Abstract
Neuropeptide Y (NPY), an inhibitory neuropeptide expressed by a moderately dense population of wide-field amacrine cells in the rat retina, acts through multiple (Y1–y6) G-protein–coupled receptors. This study determined the cellular localization of Y1 receptors and the synaptic connectivity of Y1 processes in the inner plexiform layer (IPL) of the rat retina. Specific Y1 immunoreactivity was localized to horizontal cell bodies in the distal inner nuclear layer and their processes in the outer plexiform layer. Immunoreactivity was also prominent in cell processes located in strata 2 and 4, and puncta in strata 4 and 5 of the IPL. Double-label immunohistochemical experiments with calbindin, a horizontal cell marker, confirmed Y1 immunostaining in all horizontal cells. Double-label immunohistochemical experiments, using antibodies to choline acetyltransferase and vesicular acetylcholine transporter to label cholinergic amacrine cell processes, demonstrated that Y1 immunoreactivity in strata 2 and 4 of the IPL was localized to cholinergic amacrine cell processes. Electron microscopic studies of the inner retina showed that Y1-immunostained amacrine cell processes and puncta received synaptic inputs from unlabeled amacrine cell processes (65.2%) and bipolar cell axon terminals (34.8%). Y1-immunoreactive amacrine cell processes most frequently formed synaptic outputs onto unlabeled amacrine cell processes (34.0%) and ganglion cell dendrites (54.1%). NPY immunoreactivity in the rat retina is distributed primarily to strata 1 and 5 of the IPL, and the present findings, thus, suggest that NPY acts in a paracrine manner on Y1 receptors to influence both horizontal and amacrine cells.
Indexing terms: amacrine cells, choline acetyltransferase, calcium binding protein, horizontal cells, neuropeptides
Neuropeptide Y (NPY) has multiple physiologic actions in both the central and peripheral nervous system, including effects on blood flow, memory retention, food intake, epilepsy, and pain (Lundberg et al., 1996; Munglani et al., 1996; Balasubramaniam, 1997; Blomqvist and Herzog, 1997; Thorsell et al., 2000; Furtinger et al., 2001; Naveilhan et al., 2001). These effects are mediated by means of at least five G-protein–coupled receptors, designated as Y1, Y2, Y4, Y5, and y6, which are coupled to pertussis toxin–sensitive inhibitory G-proteins (Lundberg et al., 1996; Munglani et al., 1996; Balasubramaniam, 1997; Blomqvist and Herzog, 1997; Michel et al., 1998). Y-receptors have been localized to specific regions of the central and peripheral nervous system by receptor binding autoradiography, immunohistochemistry, and in situ hybridization (Zhang et al., 1994; Bao et al., 1997; Jacques et al., 1997; Dumont et al., 1998; Hökfelt et al., 1998; Gackenheimer et al., 2001). Y1 receptor is the most studied Y-receptor and it plays key roles in many of the central and peripheral effects of NPY (Lundberg et al., 1996; Munglani et al., 1996; Balasubramaniam, 1997; Blomqvist and Herzog, 1997).
NPY immunoreactivity is present in human, cat, guinea pig, mouse, and rat retinas (Bruun et al., 1984; Tornqvist and Ehinger, 1988; Ferriero and Sagar, 1989; Straznicky and Hiscock, 1989; Li and Lam, 1990; Jen et al., 1994; Hutsler and Chalupa, 1994, 1995; Ammar et al., 1998; Kang et al., 2001; Oh et al., 2001; Sinclair and Nirenberg, 2001). This peptide is mainly localized to amacrine cells and displaced amacrine cells and, in cat and human retinas, to small ganglion cells. In the rat retina, NPY immunoreactivity is localized to moderately dense amacrine and displaced amacrine cell populations that ramify primarily to strata 1 and 5 of the inner plexiform layer (IPL; D'Angelo and Brecha, 1999; Oh et al., 2001).
The cellular distribution of Y-receptors, including the Y1 receptor, has not been determined in the retina. However, several lines of evidence suggest that NPY acts on Y-receptors in the retina: (1) NPY is localized to amacrine cells in many species, and presumably, there are NPY sites of action in the retina; (2) exogenous application of NPY to whole rabbit and chicken retinas stimulates the release of multiple neurotransmitters, including acetylcholine and γ-aminobutyric acid (GABA), in a calcium-dependent manner (Bruun and Ehinger, 1993); (3) reduced cAMP accumulation in response to exogenous NPY application to the rabbit retina suggests that NPY acts to inhibit adenylyl cyclase activity by means of G-protein–coupled Y-receptors (Bruun et al., 1994).
The aim of the present study was to determine the cellular expression of Y1 in the rat retina. Some of these observations have been reported in abstract form (D'Angelo and Brecha, 1999).
Materials and Methods
Animals
Adult Sprague-Dawley rats of either sex were used for these studies. They were housed and fed under normal conditions with a 12-hour light-dark cycle. The animals were treated according to the regulations of the Animal Research Committee of the University of California at Los Angeles, and the Animal Ethics Committee of the Catholic University of Korea, conforming to all NIH guidelines.
Tissue preparation
Rats were deeply anesthetized with an intraperitoneal injection of 30–70 mg/kg pentobarbital and transcardially perfused with 50–100 ml of 0.1 M phosphate buffered saline (PBS; pH 7.4) followed by 500 ml of 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.4) in PB at room temperature. Each eye was removed and dissected, and the posterior eyecup containing the retina was immersed in 4% PFA for 1–2 hours at room temperature then transferred to 25% sucrose in 0.1 M PB and stored overnight at 4°C. For cryostat sections, the eyecup, including the retina, was cut perpendicular to the vitreal surface at 12–16 μm. Sections were mounted onto gelatin-coated slides and stored at −20°C until immunohistochemical staining. For sliding microtome sections, the retina was isolated and cut parallel to the vitreal surface at 25 μm. Sections of the retina were stored in 0.1 M PB at 4°C until antibody staining.
Antibodies
An affinity-purified rabbit polyclonal antibody directed against a C-terminus fragment (amino acids 370-382) of the rat Y1 receptor (Ab 96106) was produced by the Antibody/RIA Core of the CURE/Digestive Diseases Research Center and generously provided by H. Wong and J.H. Walsh of the University of California, Los Angeles (Zhang et al., 1994; Bao et al., 1997; Ubink et al., 2001). Specificity of immunostaining was evaluated by preadsorbing the antibody with 10-6 M synthetic Y1370-382. Specificity of immunostaining of this Y1 antibody was also evaluated by using Y1 receptor-deficient mice (Ubink et al., 2001). Sections from Y1-deficient mice did not show any specific Y1 immunoreactivity. Mouse monoclonal antibodies directed against calbindin (CaBP; Sigma, St. Louis, MO; Katsetos et al., 1994) and choline acetyltrans-ferase (ChAT; Chemicon, Temecula, CA; Eckenstein and Thoenen, 1982) were used to identify horizontal cells and cholinergic amacrine cells, respectively. A guinea pig polyclonal antibody to vesicular acetylcholine transporter (VAChT; Chemicon; Arvidsson et al., 1997) was also used to confirm Y1 localization to cholinergic amacrine cell processes. Secondary antibodies were Alexa Fluor–conjugated affinity-purified antibodies (Molecular Probes, Eugene, OR).
Immunohistochemistry
Immunostaining was performed by using the indirect fluorescence method. Cryostat sections were rinsed in 0.1 M PB for 30 minutes, incubated 12–16 hours in Y1 (1: 3,000) antibody with 0.5% Triton X-100 in 0.1 M PB at 4°C, then rinsed for 30 minutes with 0.1 M PB and incubated in goat anti-rabbit immunoglobulin (Ig) G Alexa Fluor 488 antibody (Molecular Probes) for 1–2 hours at room temperature. Sections were washed for 30 minutes with 0.1 M PB and mounted onto gelatin-coated slides, if free floating and air-dried. Sections were cover-slipped with Prolong anti-fade solution (Molecular Probes).
For double-label studies, sections were incubated overnight in a mixture of Y1 (1:2,000) antibody and the CaBP (1:3,000) or ChAT (1:2,000) monoclonal antibodies or VAChT (1:500) polyclonal antibody with 0.5% Triton X-100 in 0.1 M PB at 4°C. Sections were rinsed for 30 minutes with 0.1 M PB and incubated in goat anti-rabbit IgG Alexa Fluor 488 antibody (Molecular Probes) and goat anti-mouse or goat anti-guinea pig IgG Alexa Fluor 568 antibody (Molecular Probes) for 1–2 hours at room temperature. Sections were washed for 30 minutes with 0.1 M PB and cover-slipped as before. To determine that the secondary antibody did not cross-react with the inappropriate primary antibody, some sections were incubated in rabbit polyclonal primary antibody followed by anti-mouse or anti-guinea pig secondary antibody, whereas other sections were incubated in mouse or guinea pig primary antibody followed by anti-rabbit secondary antibody. These sections did not show any immunostaining. Because the emission spectra of Alexa Fluor 488 and 568 partly overlap, sections were double-labeled as well as single-labeled by using each secondary antibody and compared with detect possible “bleed through.” No “bleed through” was detected at the given concentrations.
Immunostained retinas were evaluated by using a Zeiss Axioplan 2 fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a 40× 1.3 NA Zeiss objective. Immunostaining was photographed by using an Axiocam digital camera (Carl Zeiss, Inc.) and data were stored digitally in “.zvi” format at 2,600 × 2,060 resolution. Some sections were evaluated with a Zeiss 410 Laser Scanning Microscope with a krypton/argon laser. Confocal images were acquired with a Zeiss PlanApo 100× 1.3 NA objective at a magnification zoom of 1×. Usually 10–20 optical sections were taken with a z-axis of 1 μm and images were “stacked,” or combined to form a single image. Confocal data were acquired and stored digitally as acquired at 72 dpi. The digital images were scaled to final manuscript size; saved at 320 dpi in “.tiff” format; and adjusted for contrast and brightness, labeled, and formatted by using Adobe Photoshop 5.5 (Adobe Systems, Mountain View, CA).
Electron microscopy
The eyecups were fixed in a mixture of 4% PFA and 0.2% picric acid in 0.1 M PB for 30 minutes at room temperature. The retinas were dissected and small pieces from the central region were fixed for 2 hours at 4°C in the same fixative. After being washed in PB, the retinal pieces were transferred to 30% sucrose in 0.1 M PB for 6 hours, rapidly frozen in liquid nitrogen, thawed, and embedded in 4% agar in distilled water.
The retina was cut with a Vibratome at 50 μm, and sections were placed in 0.01 M PBS (pH 7.4). They were incubated in 0.01 M PBS containing 3% normal goat serum for 1 hour at room temperature to block nonspecific binding sites and were then incubated in the Y1 antibody (1:1,000) for 18 hours at 4°C. The sections were washed in 0.01 M PBS for 45 minutes, incubated in biotin-labeled goat anti-rabbit IgG for 2 hours, and washed three times in 0.01 M PBS for 45 minutes. Sections were incubated in ABC solution for 1 hour, washed in TB, and then incubated in 0.05% diaminobenzidine (DAB) solution containing 0.01% H2O2. The reaction was monitored and ended by replacing the DAB and H2O2 solution with 0.05 M TB.
The stained sections were post-fixed in 1% glutaraldehyde in 0.1 M PB for 1 hour and, after being washed in 0.1 M PB containing 4.5% sucrose for 15 minutes, they were post-fixed in 1% OsO4 in 0.1 M PB for 2 hours, dehydrated in a graded series of alcohol, and flat-embedded in Epon 812. After curing at 60°C for 3 days, well-stained areas were cut out and attached to an Epon support for further sectioning. Ultrathin sections (70–90 nm in thickness) were collected on one-hole grids coated with Formvar and evaluated by using a Jeol 1200EX electron microscope.
Results
Y1 immunoreactivity in the rat retina
Specific Y1 immunoreactivity was present in all retinal regions. In the outer retina, Y1 immunoreactivity was localized to the distal inner nuclear layer (INL) and the outer plexiform layer (OPL). In the inner retina, Y1 immunoreactivity was observed in strata 2 and 4 of the IPL, and in puncta spanning strata 4 and 5 of the IPL (Figs. 1A, 2A). Very faintly Y1-immunoreactive cell bodies were also visible in the INL, at the IPL border, and in the ganglion cell layer (GCL; Fig. 1A). These cells were small (8–9.5 μm) and widely spaced. Often, only a podium of the cell body or the main process entering the IPL was immunostained. Immunoreactivity was absent from control tissue stained with Y1 antibody incubated with synthetic Y1370-380 peptide (Fig. 1B).
Fig. 1.
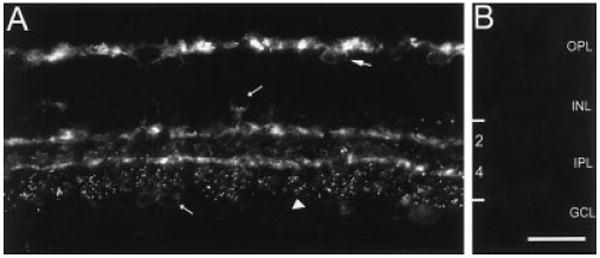
Y1 immunoreactivity in the rat retina. Fluorescence photomicrograph of a retinal section cut perpendicular to the vitreal surface at 12 μm. A: Y1 immunoreactivity in the outer retina is localized to horizontal cell bodies in the distal INL (large arrow) and to processes in the OPL. Immunoreactivity in the inner retina is localized to faintly immunoreactive cell bodies in the proximal INL and GCL (short arrows), strata 2 and 4 of the IPL, and to puncta spanning lamina 5 of the IPL (arrowhead). B: Y1 immunoreactivity is absent from a retinal section incubated overnight with Y1 antibody that was preincubated with 10-6 M synthetic Y1370-382 peptide. Cryostat section. OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 30 μm in B (applies to A,B).
Fig. 2.
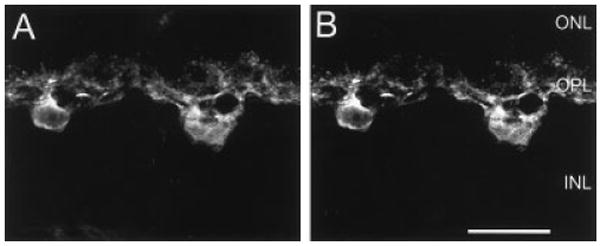
Confocal images of Y1 (A) and calbindin (CaBP; B) immunoreactivities. A,B: Y1 and CaBP immunoreactivities in the same retinal section. A: Y1 immunoreactivity is in horizontal cell bodies and processes in the OPL. B: CaBP immunoreactivity is in horizontal cell bodies and processes in the OPL. A,B are a stack of eight, 1-μm scans. ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer. Scale bar = 20 μm in B (applies to A,B).
Y1 immunoreactivity is localized to horizontal cells
Y1 immunoreactivity was evaluated by double-label histochemistry with well-characterized antibodies that mark horizontal cells. In the outer retina, Y1 immunoreactivity was localized to large cell bodies located in the distal INL and fine processes forming a dense plexus in the OPL (Fig. 2A). Double-labeling experiments using antibodies to CaBP, which label horizontal cells and their processes in the OPL (Röhrenbeck et al., 1987; Uesugi et al., 1992), demonstrated colocalization of Y1 and CaBP in horizontal cell bodies and processes (Fig. 2B). Y1 immunoreactivity was observed predominantly in horizontal cell processes and tips (Peichl and Gonzalez-Soriano, 1994; Fig. 2A). Counts of immunolabeled horizontal cells from horizontal sections cut at 25 μm, from five different retinas, and processed by double-label immunohistochemistry, showed 96% (n = 527) of the CaBP-immunoreactive cells contained Y1 immunoreactivity in the distal INL (Fig. 3). The remaining 4% likely have low levels of Y1 immunostaining and were below the level of detect ability in these preparations.
Fig. 3.
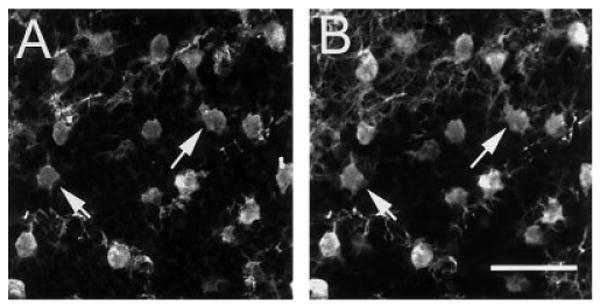
Confocal images of Y1 (A) and CaBP (B) immunoreactivities in a horizontal section through the outer retina. Y1 (A) and CaBP (B) immunoreactivities are colocalized to horizontal cells (arrows indicate colocalization in two horizontal cells). Immunolabeled processes in the image plane are also illustrated in the top portion of A and B. A,B are a stack of five, 1-μm scans through the distal inner nuclear layer. Scale bar = 40 μm in B (applies to A,B).
Y1 immunoreactivity is localized to cholinergic amacrine cells
The pattern of Y1 immunoreactivity in the inner retina was evaluated by using double-labeling immunohistochemistry with ChAT and VAChT, well-characterized antibodies to established amacrine cell populations. In the inner retina, Y1 immunoreactivity was prominent in strata 2 and 4 of the IPL and puncta that spanned strata 4 and 5 of the IPL (Fig. 4A). A few faintly immunoreactive cell bodies were in the INL and GCL (Figs. 1A, 4A). Double-label experiments with Y1 and VAChT, which label the processes and terminals of cholinergic amacrine cells in strata 2 and 4 of the IPL (Sawaguchi et al., 1999), demonstrated the colocalization of Y1 immunoreactivity with VAChT (Fig. 4A,B). These results were verified in other double-label immunohistochemical experiments with monoclonal antibodies to ChAT, a marker for cholinergic amacrine cells (Voigt, 1986; Kim et al., 2000). Y1 immunoreactivity colocalized with ChAT immunoreactivity in the processes of cholinergic amacrine cells that ramify in strata 2 and 4 of the IPL (Fig. 4C,D). ChAT immunoreactivity was similar to that of previous reports (Voigt, 1986; Kim et al., 2000) with small cell bodies in the INL and GCL, and immunoreactive processes forming dense plexuses in strata 2 and 4 of the IPL. Few cell bodies in the INL and GCL that contained Y1 immunoreactivity were detected; therefore, it was difficult to establish whether all cholinergic amacrine cells express Y1.
Fig. 4.
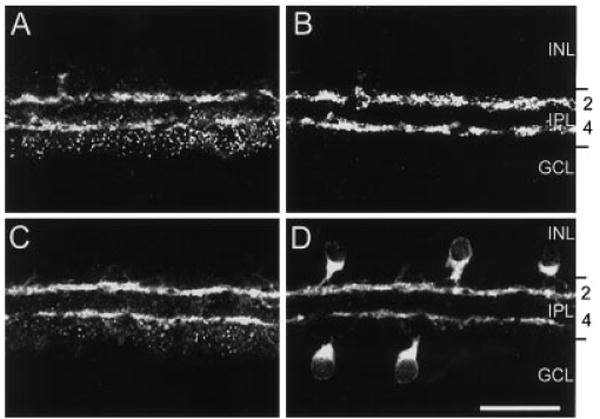
Confocal images of Y1 (A,C), vesicular acetylcholine transporter (VAChT; B), and choline acetyltransferase (ChAT; C) immunoreactivities in the inner retina. A,B: Y1 and VAChT immunoreactivities in the same section. A: Y1 immunoreactivity is localized to processes in strata 2 and 4 of the IPL and puncta primarily distributed to strata 4 and 5. B: VAChT immunoreactivity, which marks cholinergic amacrine cell terminals and processes, is mainly localized to strata 2 and 4 of the IPL. C,D: Y1 and ChAT immunoreactivities in the same retinal section. C: Y1 immunoreactivity localized to processes in the inner retina. D: ChAT immunoreactivity localized to cell bodies in the INL and GCL and processes in strata 2 and 4 of the IPL. A–D are stacks of ten 1-μm scans. INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 30 μm.
Electron microscopic evidence (given below) suggested that Y1-immunoreactive puncta spanning strata 4 and 5 of the IPL are localized to amacrine cells. However, we have not been able to determine the cell type expressing Y1-immunoreactive puncta in strata 4 and 5 of the IPL by double-label immunohistochemistry.
Synaptic connectivity of Y1 immunoreactive cells
Y1 immunoreactivity was identified ultrastructurally as an electron-dense reaction product that was mainly associated with the cell membranes, mitochondrial membranes, nuclear membranes, the amorphous cytoplasmic matrix, and synaptic vesicles. From previous descriptions of processes and synapses in the IPL, the following criteria were applied when defining the identity of the Y1-immunoreactive processes observed (Dubin, 1970; Kolb, 1979; McGuire et al., 1984, 1986): (1) terminals with synaptic ribbons were designated as bipolar cell processes, (2) processes with synaptic vesicles that had chemical synapses or electrical synapses were designated as amacrine cell processes, and (3) processes with clear cytoplasm and microtubules were designated as ganglion cell dendrites.
The synaptic contacts of Y1-immunoreactive cells were observed in the IPL in tissue samples taken from the central retina (Table 1). Y1-immunoreactive processes in the IPL were identified as amacrine cell processes due to the presence of numerous vesicles and the lack of synaptic ribbons. A total of 366 synapses were observed. Of these, 161 were synapses onto Y1-immunoreactive amacrine cell processes from unlabeled amacrine cell processes and bipolar cell axon terminals, and 189 were synapses from Y1-immunoreactive amacrine cell processes onto amacrine, ganglion, bipolar, and unidentified cell processes. A total of 16 synapses occurred between two Y1-immunoreactive amacrine cell processes. The majority of synapses were observed in strata 2, 4, and 5 of the IPL.
TABLE 1.
Kinds and Numbers of Synapses Made by Y1-Immunoreactive Cells in the IPL of the Rat Retina1
| Sublamina a | Sublamina b | Total (%) | ||||
|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | ||
| Input from | ||||||
| Amacrine cells | 3 | 55 | 0 | 33 | 14 | 105 (65.2) |
| Bipolar cells | 1 | 27 | 0 | 21 | 7 | 56 (34.8) |
| Subtotal | 4 | 82 | 0 | 54 | 21 | 161 (100) |
| Output onto | ||||||
| Amacrine cells | 0 | 20 | 0 | 24 | 7 | 51 (27.0) |
| Ganglion cells | 0 | 58 | 0 | 47 | 8 | 113 (60.0) |
| Bipolar cells | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Unidentified | 0 | 12 | 0 | 10 | 3 | 25 (13.0) |
| Subtotal | 0 | 90 | 0 | 81 | 18 | 189 (100) |
| Labeled to labeled | 0 | 7 | 0 | 6 | 3 | 16 (4.8) |
| Total | 4 | 179 | 0 | 141 | 42 | 366 |
S, stratum in the inner plexiform layer.
Postsynaptic labeling pattern
The most common synaptic input onto Y1-immunoreactive amacrine cells was from unlabeled amacrine cell processes (65.2% of all synaptic inputs in the IPL, n = 105). These synaptic inputs were observed in strata 2, 4, and 5 of the IPL. Figure 5 shows Y1-immunolabeled amacrine cell processes with densely filled synaptic vesicles, which display homogenous labeling. These amacrine cell processes receive synaptic input from unlabeled amacrine cell processes and, in Figure 5B, an immunoreactive amacrine cell process forms a reciprocal synapse with nonimmunoreactive amacrine cell process.
Fig. 5.
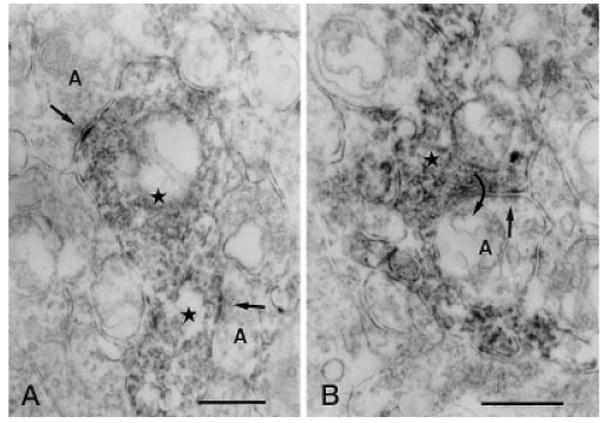
Vertical sections through the IPL of a rat retina processed for Y1 immunoreactivity. A: Two immunoreactive amacrine cell processes (stars) receive synaptic input (arrows) from two unlabeled amacrine cell processes (A) in stratum 4 of the IPL, respectively. B: An immunoreactive amacrine cell process (star) receiving synaptic input (straight arrow) from an unlabeled amacrine cell process (A) in stratum 4 of the IPL. The immunoreactive process makes synaptic output (curved arrow) back onto the unlabeled amacrine cell process. IPL, inner plexiform layer. Scale bars = 0.5 μm in A,B.
Synaptic inputs from bipolar axon terminals onto Y1-immunoreactive amacrine cell processes were also frequent in strata 2 and 4 of the IPL and compose 34.8% (n = 56) of all synaptic inputs observed (Figs. 6, 7). These synapses were, in most cases, dyads where one postsynaptic element was a Y1-immunoreactive amacrine cell process and the other was an unlabeled amacrine cell process (Figs. 6A,B, 7A). Rod bipolar cell axon terminals, identified by the presence of a ribbon and the features of their postsynaptic dyads, were never presynaptic to Y1-immunoreactive processes. This finding suggests that the bipolar cell terminals engaged in synaptic relationships with Y1-immunoreactive amacrine cell processes were ON- and OFF-type cone bipolar cells (Famiglietti and Kolb, 1975; Freed et al., 1987; Chun et al., 1993; Wässle et al., 1995).
Fig. 6.
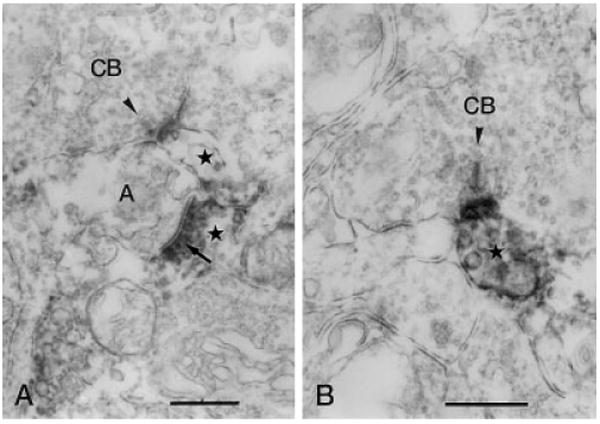
Vertical sections through the IPL stained for Y1 immunoreactivity. A: Y1-immunoreactive process (stars) forms a postsynaptic dyad with an unlabeled amacrine cell process (A), postsynaptic (arrow) to an immunoreactive amacrine cell process, at the ribbon synapse (arrowhead) of a cone bipolar axon terminal in stratum 4 of the IPL. B: Immunoreactive amacrine cell process is postsynaptic to the ribbon synapse (arrowhead) of a CB in stratum 2 of the IPL. IPL, inner plexiform layer; CB, cone bipolar axon terminal. Scale bars = 0.5 μm in A,B.
Fig. 7.
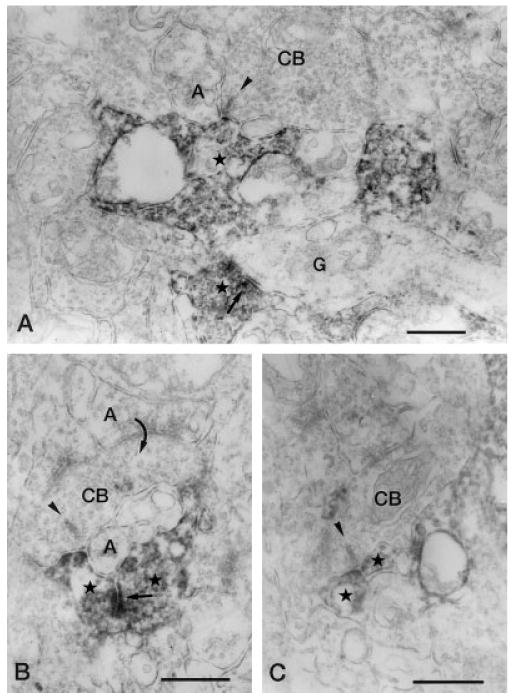
Vertical sections through the IPL stained for Y1 immunoreactivity. A: Y1-immunoreactive amacrine cell process (star) forms a postsynaptic dyad with an unlabeled amacrine cell process (A) at the ribbon synapse (arrowhead) of a CB in stratum 4 of the IPL. In the lower part of the figure, a labeled amacrine cell processes (star) that is making synaptic output (arrow) onto a ganglion cell dendrite (G) can be seen. B: A CB, postsynaptic (curved arrow) to an unlabeled amacrine cell process (upper A), makes a ribbon synapse (arrowhead) onto a postsynaptic dyad consisting of a labeled amacrine cell process (left star) and an unlabeled amacrine cell process (lower A) in stratum 4 of the IPL. The labeled process receives synaptic input (arrow) from another labeled amacrine cell process (right star). C: Two labeled amacrine cell processes (stars) form a postsynaptic dyad at the ribbon synapse of an axon terminal of a CB) in stratum 4 of the IPL. IPL, inner plexiform layer; CB, cone bipolar axon terminal. Scale bar = 0.5 μm.
Presynaptic labeling pattern
Y1-immunoreactive amacrine cell processes form conventional synapses onto unlabeled amacrine and ganglion cell processes in 164 synapses observed in our study. The synapses onto amacrine cells and ganglion cells were observed in strata 2, 4, and 5 of the IPL. Synapses onto bipolar cells were not observed in our study. Of the output synapses of Y1-immunoreactive amacrine cells, 60% (n = 113) were onto ganglion cells. Processes that contained microtubules and no synaptic vesicles in their clear cytoplasm were considered to be the dendrites of ganglion cells. Figure 8 shows an immunoreactive amacrine cell process synapsing onto ganglion cell dendrites. In Figure 8B, an immunolabeled amacrine cell process makes an output synapse onto a ganglion cell dendrite and a small process that is classified as “unidentified” (the process is too small to be identified as a ganglion cell dendrite or an amacrine cell process). The incidence of synapses onto unidentified processes was 13% of all output synapses.
Fig. 8.
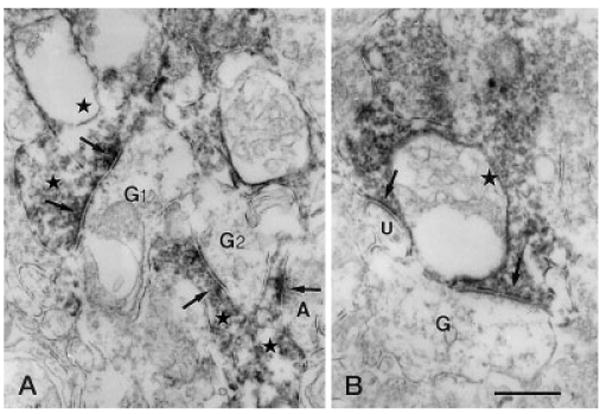
Vertical sections through the IPL of a rat retina processed for Y1 immunoreactivity. A: G1 is postsynaptic (left, upper arrows) to two immunoreactive amacrine cell processes (left, upper stars) in stratum 2 of the IPL. Another ganglion cell dendrite (G2) receiving synaptic input from an immunoreactive amacrine cell process (center star) is seen. In the right lower corner of the figure, an immunolabeled amacrine cell process (right, lower star) receives synaptic input (right, lower arrows) from an unlabeled amacrine cell process (A). B: Immunoreactive amacrine cell process (star) makes output synapses (arrow) onto an unlabeled G and an unidentified process (U) in stratum 4 of the IPL. G,G1,G2, ganglion cell dendrite; IPL, inner plexiform layer. Scale bar = 0.5 μm in B (applies to A,B).
Synapses onto unlabeled amacrine cells were observed in 51 cases (27%). Figure 9A shows that a Y1-immunoreactive amacrine cell process establishes a conventional output synapse onto an nonstained amacrine cell process. Synaptic contacts between two immunoreactive processes have been also observed in strata 2, 4, and 5 of the IPL (Fig. 9B) and made up 4.4% (n = 16) of all synapses observed in the IPL.
Fig. 9.
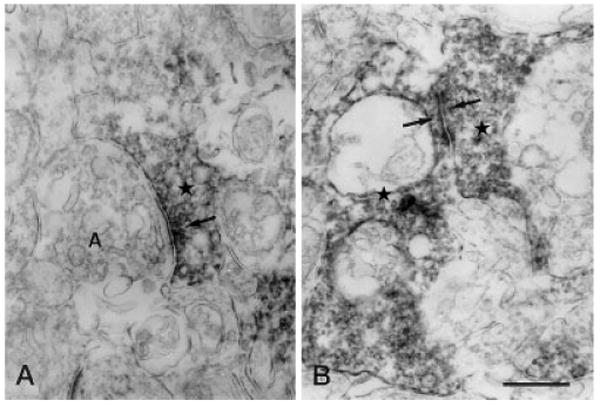
Vertical sections through the IPL of a rat retina processed for Y1 immunoreactivity. A: Immunoreactive amacrine cell process (star) makes an output synapse (arrow) onto an amacrine cell process (A) in stratum 2 of the IPL. B: Synaptic contact (arrows) between two immunoreactive amacrine cell processes (stars) is seen in stratum 4 of the IPL. IPL, inner plexiform layer. Scale bar = 0.5 μm in B (applies to A,B).
Discussion
The present study investigated the distribution of Y1 immunoreactivity in the rat retina and determined the synaptic connectivity of Y1-immunoreactive processes in the IPL by using electron microscopy. Y1 immunoreactivity was present in the outer retina in cell bodies located in the distal INL and processes in the OPL. Double-labeling with antibodies to CaBP demonstrated that Y1 immunoreactivity in the outer retina was localized to horizontal cells. In the inner retina, Y1 immunoreactivity was prominent in processes distributed to strata 2 and 4 of the IPL and puncta spanning strata 4 and 5 of the IPL. Double-labeling with antibodies to ChAT and VAChT demonstrated that Y1 immunoreactivity in the inner retina was localized to the processes of cholinergic amacrine cells. The cellular identity of the puncta distributed to strata 4 and 5 of the IPL has not been established. Electron microscopic observations of the IPL showed that Y1 immunoreactivity was confined to amacrine cell processes and that these processes were postsynaptic to unlabeled amacrine cells and cone bipolar cells, and most presynaptic outputs were onto ganglion cell dendrites and unlabeled amacrine cell processes.
Although, the distribution of NPY immunoreactivity is now established in the retinas of various mammalian species, including human (Tornqvist and Ehinger, 1988; Li and Lam, 1990; Jen et al., 1994), cat (Hutsler et al., 1993; Hutsler and Chalupa, 1994, 1995), guinea pig (Bruun et al., 1984), monkey (Marshak et al., 1986), mouse (Sinclair and Nirenberg, 2001), and rat (Ferriero and Sagar, 1989; D'Angelo and Brecha, 1999; Oh et al., 2001), this is the first study investigating the distribution of any of the Y-receptors in the retina. There are several lines of evidence suggesting that NPY may have a physiological role in the retina: (1) the presence of NPY in amacrine cells in all mammalian retinas (Bruun et al., 1984; Marshak et al., 1986; Tornqvist and Ehinger, 1988; Ferriero and Sagar, 1989; Li and Lam, 1990; Hutsler et al., 1993; Jen et al., 1994; Hutsler and Chalupa, 1994, 1995; D'Angelo and Brecha, 1999; Oh et al., 2001; Sinclair and Nirenberg, 2001) suggests that this peptide may function as a modulator or transmitter as it does in other systems (Lundberg et al., 1996; Munglani et al., 1996; Balasubramaniam, 1997; Blomqvist and Herzog, 1997; Michel et al., 1998), (2) NPY release is induced with light flashes or potassium depolarization in the frog retina in a calcium-dependent manner (Bruun et al., 1991), (3) exogenously applied NPY affected the release of [3H]glycine, [3H]dopamine, [3H]5-hydroxytryptamine, and [3H]choline chloride–derived radioactivity in rabbit retina and of [3H]GABA, [3H]5-hydroxytryptamine, and [3H]choline chloride–derived radioactivity in chicken retina (Bruun and Ehinger, 1993), and (4) exogenously applied NPY inhibits adenylyl cyclase activity in the rabbit retina (Bruun et al., 1994). The present study showed the cellular localization of the NPY receptor Y1, indicating several possible sites of action of NPY in the retina, and furthermore, suggests that NPY acts directly on cholinergic amacrine cells and horizontal cells to affect the release of acetylcholine and GABA.
Horizontal cells express Y1
Y1 immunoreactivity was observed in the rat outer retina and localized to horizontal cell bodies in the distal INL and their processes in the OPL. The localization of Y1 immunoreactivity to horizontal cells and processes was confirmed in the double-label immunohistochemical experiments using CaBP, a well-established marker for horizontal cells (Röhrenbeck et al., 1987; Uesugi et al., 1992). Nearly all (96%) CaBP-immunoreactive cells contained Y1 immunoreactivity, suggesting that all horizontal cells express Y1 receptor in the rat retina.
In other neuronal systems, Y1 receptor is primarily coupled to inhibitory G-proteins and its activation leads to the inhibition of adenylyl cyclase (Lundberg et al., 1996; Munglani et al., 1996; Balasubramaniam, 1997; Blomqvist and Herzog, 1997; Michel et al., 1998; Sun and Miller, 1999; Sun et al., 2001). Other inhibitory G-protein–coupled receptors are expressed by horizontal cells in the mammalian retina, and they have several actions, including the coupling and uncoupling of horizontal cells by modulating gap junctions (Lasater and Dowling, 1985; He et al., 2000). Dopamine receptors are expressed by mammalian horizontal cells (Lasater and Dowling, 1985; He et al., 2000) and activation of Gi/Go-coupled dopamine receptors expressed by horizontal cells decreases the electrical coupling between horizontal cells by activating adenylyl cyclase, which results in the closure of gap junctions (He et al., 2000.) Perhaps Y1 receptors could also modulate horizontal cell coupling by inhibiting adenylyl cyclase. NPY, acting on Y1 receptors, could also modulate horizontal cell properties by modulating K+ and Ca2+ channels expressed by horizontal cells, as it does in other systems (Sun and Miller, 1999; Sun et al., 2001).
Cholinergic amacrine cells express Y1
In the inner retina, Y1 immunoreactivity was localized to dense plexuses in strata 2 and 4 of the IPL. Double-label experiments with cholinergic amacrine cell markers ChAT and VAChT showed that Y1 immunoreactivity in these strata was localized to cholinergic amacrine cell processes (Voigt, 1986; Sawaguchi et al., 1999).
Y1 is coupled to inhibitory G-proteins in other tissues and in cell lines (Lundberg et al., 1996; Munglani et al., 1996; Balasubramaniam, 1997; Blomqvist and Herzog, 1997; Michel et al., 1998), and presumably, it is also coupled to Gi/Go proteins on cholinergic amacrine cell processes where it could modulate neurotransmitter release from these cells. An inhibitory action on neurotransmitter release from these cells is inconsistent with early observations that NPY stimulates the release of [3H]choline chloride from the rabbit and chicken retinas (Bruun and Ehinger, 1993). Perhaps the action of NPY is on multiple circuits and the overall effect of NPY overrides any specific inhibition the Y1 receptor has at this synapse. Alternatively, Y1 may not be coupled to inhibitory G-proteins in these cells. Cholinergic amacrine cells have been implicated in directional selectivity; however, their exact role is not fully understood (Kittila and Massey, 1997; He and Masland, 1997; Grzywacz et al., 1998; Tjepkes and Amthor, 2000; Yoshida et al., 2001). Our present findings suggest NPY acting by means of Y1 receptors could also have an influence on the response properties of these cells, perhaps to produce long-lasting changes in the cholinergic plexus.
Synaptic connectivity of Y1-immunoreactive amacrine cells in the IPL
Ultrastructurally, Y1 immunoreactivity appeared as an electron-dense reaction product that was associated with intracellular membranes, the amorphous cytoplasmic matrix and synaptic vesicles. Intracellular membrane immunolabeling occurred as expected, because the Y1 antibody is directed against an intracellular loop of the Y1 receptor. Intracellular labeling with the Y1 receptor antibody could also be due to the presumptive pool of newly synthesized Y1 receptor and any Y1 receptor internalization, which has been demonstrated for this receptor (Parker et al., 2001). Intracellular labeling is also due to the diffusion of the immunolabel associated with the postembedding processing of the retina for electron microscopy.
Y1-immunoreactive amacrine cell processes receive synaptic input from amacrine cell processes and bipolar cell axon terminals. Most synaptic inputs to Y1-immunoreactive processes are from amacrine cells (65%). Bipolar cell synaptic inputs account for 35% of the total observed. In addition, rod bipolar cell terminals, easily identified by their cytologic characteristics in mammalian retinae (Kolb and Famiglietti, 1974; Famiglietti and Kolb, 1975; Kolb, 1979; McGuire et al., 1984, 1986), did not synapse onto Y1-immunoreactive amacrine cell processes. These findings indicate that Y1-immunoreactive amacrine cell processes receive bipolar cell axonal input, although which cone bipolar cell types (Euler and Wässle, 1995; Hartveit, 1996) provide this input remains to be determined.
Y1-immunoreactive amacrine cell processes make conventional synaptic contacts onto ganglion cell dendrites (54%) and unlabeled amacrine cell processes (34%) in both the ON and OFF sublaminae of the IPL (Famiglietti et al., 1977). Y1-immunolabeled amacrine cell processes did not form synaptic contacts onto bipolar cell axon terminals. These findings indicate that Y1-expressing amacrine cells influence both ON- and OFF-type ganglion cells, either directly by means of synaptic contacts onto ganglion cells or indirectly by means of other amacrine cells. The identity of the amacrine cells that are postsynaptic to Y1-immunoreactive amacrine cell processes is unknown. More than one amacrine cell type likely receives synaptic input from Y1-immunoreactive cells because Y1 cells form synaptic connections with cell processes in several different strata of the IPL.
Double-label immunohistochemical experiments with ChAT have shown that a large portion of the Y1 immunoreactivity observed in the IPL is localized to cholinergic amacrine cell processes. Several studies have examined the cholinergic plexus ultrastructurally in the mammalian retina. For example, in the rabbit retina, the principal input to cholinergic processes in the IPL is from bipolar cell axon terminals, whereas most synaptic outputs are onto ganglion cell dendrites (Famiglietti, 1983; Brandon, 1987). Another study in the rabbit retina reported synaptic contacts between amacrine cells in the cholinergic plexus (Millar and Morgan, 1987). In the monkey retina, cholinergic amacrine cells receive synaptic inputs from bipolar cells (67%) and amacrine cells (33%) and synapse onto ganglion cells (62.5%) and amacrine cells (37%; Mariani and Hersh, 1988). Our results in the rat retina generally agree; Y1-immunoreactive processes receive synaptic input from amacrine cells and bipolar cells and form outputs onto ganglion cells and unlabeled amacrine cells. Small variations in the ratios of contacts are expected because not all Y1-immunoreactive processes in the IPL are cholinergic amacrine cell processes and we have combined in our analysis all Y1-immunoreactive profiles in the IPL. Finally, an ultrastructural analysis of the cholinergic plexus in the rat retina has not been published, and we are comparing our observations with findings from other mammalian species; there could be differences in synaptic connectivity between species.
NPY as a paracrine neuromodulator in the retina
In the rat retina, there is a difference between the distribution of NPY immunoreactivity and Y1 receptor immunoreactivity (Fig. 10). NPY immunoreactivity has been localized to amacrine cells and displaced amacrine cells and their processes that ramify primarily in strata 1 and 5 of the IPL (D'Angelo and Brecha, 1999; Oh et al., 2001). In contrast, Y1 immunoreactivity is expressed by cholinergic amacrine processes localized to strata 2 and 4 of the IPL, discrete puncta in strata 4 and 5 of the IPL, and horizontal cells in the distal INL and their processes in the OPL. This difference in the distribution of NPY and its receptor, Y1, indicates that this peptide acts at a distance from its site of release. In the mouse and bovine retinas, Y-receptors are expressed in the retinal pigment epithelium (Ammar et al., 1998). It is likely that NPY released from the retina acts in a paracrine manner on Y receptors in the pigment epithelium. Other neuroactive substances, such as dopamine, somatostatin, and substance P (Witkovsky and Schutte, 1991; Casini et al., 1997; Johnson et al., 1998, 1999), are also distributed away from their receptors in the retina. For example, in the rat retina, somatostatin is localized to amacrine and displaced amacrine cells and processes that ramify primarily in strata 1 and 5 of the IPL, whereas the somatostatin receptor 2A is localized to cone photoreceptors, horizontal cells, bipolar cells, and amacrine cells (Johnson et al., 1998, 1999, 2000) and the somatostatin receptor 4 is localized to multistratified ganglion cells (Vila and Brecha, 2001). Dopamine is expressed primarily by wide-field amacrine cells with processes that ramify in strata 1 of the IPL (Nguyen-Legros et al., 1997), whereas dopamine receptors are found throughout the retina, including horizontal cells (Firth et al., 1997; Nguyen-Legros et al., 1999). These mismatches between the localization of neuropeptides and their receptors support the hypothesis that many neuroactive substances act in a paracrine manner on targets located far from the site of their release to affect various aspects of vision, including visual processing.
Fig. 10.
Diagram comparing the distribution of NPY and Y1 immunoreactivity in the rat retina. NPY immunoreactivity is localized to amacrine cells and displaced amacrine cells and their processes that ramify primarily in strata 1 and 5 of the IPL. Some processes are also present in strata 3 of the IPL (D'Angelo and Brecha, 1999). Y1 immunoreactivity is localized to horizontal cells in the distal INL and their processes in the OPL, to cholinergic or “starburst” amacrine cell processes that ramify in strata 2 and 4 of the IPL, and to discrete puncta that are primarily localized to strata 4 and 5 of the IPL. NPY, neuropeptide Y; IPL, inner plexiform layer; OPL, outer plexiform layer.
In conclusion, Y1 immunoreactivity was localized to horizontal cells and cholinergic amacrine cells. Y1-immunoreactive amacrine cell processes in the IPL receive synaptic input from amacrine cells and bipolar cells and form synaptic outputs onto ganglion cells and other amacrine cells. This expression pattern suggests that NPY acts at multiple points in retinal circuits to modulate visual information.
Acknowledgments
The authors thank Dr. J.H. Walsh (deceased) and H. Wong for the kind gift of the Y1 antibody; Dr. C. Sternini, Dr. A. Hirano, Dr. S. Stella, and J. Cueva for their comments and assistance in preparing this article; and Rebecca Jones for her technical assistance. I.D. and N.C.B. received funding from the NEI, M.H.C. received funding from the KOSEF, and N.C.B. received funding from the VA Merit Review Fund.
Grant sponsor: NEI; Grant number: EY07026; Grant number: EY04067; Grant sponsor: KOSEF: Grant number: 95-0403-04-04-3; Grant sponsor: Veterans Administration.
Literature Cited
- Ammar DA, Hughes BA, Thompson DA. Neuropeptide Y and the retinal pigment epithelium: receptor subtypes, signaling, and bioelectrical responses. Invest Ophthalmol Vis Sci. 1998;39:1870–1878. [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Elde R, Meister B. Vesicular acetylcholine transporter (VAChT) protein: a novel and unique marker for cholinergic neurons in the central and peripheral nervous systems. J Comp Neurol. 1997;378:454–467. [PubMed] [Google Scholar]
- Balasubramaniam AA. Neuropeptide Y family of hormones: receptor subtypes and antagonists. Peptides. 1997;18:445–457. doi: 10.1016/s0196-9781(96)00347-6. [DOI] [PubMed] [Google Scholar]
- Bao L, Kopp J, Zhang X, Xu ZQ, Zhang LF, Wong H, Walsh J, Hökfelt T. Localization of neuropeptide Y Y1 receptors in cerebral blood vessels. Proc Natl Acad Sci U S A. 1997;94:12661–12666. doi: 10.1073/pnas.94.23.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist AG, Herzog H. Y-receptor subtypes: how many more? Trends Biol Sci. 1997;20:294–298. doi: 10.1016/s0166-2236(96)01057-0. [DOI] [PubMed] [Google Scholar]
- Brandon C. Cholinergic neurons in the rabbit retina: dendritic branching and ultrastructural connectivity. Brain Res. 1987;426:119–130. doi: 10.1016/0006-8993(87)90431-8. [DOI] [PubMed] [Google Scholar]
- Bruun A, Ehinger B. NPY induced neurotransmitter release from the rabbit and chicken retina. Acta Ophthalmol. 1993;71:590–596. doi: 10.1111/j.1755-3768.1993.tb04647.x. [DOI] [PubMed] [Google Scholar]
- Bruun A, Ehinger B, Sundler F, Tornqvist K, Uddman R. Neuropeptide Y immunoreactive neurons in the guinea-pig uvea and retina. Invest Ophthalmol Vis Sci. 1984;25:1113–1123. [PubMed] [Google Scholar]
- Bruun A, Ehinger B, Ekman R. Characterization of neuropeptide Y-like immunoreactivity in vertebrate retina. Exp Eye Res. 1991;53:539–543. doi: 10.1016/0014-4835(91)90171-a. [DOI] [PubMed] [Google Scholar]
- Bruun A, Edvinsson L, Ehinger B. Neuropeptide Y inhibits adenylyl cyclase activity in rabbit retina. Acta Ophthalmol (Copenh) 1994;72:326–331. doi: 10.1111/j.1755-3768.1994.tb02767.x. [DOI] [PubMed] [Google Scholar]
- Casini G, Rickman DW, Sternini C, Brecha NC. Neurokinin 1 receptor expression in the rat retina. J Comp Neurol. 1997;389:496–507. [PMC free article] [PubMed] [Google Scholar]
- Chun MH, Han SH, Chung JW, Wässle H. Electron microscopic analysis of the rod pathway of the rat retina. J Comp Neurol. 1993;332:421–432. doi: 10.1002/cne.903320404. [DOI] [PubMed] [Google Scholar]
- D'Angelo I, Brecha NC. Neuropeptide Y and neuropeptide Y1 receptor expression in the rat retina. Invest Ophthalmol Vis Sci. 1999;40:S439. [Google Scholar]
- Dubin MW. The inner plexiform layer of the vertebrate retina: a quantitative and comparative electron microscopic analysis. J Comp Neurol. 1970;140:479–505. doi: 10.1002/cne.901400406. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Jacques D, Bouchard P, Quirion R. Species differences in the expression and distribution of the neuropeptide Y Y1, Y2, Y4, and Y5 receptors in rodents, guinea pig, and primates brains. J Comp Neurol. 1998;402:372–384. [PubMed] [Google Scholar]
- Eckenstein F, Thoenen H. Production of specific antisera and monoclonal antibodies to choline acetyltransferase: characterization and use for identification of cholinergic neurons. EMBO J. 1982;1:363–368. doi: 10.1002/j.1460-2075.1982.tb01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol. 1995;36:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV., Jr On and off pathways through amacrine cells in mammalian retina: the synaptic connections of “starburst” amacrine cells. Vision Res. 1983;23:1265–1279. doi: 10.1016/0042-6989(83)90102-5. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Kolb H. A bistratified amacrine cell and synaptic circuitry in the inner plexiform layer of the retina. Brain Res. 1975;84:293–300. doi: 10.1016/0006-8993(75)90983-x. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV, Kaneko A, Tachibana M. Neuronal architecture of on and off pathways to ganglion cells of the carp retina. Science. 1977;198:1267–1269. doi: 10.1126/science.73223. [DOI] [PubMed] [Google Scholar]
- Ferriero DM, Sagar SM. Development of neuropeptide Y-immunoreactive neurons in the rat retina. Dev Brain Res. 1989;48:19–26. doi: 10.1016/0165-3806(89)90090-4. [DOI] [PubMed] [Google Scholar]
- Firth SI, Morgan IG, Boelen MK. Localization of D1 dopamine receptors in the chicken retina. Aust N Z J Ophthalmol. 1997;25(Suppl 1):S64–S66. doi: 10.1111/j.1442-9071.1997.tb01760.x. [DOI] [PubMed] [Google Scholar]
- Freed MA, Smith RG, Sterling P. Rod bipolar array in the cat retina: pattern of input from rods and GABA-accumulating amacrine cells. J Comp Neurol. 1987;266:445–455. doi: 10.1002/cne.902660310. [DOI] [PubMed] [Google Scholar]
- Furtinger S, Pirker S, Czech T, Baumgartner C, Ransmayr G, Sperk G. Plasticity of Y1 and Y2 receptors and neuropeptide Y fibers in patients with temporal lobe epilepsy. J Neurosci. 2001;21:5804–5812. doi: 10.1523/JNEUROSCI.21-15-05804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackenheimer SL, Schober DA, Gehlert DR. Characterization of neuropeptide Y Y1-like and Y2-like receptor subtypes in the mouse brain. Peptides. 2001;22:335–341. doi: 10.1016/s0196-9781(01)00335-7. [DOI] [PubMed] [Google Scholar]
- Grzywacz NM, Amthor FR, Merwine DK. Necessity of acetylcholine for retinal directionally selective responses to drifting gratings in rabbit. J Physiol. 1998;512:575–581. doi: 10.1111/j.1469-7793.1998.575be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartveit E. Membrane currents evoked by ionotropic glutamate receptor agonists in rod bipolar cells in the rat retinal slice preparation. J Neurophysiol. 1996;76:401–422. doi: 10.1152/jn.1996.76.1.401. [DOI] [PubMed] [Google Scholar]
- He S, Masland RH. Retinal direction selectivity after targeted laser ablation of starburst amacrine cells. Nature. 1997;389:378–382. doi: 10.1038/38723. [DOI] [PubMed] [Google Scholar]
- He S, Weiler R, Vaney DI. Endogenous dopaminergic regulation of horizontal cell coupling in the mammalian retina. J Comp Neurol. 2000;418:33–40. doi: 10.1002/(sici)1096-9861(20000228)418:1<33::aid-cne3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Hökfelt T, Broberger C, Zhang X, Diez M, Kopp J, Xu Z, Landry M, Bao L, Schalling M, Koistinaho J, DeArmond SJ, Prusiner S, Gong J, Walsh JH. Neuropeptide Y: some viewpoints on a multifaceted peptide in the normal and diseased nervous system. Brain Res Brain Res Rev. 1998;26:154–166. doi: 10.1016/s0165-0173(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Chalupa LM. Neuropeptide Y immunoreactivity identifies a regularly arrayed group of amacrine cells within the cat retina. J Comp Neurol. 1994;346:481–489. doi: 10.1002/cne.903460402. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, Chalupa LM. Development of Neuropeptide Y immunoreactive amacrine and ganglion cells in the pre- and postnatal cat retina. J Comp Neurol. 1995;361:152–164. doi: 10.1002/cne.903610112. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ, White CA, Chalupa LM. Neuropeptide Y immunoreactivity identifies a group of gamma-type retinal ganglion cells in the cat retina. J Comp Neurol. 1993;336:468–480. doi: 10.1002/cne.903360311. [DOI] [PubMed] [Google Scholar]
- Jacques D, Dumont Y, Fournier A, Quirion R. Characterization of neuropeptide Y receptor subtypes in the normal human brain, including the hypothalamus. Neuroscience. 1997;79:129–148. doi: 10.1016/s0306-4522(96)00639-2. [DOI] [PubMed] [Google Scholar]
- Jen PYP, Li WWY, Yew DT. Immunohistochemical localization of neuropeptide Y and somatostatin in human fetal retina. Neuroscience. 1994;60:727–735. doi: 10.1016/0306-4522(94)90500-2. [DOI] [PubMed] [Google Scholar]
- Johnson J, Wong H, Walsh JH, Brecha NC. Expression of the somatostatin subtype 2A receptor in the rabbit retina. J Comp Neurol. 1998;393:93–101. doi: 10.1002/(sici)1096-9861(19980330)393:1<93::aid-cne9>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Wu V, Wong H, Walsh JH, Brecha NC. Somatostatin receptor subtype 2A expression in the rat retina. Neuroscience. 1999;94:675–683. doi: 10.1016/s0306-4522(99)00170-0. [DOI] [PubMed] [Google Scholar]
- Johnson J, Rickman DW, Brecha NC. Somatostatin and somatostatin subtype 2A expression in the mammalian retina. Microsc Res Tech. 2000;50:103–111. doi: 10.1002/1097-0029(20000715)50:2<103::AID-JEMT2>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Kang WS, Lim MY, Lee EJ, Kim IB, Oh SJ, Brecha NC, Park CB, Chun MH. Light- and electron-microscopic analysis of neuropeptide Y-immunoreactive amacrine cells in the guinea pig retina. Cell Tissue Res. 2001;306:363–371. doi: 10.1007/s004410100460. [DOI] [PubMed] [Google Scholar]
- Katsetos CD, Jami MM, Krishna L, Jackson R, Patchefsky AS, Cooper HS. Novel immunohistochemical localization of 28,000 molecular-weight (Mr) calcium binding protein (calbindin-D28k) in enterochro-maffin cells of the human appendix and neuroendocrine tumors (carcinoids and small-cell carcinomas) of the midgut and foregut. Arch Pathol Lab Med. 1994;118:633–639. [PubMed] [Google Scholar]
- Kim IB, Lee EJ, Kim MK, Park DK, Chun MH. Choline acetyltransferase-immunoreactive neurons in the developing rat retina. J Comp Neurol. 2000;427:604–616. doi: 10.1002/1096-9861(20001127)427:4<604::aid-cne8>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Kittila CA, Massey SC. Pharmacology of directionally selective ganglion cells in the rabbit retina. J Neurophysiol. 1997;77:675–689. doi: 10.1152/jn.1997.77.2.675. [DOI] [PubMed] [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979;8:295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of the cat retina. Science. 1974;186:47–49. doi: 10.1126/science.186.4158.47. [DOI] [PubMed] [Google Scholar]
- Koulen P. Vesicular acetylcholine transporter (VAChT): a cellular marker in rat retinal development. Neuroreport. 1997;8:2845–2848. doi: 10.1097/00001756-199709080-00008. [DOI] [PubMed] [Google Scholar]
- Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci U S A. 1985;82:3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HB, Lam DMK. Localization of neuropeptide immunoreactive neurons in the human retina. Brain Res. 1990;522:30–36. doi: 10.1016/0006-8993(90)91573-y. [DOI] [PubMed] [Google Scholar]
- Lundberg JM, Modin A, Malmstrom RE. Recent developments with neuropeptide Y receptor antagonists. Trends Pharmacol Sci. 1996;17:301–304. [PubMed] [Google Scholar]
- Mariani AP, Hersh LB. Synaptic organization of cholinergic amacrine cells in the rhesus monkey retina. J Comp Neurol. 1988;267:269–280. doi: 10.1002/cne.902670209. [DOI] [PubMed] [Google Scholar]
- Marshak D, Sharp B, Taylor I. Localization of immunoreactive corticotropin releasing factor and neuropeptide Y in macaque retina. Soc Neurosci Abstr. 1986;12:640. [Google Scholar]
- McGuire BA, Stevens J, Sterling P. Microcircuitry of bipolar cells in cat retina. J Neurosci. 1984;4:2920–2938. doi: 10.1523/JNEUROSCI.04-12-02920.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire BA, Stevens J, Sterling P. Microcircuitry of beta ganglion cells in cat retina. J Neurosci. 1986;6:907–918. doi: 10.1523/JNEUROSCI.06-04-00907.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- Millar TJ, Morgan IG. Cholinergic amacrine cells in the rabbit retina synapse onto other cholinergic amacrine cells. Neurosci Lett. 1987;74:281–285. doi: 10.1016/0304-3940(87)90310-7. [DOI] [PubMed] [Google Scholar]
- Munglani R, Hudspith MJ, Hunt SP. The therapeutic potential of Neuropeptide Y. Drugs. 1996;52:371–389. doi: 10.2165/00003495-199652030-00004. [DOI] [PubMed] [Google Scholar]
- Naveilhan P, Hassani H, Lucas G, Blakeman KH, Hao JX, Xu XJ, Wiesenfeld-Hallin Z, Thoren P, Ernfors P. Reduced antinociception and plasma extravasation in mice lacking a neuropeptide Y receptor. Nature. 2001;409:513–517. doi: 10.1038/35054063. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Savy C. Dopaminergic and GABAergic retinal cell populations in mammals. Microsc Res Tech. 1997;36:26–42. doi: 10.1002/(SICI)1097-0029(19970101)36:1<26::AID-JEMT3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Versaux-Botteri C, Vernier P. Dopamine receptor localization in the mammalian retina. Mol Neurobiol. 1999;19:181–204. doi: 10.1007/BF02821713. [DOI] [PubMed] [Google Scholar]
- Oh SJ, D'Angelo I, Lee EJ, Chun MH, Brecha NC. Distribution and synaptic connectivity of neuropeptide Y (NPY) immunoreactive amacrine cells in the rat retina. J Comp Neurol. 2002;446:219–234. doi: 10.1002/cne.10184. [DOI] [PubMed] [Google Scholar]
- Parker SL, Kane JK, Parker MS, Berglund MM, Lundell IA, Li MD. Cloned neuropeptide Y (NPY) Y1 and pancreatic polypeptide Y4 receptors expressed in Chinese hamster ovary cells show considerable agonist-driven internalization, in contrast to the NPY Y2 receptor. Eur J Biochem. 2001;268:877–886. doi: 10.1046/j.1432-1327.2001.01966.x. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Röhrenbeck J, Wässle H, Heizmann CW. Immunocytochemical labeling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neurosci Lett. 1987;77:255–260. doi: 10.1016/0304-3940(87)90508-8. [DOI] [PubMed] [Google Scholar]
- Sawaguchi A, Idate Y, Ide S, Kawano Ji, Nagaike R, Oinuma T, Suganuma T. Multistratified expression of polysialic acid and its relationship to VAChT-containing neurons in the inner plexiform layer of adult rat retina. J Histochem Cytochem. 1999;47:919–928. doi: 10.1177/002215549904700709. [DOI] [PubMed] [Google Scholar]
- Sinclair JR, Nirenberg S. Characterization of neuropeptide Y-expressing cells in the mouse retina using immunohistochemical and transgenic techniques. J Comp Neurol. 2001;432:296–306. doi: 10.1002/cne.1104. [DOI] [PubMed] [Google Scholar]
- Straznicky C, Hiscock J. Neuropeptide Y-like immunoreactivity in neurons of the human retina. Vision Res. 1989;29:1041–1048. doi: 10.1016/0042-6989(89)90051-5. [DOI] [PubMed] [Google Scholar]
- Sun L, Miller RJ. Multiple neuropeptide Y receptors regulate K+ and Ca2+ channels in acutely isolated neurons from the rat arcuate nucleus. J Neurophysiol. 1999;81:1391–1403. doi: 10.1152/jn.1999.81.3.1391. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Neuropeptide Y receptors differentially modulate G-protein-activated inwardly rectifying K+ channels and high-voltage-activated Ca2+ channels in rat thalamic neurons. J Physiol. 2001;531:67–79. doi: 10.1111/j.1469-7793.2001.0067j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, Mathe AA, Heilig M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97:12852–12857. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjepkes DS, Amthor FR. The role of NMDA channels in rabbit retinal directional selectivity. Vis Neurosci. 2000;17:291–302. doi: 10.1017/s0952523800172128. [DOI] [PubMed] [Google Scholar]
- Tornqvist K, Ehinger B. Peptide immunoreactive neurons in the human retina. Invest Ophthalmol Vis Sci. 1988;29:680–686. [PubMed] [Google Scholar]
- Ubink R, Kopp J, Wong H, Walsh JH, Pedrazzini T, Hökfelt T. Transient prenatal expression of NPY-Y1 receptor in trigeminal axons innervating the mystacial vibrissae. J Comp Neurol. 2001;429:183–191. [PubMed] [Google Scholar]
- Uesugi R, Yamada M, Mizuguchi M, Baimbridge KG, Kim SU. Calbindin D-28k and parvalbumin immunohistochemistry in developing rat retina. Exp Eye Res. 1992;54:491–499. doi: 10.1016/0014-4835(92)90127-e. [DOI] [PubMed] [Google Scholar]
- Vila A, Brecha NC. Somatostatin subtype receptor 4 expression in the mammalian retina. Soc Neurosci Abstr. 2001;31:146. [Google Scholar]
- Voigt T. Cholinergic amacrine cells in the rat retina. J Comp Neurol. 1986;248:19–35. doi: 10.1002/cne.902480103. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grunert U, Chun MH, Boycott BB. The rod pathway of the macaque monkey retina: identification of AII-amacrine cells with antibodies against calretinin. J Comp Neurol. 1995;361:537–551. doi: 10.1002/cne.903610315. [DOI] [PubMed] [Google Scholar]
- Witkovsky P, Schutte M. The organization of dopaminergic neurons in vertebrate retinas. Vis Neurosci. 1991;7:113–124. doi: 10.1017/s0952523800010981. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Miller RF. The physiology of somatostatin in the rabbit retina. J Neurosci. 1990;10:383–393. doi: 10.1523/JNEUROSCI.10-02-00383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Bao L, Xu ZQ, Kopp J, Arvidsson U, Elde R, Hökfelt T. Localization of neuropeptide Y Y1 receptors in the rat nervous system with special reference to somatic receptors on small dorsal root ganglion neurons. Proc Natl Acad Sci U S A. 1994;91:11738–11742. doi: 10.1073/pnas.91.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]


