Abstract
The vesicular γ-aminobutyric acid (GABA) transporter (VGAT), which transports the inhibitory amino acid transmitters GABA and glycine, is localized to synaptic vesicles in axon terminals. The localization of VGAT immunoreactivity to mouse and rat retina was evaluated with light and electron microscopy by using well-characterized VGAT antibodies. Specific VGAT immunoreactivity was localized to numerous varicose processes in all laminae of the inner plexiform layer (IPL) and to the outer plexiform layer (OPL). Amacrine cell somata characterized by weak VGAT immunoreactivity in the cytoplasm were located in the ganglion cell layer and proximal inner nuclear layer (INL) adjacent to the IPL. In rat retina, VGAT-immunoreactive cell bodies also contained GABA, glycine, or parvalbumin (PV) immunoreactivity, suggesting vesicular uptake of GABA or glycine by these cells. A few varicose VGAT-immunoreactive processes entered the OPL from the IPL. VGAT immunoreactivity in the OPL was predominantly localized to horizontal cell processes. VGAT and calcium binding protein-28K immunoreactivities (CaBP; a marker for horizontal cells) were colocalized in processes and terminals distributed to the OPL. Furthermore, VGAT immunoreactivity overlapped or was immediately adjacent to postsynaptic density-95 (PSD-95) immunoreactivity, which is prominent in photoreceptor terminals. Preem-bedding immunoelectron microscopy of mouse and rat retinae showed that VGAT immunoreactivity was localized to horizontal cell processes and their terminals. Immunoreactivity was distributed throughout the cytoplasm of the horizontal cell processes. Taken together, these findings demonstrate VGAT immunoreactivity in both amacrine and horizontal cell processes, suggesting these cells contain vesicles that accumulate GABA and glycine, possibly for vesicular release.
Indexing terms: retina, synaptic vesicles, GABA transport, VGAT
The vesicular γ-aminobutyric acid (GABA) transporter (VGAT) concentrates GABA and glycine into vesicles (McIntire et al., 1997; Sagne et al., 1997) and is localized to synaptic vesicles in GABA-, glycine-, and GABA/ glycine-immunoreactive nerve terminals in the rat central nervous system (Chaudhry et al., 1998; Dumoulin et al., 1999). VGAT transport of these inhibitory amino acid transmitters uses both the chemical (ΔpH) and electrical (Δψ) components of a luminal proton gradient and not a Na+ gradient like the GABA plasma membrane transporters (Cammack and Schwartz, 1993; Risso et al., 1996; McIntire et al., 1997). In addition, mutant Caenorhabditis elegans with nonfunctional VGAT exhibit an increase in the level of cytoplasmic GABA and the absence of GABA function, suggesting that VGAT transport activity is restricted to the vesicular membrane (McIntire et al., 1993).
GABA and glycine are the predominant fast, inhibitory transmitters in the inner retina. Numerous amacrine and displaced amacrine cells contain GABA or glycine and express the GABA synthetic enzymes glutamic acid decarboxylase65 and 67 (GAD65 and GAD67) and the GABA plasma membrane transporters-1 and −3 (GAT-1 and GAT-3) or the glycine plasma membrane transporter-1 (GlyT-1) (Hendrickson et al., 1988; Pourcho and Owczarzak, 1991; Johnson et al., 1996; Crook and Pow, 1997; Menger et al., 1998; Vardi et al., 1998; Haverkamp and Wässle, 2000). Furthermore, GABA- and glycine-immunoreactive amacrine cell terminals have synaptic contacts with accumulations of synaptic vesicles, suggesting that these cells release GABA or glycine by conventional vesicular mechanisms (Hendrickson et al., 1988; Chun and Wässle, 1989; Grünert and Wässle, 1990; Lopez-Costa et al., 1999).
GABA and the GAD isoforms, but not glycine, are localized to horizontal cells in several mammalian species, including the cat, rabbit, and primate (Moran et al., 1986; Mosinger and Yazulla, 1987; Chun and Wässle, 1989; Wässle and Chun, 1989; Grünert and Wässle, 1990; Perez and Davanger, 1994; Vardi et al., 1994; Johnson and Vardi, 1998; Menger et al., 1998; Haverkamp and Wässle, 2000). In rabbit and primate retina, most studies report that horizontal cells contain GABA immunoreactivity and that rabbit and primate GABA-immunoreactive horizontal cells show a nonuniform distribution (Mosinger and Yazulla, 1987; Grünert and Wässle, 1990; Pow et al., 1994; Johnson and Vardi, 1998). Furthermore, in adult cat, rabbit, and primate retina, horizontal cells contain GAD65 or GAD67 immunoreactivity or mRNA (Sarthy and Fu, 1989a,b; Vardi et al., 1994; Vardi and Auerbach, 1995; Johnson and Vardi, 1998).
The presence of GABA and GAD immunoreactivities in mouse and rat horizontal cells, however, is not certain (Schnitzer and Rusoff, 1984; Kosaka et al., 1994; Yazulla et al., 1997; Koulen et al., 1998b; Haverkamp and Wässle, 2000). Prominent GAD immunoreactivity is observed in mouse horizontal cells between birth and postnatal day 12 (Schnitzer and Rusoff, 1984) but is absent in mice older than 4 weeks of age (Yazulla et al., 1997; Haverkamp and Wässle, 2000). In adult rat horizontal cells, immunohistochemical studies indicate that GABA and GAD immunoreactivity is either weak (Brecha, 1992; Fletcher and Kalloniatis, 1997; Koulen et al., 1998b) or not present (Vaughn et al., 1981; Mosinger et al., 1986; Brecha et al., 1991; Kosaka et al., 1994; Yazulla et al., 1997). However, the presence of GABAA and GABAC receptor subunit immunoreactivity in adult rodent outer retina (Greferath et al., 1993; Enz et al., 1996; Yazulla et al., 1997; Wässle et al., 1998; Haverkamp and Wässle, 2000) supports the idea that GABA is used as a transmitter in the adult mouse and rat OPL (Vardi et al., 1992; Greferath et al., 1994b; Vardi and Sterling, 1994; Wässle et al., 1998). Horizontal cells are one possible source of GABA. A second possible source is the small number of GABA-containing interplexiform cell processes that extend into the OPL, although they are likely to be a minor source of GABA (Mosinger et al., 1986; Ryan and Hendrickson, 1987; Wässle and Boycott, 1991).
Mechanisms underlying transmitter release from mammalian horizontal cells are poorly understood. GABA may be released by a plasma membrane transporter, in a manner similar to that reported for GABA release from nonmammalian horizontal cells (Schwartz, 1982, 1987; Yazulla, 1983; Yazulla and Kleinschmidt, 1983; Cammack and Schwartz, 1993; Dong et al., 1994). In addition, turtle, fish, and rabbit horizontal cells isolated from an eye-cup preparation fail to internalize significant quantities of SR 101, a fluorescent probe used to monitor activity-dependent endocytosis events (Miller et al., 2001). This finding is supportive of studies indicating that horizontal cells do not release GABA by means of a vesicular mechanism.
Alternatively, GABA could be released in a vesicular manner from mammalian horizontal cells. In human, rabbit, and cat retina, ultrastructural studies consistently demonstrate a few synaptic specializations with small clusters of synaptic vesicles between horizontal cell process and bipolar cell dendrites and scattered vesicles throughout the cytoplasm of horizontal cell processes and their tips (Dowling et al., 1966; Raviola and Gilula, 1975; Linberg and Fisher, 1988; Brandstätter et al., 1999). Furthermore, in rat and rabbit retina, proteins implicated in vesicular transmitter release such as synaptoporin, SNAP-25, and Rab3A have been localized to horizontal cells and they are also distributed throughout these processes (Brandstätter et al., 1996; Grabs et al., 1996; Koulen et al., 1999). Clearly, further studies are needed to establish the mechanisms underlying transmitter release from mammalian horizontal cells.
The present studies have focused on the localization of VGAT immunoreactivity in the mouse and rat retina to determine the sites of GABA and glycine vesicular transport. The distribution of VGAT immunoreactivity has been determined by using polyclonal antibodies to the N-and C- termini of VGAT. These studies reveal VGAT-immunoreactive somata in the ganglion cell layer (GCL) and inner nuclear layer (INL) as well as in numerous processes distributed throughout the IPL and OPL. VGAT-immunoreactive horizontal cell processes in the OPL invaginate photoreceptor terminals. Some of the findings have been presented in abstract form.
MATERIALS AND METHODS
Tissue preparation
The retinae from adult male Sprague-Dawley rats and C57BL/6J mice were used for these studies. The Animal Research Committees of UCLA, the VAGLAHS, the Federal Government of Germany, and the Max Planck Society approved the care and handling of the animals in accordance with all institutional and NIH guidelines.
Rats and mice were deeply anesthetized with a lethal dose of Nembutal (80–90 mg/kg). Some animals were perfused through the heart with 0.1 M phosphate buffered saline (PBS; pH 7.4), followed by either 2% or 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4). Each eye was removed, dissected, and the posterior eye cup containing the retina was immediately immersed in PFA fixative for 0.5, 1, or 2 hours at room temperature. In some experiments, animals were killed with a lethal dose of Nembutal and the posterior eye cup containing the retina was dissected and immediately immersed in 4% PFA for 15 minutes. The eyes were then stored overnight in 25% sucrose in 0.1 M PB at 4°C. Cryostat sections of the retina were cut at 12–15 µm, mounted on gelatin-coated slides, air-dried, and stored at −20°C. For preembedding immunoelectron microscopy, Vibratome sections of the retina were cut at 50 µm from nonperfused, immersion-fixed retina.
Antibodies
The VGAT N-terminus fusion protein was produced in Escherichia coli transformed with a DNA construct containing the predicted 99 amino acids of the rat VGAT N-terminus fused to glutathione S-transferase (Chaudhry et al., 1998). Antibodies to the VGAT N-terminus fusion protein were raised in New Zealand White rabbits (Chaudhry et al., 1998). The VGAT antibody recognizes a single protein of the expected molecular size in Western blots of rat brain and transfected cells (Chaudhry et al., 1998). A VGAT C-terminus antibody (Chaudhry et al., 1998) and a commercially available VGAT antibody (Chemicon, Temecula, CA) were also used in these studies. A mouse monoclonal antibody against CaBP (clone CL-300; Sigma, St. Louis, MO) was used to identify horizontal cells (Röhrenbeck et al., 1987). A mouse monoclonal antibody against GABA (Sigma) was used to identify GABA-immunoreactive amacrine cells; a mouse monoclonal antibody against PV was used to identify the glycine-containing AII amacrine cells in the rat retina (Wässle et al., 1993); a rat polyclonal antibody against glycine (kindly donated by Dr. D. Pow; University of Queensland, Brisbane, Australia) was used to identify glycinergic amacrine cells and a mouse polyclonal antibody against PSD-95 (Chemicon) was used to identity photoreceptor terminals.
Immunohistochemistry
Retinal sections were incubated in affinity purified goat anti-rabbit IgG Alexa 488 or goat anti-mouse IgG tetra-methyl rhodamine Red-X (Molecular Probes, Eugene, OR, or Jackson Immuno Labs, West Grove, PA) at a dilution of 1:1,000 and 1:50, respectively, for 2 hours at room temperature and washed in 0.1 M PB. Sections were mounted if free floating and cover-slipped with a glycerol-phosphate buffer-containing 2% potassium iodide (pH 7.4) to retard fading. Double-label sections were incubated 12–48 hours in a mixture of primary antibodies to VGAT and CaBP, GABA, glycine, PV, or PSD-95 with 1% normal goat serum (NGS) and 0.5% Triton X-100 at 4°C.
Specificity of VGAT immunoreactivity was assessed by preadsorbing the VGAT antibody with 10−5 M VGAT fusion protein (Chaudhry et al., 1998) overnight at 4°C and by using the preadsorbed antibody in place of the primary antibody.
Immunoelectron microscopy
Retinal sections were frozen and thawed, embedded in agar, and cut at 50–100 µm with a Vibratome. Retinal sections were then incubated for 4 days at 4°C in the VGAT antibody that was diluted to 1:2,000 in 3% NGS, 1% bovine serum albumin (BSA), and 0.1 M PB. The sections were washed and incubated in biotinylated goat anti-rabbit avidin-biotin-peroxidase complex (Vector Labs, CA). Sections were rinsed several times in PBS and 0.05% Tris-HCl, pH 7.6 (TB), and then incubated in 0.05% di-aminobenzidine (DAB) in TB with 0.01% H2O2. Processed sections were rinsed in cacodylate buffer (0.1 M, pH 7.4) and post-fixed in 2.5% glutaraldehyde followed by silver intensification to provide a particulate appearing peroxidase product. Ultrathin sections were cut and stained with uranyl acetate and lead citrate (Brandstätter et al., 1996). Detection of immunoreactivity and microscopic analysis were performed as previously described (Sassoè-Pognetto et al., 1994).
Fluorescence and confocal microscopy
VGAT-, CaBP-, GABA-, glycine-, PV-, and PSD-95–immunoreactive somata and processes were examined in transverse sections by using conventional fluorescence microscopy. Sections were also examined by using a Zeiss Laser Scanning Microscope 410 with Plan Nufluor 40× 1.3 na, 63× 1.25 na, or 100× 1.4 na oil objectives. Confocal images were acquired at 1-µm thickness. Images prepared for presentation consisted of 4 or 5 collapsed 1-µm-thick sections. Digital images were adjusted for brightness and contrast by using Adobe Photoshop 5.5 (Adobe Systems, Inc., Mountain View, CA).
RESULTS
VGAT immunoreactivity in mouse and rat retina
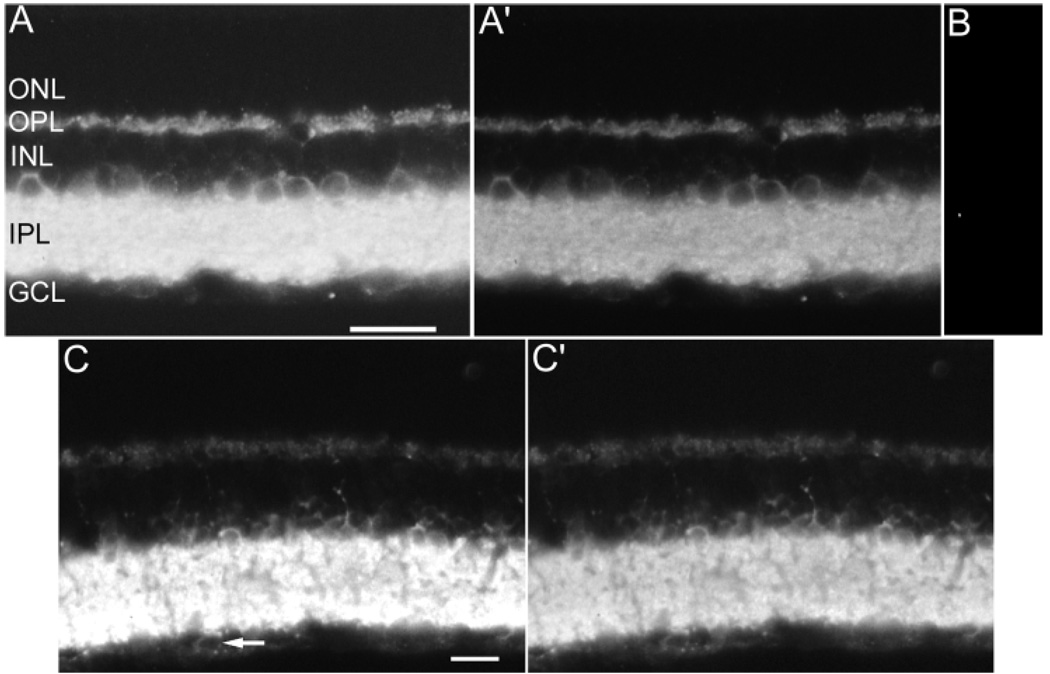
Specific VGAT immunoreactivity was present in both the inner and outer retina, and absent in the photoreceptor, outer nuclear layer, optic nerve fiber layer and optic nerve (Fig. 1A,C) in rat and mouse retinae. Immunoreactivity was observed in central and peripheral retina regions. No differences in the pattern of immunoreactivity were observed by using different fixations (2% or 4% PFA) or fixation times (0.5, 1, or 2 hours). The immunoreactive patterns with the C-terminus (Chaudhry et al., 1998) and the commercially available antibodies were identical to that obtained with the antibody to the VGAT N-terminus (data not shown). The C-terminus and commercially available antibodies produced weaker overall immunostaining than the VGAT N-terminus antibody (data not shown). The VGAT N-terminus antibody, therefore, was used throughout the study. No immunoreactivity was observed in sections incubated in the N-terminus VGAT antibody preadsorbed with the N-terminus VGAT fusion protein, further demonstrating the specificity of the VGAT antibody (Fig. 1B).
Fig. 1.
A–C′: Vesicular γ-aminobutyric acid transporter (VGAT) immunoreactivity in rat and mouse retina. A: Rat retina: VGAT immunoreactivity is present in cell somata in the INL. Immunoreactivity is also present in all laminae of the IPL and the OPL. Brightness and contrast were increased to illustrate the detail of VGAT immunoreactivity in the INL and OPL. A′: The brightness and contrast were reduced in the same rat retinal section in A to illustrate the VGAT immunoreactivity pattern in all lamina of the IPL. B: Control section; the VGAT antibody was preadsorbed with the antigen, resulting in the loss of specific immunostaining. C: Mouse retina: VGAT immunoreactivity is present with the same distribution as in rat. Brightness and contrast were maximized to show the VGAT-immunoreactivity pattern in the INL and OPL. The arrow indicates a cell body in the GCL with weak VGAT immunoreactivity. C′: The brightness and contrast were minimized in the same rat retinal section in C to illustrate the VGAT-immunoreactivity pattern in all laminae of the IPL. GCL, ganglion cell layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer. Scale bars = 20 µm in A (applies to A–B), in C (applies to C,C).
Localization of VGAT immunoreactivity to the inner retina
VGAT immunoreactivity was strongest in the IPL with numerous immunostained processes and puncta in all laminae of the IPL in both rat and mouse (Fig. 1A,C). VGAT-immunoreactive processes were densely distributed across the IPL laminae, suggesting the expression of VGAT immunoreactivity by multiple cell types with processes in one or more laminae of the IPL. In ultrastructural preparations, VGAT immunoreactivity was localized to amacrine cell processes in the IPL (data not shown).
The VGAT immunolabeled cells in the proximal INL were weakly immunostained and identified as amacrine cells based on their small size and position in the INL adjacent to the IPL (Figs. 1A,C, 2A,C,E, 4A). Rarely occurring and weakly labeled small cell bodies were in the GCL and identified as displaced amacrine cells on the basis of their size (Figs. 1C, arrow). VGAT immunoreactivity was not observed in medium or large cell bodies in the GCL.
Fig. 2.
A–D: Vesicular γ-aminobutyric acid (GABA) transporter (VGAT) immunoreactivity is present in GABA- and glycine-containing amacrine cells. Confocal fluorescent photomicrographs of vertical sections of the rat retina double labeled with antibodies for VGAT and GABA (A,B), VGAT and glycine (C,D), and VGAT and PV (E,F). VGAT (A) and GABA (B) immunoreactivity are colocalized in somata located in the proximal inner nuclear layer (INL) and ganglion cell layer (GCL). VGAT (C) and glycine (D) immunoreactivity are colocalized in two layers of amacrine cells in the proximal INL. VGAT (E) and PV (F) immunoreactivity are colocalized in glycine-containing AII amacrine cell somata and processes in the inner plexiform layer. Scale bars = 20 µm.
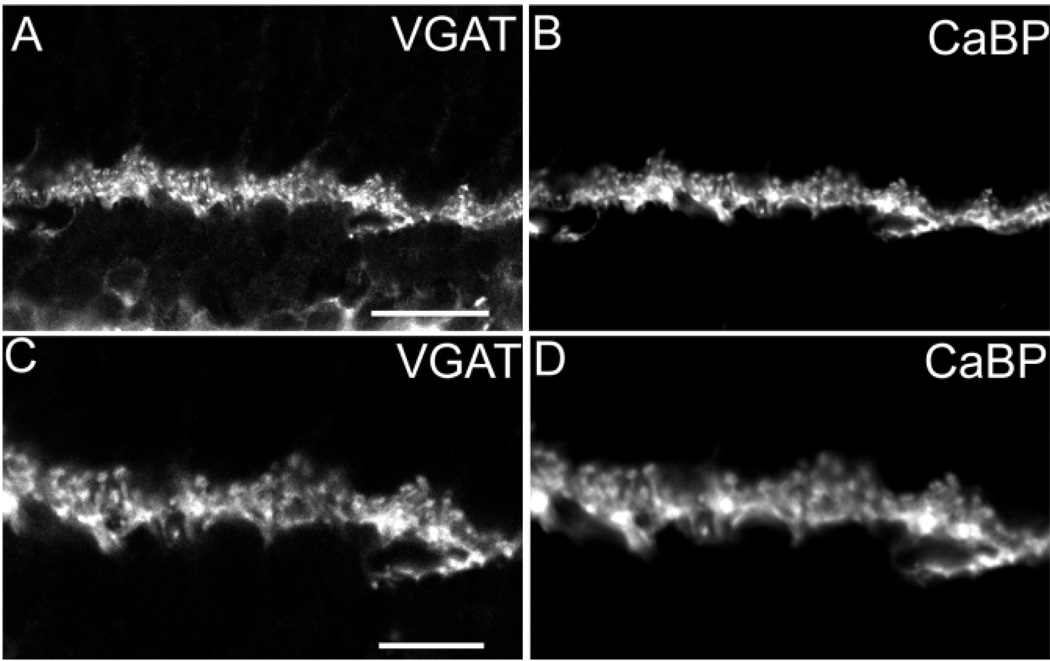
Fig. 4.
A–D: Vesicular γ-aminobutyric acid transporter (VGAT) immunoreactivity in the outer retina. Confocal fluorescent photomicrographs of a vertical section through a rat retina double labeled with antibodies to VGAT and calcium binding protein-28K (CaBP). A: Punctate VGAT immunoreactivity is present in the outer plexiform layer (OPL). B: CaBP immunoreactivity has a punctate staining pattern in the OPL virtually identical to the VGAT immunoreactivity pattern. C,D: Higher magnification of VGAT (C) and CaBP (D) immunoreactivity colocalization in the OPL. Scale bars = 20 µm in A (applies to A,B), 10 µm in C (applies to C,D).
To better demonstrate the faintly VGAT-immunoreactive amacrine cell bodies in the INL and processes in the OPL, the contrast and brightness in Figure 1A and C were increased. To better illustrate the distribution of VGAT immunoreactivity to the IPL, the contrast and brightness in Figure 1A and C were decreased. Further documentation of VGAT immunoreactivity in amacrine cell bodies is provided in Figures 2 and 3.
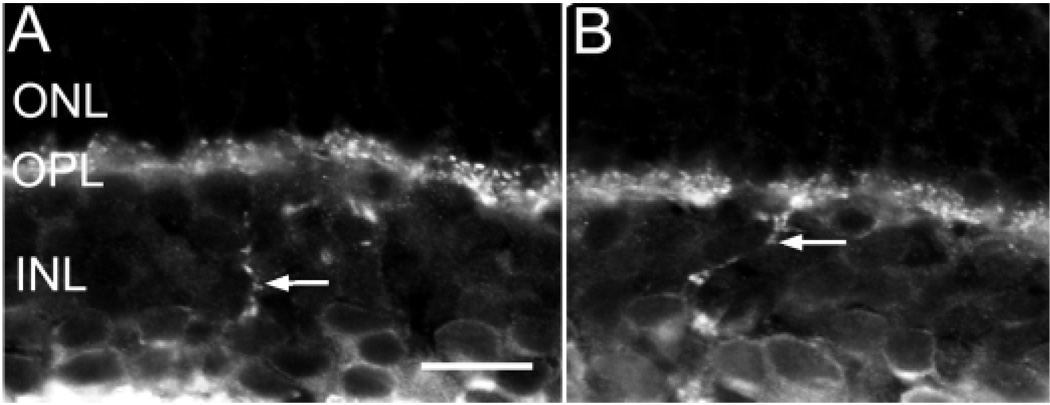
Fig. 3.
A,B: Vesicular γ-aminobutyric acid transporter (VGAT) immunoreactivity is present in amacrine cell somata and interplexiform processes (arrows) in the INL. INL, inner plexiform layer; OPL, outer plexiform layer; ONL, outer nuclear layer. Scale bar = 20 µm.
Some VGAT-immunoreactive processes were also observed to cross the INL (Fig. 3 A,B, arrow) into the OPL where they ramified in this layer. These processes were observed in all retinal regions. These observations indicate the presence of VGAT-immunoreactive interplexi-form cells.
VGAT colocalized with GABA, glycine, and PV immunoreactivity in processes and somata in the inner retina of the rat (Fig. 2A – F). Strong VGAT immunoreactivity was present in all laminae of the IPL, making the use of light microscopy to colocalize VGAT to GABA- or glycine-containing amacrine cell processes difficult. To address this problem, we focused on amacrine somata with immu-noreactivity to VGAT and one of the following antibodies: GABA, glycine, or PV. There was colocalization of weak VGAT immunoreactivity and GABA immunoreactivity in amacrine cell bodies in the INL. Some VGAT-immuno-reactive somata did not contain GABA immunoreactivity.
Double-labeling experiments were performed with VGAT and glycine (Fig. 2C,D) and with VGAT and PV (Fig. 2E,F) antibodies to further characterize the expression of VGAT in the inner retina. Glycine immunoreactivity was present in two layers of amacrine cell somata in the inner margin of the INL (Chaudhry et al., 1998; Menger et al., 1998). ON-type bipolar cells in the distal INL that receive glycine through gap junctions formed with AII amacrine cells (Vaney et al., 1998) contained weak glycine immunoreactivity. Many VGAT-immunoreactive cell somata also contained glycine immunoreactivity and all the glycine-immunoreactive amacrine cells contained VGAT immunoreactivity. No glycine-immunoreactive bipolar cells contained VGAT immunoreactivity. PV antibodies mark glycine-containing AII amacrine cells (Wässle et al., 1993). A subset of VGAT-immunoreactive amacrine cell somata contained PV immunoreactivity and all PV-immunoreactive amacrine cell somata contained VGAT immunoreactivity (Fig. 2E,F).
Localization of VGAT immunoreactivity to the outer retina
VGAT immunoreactivity in the outer retina was distributed along the distal border of the OPL (Figs. 1A, 4A,C). Immunoreactivity was localized to multiple, laterally running processes, many fine caliber processes and tips.
Individual immunoreactive puncta, presumably corresponding to horizontal cell process terminals, were located along the border of the OPL and ONL. This pattern suggests the presence of horizontal cell terminals that invaginate photoreceptor terminals (Fig. 4A,C).
Other studies have shown that CaBP immunoreactivity is localized to horizontal cell bodies and processes in the mouse and rat retina (Röhrenbeck et al., 1987; Peichl and Gonzalez-Soriano, 1994). To confirm the presence of VGAT immunoreactivity in horizontal cells, retinal sections were double labeled with antibodies to VGAT and CaBP. VGAT and CaBP immunoreactivity colocalized in primary and secondary processes and smaller processes and puncta in the most distal region of the OPL (Fig. 4A – D). VGAT immunoreactivity was in all CaBP-immunoreactive puncta, indicating the presence of VGAT in all horizontal cell processes and terminals.
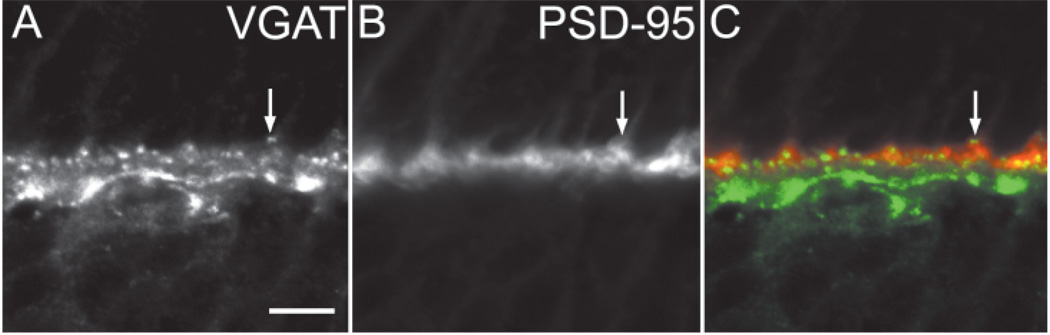
In the outer rat retina, PSD-95 immunoreactivity is localized to rod spherules (Koulen et al., 1998a). Double-label experiments with antibodies to VGAT and PSD-95 were performed to characterize the distribution of VGAT immunoreactivity in horizontal cell tips. VGAT-immunoreactive horizontal cell puncta are localized within (Fig. 5A–C, arrow) and adjacent to PSD-95–immunoreactive photoreceptor terminals (Fig. 5A – C), suggesting that VGAT is in horizontal cell tips that are embedded in photoreceptor terminals.
Fig. 5.
A–C: Vesicular γ-aminobutyric acid transporter (VGAT) and postsynaptic density-95 (PSD-95) immunoreactivity in the outer retina. VGAT immunoreactivity is found immediately adjacent to or within PSD-95 immunoreactivity. There is overlap between the distal VGAT puncta in horizontal cells and PSD-95 immunoreactivity in photoreceptor terminals (C). The arrows point to a region of the outer plexiform layer with overlap of VGAT and PSD-95 immunoreactivity. Scale bar = 10 µm in A (applies to A–C).
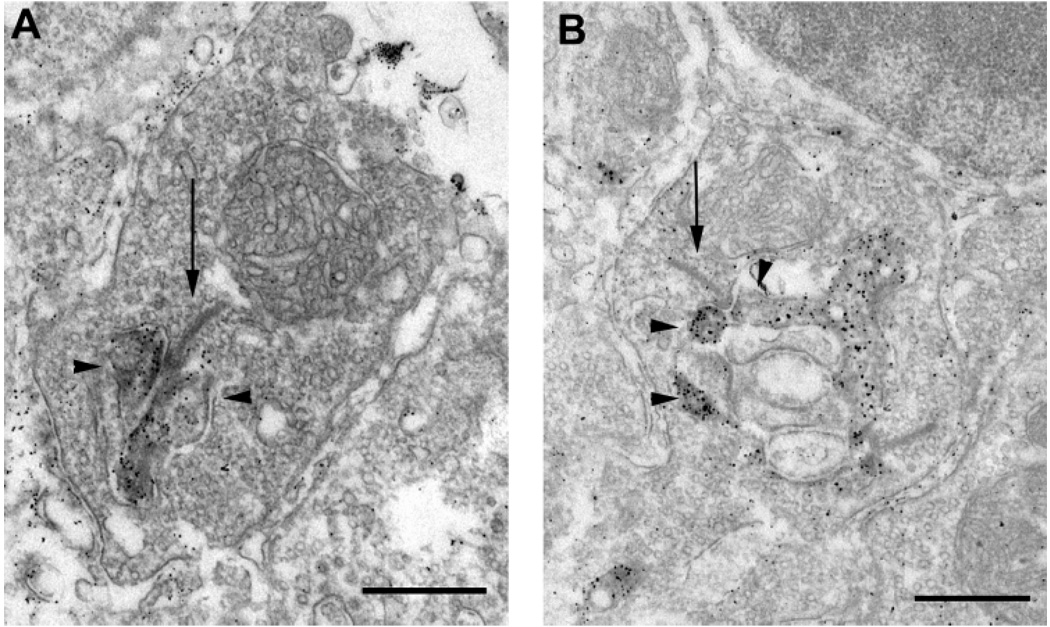
VGAT immunoreactivity was present in mouse and rat horizontal cell processes and their tips in rod spherules as demonstrated at the electron microscopic level (Fig. 6A,B). In ultrastructural preparations, VGAT immunoreactivity was characterized by a particulate appearance after silver intensification of the DAB reaction product. VGAT immunoreactivity in horizontal cell processes was restricted to the cytoplasm, and it was located near the plasma membrane. VGAT immunoreactivity was detected in horizontal cell processes that invaginated rod terminals, and they were located as lateral elements below the terminal. Neither photoreceptor terminals nor putative bipolar cell dendrites contained VGAT immunoreactivity.
Fig. 6.
A,B: Vesicular γ-aminobutyric acid transporter (VGAT) immunoreactivity in horizontal cell tips. Electron photomicrographs illustrate VGAT immunoreactivity in terminals of horizontal cells in the OPL of the mouse (A) and rat (B) retina. VGAT immunoreactivity is concentrated in the cytoplasm of both horizontal cell processes that invaginate rod spherules. Arrows indicate synaptic ribbons. Arrow-heads indicate horizontal cells. Scale bars = 0.5 µm in A,B.
The appearance of the reaction product was similar to that seen in other preembedding electron microscopy preparations by using antibodies to synaptoporin (Brand-stätter et al., 1996). Because the retina tissue structure was compromised in our preparations due to the light fixation and preembedding protocol used to demonstrate VGAT immunoreactivity, it was not possible to reliably localize VGAT immunoreactivity to cellular organelles and other intracellular structures in these preparations.
DISCUSSION
VGAT immunoreactivity is localized to displaced amacrine, amacrine, and horizontal cells in mouse and rat retina. In rabbit and primate retina, we have also observed VGAT immunoreactivity in amacrine cells and horizontal cell processes and terminals (Haverkamp et al., 2000; unpublished observations). VGAT immunoreactivity in the inner retina is most prominent in the IPL and localized to amacrine cell processes that are distributed to all laminae of the IPL. Interplexiform cells, which are considered an amacrine cell variant (Wässle and Boycott, 1991), also contain VGAT immunoreactivity. Double-label studies show that VGAT is localized to both GABA- and glycine-containing amacrine cells. Finally, the small VGAT-immunoreactive cells in the GCL are likely to be displaced amacrine cells and likely correspond to displaced amacrine cells that also express GABA, GAT-1, and GAT-3 immunoreactivity (Kosaka et al., 1994; Johnson et al., 1996; Haverkamp and Wässle, 2000). A few sparsely occurring GABA-containing ganglion cells have been reported in rat retina (Caruso et al., 1989), and we cannot completely rule out that some cells identified as displaced amacrine cells may be small ganglion cells. In the outer retina, horizontal cell processes and their tips located within photoreceptor terminals contain VGAT immunoreactivity.
The specificity of the VGAT immunoreactivity in the mouse and rat retina was confirmed by the absence of immunoreactivity observed in sections incubated with antibody preadsorbed with excess fusion peptide. In addition, the same immunoreactivity pattern was observed by using two other antibodies to VGAT, providing additional assurance of the specificity of the VGAT immunoreactivity. An additional control for the specificity of the N-terminus VGAT antibody was conducted by a BLAST search during March of 2001 by using the VGAT N-terminus protein sequence used to produce the antibody. This search did not reveal other proteins with a similar amino acid sequence.
VGAT is a membrane protein that seems to be predominately if not exclusively localized to synaptic vesicles in neuronal processes based on several different experimental observations (Chaudhry et al., 1998; Dumoulin et al., 1999). There is a similar distribution in Caenorhabditis elegans of a green fluorescent protein-tagged VGAT construct and the synaptic vesicle-related proteins, synaptobrevin, synaptotagmin, and Rab3A (McIntire et al., 1997). In addition, postembedding immunogold electron microscopic studies of inhibitory terminals in the rat central nervous system demonstrate that VGAT immunoreactivity is associated with synaptic vesicles and that it is not localized freely within the cytoplasm or to the plasma membrane (Chaudhry et al., 1998; Dumoulin et al., 1999). Furthermore, VGAT has been localized to both GABA-and glycine-immunoreactive axon terminals in double-label studies (Chaudhry et al., 1998; Dumoulin et al., 1999). Finally, antibodies to VGAT have been used to immunoisolate GABA-containing synaptic vesicles from brain homogenates, and these immunopurified vesicles transport GABA, but not other amino acids, including glutamate (Takamori et al., 2000). Other studies have shown that VGAT transports the inhibitory amino acid transmitters GABA and glycine into vesicles by using an outward H+gradient (McIntire et al., 1997). Taken together, these findings indicate that VGAT transports GABA and glycine from the cytoplasm into synaptic vesicles.
VGAT immunoreactivity is localized to amacrine cells
The general pattern of VGAT immunoreactivity in the inner retina is similar, although not identical, to the reported patterns of GABA, glycine, GAD isoforms, GAT-1 and −3, and GlyT-1 immunoreactivity in the inner retina (Brandon, 1985; Mosinger et al., 1986; Johnson et al., 1996; Menger et al., 1998). For example, VGAT immunoreactivity in amacrine cell somata in the GCL and proximal INL is very weak, with immunoreactivity restricted to the cytoplasm. In contrast, GABA and glycine immunoreactivity is prominent in the somata, and GAT-1 and GlyT-1 immunoreactivity is primarily located near or at the plasma membrane (Brecha and Weigmann, 1994; Johnson et al., 1996; Pow and Hendrickson, 1999). In the IPL, VGAT, GABA, GAD isoforms, GAT-1– and −3–, glycine-, and GlyT-1–immunoreactive processes are distributed to all laminae of the IPL, with some variations in the density of immunoreactivity in different IPL laminae (Chun and Wässle, 1989; Wässle and Chun, 1989; Vardi et al., 1994; Johnson et al., 1996; Vaney et al., 1998; Pow and Hendrickson, 1999). The VGAT-immunoreactivity pattern in the IPL is consistent with ultrastructural studies showing the presence of synaptic vesicles at chemical synapses in amacrine cell processes containing GAD, GABA, or glycine immunoreactivity (Vaughn et al., 1981; Marc and Liu, 1985; Cohen and Sterling, 1986; Hendrickson et al., 1988; Mandell et al., 1992).
The presence of VGAT immunoreactivity in glycine-immunoreactive amacrine cell bodies and processes is consistent with reports of VGAT immunoreactivity in glycine-immunoreactive processes elsewhere in the nervous system (Chaudhry et al., 1998; Dumoulin et al., 1999). Furthermore, AII amacrine cells, which contain both glycine and PV immunoreactivities in the rat retina, also contain VGAT immunoreactivity (Menger et al., 1998; Wässle et al., 1998).
Finally, the presence of VGAT immunoreactivity in fibers crossing the INL and entering the OPL are likely to originate from interplexiform cells. Interplexiform cells are also reported to contain GABA and GAT-1 immunoreactivity in the rat retina (Mosinger et al., 1986; Johnson et al., 1996).
VGAT immunoreactivity is localized to horizontal cells
GABA and GAD immunoreactivity in adult mouse and rat horizontal cells is reported as weak or not present (Brandon, 1985; Brecha, 1992; Kosaka et al., 1994; Fletcher and Kalloniatis, 1997; Yazulla et al., 1997; Koulen et al., 1998b; Haverkamp and Wässle, 2000). Furthermore, some studies have failed to detect evidence of either GAD or GABA immunoreactivity or GAD67 mRNA (Vaughn et al., 1981; Brecha et al., 1991; Kosaka et al., 1994). However, the localization of VGAT immunoreactivity to adult mouse and rat horizontal cells provides evidence for the presence of GABA in adult rodent horizontal cells. The presence of GABA in mouse and rat horizontal cells is consistent with the well-established localization of GABA to other “nonrodent” mammalian horizontal cells (Agardh et al., 1987; Mosinger and Yazulla, 1987; Ryan and Hendrickson, 1987; Chun and Wässle, 1989; Pourcho and Owczarzak, 1989; Sarthy and Fu, 1989a,b; Wässle and Chun, 1989; Grünert and Wässle, 1990; Perez and Davanger, 1994; Vardi et al., 1994; Vardi and Auerbach, 1995; Johnson and Vardi, 1998; Menger et al., 1998). Glycine has not been reported in horizontal cells of any mammalian species (Hendrickson et al., 1988; Grünert and Wässle, 1993; Greferath et al., 1994a; Grünert and Wässle, 1996; Fletcher and Kalloniatis, 1997; Pow and Hendrickson, 1999; Haverkamp and Wässle, 2000).
VGAT immunoreactivity is present in horizontal cell puncta and laterally extending processes. The colocalization of VGAT immunoreactivity with the horizontal cell marker, CaBP (Röhrenbeck et al., 1987) confirms the presence of VGAT in horizontal cell processes and tips. The double labeling of rat retina with VGAT and PSD-95, used as a marker for rod photoreceptor terminals (Koulen et al., 1998a), suggests the invagination of photoreceptor terminals by VGAT-labeled horizontal cell tips. VGAT immunoreactive horizontal cell processes are also near the base of photoreceptor terminals. Ultrastructural localization of VGAT immunoreactivity confirmed the presence of VGAT in horizontal cell processes and also showed that these processes invaginate photoreceptor terminals.
The present studies indicate that VGAT is localized to horizontal cells and is prominent in horizontal cell tips that would be in contact with photoreceptor terminals and bipolar cell dendrites. The observation of synaptic specializations between horizontal cell processes and bipolar cell dendrites, and vesicles in horizontal cell processes (Dowling et al., 1966; Raviola and Gilula, 1975; Linberg and Fisher, 1988; Brandstätter et al., 1999), although reported to be few, suggests that VGAT could transport GABA into vesicles in horizontal cells. Observations in brain and spinal cord provide support for the hypothesis that GABA is transported into vesicles in mammalian horizontal cells; these studies show that (1) VGAT immunoreactivity is localized predominantly to synaptic vesicles and not to the plasma membrane in inhibitory axon terminals, (2) VGAT antibodies immunoisolate GABA-accumulating synaptic vesicles from the nervous system (McIntire et al., 1997; Chaudhry et al., 1998; Dumoulin et al., 1999; Takamori et al., 2000), and (3) biophysical findings show that VGAT uses the electrical and chemical components of a vesicular proton gradient to transport inhibitory amino acid transmitters in vesicles (McIntire et al., 1997). Therefore, VGAT transport activity is likely restricted to cellular structures capable of establishing and maintaining a localized concentration of protons. Taken together, these findings suggest that mammalian horizontal cells likely concentrate GABA into vesicles, thus providing the possibility that GABA is released through a vesicular mechanism.
An alternative explanation is that mammalian horizontal cells might release GABA through a plasma membrane transporter-mediated mechanism. This model, based on studies in nonmammalian horizontal cells, show that Ca2+-independent, nonvesicular release occurs by means of a GABA plasma membrane transporter with pharmacology similar to GAT-1 (Schwartz, 1982, 1987; Yazulla, 1983; Yazulla and Kleinschmidt, 1983; Attwell et al., 1993; Cammack and Schwartz, 1993; Dong et al., 1994). Physiological and imaging studies indicate that a GABA plasma membrane transporter may transport GABA both into and out of the cytoplasm of horizontal cells; for example, (1) catfish horizontal cells that contain intracellular GABA, produce an outward current when depolarized (Cammack and Schwartz, 1993), (2) cell lines engineered to express GAT-1 transport GABA into and out of the cell, give an outward current at depolarized potentials that is correlated to the transport of GABA out of the cell (Cammack et al., 1994), and (3) rabbit horizontal cell processes seem to lack significant activity-dependent endocytotic events (Miller et al., 2001). However, the GABA plasma membrane transporters GAT-1, GAT-2, and GAT-3 are not localized to mouse, rat, rabbit, or primate horizontal cells (Brecha and Weigmann, 1994; Johnson et al., 1996; Hu et al., 1999). This finding is consistent with the failure to detect the uptake of GABA or the GABA agonist, muscimol, by mammalian horizontal cells (Pourcho, 1980; Hendrickson et al., 1985; Chun et al., 1988). It cannot be excluded that a plasma membrane transporter that differs from GAT-1, GAT-2, GAT-3, and VGAT could exist and mediate GABA release from horizontal cells.
Future studies are necessary to determine the function of VGAT and the mechanism of GABA release in mammalian horizontal cells. Evidence for a vesicular GABA release mechanism from horizontal cells would raise the intriguing possibility that there are different mechanisms underlying GABA release from mammalian and some nonmammalian horizontal cells, or that horizontal cells use both a vesicular and nonvesicular mechanism to release GABA.
ACKNOWLEDGMENTS
We thank M. Caravelli, I. D’Angelo, G. S. Nam, and W. Hofer for excellent technical assistance. We also thank M. Caravelli, Dr. A. Hirano, Dr. S. Stella, and Dr. C. Sternini for critical reading of the manuscript. N.C.B. and J.G.C. received funding from the NEI, N.C.B. also received a VA Career Scientist Award, J.G.C. received funding from the American Psychological Association and a Fight for Sight Research Fellowship, and H.W. was funded by the Deutsche Forschungsgemeinschaft.
Grant sponsor: NEI; Grant number: EY 04067; Grant number: EY 07026; Grant sponsor: the American Psychological Association; Grant sponsor: The Fight for Sight research division of Prevent Blindness America; Grant number: SF20015; Grant sponsor: Deutsche Forschungsgemeinschaft; Grant number: SFB 269/B4.
LITERATURE CITED
- Agardh E, Bruun A, Ehinger B, Ekstrom P, van Veen T, Wu JY. Gamma-aminobutyric acid- and glutamic acid decarboxylase-immunoreactive neurons in the retina of different vertebrates. J Comp Neurol. 1987;258:622–630. doi: 10.1002/cne.902580411. [DOI] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Brandon C. Retinal GABA neurons: localization in vertebrate species using an antiserum to rabbit brain glutamate decarboxylase. Brain Res. 1985;344:286–295. doi: 10.1016/0006-8993(85)90806-6. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Lohrke S, Morgans CW, Wässle H. Distributions of two homologous synaptic vesicle proteins, synaptoporin and synapto-physin, in the mammalian retina. J Comp Neurol. 1996;370:1–10. doi: 10.1002/(SICI)1096-9861(19960617)370:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Fletcher EL, Garner CC, Gundelfinger ED, Wässle H. Differential expression of the presynaptic cytomatrix protein bassoon among ribbon synapses in the mammalian retina. Eur J Neurosci. 1999;11:3683–3693. doi: 10.1046/j.1460-9568.1999.00793.x. [DOI] [PubMed] [Google Scholar]
- Brecha NC. Expression of GABAA receptors in the vertebrate retina. Prog Brain Res. 1992;90:3–28. doi: 10.1016/s0079-6123(08)63606-7. [DOI] [PubMed] [Google Scholar]
- Brecha NC, Weigmann C. Expression of GAT-1, a high-affinity gamma-aminobutyric acid plasma membrane transporter in the rat retina. J Comp Neurol. 1994;345:602–611. doi: 10.1002/cne.903450410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecha NC, Sternini C, Humphrey MF. Cellular distribution of L-glutamate decarboxylase (GAD) and gamma- aminobutyric acid A (GABAA) receptor mRNAs in the retina. Cell Mol Neurobiol. 1991;11:497–509. doi: 10.1007/BF00734812. [DOI] [PubMed] [Google Scholar]
- Cammack JN, Schwartz EA. Ions required for the electrogenic transport of GABA by horizontal cells of the catfish retina. J Physiol (Lond) 1993;472:81–102. doi: 10.1113/jphysiol.1993.sp019938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack JN, Rakhilin SV, Schwartz EA. A GABA transporter operates asymmetrically and with variable stoichiometry. Neuron. 1994;13:949–960. doi: 10.1016/0896-6273(94)90260-7. [DOI] [PubMed] [Google Scholar]
- Caruso DM, Owczarzak MT, Goebel DJ, Hazlett JC, Pourcho RG. GABA-immunoreactivity in ganglion cells of the rat retina. Brain Res. 1989;476:129–134. doi: 10.1016/0006-8993(89)91544-8. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Bellocchio EE, Danbolt NC, Osen KK, Edwards RH, Storm-Mathisen J. The vesicular GABA transporter, VGAT, localizes to synaptic vesicles in sets of glycinergic as well as GABAergic neurons. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun MH, Wässle H. GABA-like immunoreactivity in the cat retina: electron microscopy. J Comp Neurol. 1989;279:55–67. doi: 10.1002/cne.902790106. [DOI] [PubMed] [Google Scholar]
- Chun MH, Wässle H, Brecha N. Colocalization of [3H]muscimol uptake and choline acetyltransferase immunoreactivity in amacrine cells of the cat retina. Neurosci Lett. 1988;94:259–263. doi: 10.1016/0304-3940(88)90027-4. [DOI] [PubMed] [Google Scholar]
- Cohen E, Sterling P. Accumulation of (3H)glycine by cone bipolar neurons in the cat retina. J Comp Neurol. 1986;250:1–7. doi: 10.1002/cne.902500102. [DOI] [PubMed] [Google Scholar]
- Crook DK, Pow DV. Analysis of the distribution of glycine and GABA in amacrine cells of the developing rabbit retina: a comparison with the ontogeny of a functional GABA transport system in retinal neurons. Vis Neurosci. 1997;14:751–763. doi: 10.1017/s0952523800012700. [DOI] [PubMed] [Google Scholar]
- Dong CJ, Picaud SA, Werblin FS. GABA transporters and GABAC-like receptors on catfish cone- but not rod-driven horizontal cells. J Neurosci. 1994;14:2648–2658. doi: 10.1523/JNEUROSCI.14-05-02648.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Brown JE, Major D. Synapses of horizontal cells in rabbit and cat retinas. Science. 1966;153:1639–1641. doi: 10.1126/science.153.3744.1639. [DOI] [PubMed] [Google Scholar]
- Dumoulin A, Rostaing P, Bedet C, Levi S, Isambert MF, Henry JP, Triller A, Gasnier B. Presence of the vesicular inhibitory amino acid transporter in GABAergic and glycinergic synaptic terminal boutons. J Cell Sci. 1999;112:811–823. doi: 10.1242/jcs.112.6.811. [DOI] [PubMed] [Google Scholar]
- Enz R, Brandstätter JH, Wässle H, Bormann J. Immunocytochemical localization of the GABA c receptor rho subunits in the mammalian retina. J Neurosci. 1996;16:4479–4490. doi: 10.1523/JNEUROSCI.16-14-04479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher EL, Kalloniatis M. Localisation of amino acid neurotransmitters during postnatal development of the rat retina. J Comp Neurol. 1997;380:449–471. doi: 10.1002/(sici)1096-9861(19970421)380:4<449::aid-cne3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Grabs D, Bergmann M, Urban M, Post A, Gratzl M. Rab3 proteins and SNAP-25, essential components of the exocytosis machinery in conventional synapses, are absent from ribbon synapses of the mouse retina. Eur J Neurosci. 1996;8:162–168. doi: 10.1111/j.1460-9568.1996.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Greferath U, Müller F, Wässle H, Shivers B, Seeburg P. Localization of GABAA receptors in the rat retina. Vis Neurosci. 1993;10:551–561. doi: 10.1017/s0952523800004764. [DOI] [PubMed] [Google Scholar]
- Greferath U, Brandstätter JH, Wässle H, Kirsch J, Kuhse J, Grünert U. Differential expression of glycine receptor subunits in the retina of the rat: a study using immunohistochemistry and in situ hybridization. Vis Neurosci. 1994a;11:721–729. doi: 10.1017/s0952523800003023. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Müller F, Wässle H. Localization of GABAA receptors in the rabbit retina. Cell Tissue Res. 1994b;276:295–307. doi: 10.1007/BF00306115. [DOI] [PubMed] [Google Scholar]
- Grünert U, Wässle H. GABA-like immunoreactivity in the macaque monkey retina: a light and electron microscopic study. J Comp Neurol. 1990;297:509–524. doi: 10.1002/cne.902970405. [DOI] [PubMed] [Google Scholar]
- Grünert U, Wässle H. Immunocytochemical localization of glycine receptors in the mammalian retina. J Comp Neurol. 1993;335:523–537. doi: 10.1002/cne.903350405. [DOI] [PubMed] [Google Scholar]
- Grünert U, Wässle H. Glycine receptors in the rod pathway of the macaque monkey retina. Vis Neurosci. 1996;13:101–115. doi: 10.1017/s0952523800007161. [DOI] [PubMed] [Google Scholar]
- Haverkamp S, Wässle H. Immunocytochemical analysis of the mouse retina. J Comp Neurol. 2000;424:1–23. [PubMed] [Google Scholar]
- Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- Hendrickson A, Ryan M, Noble B, Wu JY. Colocalization of [3H]muscimol and antisera to GABA and glutamic acid decarboxylase within the same neurons in monkey retina. Brain Res. 1985;348:391–396. doi: 10.1016/0006-8993(85)90464-0. [DOI] [PubMed] [Google Scholar]
- Hendrickson AE, Koontz MA, Pourcho RG, Sarthy PV, Goebel DJ. Localization of glycine-containing neurons in the Macaca monkey retina. J Comp Neurol. 1988;273:473–487. doi: 10.1002/cne.902730404. [DOI] [PubMed] [Google Scholar]
- Hu M, Bruun A, Ehinger B. Expression of GABA transporter subtypes (GAT1, GAT3) in the adult rabbit retina. Acta Ophthalmol Scand. 1999;77:255–260. doi: 10.1034/j.1600-0420.1999.770302.x. [DOI] [PubMed] [Google Scholar]
- Johnson J, Chen TK, Rickman DW, Evans C, Brecha NC. Multiple gamma-aminobutyric acid plasma membrane transporters (GAT-1, GAT-2, GAT-3) in the rat retina. J Comp Neurol. 1996;375:212–224. doi: 10.1002/(SICI)1096-9861(19961111)375:2<212::AID-CNE3>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MA, Vardi N. Regional differences in GABA and GAD immunoreactivity in rabbit horizontal cells. Vis Neurosci. 1998;15:743–753. doi: 10.1017/s0952523898154135. [DOI] [PubMed] [Google Scholar]
- Kosaka J, Morii E, Taniguchi M, Kitamura Y, Nomura S, Fukuda Y. Expression and localization of gamma-aminobutyric acid A (GABAA) receptor alpha 1 subunit and L-glutamate decarboxylase (GAD) mRNAs in rat retina: an analysis by in situ hybridization. Brain Res Mol Brain Res. 1994;25:163–167. doi: 10.1016/0169-328x(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Koulen P, Malitschek B, Kuhn R, Bettler B, Wässle H, Brandstätter JH. Presynaptic and postsynaptic localization of GABA(B) receptors in neurons of the rat retina. Eur J Neurosci. 1998b;10:1446–1456. doi: 10.1046/j.1460-9568.1998.00156.x. [DOI] [PubMed] [Google Scholar]
- Koulen P, Fletcher EL, Craven SE, Bredt DS, Wässle H. Immunocytochemical localization of the postsynaptic density protein PSD-95 in the mammalian retina. J Neurosci. 1998a;18:10136–10149. doi: 10.1523/JNEUROSCI.18-23-10136.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen P, Kuhn R, Wässle H, Brandstätter JH. Modulation of the intracellular calcium concentration in photoreceptor terminals by a presynaptic metabotropic glutamate receptor. Proc Natl Acad Sci U S A. 1999;96:9909–9914. doi: 10.1073/pnas.96.17.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linberg KA, Fisher SK. Ultrastructural evidence that horizontal cell axon terminals are presynaptic in the human retina. J Comp Neurol. 1988;268:281–297. doi: 10.1002/cne.902680211. [DOI] [PubMed] [Google Scholar]
- Lopez-Costa JJ, Goldstein J, Pecci-Saavedra J, Della Maggiore VM, De Las Heras MA, Sarmiento MI, Rosenstein RE. GABA release mechanism in the golden hamster retina. Int J Neurosci. 1999;98:13–25. doi: 10.3109/00207459908994792. [DOI] [PubMed] [Google Scholar]
- Mandell JW, Czernik AJ, De Camilli P, Greengard P, Townes-Anderson E. Differential expression of synapsins I and II among rat retinal synapses. J Neurosci. 1992;12:1736–1749. doi: 10.1523/JNEUROSCI.12-05-01736.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc RE, Liu WL. (3H) glycine-accumulating neurons of the human retina. J Comp Neurol. 1985;232:241–260. doi: 10.1002/cne.902320209. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Horvitz HR. Genes required for GABA function in Caenorhabditis elegans. Nature. 1993;364:334–337. doi: 10.1038/364334a0. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature. 1997;389:870–876. doi: 10.1038/39908. [DOI] [PubMed] [Google Scholar]
- Menger N, Pow DV, Wässle H. Glycinergic amacrine cells of the rat retina. J Comp Neurol. 1998;401:34–46. doi: 10.1002/(sici)1096-9861(19981109)401:1<34::aid-cne3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Miller RF, Fagerson MH, Staff NP, Wolfe R, Doerr T, Gottesman J, Sikora MA, Schuneman R. Structure and functional connections of presynaptic terminals in the vertebrate retina revealed by activity-dependent dyes and confocal microscopy. J Comp Neurol. 2001;437:129–155. doi: 10.1002/cne.1275. [DOI] [PubMed] [Google Scholar]
- Moran J, Pasantes-Morales H, Redburn DA. Glutamate receptor agonists release [3H]GABA preferentially from horizontal cells. Brain Res. 1986;398:276–287. doi: 10.1016/0006-8993(86)91487-3. [DOI] [PubMed] [Google Scholar]
- Mosinger J, Yazulla S. Double-label analysis of GAD- and GABA-like immunoreactivity in the rabbit retina. Vision Res. 1987;27:23–30. doi: 10.1016/0042-6989(87)90139-8. [DOI] [PubMed] [Google Scholar]
- Mosinger JL, Yazulla S, Studholme KM. GABA-like immunoreactivity in the vertebrate retina: a species comparison. Exp Eye Res. 1986;42:631–644. doi: 10.1016/0014-4835(86)90052-7. [DOI] [PubMed] [Google Scholar]
- Peichl L, Gonzalez-Soriano J. Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci. 1994;11:501–517. doi: 10.1017/s095252380000242x. [DOI] [PubMed] [Google Scholar]
- Perez MT, Davanger S. Distribution of GABA immunoreactivity in kainic acid-treated rabbit retina. Exp Brain Res. 1994;100:227–238. doi: 10.1007/BF00227193. [DOI] [PubMed] [Google Scholar]
- Pourcho RG. Uptake of [3H]glycine and [3H]GABA by amacrine cells in the cat retina. Brain Res. 1980;198:33–46. doi: 10.1016/0006-8993(80)90748-9. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Owczarzak MT. Distribution of GABA immunoreactivity in the cat retina: a light- and electron-microscopic study. Vis Neurosci. 1989;2:425–435. doi: 10.1017/s0952523800012323. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Owczarzak MT. Glycine receptor immunoreactivity is localized at amacrine synapses in cat retina. Vis Neurosci. 1991;7:611–618. doi: 10.1017/s0952523800010397. [DOI] [PubMed] [Google Scholar]
- Pow DV, Hendrickson AE. Distribution of the glycine transporter glyt-1 in mammalian and nonmammalian retinae. Vis Neurosci. 1999;16:231–239. doi: 10.1017/s0952523899162047. [DOI] [PubMed] [Google Scholar]
- Pow DV, Crook DK, Wong RO. Early appearance and transient expression of putative amino acid neurotransmitters and related molecules in the developing rabbit retina: an immunocytochemical study. Vis Neurosci. 1994;11:1115–1134. doi: 10.1017/s0952523800006933. [DOI] [PubMed] [Google Scholar]
- Raviola E, Gilula NB. Intramembrane organization of specialized contacts in the outer plexiform layer of the retina. A freeze-fracture study in monkeys and rabbits. J Cell Biol. 1975;65:192–222. doi: 10.1083/jcb.65.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risso S, DeFelice LJ, Blakely RD. Sodium-dependent GABA-induced currents in GAT1-transfected HeLa cells. J Physiol (Lond) 1996;490:691–702. doi: 10.1113/jphysiol.1996.sp021178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röhrenbeck J, Wässle H, Heizmann CW. Immunocytochemical labeling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neurosci Lett. 1987;77:255–260. doi: 10.1016/0304-3940(87)90508-8. [DOI] [PubMed] [Google Scholar]
- Ryan MK, Hendrickson AE. Interplexiform cells in macaque monkey retina. Exp Eye Res. 1987;45:57–66. doi: 10.1016/s0014-4835(87)80078-7. [DOI] [PubMed] [Google Scholar]
- Sagne C, El Mestikawy S, Isambert MF, Hamon M, Henry JP, Giros B, Gasnier B. Cloning of a functional vesicular GABA and glycine transporter by screening of genome databases. FEBS Lett. 1997;417:177–183. doi: 10.1016/s0014-5793(97)01279-9. [DOI] [PubMed] [Google Scholar]
- Sarthy PV, Fu M. Localization of L-glutamic acid decarboxylase mRNA in cat retinal horizontal cells by in situ hybridization. J Comp Neurol. 1989a;288:593–600. doi: 10.1002/cne.902880406. [DOI] [PubMed] [Google Scholar]
- Sarthy PV, Fu M. Localization of L-glutamic acid decarboxylase mRNA in monkey and human retina by in situ hybridization. J Comp Neurol. 1989b;288:691–697. doi: 10.1002/cne.902880413. [DOI] [PubMed] [Google Scholar]
- Sassoè-Pognetto M, Wässle H, Grünert U. Glycinergic synapses in the rod pathway of the rat retina: cone bipolar cells express the alpha 1 subunit of the glycine receptor. J Neurosci. 1994;14:5131–5146. doi: 10.1523/JNEUROSCI.14-08-05131.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J, Rusoff AC. Horizontal cells of the mouse retina contain glutamic acid decarboxylase-like immunoreactivity during early developmental stages. J Neurosci. 1984;4:2948–2955. doi: 10.1523/JNEUROSCI.04-12-02948.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA. Calcium-independent release of GABA from isolated horizontal cells of the toad retina. J Physiol (Lond) 1982;323:211–227. doi: 10.1113/jphysiol.1982.sp014069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz EA. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 1987;238:350–355. doi: 10.1126/science.2443977. [DOI] [PubMed] [Google Scholar]
- Takamori S, Riedel D, Jahn R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J Neurosci. 2000;20:4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaney DI, Nelson JC, Pow DV. Neurotransmitter coupling through gap junctions in the retina. J Neurosci. 1998;18:10594–10602. doi: 10.1523/JNEUROSCI.18-24-10594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N, Auerbach P. Specific cell types in cat retina express different forms of glutamic acid decarboxylase. J Comp Neurol. 1995;351:374–384. doi: 10.1002/cne.903510305. [DOI] [PubMed] [Google Scholar]
- Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Res. 1994;34:1235–1246. doi: 10.1016/0042-6989(94)90198-8. [DOI] [PubMed] [Google Scholar]
- Vardi N, Masarachia P, Sterling P. Immunoreactivity to GABAA receptor in the outer plexiform layer of the cat retina. J Comp Neurol. 1992;320:394–397. doi: 10.1002/cne.903200310. [DOI] [PubMed] [Google Scholar]
- Vardi N, Kaufman DL, Sterling P. Horizontal cells in cat and monkey retina express different isoforms of glutamic acid decarboxylase. Vis Neurosci. 1994;11:135–142. doi: 10.1017/s0952523800011172. [DOI] [PubMed] [Google Scholar]
- Vardi N, Morigiwa K, Wang TL, Shi YJ, Sterling P. Neurochemistry of the mammalian cone “synaptic complex.”. Vision Res. 1998;38:1359–1369. doi: 10.1016/s0042-6989(98)00007-8. [DOI] [PubMed] [Google Scholar]
- Vaughn JE, Famiglietti EV, Jr, Barber RP, Saito K, Roberts E, Ribak CE. GABAergic amacrine cells in rat retina: immunocytochemical identification and synaptic connectivity. J Comp Neurol. 1981;197:113–127. doi: 10.1002/cne.901970109. [DOI] [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wässle H, Chun MH. GABA-like immunoreactivity in the cat retina: light microscopy. J Comp Neurol. 1989;279:43–54. doi: 10.1002/cne.902790105. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Röhrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parval-bumin. J Comp Neurol. 1993;332:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- Wässle H, Koulen P, Brandstätter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430. doi: 10.1016/s0042-6989(97)00300-3. [DOI] [PubMed] [Google Scholar]
- Yazulla S. Stimulation of GABA release from retinal horizontal cells by potassium and acidic amino acid agonists. Brain Res. 1983;275:61–74. doi: 10.1016/0006-8993(83)90417-1. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Kleinschmidt J. Carrier-mediated release of GABA from retinal horizontal cells. Brain Res. 1983;263:63–75. doi: 10.1016/0006-8993(83)91201-5. [DOI] [PubMed] [Google Scholar]
- Yazulla S, Studholme KM, Pinto LH. Differences in the retinal GABA system among control, spastic mutant and retinal degeneration mutant mice. Vision Res. 1997;37:3471–3482. doi: 10.1016/S0042-6989(96)00223-4. [DOI] [PubMed] [Google Scholar]