science.2429366. [DOI] [PubMed] [Google Scholar]