Abstract
Purpose: The α-to-β (α/β) ratio for prostate tumor is likely lower than that for the surrounding normal organs, such as rectum and bladder (∼3 Gy). As a result, hypofractionation is expected to improve the therapeutic ratio in prostate radiation therapy. However, with the use of fewer, larger fractions, the accuracy of treatment dose delivery becomes more influenced by the physical uncertainties resulting from motion and radiobiological uncertainties in the α/β ratio of the prostate tumor. The purpose of this study is to evaluate the impact of interfractional motion on treatment dose delivery within the likely range of the tumor α/β ratio.
Methods: Serial CT images acquired at simulation and daily treatment for three prostate patients were studied retrospectively. A conventional 3D-conformal proton plan was created for each patient, delivering 25 fractions of 2 Gy to ITV1 (internal target volume, expanded from the prostate and clinically involved seminal vesicles) followed by 14 fractions to ITV2 (expanded from the prostate). The plans were renormalized for a series of hypofractionated protocols of between five and 28 fractions. The fractional doses were computed on daily CT and were mapped onto simulation CT using deformable registration. In each course, the doses from the fractions with the lowest D97% of the ITV2 were summed to approximate the lower limit (worst case) of target coverage. The uncertainty in dose and coverage was estimated as the deviation of the worst case from the nominal plan.
Results: For treatments in 28 to five fractions, the uncertainty arising from interfractional motion ranged from ∼1% to 4% for V100% and ∼2% to 6% for D100% of the ITV2. The uncertainties in V95% and D95% were both minimal (<1%) for all protocols. For tumors with a low α/β of 1.0 Gy, the treatment in five fractions could deliver an additional 21.0 and 17.4 GyEQD2 to 95% and 100% of the ITV2, respectively, compared to that in 28 fractions. This advantage disappeared for tumors with α/β > 2.5 Gy, assuming the worst case for interfractional motion.
Conclusions: In hypofractionated proton therapy for prostate cancer, the dosimetric uncertainties due to interfractional motion were minimal for the ITV2 coverage at 95% isodose level and the dose received by 95% of the ITV2. Although hypofractionation could yield an increase in equivalent dose to the target for tumors with low α/β, the gain was cancelled out by the uncertainty due to interfractional motion for tumors with α/β > 2.5 Gy.
Keywords: prostate cancer, proton therapy, hypofractionation, interfractional motion, α/β ratio, dose delivery uncertainty
INTRODUCTION
Radiation therapy for prostate cancer is conventionally delivered in 1.8- or 2.0-Gy fractions. With these standard dose fractionations, the delivery of a curative dose typically spans 8–9 weeks. Unlike most solid tumors which have a high α-to-β (α/β) ratio similar to early responding normal organs (∼10 Gy), the prostate cancer may have an α/β ratio even lower than late-responding normal organs such as rectum and bladder (∼3 Gy).1, 2 Based on the analysis of numerous clinical tumor control data, Ritter suggested a low α/β ratio for the prostate tumor of the order of 1–3 Gy.2 This unique radiobiological characteristic has motivated numerous clinical trials on hypofractionation,1, 2, 3, 4, 5, 6, 7, 8 aiming to achieve more efficient elimination of cancer cells while maintaining the similar risk of toxicity to the surrounding normal organs (e.g., rectum and bladder). Despite the radiobiological advantage, the clinical adoption of hypofractionation has been challenged by a number of issues, among which motion management is one of the most important. On the one hand, the increasing fractional dose usually requires a longer treatment time, allowing for a larger intrafractional motion, which may require an increased internal margin.9, 10 On the other hand, the reduced number of fractions magnifies the impact of any fractional dose deviations resulting from interfractional organ motion and setup uncertainty. Due to the scarcity of imaging data describing the daily variations in patient's anatomy, the effect of interfractional motion has not been sufficiently evaluated to date.
Using serial CT images acquired at simulation and daily treatment, this study aimed to quantify the dose delivery uncertainties arising from interfractional motion for various hypofractionated protocols evaluated in various clinical trials.3, 4, 5, 6, 7, 8 Furthermore, the dose uncertainties were evaluated for a range of most likely values of α/β ratio for the prostate tumor (1–3 Gy).
METHODS AND MATERIALS
Treatment planning
This study used simulation and daily serial CT images from three patients.11 The simulation CT was obtained using a Siemens CT simulator, and the daily CT was acquired using a CT-on-rail system employing a Siemens CT scanner. The axial resolution of the CT images was approximately 1 mm and the slice thickness was 5 mm. The prostate, seminal vesicles, bladder, and anterior rectal wall were manually contoured for each fraction. The gross tumor volume (GTV) included the prostate gland, and the clinical target volume (CTV) contained the GTV and the clinically involved seminal vesicles (1 cm superior to the prostate). An internal margin of 5 mm was used to account for intrafractional motion. The internal target volume (ITV) expanded from CTV and GTV would be referred to as ITV1 and ITV2, respectively.
A standard-fractionation 3D-conformal proton plan was created for each patient, using a commercial treatment planning system-–XiO Proton (CMS, St. Louis, MO). The treatment consisted of a full-field course of 25 fractions to ITV1, followed by a boost of 14 fractions to ITV2. Each fraction delivered 2 Gy by a pair of opposed lateral beams. Here and below, Gy refers to the dose corrected to reflect the higher relative radiobiological effectiveness (RBE) of protons, i.e., Gy(RBE), assuming the RBE factor of 1.1. The full-field beams are designed to assure that the ITV1 gets its full prescription dose of 50 Gy in the first 25 fractions of the course. This objective is typically easily achieved. The additional contributions from the boost course are not considered when planning the full-field course. As a result, the dosimetric evaluation on ITV1 is of little practical value given the large spill-over from the boost course. The impact of interfractional motion was only assessed for the ITV2. The range compensator was smeared by 10 mm to prevent underdosing due to the change of proton range resulting from the misalignment, in daily setup, between the bones and the prostate. The spread-out Bragg peak (SOBP) of the proton fields was expanded by 3.5% to account for the proton range uncertainty, which mainly arises from the uncertainty in the conversion of CT number to proton stopping power.12 The range compensator smearing and the expansion of the SOBP are both essential for producing clinically meaningful plans with the current technology, the result is that the treated volume extends beyond the target volume both distally and proximally, which mitigates the impact of the lateral motion of the target volume. Our previous report showed that the most significant motion of the prostate observed in these datasets was caused by the variation of the filling status of the bladder and the rectum, manifested as AP and SI shifts, as well as the gland deformation and its rotation around the lateral axis.13
Hypofractionated delivery protocols
Since the late 1990s, the safety and effectiveness of hypofractionated photon therapy for prostate cancer have been investigated in numerous clinical trials using between five and 28 fractions.3, 4, 5, 6, 7, 8 A large-scale, prospective clinical trial involving proton therapy has been initiated by the RTOG.14 While many photon trials only treated the low-risk prostate tumor to the full dose in one course, it was our intention to consider the two-stage courses, per current practice in proton therapy for low- and intermediate-risk patients, by also treating the clinically involved seminal vesicles to a dose lower than the gland. Table 1 summarizes the hypothetical proton delivery protocols evaluated in this study. The biological equivalent dose delivered by different fractionation schemes was evaluated using the concept of equivalent dose in 2-Gy fractions (EQD2), calculated by5
| (1) |
where n is the number of fractions and d is the fraction size (Gy/fraction). In Table 1, the α/β ratio was assumed to be 1.5 Gy for the prostate tumor and 3.0 Gy for the rectum. The fraction size was selected to maintain the maximum EQD2 received by the rectum constant at 78 GyEQD2. These fraction sizes were within 5% of those previously reported in the various clinical trials.3, 4, 5, 6, 7, 8
Table 1.
Summary of the standard (39 fractions) and hypofractionated proton delivery protocols. The equivalent doses were estimated assuming α/β = 1.5 Gy for the prostate tumor and 3.0 Gy for the rectum.
| No. of fractions | 39 | 28 | 26 | 23 | 22 | 20 | 16 | 12 | 7 | 5 |
| No. of full-field fractions | 25 | 18 | 17 | 15 | 14 | 13 | 10 | 8 | 4 | 3 |
| No. of boost fractions | 14 | 10 | 9 | 8 | 8 | 7 | 6 | 4 | 3 | 2 |
| Fraction size (Gy/fx) | 2.00 | 2.52 | 2.65 | 2.88 | 2.97 | 3.16 | 3.66 | 4.39 | 6.11 | 7.46 |
| Prescription dose (Gy) | 78.0 | 70.6 | 69.0 | 66.3 | 65.3 | 63.3 | 58.6 | 52.7 | 42.8 | 37.3 |
| Max rectal dose (GyEQD2) | 78.0 | 78.0 | 78.0 | 78.0 | 78.0 | 78.0 | 78.0 | 78.0 | 78.0 | 78.0 |
| Tumor dose (GyEQD2) | 78.0 | 81.2 | 81.9 | 83.0 | 83.4 | 84.3 | 86.3 | 88.8 | 93.1 | 95.4 |
Deformable dose accumulation
The beam fluence and hardware designed for the original plan were applied to each daily CT for fractional dose calculation in XiO. The isocenter of the proton beams was placed at the center of the GTV. The daily dose was registered to the simulation CT using a publicly available software—Plastimatch.15, 16 The accuracy of the deformable registration for these three patients has been validated in our previous report.13 Each patient had at least 33 fractions with a dose deviation of less than 3% (evaluated by comparing the D97% for the ITV2 calculated before and after the deformable registration). To facilitate direct comparison with the standard fractionation, the hypofractionated treatments were delivered in two courses with a similar ratio of roughly 25:14 for full-field to boost fractions. From each course of the standard treatment, the fractions with the lowest D97% for the ITV2 were summed to represent the worst-case scenario for accumulated target dose in a hypofractionated treatment. For example, for the protocol with 28 fractions, the 18 fractions with the lowest target dose in the full-field course of the standard 2-Gy-per-fraction treatment were renormalized to the corresponding fraction size (2.52 Gy) and summed. Similarly, the 10 fractions with the lowest target dose in the boost course were renormalized and summed. The summed dose from the full-field course (45.36 Gy) was renormalized to 64% (= 25/39), and that from the boost course (25.20 Gy) to 36%, of the prescription dose (70.56 Gy). The normalized doses from the two courses were summed to yield the accumulated dose.
RESULTS
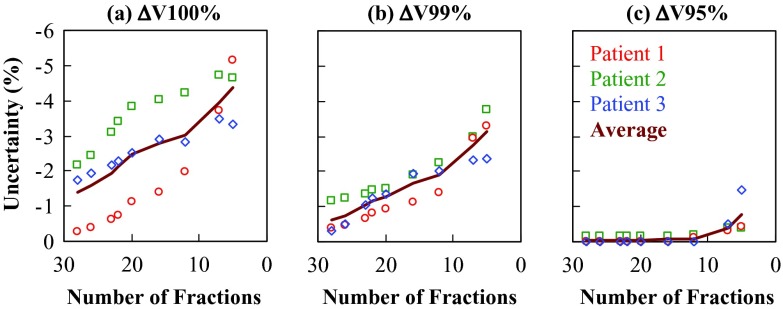
Figure 1 shows the uncertainty in ITV2 coverage, expressed in ΔV100%, ΔV99%, and ΔV95%, as a function of the number of fractions in a hypofractionated protocol. The uncertainty represents the maximum deficit of target coverage (i.e., the minimum delivered value minus the planned value). The magnitude of the uncertainty generally increased in treatments using fewer fractions. On average, when the treatment was shortened from 28 to five fractions, the magnitude of ΔV100% increased from 1.4% to 4.4%, of ΔV99% from 0.6% to 3.1%, and of ΔV95% from 0.05% to 0.8%. The uncertainties for patient 1 were generally the lowest at 28 fractions, but increased most rapidly as the number of fractions decreased, suggesting a more random distribution of underdosing from fraction to fraction. In contrast, the uncertainties for patients 2 and 3 were higher at 28 fractions, but the rate of increase was generally less significant, suggesting a large contribution from systematic dosimetric difference between the simulation and the treatment.
Figure 1.
The uncertainty in target coverage, expressed in (a) ΔV100%, (b) ΔV99%, and (c) ΔV95% of the ITV2, as a function of the number of fractions in a hypofractionated protocol. The solid line shows the averaged results over the three patients.
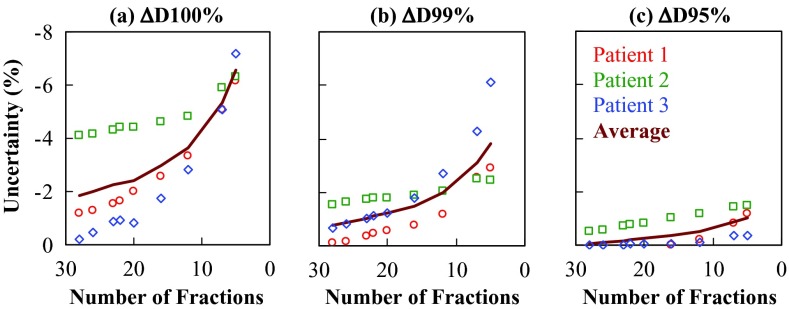
Figure 2 shows the uncertainty in the dose received by the ITV2, expressed in ΔD100%, ΔD99%, and ΔD95%, as a function of the number of fractions. On average, when the treatment was shortened from 28 to five fractions, the magnitude of ΔD100% increased from 1.8% to 6.6%, of ΔV99% from 0.8% to 3.8%, and of ΔV95% from 0.1% to 1.0%. The uncertainty in D100% was more than 5% for all three patients when treated in seven and five fractions. The results shown in Figs. 12 suggested that the impact of interfractional motion was minimal for the 95% isodose coverage (V95%) and the dose received by 95% (D95%) of the ITV2.
Figure 2.
The uncertainty in isodose received by the target, expressed in (a) ΔD100%, (b) ΔD99%, and (c) ΔD95% of the ITV2, as a function of the number of fractions in a hypofractionated protocol. The solid line shows the averaged results over the three patients.
The results shown in Figs. 12 are independent of the α/β ratio of the prostate tumor. Based on the results averaged among the three patients (displayed as solid lines in Fig. 2), Table 2 shows how the variations in tumor α/β ratio affected the minimum D100%, D99%, and D95% of the ITV2 (calculated using the fractions with the lowest D97% of the ITV2 as described in Sec. 2C.). The dosimetric advantage of hypofractionation decreased with increasing tumor α/β ratio. At α/β = 1.0 Gy, the shortening of the treatment from 28 to five fractions could result in an increase of 17.4 GyEQD2 for D100% and 21.0 GyEQD2 for D95%. The gain was reduced to 10.2 and 13.2 GyEQD2 at α/β = 1.5 Gy, and to 4.8 and 7.3 GyEQD2 at α/β = 2.0 Gy. Thus, the size of the estimated gain in GyEQD2 decreased for higher α/β. For prostate tumors with α/β > 2.5 Gy, hypofractionation may not provide any substantial gain on target equivalent dose as a result of the uncertainty due to interfractional motion. The dose gradient in the peripheral 1% (D99%–D100%) and 5% (D95%–D100%) of the ITV2 is steeper for tumor with lower α/β and treatment in fewer fractions. At α/β = 1.0 Gy, the gradient was 5.4 and 8.2 GyEQD2 for the peripheral 1% and 5% of the ITV2, respectively, for the treatment in five fractions.
Table 2.
The minimum (a) D100%, (b) D99%, and (c) D95% of the ITV2 (in GyEQD2) as a function of the α/β ratio (in Gy) of the prostate tumor. The data were shown for the hypofractionated protocols with 28 (bold), 20 (italic), 12 (underlined), and 5 (bold italic) fractions.
| D100% |
D99% |
D95% |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α/β | 28 fx | 20 fx | 12 fx | 5 fx | 28 fx | 20 fx | 12 fx | 5 fx | 28 fx | 20 fx | 12 fx | 5 fx |
| 1.0 | 78.9 | 83.1 | 88.9 | 96.3 | 81.7 | 86.2 | 92.7 | 101.7 | 83.5 | 88.2 | 94.9 | 104.5 |
| 1.5 | 77.2 | 79.8 | 83.3 | 87.4 | 80.0 | 82.8 | 86.8 | 92.3 | 81.7 | 84.7 | 88.9 | 94.9 |
| 2.0 | 76.0 | 77.3 | 79.0 | 80.8 | 78.7 | 80.2 | 82.4 | 85.3 | 80.4 | 82.0 | 84.4 | 87.7 |
| 2.5 | 75.0 | 75.3 | 75.8 | 75.6 | 77.7 | 78.2 | 79.0 | 79.8 | 79.4 | 80.0 | 80.9 | 82.1 |
| 3.0 | 74.2 | 73.8 | 73.1 | 71.4 | 76.9 | 76.6 | 76.2 | 75.4 | 78.5 | 78.3 | 78.1 | 77.6 |
DISCUSSION
This study used serial CT images acquired prior to each fraction to reveal the impact of interfractional motion on target dose delivery in hypofractionated proton therapy for prostate cancer. The results suggested that the uncertainty resulting from the interfractional motion was minimal for the V95% and D95% of the ITV2. Sharp dose shortfall (up to ∼8 GyEQD2) only existed in the peripheral 5% of the ITV2. If the range of internal motion is indeed within 5 mm, less than 5% of GTV could appear in the peripheral 5% of the ITV2 with sharp dose gradients during all treatment sessions. Moreover, the underdosing resulting from the dose gradients would be spatially smeared by motion. As a result, the dosimetric impact of interfractional motion is also expected to be minimal for at least 95% of the GTV for hypofractionated treatments delivered in more than five fractions.
While many proton clinics, including ours, use orthogonal x-ray images and fiducial markers to setup prostate patients, this practice could not be simulated using the available imaging data which did not include fiducial makers. Alternatively, the target dose uncertainty was assessed based on in-room CT imaging, which has not been widely available in existing proton centers, but has become a very popular option for new constructions. Therefore, it is reasonable to expect that the results from this study would provide important guidance in selecting an optimal fractionation schemes for prostate proton therapy.
CONCLUSIONS
In hypofractionated prostate proton therapy, the impact of interfractional motion was found minimal for the central 95% of the target and only resulted in large dosimetric uncertainty in the peripheral 5% of the target. Although hypofractionation could substantially increase the equivalent dose to the target for tumors with low α/β ratios, this advantage diminished for tumors with α/β > 2.5 Gy assuming the worst case scenario for interfractional motion.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to James R. Wong, M.D., and Scott Merrick (Morristown Memorial Hospital, Morristown, NJ) for providing the patient data, and Dr. I. Frank Ciernik for his help with data processing. This study was supported by the Federal Share of program income earned by the Massachusetts General Hospital on C06-CA059267, Proton Therapy Research and Treatment Center.
References
- Miles E. F. and Lee W. R., “Hypofractionation for prostate cancer: A critical review,” Semin. Radiat. Oncol. 18, 41–47 (2008). 10.1016/j.semradonc.2007.09.006 [DOI] [PubMed] [Google Scholar]
- Ritter M., “Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer,” Semin. Radiat. Oncol. 18, 249–256 (2008). 10.1016/j.semradonc.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji H., Yanagi T., Ishikawa H., Kamada T., Mizoe J. E., Kanai T., Morita S., and Tsujii H., “Hypofractionated radiotherapy with carbon ion beams for prostate cancer,” Int. J. Radiat. Oncol., Biol., Phys. 63, 1153–1160 (2005). 10.1016/j.ijrobp.2005.04.022 [DOI] [PubMed] [Google Scholar]
- Soete G., Arcangeli S., De Meerleer G., Landoni V., Fonteyne V., Arcangeli G., De Neve W., and Storme G., “Phase II study of a four-week hypofractionated external beam radiotherapy regimen for prostate cancer: Report on acute toxicity,” Radiother. Oncol. 80, 78–81 (2006). 10.1016/j.radonc.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Ritter M. A., Forman J. D., Petereit D. G., Kupelian P., Wang D., Walker W., Fowler J., Chappell R., and Tame W., “Dose-per-fraction escalation for localized prostate cancer: A multi-institutional phase I/II trial,” Int. J. Radiat. Oncol., Biol., Phys. 66, S11 (2006). 10.1016/j.ijrobp.2006.07.041 [DOI] [Google Scholar]
- Pollack A., Hanlon A. L., Horwitz E. M., Feigenberg S. J., Konski A. A., Movsas B., Greenberg R. E., Uzzo R. G., Ma C. M., McNeeley S. W., Buyyounouski M. K., and R. A.Price, Jr., “Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial,” Int. J. Radiat. Oncol., Biol., Phys. 64, 518–526 (2006). 10.1016/j.ijrobp.2005.07.970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen B. L., His R. A., Pham H. T., Fowler J. F., Esagui L., and Corman J., “Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.4 Gy in five fractions for localized disease: First clinical trial results,” Int. J. Radiat. Oncol., Biol., Phys. 67, 1099–1105 (2007). 10.1016/j.ijrobp.2006.10.050 [DOI] [PubMed] [Google Scholar]
- Martin J. M., Rosewall T., Bayley A., Bristow R., Chung P., Crook J., Gospodarowicz M., McLean M., Ménard C., Milosevic M., Warde P., and Catton C., “Phase II trial of hypofractionated image-guided intensity-modulated radiotherapy for localized prostate adenocarcinoma,” Int. J. Radiat. Oncol., Biol., Phys. 69, 1084–1089 (2007). 10.1016/j.ijrobp.2007.04.049 [DOI] [PubMed] [Google Scholar]
- Xie Y., Djajaputra D., King C. R., Hossain S., Ma L., and Xing L., “Intrafractional motion of the prostate during hypofractionated radiotherapy,” Int. J. Radiat. Oncol., Biol., Phys. 72, 236–246 (2008). 10.1016/j.ijrobp.2008.04.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson J., Wu Q., and Yan D., “Dosimetric effect of intrafraction motion and residual setup error for hypofractionated prostate intensity-modulated radiotherapy with online cone beam computed tomography image guidance,” Int. J. Radiat. Oncol., Biol., Phys. 80, 453–61 (2011). 10.1016/j.ijrobp.2010.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofimov A. V., Nguyen P. L., Efstathiou J. A., Wang Y., Lu H., Engelsman M., Merrick S., Cheng C., Wong J. R., and Zietman A. L., “Interfractional variations in the setup of pelvic bony anatomy and soft tissue, and their implications on the delivery of proton therapy for localized prostate cancer,” Int. J. Radiat. Oncol., Biol., Phys. 80, 928–937 (2011). 10.1016/j.ijrobp.2010.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trofimov A. V., Nguyen P. L., Coen J. J., Doppke K. P., Schneider R. J., Adams J. A., Bortfeld T. R., Zietman A. L., DeLaney T. F., and Shipley W. U., “Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: A treatment planning study,” Int. J. Radiat. Oncol., Biol., Phys. 69, 444–453 (2007). 10.1016/j.ijrobp.2007.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Efstathiou J. A., Sharp G. C., Lu H., Ciernik I. F., and Trofimov A. V., “Evaluation of the dosimetric impact of interfractional anatomical variations on prostate proton therapy using daily in-room CT images,” Med. Phys. 38, 4623–4633 (2011). 10.1118/1.3604152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukka H., Bahary J., Lawton C., Efstathiou J. A., Bruner D. W., Kudchadker R. J., Ponsky L. E., and Shook S., “A randomized phase ii trial of hypofractionated radiotherapy for favorable risk prostate cancer,” RTOG Protocol 0938, Version September 1, 2011.
- See http://plastimatch.org.
- Wu Z., Rietzel E., Boldea V., Sarrut D., and Sharp G. C., “Evaluation of deformable registration of patient lung 4DCT with subanatomical region segmentations,” Med. Phys. 35, 775–781 (2008). 10.1118/1.2828378 [DOI] [PubMed] [Google Scholar]