Abstract
There is increasing evidence that genomic imprinting, a process by which certain genes are expressed in a parent-of-origin-specific manner, can influence neurogenetic and psychiatric manifestations. While some data suggest possible imprinting effects of the X chromosome on physical and cognitive characteristics in humans, there is no compelling evidence that X-linked imprinting affects brain morphology. To address this issue, we investigated regional cortical volume, thickness, and surface area in 27 healthy controls and 40 prepubescent girls with Turner syndrome (TS), a condition caused by the absence of one X chromosome. Of the young girls with TS, 23 inherited their X chromosome from their mother (Xm) and 17 from their father (Xp). Our results confirm the existence of significant differences in brain morphology between girls with TS and controls, and reveal the presence of a putative imprinting effect among the TS groups: girls with Xp demonstrated thicker cortex than those with Xm in the temporal regions bilaterally, while Xm individuals showed bilateral enlargement of gray matter volume in the superior frontal regions compared with Xp. These data suggest the existence of imprinting effects of the X chromosome that influence both cortical thickness and volume during early brain development, and help to explain variability in cognitive and behavioral manifestations of TS with regard to the parental origin of the X chromosome.
Introduction
Genomic imprinting is a process by which genes are preferentially expressed depending on their parental origin. Although the number of genes subjected to genomic imprinting effects is likely limited (<1%; Tycko and Morison, 2002), they seem to have a significant impact on physiology and behavior, as many are expressed predominantly in the brain (Davies, 2010) and a subset have been implicated in the etiology of neuropsychiatric disorders (Wilkinson et al., 2007). Sex chromosomes are an interesting case of genomic imprinting, as expression of imprinted genes on the X chromosome may occur in a sex-specific manner and contribute to sexual dimorphism (Davies, 2010; Gregg et al., 2010). Animal models have revealed the existence of a cluster of imprinted genes on the X chromosome (Raefski and O'Neill, 2005), and recent evidence suggests that a number of genes are expressed in a sex-specific manner in the mammalian brain (Gregg et al., 2010; DeVeale et al., 2012).
In humans, evidence in favor of genomic imprinting effects on the X chromosome comes mainly from studies of sex chromosomal aneuploidies such as Turner syndrome (TS), a disorder caused by the absence of one copy of the X chromosome in females. Given that individuals with TS carry only one X chromosome in each cell, the typical process of random inactivation of one sex chromosome is bypassed, resulting in an exclusive pattern of either paternally (Xp) or maternally (Xm) inherited X chromosomes. To date, a number of studies have suggested the existence of a parent-of-origin effect of the X chromosome on cognitive (Skuse et al., 1997; Loesch et al., 2005; Lepage et al., 2012a,b) and physical (Chu et al., 1994; Van et al., 2006; Sagi et al., 2007) characteristics in TS. A few studies have also looked at the effect of genomic imprinting on brain morphology in TS with conflicting results (Brown et al., 2002; Good et al., 2003; Kesler et al., 2003, 2004; Cutter et al., 2006). While some have reported the existence of genomic imprinting effect on brain structure in the temporal lobe (Kesler et al., 2003; Cutter et al., 2006) and caudate nucleus (Cutter et al., 2006), others found no clear effects of imprinting (Brown et al., 2002; Good et al., 2003; Kesler et al., 2004).
These inconsistencies may be in part due to small sample sizes (Mullaney and Murphy, 2009) and heterogeneity of the TS sample regarding exogenous estrogen treatment and age. Moreover, given that previous imaging studies relied on voxel-based morphometry and manual segmentation to measure cortical volume, the potential influence of genomic imprinting on cortical morphology in terms of surface area and cortical thickness, which have distinct genetic etiologies (Panizzon et al., 2009), has been overlooked. Here, we used surface-based analysis (SBA) to assess the impact of genomic imprinting on cortical volume, thickness and surface area in a large, homogenous cohort of young girls with complete X monosomy who have never been exposed to exogenous estrogen therapy and age-matched controls.
Materials and Methods
Participants.
TS participants were recruited through the national Turner Syndrome Society, the Turner Syndrome Foundation, a local network of physicians, and advertisement on the Stanford University School of Medicine website. Control participants were recruited through local print media and parent networks. All participants were screened for magnetic resonance imaging (MRI) contraindications as well as past medical history to ensure that there were no instances of premature birth (i.e., <34 weeks gestation), neurological injury, psychiatric illness, or disease, except those commonly associated with TS. Only individuals with TS showing complete X monosomy were included in the present study; we excluded subjects with verbal IQ outside of the normal range (70–130). Controls were included if they presented a full-scale IQ (FSIQ) within the normal limit. The local Institutional Review Board at the Stanford University School of Medicine approved this study and informed written consent was obtained from the legal guardian for all participants.
Twenty-seven controls (mean age: 8.17, SD: 2.93), and 40 girls with TS met the inclusion criteria for the study. Among the participants with TS, 23 had an Xm (mean age: 7.93, SD 2.63), and 17 Xp (mean age: 9.25, SD 2.61). Thirty-four individuals with TS were taking growth hormone at the time of testing (20 Xm, 14 Xp; Pearson's χ2, p = 0.339, n.s.) and none were receiving exogenous estrogen treatment. The sample used in the present study overlaps with previously published work (Lepage et al., 2012a,b). The population characteristics are summarized in Table 1.
Table 1.
Participants' characteristics and whole brain measures
| Control (n = 27) | Turner |
||
|---|---|---|---|
| Xm (n = 23) | Xp (n = 17) | ||
| Demographics | |||
| Age | 8.17 (2.93) | 7.93 (2.63) | 9.25 (2.61) |
| Growth hormone | — | 20 (87%) | 14 (76%) |
| Cognitive measures | |||
| FSIQabc | 113.63 (8.57) | 97.09 (14.05) | 88.88 (12.46) |
| PRI/PIQabc | 114.19 (11.78) | 97.48 (15.10) | 86.12 (13.16) |
| VCI/VIQab | 113.63 (13.33) | 105.00 (12.13) | 99.76 (14.36) |
| WMIab | 102.16 (10.66) | 86.19 (13.30) | 87.13 (13.55) |
| PSI/PSQab | 108.84 (10.65) | 92.48 (12.82) | 88.11 (13.27) |
| Whole- brain measures | |||
| Total brain volume | 1029.76 (98.29) | 1053.21 (82.02) | 1004.51 (109.81) |
| Total GMV | 593.24 (47.77) | 616.34 (53.08) | 579.56 (44.85) |
| Total WMV | 436.98 (32.65) | 425.98 (36.15) | 429.67 (44.50) |
| Total surface areab | 1716.72 (187.9) | 1734.29 (156.26) | 1616.91 (170.98) |
| Mean cortical thickness | 2.77 (0.12) | 2.79 (0.13) | 2.82 (0.11) |
Xm, X chromosome from maternal origin; Xp, paternal origin. FSIQ, Full-Scale Intelligence Quotient; PRI/PIQ, Performance Intelligence Quotient; VCI/VIQ, Verbal Intelligence Quotient. Significant post hoc difference (p < 0.05) between groups are indicated by letters in superscript:
aControls versus Xm,
bControls versus Xp, and
cXm versus Xp. Pearson χ2 was performed on growth hormone usage (p = 0.339, n.s.). ANCOVAs were used to assess between-group differences in total brain volume (age as covariate), total surface area, and mean cortical thickness (age and total brain volume as covariates). Volumes are expressed in cm3, area in cm2, and thickness in mm. Numbers represent mean values with standard deviation in parentheses.
Genetic analysis.
X monosomy was established through standard karyotype analysis of at least 20 cells, which allows exclusion of 11% mosaicism or greater with 0.90 confidence (Hook, 1977). Parental origin of the X chromosome was determined by comparison of amplification patterns of four polymorphic markers located exclusively on the X chromosome (DXS6807 at 14 cM, DXS993 at 42 cM, DXS1106 at 67cM, and the Androgen receptor at 90cM) and one marker in the pseudo-autosomal region (Amelogenin) between the proband and mother.
Cognitive assessment.
Participants were administered cognitive assessments appropriate for their age: the Wechsler Preschool and Primary Scale of Intelligence,Third Edition (WPPSI-III) was administered for children younger than 5years old; and the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) for girls older than 6 years of age. These tests measured participants' FSIQ, Verbal Comprehension Index/Verbal IQ (VCI/VIQ), Perceptual Reasoning Index/Performance IQ (PRI/PIQ), and Processing Speed Index/Quotient (PSI/PSQ), as well as the Working Memory Index (WMI) for participants who took the WISC-IV.
MR acquisition.
All imaging data were acquired at the Stanford University Lucas Center for Medical Imaging. MRI images of the young cohort were collected between 2006 and 2011 on a GE Signa HDxt 3.0 T whole-body MR system (GE Medical Systems) using a standard birdcage head coil. A fast spoiled gradient recalled echo pulse sequence was used to obtain a high-resolution T1 anatomical brain image of each subject (124 coronal slices, repetition time [TR]/echo time [TE] = 6.4/2.1 ms, inversion time [TI] = 300 ms, flip angle = 15°, NEX = 3, FOV = 22 × 22 cm, matrix = 256 × 256, 1.5 mm thickness, acquisition time = 14 min 43 s).
Morphometric analysis.
MRI data were first visually inspected to eliminate scans with significant head motion or flow artifacts. All scans were preprocessed using bias field correction methods available with SPM8 before processing with the FreeSurfer SBA pipeline. Cortical reconstruction and volumetric segmentation was performed with the FreeSurfer version 5.0 image analysis suite. The technical details of the procedures used are extensively described in prior publications (Dale et al., 1999; Fischl and Dale, 2000; Fischl et al., 2004). Briefly, this processing includes removal of nonbrain tissue, segmentation of subcortical white matter and deep gray matter volumetric structures, intensity normalization, and tessellation of the gray matter–white matter boundary. The gray-white and pial surfaces were visually inspected, and where needed, appropriate manual corrections were performed. All raters were trained to achieve inter-rater reliability of ≥0.95 (intraclass correlation coefficient) with gold-standard datasets developed in our laboratory for volumetric regions of interest. Once cortical models were complete, brain surfaces for each hemisphere were parcellated into 34 distinct regions, for which FreeSurfer calculates gray matter volume (GMV), surface area of the gray–white boundary, mean cortical thickness, and white matter volume (WMV) (Fischl et al., 2004; Desikan et al., 2006). Procedures for measurement of cortical thickness have been validated against histological analysis (Rosas et al., 2002) and manual measurements (Salat et al., 2004). The FreeSurfer image processing pipeline has been shown to be accurate for children as young as four years of age (Phillips et al., 2011).
Statistical analysis.
For demographic and cognitive data, one-way ANOVAs were used to assess differences between groups (Xm, Xp, controls). For structural data, whole-brain characteristics including total cortical GMV, subcortical GMV, total WMV, total surface area, mean cortical thickness, and cerebellar volume were investigated using ANCOVA with age and total brain volume as covariates. Total brain volume was defined as total gray and white matter tissue within the cerebrum to exclude the larger ventricles sometimes observed in the TS population (Marzelli et al., 2011). Results of the individual cortical regions pertaining to GMV, WMV, surface area, and cortical thickness were investigated using ANCOVAs with group as a fixed factor, and age, total brain volume, and FSIQ as covariates. The same model was used to analyze the 16 subcortical structures defined in the FreeSurfer atlas. Multiple comparisons were controlled using the false discovery rate (FDR; Storey, 2002) applied independently for each measure (GMV, WMV, surface area, cortical thickness, subcortical structures) on the group factor term. Results were considered statistically significant if they passed the FDR threshold q < 0.05 for multiple comparisons. If this occurred, two-group comparisons were further investigated with Bonferroni corrected post hoc t tests.
Considering that global size is the most consistent difference found between males and females regarding brain anatomy (Paus, 2010), the potential contribution of genomic imprinting effects to sexual dimorphism of the brain was examined with exploratory trend analyses conducted on whole-brain measures after controlling for age. Presumably, genomic imprinting effects would make the brain of Xm more similar to males, while Xp would potentially present exacerbated female-like characteristics, with typical females in between.
Results
Groups did not differ regarding age (F = 1.242; p = 0.296) or the frequency of growth hormone treatment between TS groups (χ2; (1, 40) = 0.744, p = 0.388). ANOVAs performed on neuropsychological measures revealed that both TS groups scored significantly lower than controls on FSIQ (p < 0.001), PRI/PIQ (p < 0.001), VCI/VIQ (p ≤ 0.025), WMI (p ≤ 0.001), and PSI (p < 0.001). Girls with Xp scored lower than Xm for FSIQ (p = 0.032) and PIQ (p = 0.002), but did not differ on other measures.
Regarding whole-brain measures, groups did not differ on total brain volume (F = 2.162; p = 0.124), mean cortical thickness (F = 1.737; p = 0.185), total WMV (F = 2.712; p = 0.074), and total GMV (F = 2.710; p = 0.074), when covarying for age and total brain volume. However, the ANCOVA for total surface area was significant with Xp showing smaller total surface area than controls (F = 4.088; p = 0.021; post hoc, p = 0.006). Trend analysis showed the presence of significant linear trends for total brain volume (F = 4.184; p = 0.045), total GMV (F = 5.047; p = 0.028), and total surface area (F = 6.486; p = 0.013). For all three measures, the Xm group had the largest values, followed by controls, then by the Xp group.
ANCOVAs performed on segmented regions revealed a significant effect of the group factor for 16 regions for cortical thickness, 15 regions for GMV, 10 regions for WMV, 20 regions for surface area, and seven subcortical structures (FDR corrected; all ps < 0.05).
Xm versus Xp
Regarding genomic imprinting effects, we found that compared with girls with Xm, those with Xp show bilateral increase in cortical thickness in the inferior (p ≤ 0.002) and middle (p < 0.001) temporal regions, as well as in the left superior temporal gyrus (p < 0.001), the right superior temporal sulcal bank (p < 0.001), and the right inferior parietal cortex (p = 0.004). Girls with Xm showed increased GMV in the superior frontal region bilaterally (p ≤ 0.011), decreased GMV in the right superior temporal sulcal bank (p = 0.016), and increased surface area in the right entorhinal cortex (p = 0.015), the left parahippocampal regions (p = 0.011), and the left superior temporal region (p = 0.016). Individuals with Xm displayed reduced WMV in the pericalcarine region bilaterally (p ≤ 0.015) compared with Xp (Fig. 1A). Groups did not differ in measures of subcortical anatomy.
Figure 1.
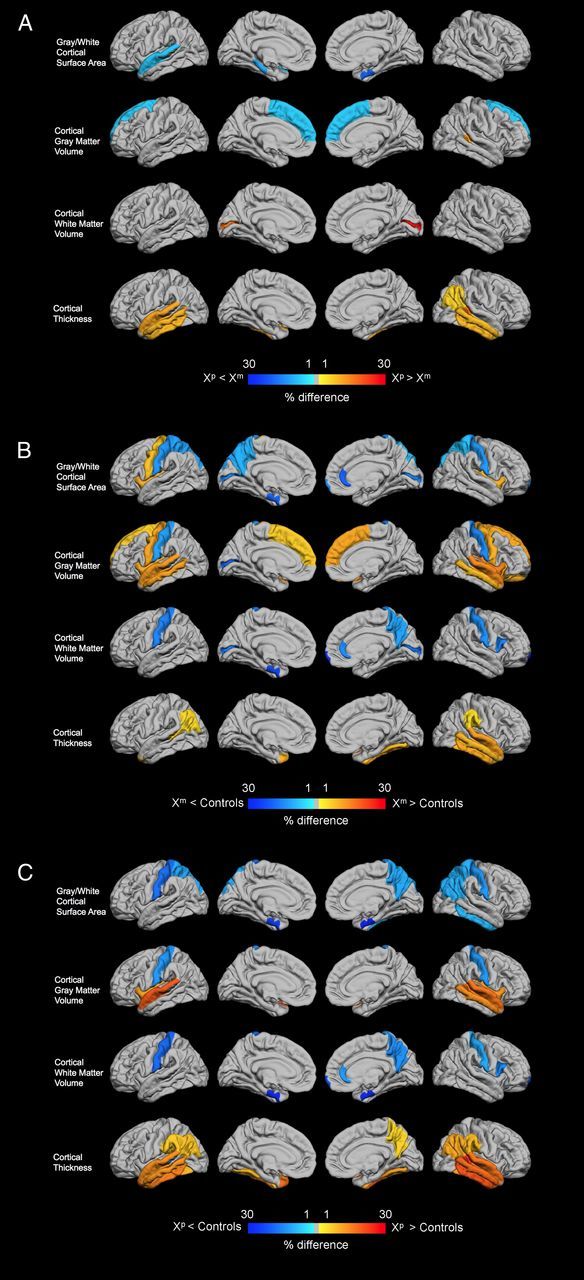
Cortical regions showing significant differences between groups. A, Xp versus Xm. B, Xm versus Controls. C, Xp versus Controls. Colors show the percentage of difference between groups for each significant region. For comparisons with the control group, results are expressed in percentage change relative to controls. For comparisons between Xm and Xp, the comparison was made using Xm values as the baseline. Genomic imprinting effects are shown in A, where cold colors represent smaller values for individuals with Xp compared with Xm, and warm colors the opposite pattern. For B and C, cold and warm colors indicate, respectively, significantly smaller and larger values for Xm and Xp compared with controls. The rows of the figure correspond from top to bottom, to cortical surface area, GMV, WMV, and cortical thickness.
Xm versus controls
Compared with controls, girls with Xm showed increased cortical thickness in the left temporal pole (p = 0.012), superior temporal sulcal bank (p = 0.002), and inferior parietal cortex (p = 0.013). In the right hemisphere, cortical thickening for the Xm group was also observed in the inferior, middle, and superior temporal regions (p ≤ 0.001), as well as in the supramarginal (p = 0.016) and fusiform (p = 0.002) regions. In the Xm group, significant increase in GMV was observed bilaterally in the middle (p ≤ 0.013) and superior (p ≤ 0.001) temporal regions; the insula (p < 0.001), the precentral (p ≤ 0.004), and superior frontal (p ≤ 0.010) regions; and in right lateral orbitofrontal cortex (p = 0.003). Conversely, Xm showed a reduction in GMV in the postcentral region bilaterally (p = 0.001) and in the left pericalcarine cortex (p = 0.001). Regarding WMV, Xm presented significant reduction compared with controls in multiple regions, including postcentral (p ≤ 0.001) and pericalcarine (p ≤ 0.010) regions bilaterally; the left entorhinal cortex (p < 0.001); and the precuneus (p = 0.002), pars opercularis (p < 0.001), frontal pole (p = 0.001), and rostral anterior cingulate (p = 0.001) regions of the right hemisphere.
Girls with Xm displayed a reduction bilaterally in surface area of the postcentral, pericalcarine, and superior parietal regions (all p ≤ 0.011), while an increase in surface area was seen in the bilateral insula (p ≤ 0.007) and left precentral regions (p = 0.008). The Xm group also showed a reduction in surface area of the left precuneus (p = 0.007), entorhinal (p = 0.001) cortex, and in the rostral anterior cingulate (p = 0.001) and frontal pole (p = 0.013) of the right hemisphere (Fig. 1B). Regarding subcortical structures, Xm girls showed increased volume compared with controls in the fourth ventricle (p < 0.001), left hippocampus (p = 0.004), left (p < 0.001) and right (p = 0.006) amygdala, and cerebellum GMV of the right hemisphere (p = 0.001).
Xp versus controls
Compared with controls, girls with Xp showed large areas of increased cortical thickness bilaterally, covering the inferior (p < 0.001), middle (p < 0.001), and superior (p < 0.001) temporal regions and the fusiform (p ≤ 0.001), inferior parietal (p ≤ 0.002) and supramarginal regions (p ≤ 0.003). Increase cortical thickness was also present in the sulcal bank superior of the right superior temporal sulcal bank, right precuneus, and left temporal pole (all p ≤ 0.003). Similarly to what was observed in Xm, girls with Xp displayed a reduction in GMV in the postcentral regions (p ≤ 0.011), with an increase present in the insular (p ≤ 0.002) and superior temporal cortex (p < 0.001) bilaterally and in the right middle temporal (p = 0.001) and superior temporal sulcal bank (p = 0.005). Reductions in WMV were also present in Xp compared with controls. These alterations were observed in the postcentral (p ≤ 0.004), entorhinal (p ≤ 0.002) regions bilaterally and in the right hemisphere in the pars opercularis (p = 0.009), precuneus (p = 0.009), frontal pole (p = 0.011), and rostral anterior cingulate (p = 0.015), in parallel to findings observed in the Xm group. Girls with Xp showed bilateral reduction of surface area in the postcentral (p ≤ 0.001), superior parietal (p ≤ 0.003), and entorhinal (p < 0.001) regions, as well as in the inferior parietal (p = 0.005), inferior temporal (p = 0.008), and precuneus (p = 0.010) of the right hemisphere (Fig. 1C). Regarding subcortical structures, Xp displayed the same between-group pattern observed between Xm and controls, namely increased volume in the fourth ventricle (p < 0.001), left hippocampus (p = 0.004), and left (p < 0.001) and right (p = 0.004) amygdala, but also presented increased volume in the left (p = 0.001) and right (p = 0.004) putamen (Table 2).
Table 2.
Significant results for subcortical structures
| Controls | Turner |
Xm versus controls (%) | Xp versus controls (%) | Xp versus Xm (%) | ||
|---|---|---|---|---|---|---|
| Xm | Xp | |||||
| Left amygdala | 1309.71 (200.05) | 1507.86 (192.07) | 1544.16 (194.94) | 15.13** | 17.90** | 2.41 |
| Left hippocampus | 3994.97 (440.84) | 4362.57 (388.03) | 4458.35 (444.84) | 9.20* | 11.60* | 2.20 |
| Left putamen | 5603.53 (574.07) | 5938.46 (501.21) | 6371.53 (559.46) | 5.98 | 13.71** | 7.29 |
| Right amygdala | 1362.97 (220.06) | 1540.42 (192.07) | 1588.34 (214.44) | 13.02* | 16.54* | 3.11 |
| Right cerebellum cortex | 60684.46 (5781.10) | 66227.47 (5046.98) | 64038.79 (5633.81) | 9.13** | 5.53 | −3.30 |
| Right putamen | 5380.32 (597.25) | 5735.17 (519.34) | 5986.78 (579.74) | 6.60 | 11.27* | 4.39 |
| Fourth ventricle | 1763.31 (800.73) | 2738.32 (699.04) | 2887.77 (780.29) | 55.29** | 63.77** | 5.46 |
Significant between-group results for subcortical structures. For comparisons with the control group, results are expressed as percentage change relative to controls. For comparisons between Xm and Xp, the comparison was made using Xm as a reference. Statistical measures (means and SDs) account for covariates of age, total brain volume, and FSIQ.
*p ≤ 0.01;
**p ≤ 0.001.
Brain-behavior analysis
To establish within-group brain-behavior associations with regard to imprinting effects, we conducted exploratory correlation analyses between brain variables showing significant differences between Xm and Xp and neuropsychological measures (FSIQ, VIQ, PIQ). Partial correlations, using age and total brain volume as covariates, were conducted separately for each of the three groups. Significant correlations (FDR corrected) were observed only in the Xp group, where cortical thickness of the right middle temporal region was positively associated with all three cognitive scales (FSIQ, VIQ, and PIQ; all p ≤ 0.001), and cortical thickness of the right inferior parietal region was also positively related to FSIQ and PIQ (respectively, p = 0.002, p < 0.001). Significance of the difference between the correlation coefficients in Xp, Xm, and controls was assessed using Fisher r-to-z transformation. All three correlations found in Xp between cortical thickness of the middle temporal region and cognitive measures differed significantly from those seen in both Xm and controls (FSIQ: Xp, r = 0.774; Xm, r = 0.281; controls, r = 0.138; p ≤ 0.036; VIQ: Xp, r = 0.790; Xm, r = 0.050; controls, r = 0.092; p ≤ 0.004; and PIQ: Xp, r = 0.817; Xm, r = 0.286; controls, r = 0.101; p ≤ 0.016). Similarly, the relationship observed in Xp between cortical thickness of the inferior parietal region and PIQ differed significantly from those seen in Xm and controls (Xp, r = 0.790; Xm, r = 0.288; controls, r = 0.074; p ≤ 0.026). Although there was a trend in that same direction, the correlation between FSIQ and inferior parietal thickness did not differ between the Xp group (r = 0.703), Xm (r = 0.201; p = 0.055), and the controls (r = 0.325; p = 0.111).
Discussion
The main objective of the present study was to establish potential X-chromosome genomic imprinting effects on brain morphology by examining brain structure in young individuals with X monosomy who have not been exposed to estrogen treatment. To our knowledge, this is the first study to look at the impact of genomic imprinting on cortical thickness and surface area, in addition to cortical and subcortical volumes, in young girls with TS. We demonstrated the existence of putative imprinting effects of the X chromosome, which impact cortical thickness, surface area, and cortical volume in TS, and provide preliminary evidence in favor of genomic imprinting effects of the X chromosome on sexual dimorphism of the brain.
Overall, our results replicate most previous findings regarding structural abnormalities seen in adults and adolescents with TS, namely enlargement of the hippocampus and amygdala (Good et al., 2003; Kesler et al., 2004), reductions of GMV and WMV in the parieto-occipital and postcentral cortical regions (Brown et al., 2004; Cutter et al., 2006; Holzapfel et al., 2006; Marzelli et al., 2011), and enlargement of the temporal gyri and insula (Kesler et al., 2003; Cutter et al., 2006; Marzelli et al., 2011), as well as increased cortical thickness in the temporal regions (Raznahan et al., 2010: Lepage et al., 2012a,b). Our study also shows the existence of abnormal surface area in TS, as reduced surface area is observed in both TS groups in the postcentral, superior parietal, and entorhinal regions, coupled with an increase of surface area in the insular cortex visible in the Xm group. The results of our cognitive tests, where controls obtained higher scores than both TS cohorts, are also in line with the extant literature (Rovet, 1990; Hong et al., 2009). Together, these results support the validity of the current data. Specifically, the fact that highly similar alterations are observed in both Xm and Xp groups suggests the existence of an overarching effect of X monosomy on brain morphology occurring early in development.
Regarding genomic imprinting effects on cortical morphology, we identified different patterns of abnormalities depending on parent of origin in cortical thickness, surface area, and cortical volume. While Xm show anomalies in volume in superior frontal and pericalcarine regions, Xp seem to present with more aberrant cortical thickness. Indeed, one of the most striking results is a prominent increase in cortical thickness within the temporal lobes of individuals with Xp compared with both Xm and controls. This effect is mostly bilateral, although extending to the inferior parietal cortex in the right hemisphere. Genomic imprinting effects have previously been reported in the temporal regions (Kesler et al., 2003; Cutter et al., 2006). However, while we observe an increase in cortical thickness in the temporal lobes of Xp and an increase in surface area in the left superior temporal gyrus of Xm, Kesler et al. (2003) reported increased GMV and WMV in the left, and increased WMV in the right STG of Xm individuals (Kesler et al., 2003). In contrast, Cutter et al. (2006) reported bilateral decrease in WMV in the temporal lobe of adult Xm individuals in comparison with Xp. A number of different factors may explain these conflicting results, such as the use of different methodologies (voxel based vs SBA), the small sample size of the Xp group in both previous studies (n of 10 and 7, respectively), as well as the inclusion of participants of different ages and hormonal treatment history. In comparison, the current study used a much larger group of participants with Xp (n = 17), groups with small ranges of age, and only included participants who were not administered estrogen treatment. Also, considering that estrogen treatment possibly influences brain development in girls with TS (Lepage et al., 2012a,b), it is plausible that hormonal treatment interacts with genomic imprinting, which may partly explain differences in imprinting effects observed in children compared with adults with TS.
The current study also demonstrates significant differences in superior frontal regions, where Xm display more GMV than their Xp counterparts, and in the pericalcarine region (corresponding approximately to V1), where Xm show decreased WMV. Given that individuals with Xm comprise the largest portion of the TS population, it is plausible that previous observations of reduced WMV in the occipital cortex were primarily driven by individuals with Xm (Brown et al., 2002; Cutter et al., 2006). However, both increases (Good et al., 2003; Molko et al., 2003) and decreases (Molko et al., 2004; Cutter et al., 2006) in GMV and surface area (Raznahan et al., 2010) have been observed in frontal regions of TS. With regard to genomic imprinting, and given the relatively modest association with our cognitive measures, it is difficult to assess the functional and clinical relevance of these putative imprinting effects on the brain. Table 3 summarizes sample characteristics, imaging techniques, and main findings of all studies, including the current one, investigating genomic imprinting effects in TS.
Table 3.
Main findings of neuroimaging studies investigating genomic imprinting in Turner syndrome
| Study | Sample | Hormonal status | Scan type, technique, ROI | Main findings regarding imprinting effects |
|---|---|---|---|---|
| Brown et al., 2002 | 26 non-mosaic TS (17Xm, 9Xp); 26 female controls (mean age 13.2 ± 4.3) | Not reported | 1.5T, semi-automated segmentation | ↑ Cerebellar GM and ↓ occipital WM in Xm compared to controls; no significant difference between Xm and Xp |
| Good et al., 2003 | 21 non-mosaic TS (11 Xm, 10 Xp; mean age 23.3 ± 7.1); 42 healthy controls (17 females; mean age 26.25 ± 8.4) | All TS used sex steroid replacement | 2T, VBM, amygdala, orbitofrontal cortex | No significant difference between Xm and Xp |
| Kesler et al., 2003 | 30 non-mosaic TS (20 Xm, 10 Xp, mean age 14.73 ± 6.41; range 7.56–33.30) | Not reported | Multiple scanners, manual tracing, STG | ↑ GM and total volume of left STG in Xm compared to Xp;↑ total right STG volume in Xm compared to Xp; ↑ WM of right STG in Xm compared to controls |
| 30 healthy females (mean age 14.63 ± 5.90; range 6.35–32.65) | ||||
| Kesler et al., 2004 | 30 non-mosaic TS (20 Xm, 10 Xp, mean age 14.7 ± 6.4; range 7.6–33.3) | Not reported | Multiple scanners, manual tracing, amygdala, hippocampus | No significant difference between Xm and Xp |
| 30 healthy females (mean age 14.8 ± 5.9; range 6.4–32.7) | ||||
| Cutter et al., 2006 | 25 non-mosaic TS (18 Xm, 7 Xp, mean age 27 ± 8); 30 healthy females (mean age 27 ± 7) | All TS used estrogen, 13 GH, 12 oxandrolone | 1.5T, VBM and manual tracing, cerebral lobes, amygdala, caudate nucleus, hippocampus, putamen | ↓ GM volume of bilateral caudate nuclei in Xm compared to Xp'; ↓ WM volume in temporal lobes bilaterally in Xm compared to Xp |
| Present study | 40 non-mosaic TS (23 Xm, 17 Xp, mean age 8.49 ± 2.62); 27 healthy females (mean age 8.17 ± 2.93) | No TS used estrogen, 20 Xm and 17 Xp used GH | 3 T, automated surface-based segmentation | ↑ Cortical thickness in temporal lobes bilaterally and right inferior parietal region in Xp compared to Xm; ↑ GM volume in superior frontal regions bilaterally and right bank of STS in Xm compared to Xp; ↑ surface area in right entorhinal, left parahippocampal, and left superior temporal region in Xm compared to Xp; ↓ WM volume in bilateral pericalcarine regions in Xm compared to Xp |
Summary of findings from previous studies examining genomic imprinting effects on brain morphology in Turner syndrome. GH, growth hormone; GM, gray matter; STG, superior temporal gyrus; STS, superior temporal sulcus; TS, Turner syndrome; VBM, voxel-based morphometry; WM, white matter; Xm or Xp, X chromosome from maternal (m) or paternal (p) origin; ROI, region of interest.
The results of our cognitive tests show that individuals with TS present lower global intellectual performance than controls. While this is in line with several previous reports (Hong et al., 2009), it is possible that the observed differences seen here were accentuated by the fact that the control group presents with global intellectual functioning in the high-average range. It is interesting to note that girls with Xp were more severely affected than Xm in the visuospatial domain. This detrimental effect of the Xp chromosome is in agreement with recent work conducted in TS (Skuse et al., 1997; Bishop et al., 2000; Loesch et al., 2005; Lepage et al., 2012a,b) and Klinefelter syndrome (Stemkens et al., 2006; Bruining et al., 2010). However, neural correlates for this effect remain elusive. Our correlational analyses suggest a link between cortical thickness abnormalities in the temporal and parietal lobes of individuals with Xp and exacerbation of cognitive difficulties associated with this genotype. Considering the positive relationship between cortical thickness and cognitive measures in this group, it is possible that larger cortical thickness values reflect the outcome of compensatory neurodevelopmental mechanisms related to impaired cognitive skills present in Xp.
Genomic imprinting effects and sexual dimorphism
The present findings have direct implications for our understanding of the epigenetic mechanisms involved in sexual dimorphism of the brain that are taking place early in development. Due to their inherent relationships, it is difficult to establish the relative contribution of hormonal, genetic, and epigenetic factors on sexual differentiation of the brain during maturation. The present sample provides a rare opportunity to assess the potential contribution of genomic imprinting of the X chromosome in that process.
By far, the most consistent finding with regard to sex differences in brain anatomy is the larger brain volume found in males compared with females (Paus, 2010). Although our groups did not differ on most whole-brain measures, our analyses revealed the existence of significant trends on total brain volume, GMV, and surface area, where these variables increased linearly from the Xp group being smallest, to the Xm group being largest, with typically developing girls in between. Considering that typically developing males invariably inherit the maternal X chromosome, while typically developing females inherit both and randomly express one of them in each cell, a linear increase in brain volume as seen in the present study is in agreement with what would be expected if imprinted genes located on the X chromosome were involved in brain size determination. It is also possible that X-chromosome imprinted genes have more localized effects, such as altering the volume of subcortical structures (Fischl et al., 2002; Fjell et al., 2009); however, the effect sizes are likely to be small and would be difficult to detect with the present sample size.
Potential genetic basis
The precise genetic basis for the observed effects between TS and controls is not well understood, but haploinsufficiency resulting from the absence of genes that would otherwise escape the X-inactivation process is thought to play a role (Zinn and Ross, 1998). Potential candidate genes have been put forth to explain some aspects of the cognitive and physical phenotype of TS (Ross et al., 2006, Zinn et al., 2007), but their precise impact on cortical morphology remains unknown. Among the genes that could be involved in the TS neurophenotype, a plausible candidate is the MECP2 gene, which has been shown explicitly to influence brain morphology in humans (Joyner et al., 2009). Carriers of the minor allele of SNP rs2239464 showed reduction in surface area in multiple regions that are also markedly affected in TS, including the fusiform, cuneus, precuneus, and the posterior cingulate (Joyner et al., 2009; Lepage et al., 2012a,b). Interestingly, this effect, observed in two independent cohorts, was only present in males (Joyner et al., 2009). Given that the MECP2 is subject to X inactivation, it has been hypothesized that the gender bias reflects redundancy mechanisms related to the presence of the extra chromosome that would protect from detrimental mutations in typical females (Joyner et al., 2009). Considering that individual variation in cortical volumes is mainly attributable to changes in surface area rather than cortical thickness (Rakic, 1988; Im et al., 2008), it is possible that many differences in cortical volumes observed in the present study, which overlaps partly with alterations in surface area, share a common genetic basis such as haploinsufficiency of the MECP2 or other X-linked genes (Zinn et al., 2007).
Although the current study provides further evidence in favor of the existence of imprinted genes on the X chromosome, it does not establish the precise genetic basis of imprinting effects, and no X-linked imprinted candidate has been identified in humans to date (Davies, 2010). Animal models have shown that a number of genes on the X chromosome are expressed differently in the brain depending on their parental origin (Davies et al., 2005; Gregg et al., 2010; DeVeale et al., 2012). Recent data also suggest that within the brain, genomic imprinting effects on gene expression of the X chromosome may be altered substantially depending on brain regions. For example, in the mouse, the YIPF6 gene shows a maternal bias in the preoptic area of the thalamus, and a paternal bias in the medial prefrontal cortex (Gregg et al., 2010). The functional significance of this expression pattern is unknown, but it illustrates the undeniably complex processes by which genomic imprinting could affect brain development.
In summary, we demonstrate the existence of genomic imprinting effects of the X chromosome on brain morphology in young girls with TS. The effects observed are cortical in origin, predominantly bilateral, affect specific regions, and seem to differentially modulate cortical thickness, surface area, and cortical volume. Longitudinal studies using larger sample sizes are needed to track potential interaction effects of genomic imprinting with growth hormone and estrogen treatment during brain maturation across development.
Footnotes
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD049653), National Institute of Mental Health (MH050047), Chain of Love Foundation, and Genentech. J.F.L. is supported by a Postdoctoral Fellowship from the Canadian Institutes of Health Research. D.S.H. is supported by an award from the National Institute of Mental Health (MH097120). We thank the reviewers for their valuable input to this work.
J.H. is co-investigator on a grant from the National Institute of Mental Health. A.L.R. received grants from NICHD and Genentech, and is an unpaid medical advisor for the Turner Syndrome Society and Turner Syndrome Foundation.
References
- Bishop DV, Canning E, Elgar K, Morris E, Jacobs PA, Skuse DH. Distinctive patterns of memory function in subgroups of females with Turner syndrome: evidence for imprinted loci on the X-chromosome affecting neurodevelopment. Neuropsychologia. 2000;38:712–721. doi: 10.1016/S0028-3932(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht M, Patwardhan A, Ross JL, Neely EK, Zeng SM, Yankowitz J, Reiss AL. Brain development in Turner syndrome: a magnetic resonance imaging study. Psychiatry Res. 2002;116:187–196. doi: 10.1016/S0925-4927(02)00086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WE, Kesler SR, Eliez S, Warsofsky IS, Haberecht M, Reiss AL. A volumetric study of parietal lobe subregions in Turner syndrome. Dev Med Child Neurol. 2004;46:607–609. doi: 10.1017/s0012162204001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruining H, van Rijn S, Swaab H, Giltay J, Kates W, Kas MJ, van Engeland H, de Sonneville L. The Parent-of-origin of the extra X chromosome may differentially affect psychopathology in Klinefelter syndrome. Biol Psychiatry. 2010;68:1156–1162. doi: 10.1016/j.biopsych.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CE, Donaldson MD, Kelnar CJ, Smail PJ, Greene SA, Paterson WF, Connor JM. Possible role of imprinting in the Turner phenotype. J Med Genet. 1994;31:840–842. doi: 10.1136/jmg.31.11.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutter WJ, Daly EM, Robertson DMW, Chitnis XA, van Amelsvoort TA, Simmons A, Ng VW, Williams BS, Shaw P, Conway GS, Skuse DH, Collier DA, Craig M, Murphy DG. Influence of X chromosome and hormones on human brain development: a magnetic resonance imaging and proton magnetic resonance spectroscopy study of Turner syndrome. Biol Psychiatry. 2006;59:273–283. doi: 10.1016/j.biopsych.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davies W. Genomic imprinting on the X chromosome: implications for brain and behavioral phenotypes. Ann N Y Acad Sci. 2010;1204(Suppl):E14–E19. doi: 10.1111/j.1749-6632.2010.05567.x. [DOI] [PubMed] [Google Scholar]
- Davies W, Isles A, Smith R, Karunadasa D, Burrmann D, Humby T, Ojarikre O, Biggin C, Skuse D, Burgoyne P, Wilkinson L. Xlr3b is a new imprinted candidate for X-linked parent-of-origin effects on cognitive function in mice. Nat Genet. 2005;37:625–629. doi: 10.1038/ng1577. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- DeVeale B, van der Kooy D, Babak T. Critical evaluation of imprinted gene expression by RNA-Seq: a new perspective. PLoS Genet. 2012;8:e1002600. doi: 10.1371/journal.pgen.1002600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Walhovd KB. Minute effects of sex on the aging brain: a multisample magnetic resonance imaging study of healthy aging and Alzheimer's disease. J Neurosci. 2009;29:8774–8783. doi: 10.1523/JNEUROSCI.0115-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Lawrence K, Thomas NS, Price CJ, Ashburner J, Friston KJ, Frackowiak RS, Oreland L, Skuse DH. Dosage-sensitive X-linked locus influences the development of amygdala and orbitofrontal cortex, and fear recognition in humans. Brain. 2003;126:2431–2446. doi: 10.1093/brain/awg242. [DOI] [PubMed] [Google Scholar]
- Gregg C, Zhang J, Butler JE, Haig D, Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329:682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel M, Barnea-Goraly N, Eckert MA, Kesler SR, Reiss AL. Selective alterations of white matter associated with visuospatial and sensorimotor dysfunction in turner syndrome. J Neurosci. 2006;26:7007–7013. doi: 10.1523/JNEUROSCI.1764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong D, Scaletta Kent J, Kesler S. Cognitive profile of Turner syndrome. Dev Disabil Res Rev. 2009;15:270–278. doi: 10.1002/ddrr.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook EB. Exclusion of chromosomal mosaicism: tables of 90%, 95% and 99% confidence limits and comments on use. Am J Hum Genet. 1977;29:94–97. [PMC free article] [PubMed] [Google Scholar]
- Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI. Brain size and cortical structure in the adult human brain. Cereb Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Joyner AH, J CR, Bloss CS, Bakken TE, Rimol LM, Melle I, Agartz I, Djurovic S, Topol EJ, Schork NJ, Andreassen OA, Dale AM. A common MECP2 haplotype associates with reduced cortical surface area in humans in two independent populations. Proc Natl Acad Sci U S A. 2009;106:15483–15488. doi: 10.1073/pnas.0901866106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Blasey CM, Brown WE, Yankowitz J, Zeng SM, Bender BG, Reiss AL. Effects of X-monosomy and X-linked imprinting on superior temporal gyrus morphology in Turner syndrome. Biol Psychiatry. 2003;54:636–646. doi: 10.1016/S0006-3223(03)00289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Garrett A, Bender B, Yankowitz J, Zeng SM, Reiss AL. Amygdala and hippocampal volumes in Turner syndrome: a high-resolution MRI study of X-monosomy. Neuropsychologia. 2004;42:1971–1978. doi: 10.1016/j.neuropsychologia.2004.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage JF, Hong DS, Hallmayer J, Reiss AL. Genomic imprinting effects on cognitive and social abilities in prepubertal girls with Turner syndrome. J Clin Endocrinol Metab. 2012a;97:E460–E464. doi: 10.1210/jc.2011-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage JF, Mazaika PK, Hong DS, Raman M, Reiss AL. Cortical brain morphology in young, estrogen-naive, and adolescent, estrogen-treated girls with Turner syndrome. Cereb Cortex. 2012b doi: 10.1093/cercor/bhs195. doi: 10.1093/cercor/bhs195. Advance online publication. July 17, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Kelso W, Huggins RM, Slater H, Warne G, Bergman PB, Bergman P, Rodda C, Mitchell RJ, Prior M. Effect of Turner's syndrome and X-linked imprinting on cognitive status: analysis based on pedigree data. Brain Dev. 2005;27:494–503. doi: 10.1016/j.braindev.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Marzelli MJ, Hoeft F, Hong DS, Reiss AL. Neuroanatomical spatial patterns in Turner syndrome. Neuroimage. 2011;55:439–447. doi: 10.1016/j.neuroimage.2010.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molko N, Cachia A, Rivière D, Mangin JF, Bruandet M, Le Bihan D, Cohen L, Dehaene S. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40:847–858. doi: 10.1016/S0896-6273(03)00670-6. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, LeBihan D, Cohen L, Dehaene S. Brain anatomy in Turner syndrome: evidence for impaired social and spatial-numerical networks. Cereb Cortex. 2004;14:840–850. doi: 10.1093/cercor/bhh042. [DOI] [PubMed] [Google Scholar]
- Mullaney R, Murphy D. Turner syndrome: neuroimaging findings: structural and functional. Dev Disabil Res Rev. 2009;15:279–283. doi: 10.1002/ddrr.87. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, Jacobson K, Lyons MJ, Grant MD, Franz CE, Xian H, Tsuang M, Fischl B, Seidman L, Dale A, Kremen WS. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T. Sex differences in the human brain: a developmental perspective. Prog Brain Res. 2010;186:13–28. doi: 10.1016/B978-0-444-53630-3.00002-6. [DOI] [PubMed] [Google Scholar]
- Phillips JP, Montague EQ, Aragon M, Lowe JR, Schrader RM, Ohls RK, Caprihan A. Prematurity affects cortical maturation in early childhood. Pediatr Neurol. 2011;45:213–219. doi: 10.1016/j.pediatrneurol.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raefski AS, O'Neill MJ. Identification of a cluster of X-linked imprinted genes in mice. Nat Genet. 2005;37:620–624. doi: 10.1038/ng1567. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Cutter W, Lalonde F, Robertson D, Daly E, Conway GS, Skuse DH, Ross J, Lerch JP, Giedd JN, Murphy DD. Cortical anatomy in human X monosomy. Neuroimage. 2010;49:2915–2923. doi: 10.1016/j.neuroimage.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/WNL.58.5.695. [DOI] [PubMed] [Google Scholar]
- Ross J, Roeltgen D, Zinn A. Cognition and the sex chromosomes: studies in Turner syndrome. Horm Res. 2006;65:47–56. doi: 10.1159/000090698. [DOI] [PubMed] [Google Scholar]
- Rovet JF. The cognitive and neuropsychological characteristics of females with Turner syndrome. In: Berch DB, Berger BG, editors. Sex chromosome abnormalities and human behavior. Boulder, CO: Westview; 1990. pp. 38–77. [Google Scholar]
- Sagi L, Zuckerman-Levin N, Gawlik A, Ghizzoni L, Buyukgebiz A, Rakover Y, Bistritzer T, Admoni O, Vottero A, Baruch O, Fares F, Malecka-Tendera E, Hochberg Z. Clinical significance of the parental origin of the X chromosome in Turner syndrome. J Clin Endocrinol Metab. 2007;92:846–852. doi: 10.1210/jc.2006-0158. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Skuse DH, James RS, Bishop DV, Coppin B, Dalton P, Aamodt-Leeper G, Bacarese-Hamilton M, Creswell C, McGurk R, Jacobs PA. Evidence from Turner's syndrome of an imprinted X-linked locus affecting cognitive function. Nature. 1997;387:705–708. doi: 10.1038/42706. [DOI] [PubMed] [Google Scholar]
- Stemkens D, Roza T, Verrij L, Swaab H, van Werkhoven MK, Alizadeh BZ, Sinke RJ, Giltay JC. Is there an influence of X-chromosomal imprinting on the phenotype in Klinefelter syndrome? A clinical and molecular genetic study of 61 cases. Clin Genet. 2006;70:43–48. doi: 10.1111/j.1399-0004.2006.00635.x. [DOI] [PubMed] [Google Scholar]
- Storey JD. A direct approach to false discovery rates. J R Stat Soc Ser B. 2002;64:479–498. doi: 10.1111/1467-9868.00346. [DOI] [Google Scholar]
- Tycko B, Morison IM. Physiological functions of imprinted genes. J Cell Physiol. 2002;192:245–258. doi: 10.1002/jcp.10129. [DOI] [PubMed] [Google Scholar]
- Van PL, Bakalov VK, Zinn AR, Bondy CA. Maternal X chromosome, visceral adiposity, and lipid profile. JAMA. 2006;295:1373–1374. doi: 10.1001/jama.295.12.1373. [DOI] [PubMed] [Google Scholar]
- Wilkinson LS, Davies W, Isles AR. Genomic imprinting effects on brain development and function. Nat Rev Neurosci. 2007;8:832–843. doi: 10.1038/nrn2235. [DOI] [PubMed] [Google Scholar]
- Zinn AR, Ross JL. Turner syndrome and haploinsufficiency. Curr Opin Genet Dev. 1998;8:322–327. doi: 10.1016/S0959-437X(98)80089-0. [DOI] [PubMed] [Google Scholar]
- Zinn AR, Roeltgen D, Stefanatos G, Ramos P, Elder FF, Kushner H, Kowal K, Ross JL. A Turner syndrome neurocognitive phenotype maps to Xp22.3. Behav Brain Funct. 2007;3:24. doi: 10.1186/1744-9081-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]


