Abstract
Purpose
To determine the expression pattern of the predominant γ-aminobutyric acid (GABA) plasma membrane transporter GAT-1 in Old World monkey (Macaca mulatta) and human retina.
Methods
GAT-1 was localized in retinal sections by using immunohistochemical techniques with fluorescence and confocal microscopy. Double-labeling studies were performed with the GAT-1 antibody using antibodies to GABA, vasoactive intestinal polypeptide (VIP), tyrosine hydroxylase (TH), and the bipolar cell marker Mab115A10.
Results
The pattern of GAT-1 immunostaining was similar in human and monkey retinas. Numerous small immunoreactive somata were in the inner nuclear layer (INL) and were present rarely in the inner plexiform layer (IPL) of all retinal regions. Medium GAT-1 somata were in the ganglion cell layer in the parafoveal and peripheral retinal regions. GAT-1 fibers were densely distributed throughout the IPL. Varicose processes, originating from both the IPL and somata in the INL, arborized in the outer plexiform layer (OPL), forming a sparse network in all retinal regions, except the fovea. Sparsely occurring GAT-1 processes were in the nerve fiber layer in parafoveal regions and near the optic nerve head but not in the optic nerve. In the INL, 99% of the GAT-1 somata contained GABA, and 66% of the GABA immunoreactive somata expressed GAT-1. GAT-1 immunoreactivity was in all VIP-containing cells, but it was absent in TH-immunoreactive amacrine cells and in Mab115A10 immunoreactive bipolar cells.
Conclusions
GAT-1 in primate retinas is expressed by amacrine and displaced amacrine cells. The predominant expression of GAT-1 in the inner retina is consistent with the idea that GABA transporters influence neurotransmission and thus participate in visual information processing in the retina.
Gamma-aminobutyric acid (GABA) is one of the predominant inhibitory neurotransmitters in the retina. GABA is transported by multiple Na+-dependent GABA plasma membrane transporters (GATs), that mediate high-affinity GABA uptake from the synaptic cleft and extracellular space into neurons and glia.1–3 GATs also mediate nonvesicular, Ca2+-independent GABA release from horizontal cells in fish and toad retina, from amacrine cells in the rabbit retina,4 – 6 and from neurons and glia in the mammalian nervous system.7–9
Three GATs (GAT-1, -2, and -3) and a betaine glycine transporter that also transports GABA with high affinity have been isolated and cloned. They have different pharmacological properties10 –18 and tissue distributions,19 –26 which suggests that they have different functional properties. In general, GAT-1 immunoreactivity in the brain is localized to axonal terminals and astrocytes, whereas GAT-3 immunoreactivity is predominantly localized to astrocytes.19 –23 In contrast, GAT-2 mRNA and immunoreactivity have been detected in the leptomeninges and in a few neuronal profiles and glia.24 –26
GABA neurotransmission is influenced by GABA transport mediated by GATs.27 Blocking of GAT-1-mediated transport with specific GABA uptake inhibitors prolongs the decay of GABAA-mediated postsynaptic potentials and enhances these potentials in the hippocampus and retina, probably due to increased levels of GABA in the vicinity of GABAA receptors.28 –35 In addition, GATs efficiently clear diffusely distributed GABA from the extracellular space, thereby limiting GABA action at high-affinity receptors that are located away from GABA release sites in several structures, including the retina, hippocampus, and cerebellar cortex.31,33,36,37
GAT-1, -2, and -3 mRNAs have been detected in rat retina using RT-PCR and Northern blot analysis.12,38 Furthermore, they have been reported in developing and adult rat optic nerve by Northern blot analysis.39 GAT-1 mRNA and immunoreactivity are mainly localized to amacrine and displaced amacrine cells in mouse, rat, guinea pig, and rabbit retinas.25,38,40 – 46 GAT-1 mRNA is also in a few ganglion cells of the rat retina,38 and both GAT-1 mRNA and immunoreactivity are expressed by Müller cells in the mouse, rat, and guinea pig retina.38,40,42,46 In contrast, GAT-3 expression is predominantly localized to Müller cells in mouse, rat, and rabbit retinas,38,42,43 although a small number of GAT-3-immunoreactive amacrine cells are also found in these retinas.42,43,46 Finally, GAT-2 immunoreactivity is predominantly distributed to the retinal pigment and ciliary epithelia,41,42,46 congruent with findings that cultured bovine retinal pigment epithelia accumulate GABA.47 Together, these findings show a prominent expression of GATs in the retina and are consistent with earlier studies showing high-affinity GABA and GABA analogue uptake by amacrine and Müller cells in many different species.4,48 –52 These findings are also consistent with the influence of GATs on the response properties of both bipolar and ganglion cells.33
A recent study reported significant differences in GAT expression patterns between rodent and primate brains, suggesting a differential regulation of extracellular GABA levels.53 As just noted, several investigations have established the localization of GATs in rodent and rabbit retina, but information on the distribution of GABA transporters in primate retinas is not available. In the present investigation, we systematically evaluated GAT-1 expression in macaque monkey and human retina. The results showed that GAT-1 expression in the primate retina is consistent with that reported in other mammalian retinas, with the notable exception that, similar to rabbit and different from rodent retinas, GAT-1 is not expressed by Müller cells in the primate retina. A brief description of preliminary observations has been presented in a prior publication (Trasarti L, et al. IOVS 1999;40:ARVO Abstract 2311).
Methods
This research adhered to the tenets of the Declaration of Helsinki, to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and to the Policies on the Use of Animals and Humans in Neuroscience Research of the Society for Neuroscience.
Tissue Preparation
Eyes of six adult Macaca mulatta monkeys of both sexes were obtained from Convance Research, Products, Inc. (Alice, TX). The animals were anesthetized with ketamine (5–10 mg/kg) and xylazine (1–2 mg/kg) followed by a lethal dose of Euthasol (Virbac Corp., Fort Worth, TX). The eyes were removed, the anterior segments were dissected, and the posterior eyecups containing the retinas were immediately immersed in either 2% or 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB; pH 7.4) or in 2% or 4% PFA with 1.37% poly-L-lysine and 0.214% sodium meta-periodate in PB (PLP) for 1 or 2 hours at room temperature. They were subsequently transferred to 25% sucrose in 0.1 M PB and stored overnight at 4°C. The eyecups were shipped by overnight courier service to University of California, Los Angeles (UCLA) and on receipt were transferred to fresh 25% sucrose in 0.1 M PB. Two normal human retinas were obtained within 2 to 4 hours of death and were kindly provided by Gregory S. Hageman (Department of Ophthalmology and Visual Sciences, University of Iowa, Iowa City, IA). Human donor eyes were obtained after informed consent and approval by the institutional review boards of the University of Iowa. The retinas were fixed with 4% PFA for 2 hours and then transferred to 25% sucrose in 0.1 M PB.
Sections of both monkey and human retinas were cut either perpendicular (vertical sections) or parallel (horizontal sections) to the vitreous surface with a cryostat or sliding microtome, respectively. Cryostat sections were cut at 10 to 15 μm, mounted on gelatin-coated slides, and stored at −20°C. Horizontal sections were cut on the sliding microtome at 20 to 25 μm and stored in 0.1 M PB at 4°C.
Antibodies
A well-characterized, affinity-purified rabbit polyclonal antibody directed to the C terminus of rat GAT-1 (cat. no. AB1570; Chemicon Inc., Temecula, CA) was used for these studies.42 This antibody does not cross-react with either GAT-2 or -3 C-terminal peptides.10,12,15,42 In double-labeling experiments, the GAT-1 antiserum was used in conjunction with monoclonal antibodies directed to GABA (clone GB-69, cat. no. A0310; Sigma-Aldrich, St. Louis, MO),54 vasoactive intestinal polypeptide (VIP; CURE-UCLA Antibody Core, Los Angeles, CA),55 or tyrosine hydroxylase (TH, clone 2/40/15; cat. no. Mab5280; Chemicon, Inc.)56 or with Mab115A10 (donated by Shinobu C. Fujita, Mitsubishi Kasei Institute of Life Sciences, Tokyo, Japan),57 which labels rod and cone bipolar cells.57,58
Immunohistochemistry
Sections were washed in 0.1 M PB and incubated in GAT-1 antibody diluted 1:500 to 1:2000 in 0.1 M PB containing 0.5% Triton X-100 for 12 to 48 hours at 4°C. The sections were then washed in 0.1 M PB and incubated in affinity-purified donkey anti-rabbit IgG conjugated to fluorescein isothiocyanate (FITC; Jackson ImmunoResearch Laboratories, West Grove, PA) at 1:100 dilution for 2 hours at room temperature. Free-floating sections were mounted on gelatin-coated slides, and all slides were coverslipped with glycerol-phosphate buffer containing 2% potassium iodide to retard fading. For double-label immunofluorescence staining, sections were incubated in a mixture containing GAT-1 (1:500) and GABA (1:400), VIP (1:500), TH (1:200), or Mab115A10 (1:2000) antibodies followed by the appropriate secondary antibodies conjugated with FITC, tetramethylrhodamine isothiocyanate (TRITC; Jackson ImmunoResearch), or Alexa Fluor 546 and Alexa Fluor 488 (Molecular Probes, Eugene, OR).
The specificity of the GAT-1 antibody has been described in rat brain,19 rat retina,42 and primate brain.59 Western blot studies indicate that this antiserum reacts with a single band at 67 kDa, consistent with the predicted size of GAT-1.60 In our studies, specificity of the immune reaction was assessed by preadsorbing the GAT-1 antibody with 10−5 M GAT-1 C-terminal peptide overnight at 4°C and using the adsorbed antiserum in place of the primary antibody. Nonspecific immunoreactivity was in cells that resembled microglial cells located in the outer plexiform layer (OPL).
Morphometric Measurements
Morphometric studies were performed on three monkey retinas from different animals. The number and the mean diameter of GAT-1-immunoreactive cells were measured in both coronal and horizontal sections with a computer-assisted image-analysis system (NIH Image; available by ftp at zippy.nimh.nih.gov/ or at http://rsb.info.nih.gov/nih-image; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD). Cells were measured at their maximum diameter.
Imaging
Immunostained sections were analyzed with both conventional fluorescence and confocal microscopy. Using conventional fluorescence microscopy, we photographed sections (T-Max 400 photographic film; Eastman Kodak, Rochester, NY), and the negatives were scanned (Sprint Scan 35 Scanner; Polaroid, Cambridge, MA) at 2700 dpi. Images were enlarged, adjusted for brightness and contrast, labeled with photo editing software (Photoshop 5.0; Adobe Systems, Inc., Mountain View, CA), and saved at 600 dpi. With a confocal microscope (model 410; Carl Zeiss Meditec, Inc., Dublin, CA), digital sections were obtained with a z-axis of 0.5 or 1 μm. Images were adjusted for brightness and contrast, labeled (Photoshop 5.0; Adobe Systems, Inc.), and saved at 600 dpi.
Results
General GAT-1 Expression Pattern in the Monkey and Human Retina
The GAT-1-immunoreactive pattern was similar in monkey and human retinas (Figs. 1, 3). Specific immunoreactivity was usually at or near the plasma membrane of numerous amacrine cell bodies located in the inner nuclear layer (INL) near the inner plexiform layer (IPL; Figs. 1A, 1B). These cells gave rise to processes that entered the IPL. Some GAT-1-immunoreactive cell bodies were in the IPL, and they were surrounded by numerous immunoreactive processes (Fig. 2B). These cells are often referred to as interstitial amacrine cells.61 In addition, immunoreactive displaced amacrine cells, identified on the basis of their cell size and position, were in the ganglion cell layer (GCL; Fig. 1A). GAT-1 immunoreactivity was not observed in the distal portion of the INL. Fine, strongly immunostained varicosities and fibers were densely packed in the IPL in all retinal regions, and they were evenly distributed across the IPL (Fig. 3C). In some cases, varicose processes from the IPL or from GAT-1-immunoreactive somata crossed the INL to ramify in the OPL (Fig. 1C). Varicose immunoreactive processes were in the GCL and they also formed a sparse network in the nerve fiber layer (NFL) of the central retina (Fig. 3D). The immunoreactive fibers in the NFL (see Fig. 5C) appeared similar to other amacrine cell processes within the IPL, and different from the thin-caliber, smooth-appearing ganglion cell axons.
Figure 1.
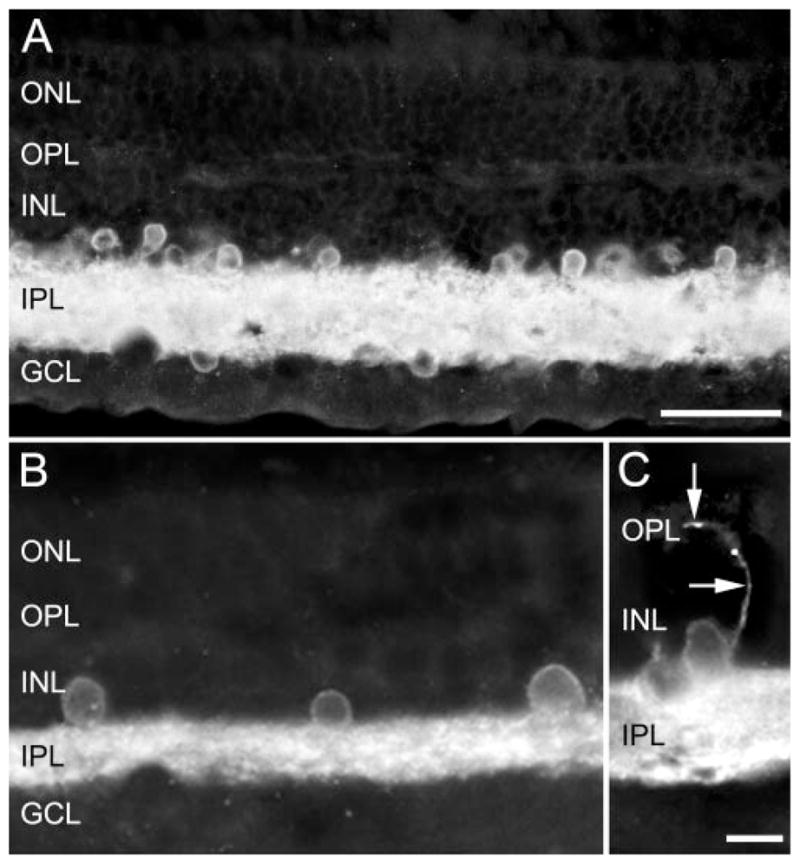
Photomicrographs showing the GAT-1 immunostaining pattern in the primate retina, which is characterized by a high density of immunoreactive processes in the IPL and immunoreactive cell bodies in the INL and GCL. (A) Vertical section of monkey retina and (B, C) human retina. (C, arrows) A GAT-1-immunoreactive process originating from a cell body in the proximal INL that crossed the INL and entered the OPL. This immunostaining pattern was consistent with the expression of GAT-1 immunoreactivity in amacrine, displaced amacrine, and interplexiform cells. Scale bars: (A) 30 μm; (B, C) 10 μm.
Figure 3.
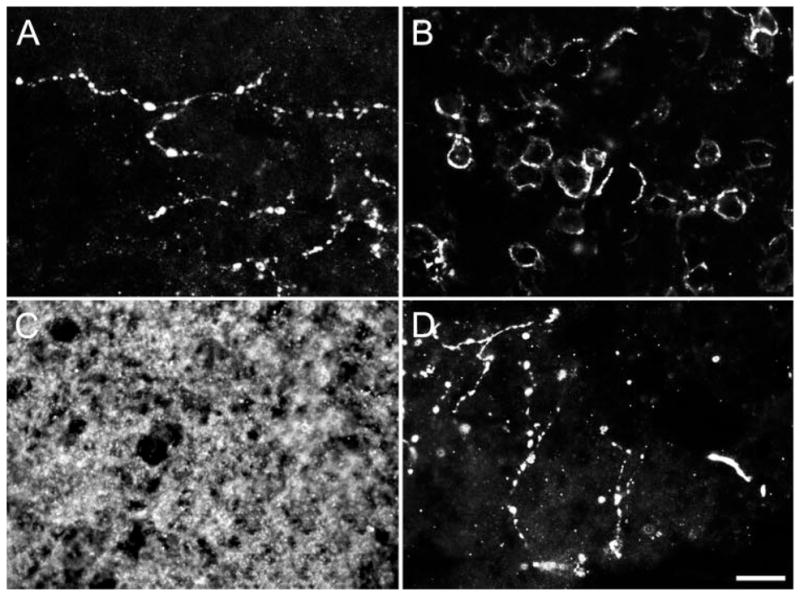
GAT-1 immunoreactivity in horizontal sections from the parafovea of a human retina. (A) A sparse meshwork of varicose, GAT-1-immunoreactive processes in the OPL. (B) GAT-1 immunoreactivity encircled cell bodies in the proximal INL. (C) A dense plexus of GAT-1-immunoreactive processes in the IPL. (D) Sparse, varicose-immunoreactive processes in the NFL. Scale bar, 10 μm.
Figure 2.
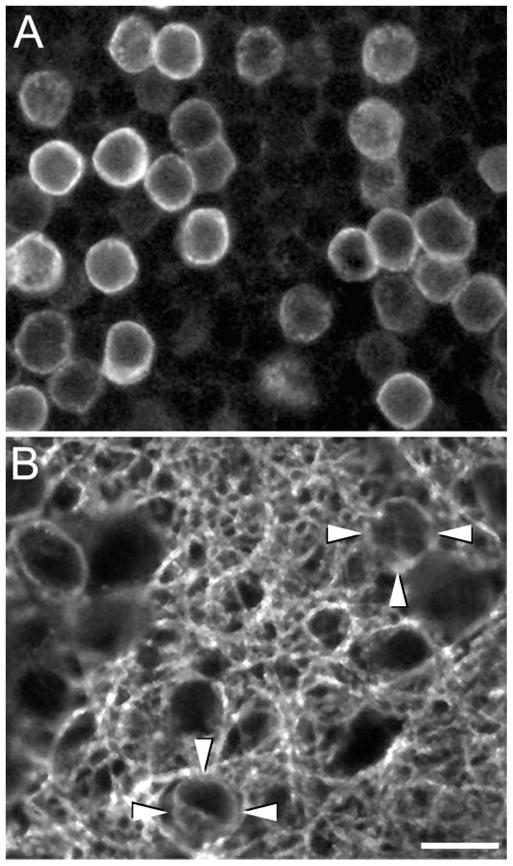
Confocal images (1 μm-thick digital sections) of horizontal sections of a monkey retina showing GAT-1 immunoreactive somata in (A) the proximal INL and (B) the IPL. GAT-1 immunoreactivity outlines the cell somata, indicating that GAT-1 is localized at or near the plasma membrane. (B, arrowheads) Two GAT-1-immunoreactive interstitial cell bodies in the IPL. Scale bar, 10 μm.
Figure 5.

Photomicrographs showing horizontal sections of the monkey retina. (A) GAT-1 immunoreactive processes in the OPL. (B) Higher-power image of immunoreactive processes in the OPL displaying varicosities. (C) A varicose GAT-1 immunoreactive process in the NFL near the optic nerve head. Scale bar: (A, C) 15 μm; (B) 5 μm.
Specific immunoreactivity was absent from control tissue stained with GAT-1 antibody incubated with its cognate peptide. Furthermore, GAT-1 immunoreactivity was not observed in the outer nuclear layer (ONL) or in the photoreceptor outer segments. Overall, this immunostaining pattern is consistent with GAT-1 expression by amacrine cells and its variants, including displaced and interstitial amacrine cells, and interplexiform cells in the primate retina.
GAT-1-Immunoreactive Pattern in the Monkey Retina
Distribution of GAT-1 Expressing Somata
GAT-1 immunoreactive cell bodies in the INL were distributed in all regions of the monkey retina. In the fovea, parafovea, and regions near the optic nerve head, immunoreactive cells were mainly located in the first two cellular rows of the INL adjacent to the IPL (Fig. 4A–C). In contrast, in peripheral retina they were confined to the cellular row of the INL adjacent to the IPL (Fig. 4D).
Figure 4.
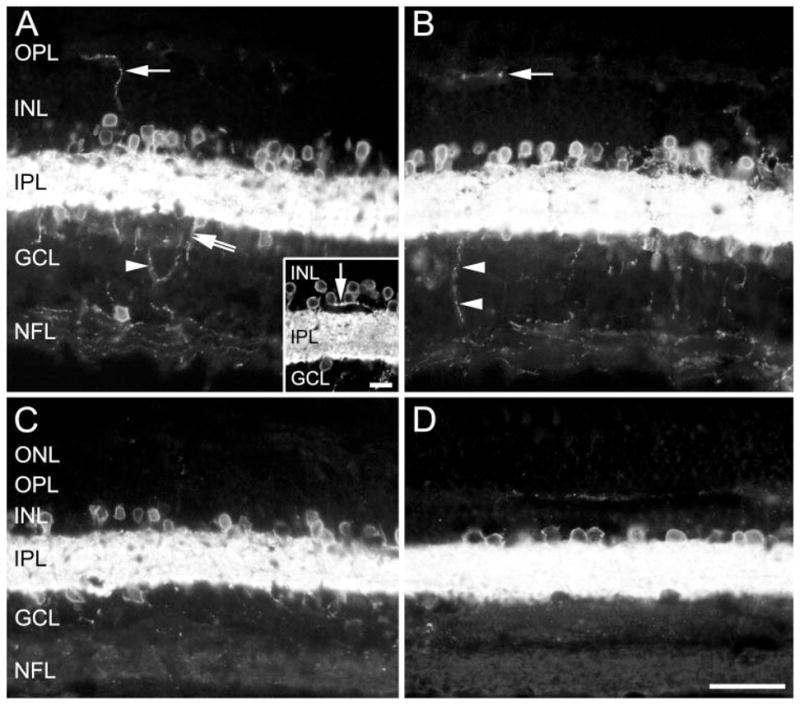
Photomicrographs showing the distribution of GAT-1 immunoreactivity in a section near (A) the optic nerve head, (B) parafovea, (C) fovea, and (D) the periphery of the monkey retina. (A) Arrow: a GAT-1-immunoreactive process in the INL and OPL. Double arrow: a process originating from an immunoreactive cell body in the GCL; arrowhead: an immunoreactive process originating from the immunoreactive plexus in the IPL and returning to the IPL after looping through the GCL without reaching the NFL. (A, inset) A bundle of immunoreactive fibers (arrow) emerging from the IPL and running within the proximal INL for a short distance. (B) Single arrow: a varicose GAT-1-immunoreactive process in the OPL; arrowheads: an immunoreactive process leaving the IPL and entering the NFL. Scale bar: (A–C) 30 μm; (A, inset) 10 μm.
Immunoreactive somata in the INL were round to pyriform (Fig. 2A), with mean diameters ranging from 5 to 12 μm and an overall mean of 7.84 ± 0.3 μm (n = 810). These small- and medium-sized cells were distributed over the whole retina. The variable size of the immunoreactive cells suggests the presence of multiple types of GAT-1-expressing amacrine cells.
GAT-1 somata in the IPL (Fig. 2B) occurred rarely. The somal sizes of these interstitial cells were in the range of the GAT-1 cells in the INL. An estimate of the density of these cells was not determined. Because they were embedded in a dense plexus of immunostained fibers, it was difficult to visualize them completely.
GAT-1 immunoreactive cells located in the GCL were less numerous than those in the INL. These cells were adjacent to the IPL, and a very few of them were near the NFL (Fig. 4A). Most of the GAT-1 somata in the GCL were small (7.02 ± 0.5 μm in diameter, n = 100) and a few were medium-sized (10.92 ± 0.3 μm in diameter, n = 100). The small cell bodies were distributed throughout the retina, whereas the medium-sized cells were mostly located in the parafovea and the retinal region surrounding the optic nerve head. In some cases, GAT-1 somata in the GCL gave rise to a process directed toward the NFL (Fig. 4A).
Distribution of GAT-1 Immunoreactive Processes
The distribution of GAT-1 processes varied in different regions of the monkey retina. In the parafovea and regions near the optic nerve head, GAT-1 fibers were in the OPL, IPL, GCL, and NFL (Figs. 4A, 4B). In contrast, in the fovea, they were mainly confined to the IPL (Fig. 4C), whereas in the peripheral retina GAT-1 processes were consistently observed in both the IPL and OPL (Fig. 4D).
GAT-1 immunoreactivity was most abundant in the IPL, and it was characterized by intensely stained puncta and varicose processes. Some GAT-1 processes entered the INL and, for short distances (20 –25 μm), ran parallel to the IPL (Fig. 4A, inset). GAT-1-immunoreactive processes originated from amacrine, displaced, and interstitial amacrine cells and interplexiform cells.
Immunoreactive processes innervating the OPL originated either from cell bodies in the proximal INL or from the GAT-1 fiber plexus in the IPL. The GAT-1 processes in the OPL were characterized by large varicosities (Fig. 5B). These processes ran for long distances without branching into secondary collaterals (Fig. 5A) and they formed a loose meshwork covering most of the OPL. These GAT-1 processes in the OPL were in all retinal regions except the fovea. Immunoreactive processes were distributed along the foveal perimeter.
GAT-1 processes were in the GCL and NFL of retinal regions near the optic nerve head and in the parafovea (Figs. 4A, 4B). These processes were characterized by varicosities (Fig. 5C), and they originated either from the small immunoreactive cell bodies in the GCL (Fig. 4A) or from the immunoreactive fiber plexus in the IPL (Fig. 4B). Some GAT-1 processes entered the GCL from the IPL, coursed a short distance through the GCL and subsequently re-entered the IPL (Fig. 4A). In the NFL, GAT-1 processes were distributed in a layer adjacent to the GCL (Figs. 4A, 4B). Immunostained processes were not observed in the optic nerve head or in the optic nerve.
Double-Labeling Experiments
Double-labeling experiments were performed with the monkey retina. GAT-1 antibodies were used in conjunction with GABA antibodies to assess the proportion of GABA-containing cells expressing this transporter. In addition, the presence of GAT-1 was tested in two distinct subpopulations of GABAergic neurons, such as the VIP- and TH-immunoreactive amacrine cells.62,63 Finally, because there are reports of GABA immunoreactivity in primate bipolar cells,64 – 69 double labeling with GAT-1 and Mab115A10 antibodies was performed to assess the possible expression of GAT-1 immunoreactivity by bipolar cells.
GAT-1 and GABA immunoreactive somata were counted in horizontal sections of different retinal regions. Nearly all (99%) of the GAT-1-immunolabeled cells in the proximal INL displayed GABA immunoreactivity (Fig. 6), whereas the majority (66%) of the GABA-immunolabeled cells contained GAT-1 immunoreactivity. All the VIP-immunoreactive amacrine cells also contained GAT-1 immunoreactivity (Fig. 7). In contrast, the TH-immunoreactive amacrine cells did not contain GAT-1 immunoreactivity (Fig. 8). Finally, GAT-1 immunoreactivity was not in Mab115A10-immunoreactive bipolar cells (data not shown).
Figure 6.

GAT-1/GABA double labeling in a horizontal section of the monkey retina. (A) GAT-1 immunoreactive somata in the proximal INL. (B) The same field as in (A) showing GABA immunoreactive somata. (C) Overlay image of (A) and (B). Confocal images, Scale bar, 10 μm.
Figure 7.
Confocal images showing GAT-1/VIP double labeling in vertical sections of the monkey retina. (A, B) GAT-1 immunostaining. (C, D) The same fields as in (A) and (B), respectively, showing VIP immunoreactivity. Arrows: a double-labeled amacrine cell (A, C) and a double-labeled displaced amacrine cell (B, D). Scale bar, 10 μm.
Figure 8.

Confocal images of GAT-1/TH double labeling in a vertical section of the monkey retina. (A) GAT-1 immunostaining. (B) The same field as in (A) showing TH immunoreactivity. Arrows: location of a TH-immunoreactive cell soma. Scale bar, 10 μm.
Discussion
GAT-1 Expression in Primate Retina
In this study GAT-1 was abundantly expressed in the primate retina and the distribution of this transporter was similar in both Old World monkey and human retinas. GAT-1-immu-noreactive cells were amacrine cells, including displaced and interstitial amacrine cells, and interplexiform cells. This transporter is not expressed by horizontal or bipolar cells or by photoreceptors. The pattern of GAT-1 expression in the primate retina is consistent with that reported in the retinas of other mammals, including mice, rats, guinea pigs, and rabbits.25,38,40 – 46,70 The only exception to this generality is the expression of GAT-1 immunoreactivity by Müller cells. In mouse, rat, and guinea pigs, GAT-1 is weakly expressed in Müller cells, whereas in rabbit and primate retinas Müller cells do not express GAT-1 immunoreactivity.38,41– 43,46
Overall, the distribution of GAT-1 immunoreactivity in monkey and human retinas is similar to the distribution of GABA and L-glutamic acid decarboxylase (GAD) immunoreactivities, and of GABA or GABA analogue uptake in the primate retina.50,52,65,69,71–76 Although most of the GABA-containing cells prominently express GAT-1 immunoreactivity, many GABA-containing amacrine cells do not express this transporter. These cells could express other GATs, such as GAT-3, which is localized to some amacrine cells in mouse, rat, guinea pig, and rabbit retina.42,43,46 In addition, GABA-containing cells could express GAT-1 on their processes, but not on their cell bodies, as commonly observed for neurons in the cortex and hippocampus.19,21 In addition, in an effort to define the GAT-1 population more narrowly, in the double-label studies we have identified the VIP-containing amacrine cells as one of the sub-populations of GABAergic neurons that express GAT-1, and the TH-containing amacrine cells as one of the subpopulations of GABAergic neurons that apparently do not express GAT-1 immunoreactivity.
These findings indicate that GAT-1 is likely to influence GABA neurotransmission in the inner retina of primates, similar to reports of GAT-1 modulation of inhibitory neurotransmission in the salamander retina.33
GAT-1 in the Outer Retina
Interplexiform cells, which are an amacrine cell variant, express GAT-1 in primate retinas as well as in mouse, rat, and rabbit retinas.40,42,44 These cells are likely to be the same as the GABA-containing interplexiform cells reported in previous studies of primate retina.65,69,72 GABA in the OPL may be taken up by GAT-1 expressing processes of interplexiform cells, thereby influencing GABA neurotransmission in the outer retina.
The lack of GAT-1 expression in horizontal cells is congruent with studies showing that these cells do not accumulate exogenous GABA or GABA analogues.50,52,71 Furthermore, in rat, guinea pig and rabbit retina, horizontal cells also lack GAT-1 and -3 expression.42,43,46 Of interest, horizontal cells in several mammalian species including primates express GABA and GAD immunoreactivities65,69,77,78 and GAD mRNA.79 Together, these observations suggest that horizontal cells synthesize and release GABA, but they do not have the cellular mechanisms to transport GABA from the synapse or extracellular space. The Purkinje cell of the cerebellum is another example of a well-studied neuronal cell type that expresses GABA and GAD immunoreactivity, but does not take up GABA or expresses GATs.80 – 84
The double-label studies with Mab115A10, a rod and cone bipolar cell marker, suggest that GAT-1 immunoreactivity is not expressed by primate bipolar cells. These findings are consistent with uptake studies that indicate that bipolar cells do not accumulate GABA or GABA agonists.50,71,85 Furthermore, GAD67 mRNA has not been reported in primate bipolar cells,73 and there is scant evidence of GAD immunostaining in these cells.64 Although there are conflicting reports of the presence64 – 69 or the absence72,74,85– 87 of GABA immunoreactivity in monkey and human bipolar cells, our observations indicate that these cells are not likely to take up GABA from the extracellular space.
GAT-1 in Ganglion Cells and in the NFL
GABA-containing ganglion cells have been identified in monkey and human retina69,74,75 and GABA-immunoreactive ganglion cell axons have been observed in the optic nerve and optic tract.88 In contrast, GAT-1-immunoreactive fibers are not present in the optic nerve head of primates, suggesting that GAT-1 is not localized to ganglion cell axons. GABA-containing ganglion cells, if they express this transporter, are therefore likely to express GAT-1 immunoreactivity at their preterminal axonal processes and axonal terminals in retinal recipient nuclei, similar to the expression of this transporter on axonal terminals of hippocampal and cortical neurons.19,21
GAT-1 immunoreactive processes in the NFL are likely to originate from displaced amacrine cells localized to the GCL. Indeed, similar processes originating from GABA-immunoreactive displaced amacrine cells have been reported in monkey and human retinas.86 A similar plexus of GAT-1 processes is present in the rabbit retina (our unpublished observation, 2005).
Functional Implications of the Differential Expression of GATs
The inhibition of GABA uptake mediated by GAT-1 in different regions of the central nervous system (CNS), including the retina, invariably results in increased tonic inhibition,27–37 suggesting that this transporter is a key player in the regulation of GABA neurotransmission. GAT-3 is also prominently distributed in the mammalian retina.42,46 GAT-1 and -3 have different functional and pharmacological properties, including different ionic dependences and inhibitor sensitivities, that influence GABA uptake (see Ref. 24 for review). In addition, GATs may transport GABA into the extracellular space in a Ca2+-independent, nonvesicular manner,4 –9 and the different GATs may have different reverse transport profiles. This pharmacological and functional heterogeneity is likely to provide considerable flexibility in the control of the extracellular levels of GABA in different physiological states.
In the primate retina, GAT-1 is expressed by amacrine cells, whereas GAT-3, is likely to be predominantly localized to Müller cells, similar to mouse, rat, and rabbit retinas.38,42,43 GAT-1, expressed by neurons, is likely to be involved in GABA removal from the synaptic cleft and extracellular space and perhaps in GABA release by a Ca2+ independent mechanism. GAT-1 would thus modulate GABA levels and its action at GABA receptors.4 – 6,31,77 GAT-3, expressed by Müller cells, could also influence the levels of GABA, because they span the entire neural retina and probably limit the spread of GABA within the retina.31
Acknowledgments
Supported by the Italian Ministry of Education; National Eye Institute Grants EY04067 and EY15573, and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK41301; VA Medical Research Funds; and a VA Senior Career Scientist Award.
The authors thank Luigi Trasarti for his contributions to the initial studies reported in the article, Gregory S. Hageman for providing human retinas, The CURE Antibody Core of the UCLA, for providing antibody CURE.V55; and Arlene A. Hirano and Catia Sternini for thoughtful input on these studies and this manuscript.
Footnotes
Disclosure: G. Casini, None; D.W. Rickman, None; N.C. Brecha, None
References
- 1.Borden LA. GABA transporter heterogeneity: pharmacology and cellular localization. Neurochem Int. 1996;29:335–356. doi: 10.1016/0197-0186(95)00158-1. [DOI] [PubMed] [Google Scholar]
- 2.Palacín M, Estèvez R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Phys Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- 3.Cherubini E, Conti F. Generating diversity at GABAergic synapses. Trends Neurosci. 2001;24:155–162. doi: 10.1016/s0166-2236(00)01724-0. [DOI] [PubMed] [Google Scholar]
- 4.O’Malley DM, Sandell JH, Masland RH. Co-release of acetylcholine and GABA by the starburst amacrine cells. J Neurosci. 1992;12:1394–1408. doi: 10.1523/JNEUROSCI.12-04-01394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz EA. Transport-mediated synapses in the retina. Phys Rev. 2002;82:875–891. doi: 10.1152/physrev.00010.2002. [DOI] [PubMed] [Google Scholar]
- 6.Yu D, Eldred WD. Nitric oxide stimulates γ-aminobutyric acid release and inhibits glycine release in retina. J Comp Neurol. 2005;483:278–291. doi: 10.1002/cne.20416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pin JP, Bockaert J. Two distinct mechanisms differentially affected by excitatory amino acids trigger GABA release from fetal mouse striatal neurons in primary culture. J Neurosci. 1989;9:648–656. doi: 10.1523/JNEUROSCI.09-02-00648.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaspary HL, Wang W, Richerson GB. Carrier-mediated GABA release activates GABA receptors on hippocampal neurons. J Neurophysiol. 1998;80:270–281. doi: 10.1152/jn.1998.80.1.270. [DOI] [PubMed] [Google Scholar]
- 9.Barakat L, Bordey A. GAT-1 and reversible GABA transport in Bergmann glia in slices. J Neurophysiol. 2002;88:1407–1419. doi: 10.1152/jn.2002.88.3.1407. [DOI] [PubMed] [Google Scholar]
- 10.Guastella J, Nelson N, Nelson H, et al. Cloning and expression of a rat brain GABA transporter. Science. 1990;249:1303–1306. doi: 10.1126/science.1975955. [DOI] [PubMed] [Google Scholar]
- 11.Nelson H, Mandiyan S, Nelson N. Cloning of the human brain GABA transporter. FEBS Lett. 1990;269:181–184. doi: 10.1016/0014-5793(90)81149-i. [DOI] [PubMed] [Google Scholar]
- 12.Borden LA, Smith KE, Hartig PR, Branchek TA, Weinshank RL. Molecular heterogeneity of the γ-aminobutyric acid GABA transport system: cloning of two novel high affinity GABA transporters from rat brain. J Biol Chem. 1992;267:21098–21104. [PubMed] [Google Scholar]
- 13.Borden LA, Dhar TG, Smith KE, Branchek TA, Gluchowski C, Weinshank RL. Cloning of the human homologue of the GABA transporter GAT-3 and identification of a novel inhibitor with selectivity for this site. Recept Channels. 1994;2:207–213. [PubMed] [Google Scholar]
- 14.Borden LA, Smith KE, Gustafson EL, Branchek TA, Weinshank RL. Cloning and expression of a betaine/GABA transporter from human brain. J Neurochem. 1995;64:977–984. doi: 10.1046/j.1471-4159.1995.64030977.x. [DOI] [PubMed] [Google Scholar]
- 15.Clark JA, Deutch AY, Gallipoli PZ, Amara SG. Functional expression and CNS distribution of a β-alanine-sensitive neuronal GABA transporter. Neuron. 1992;9:337–348. doi: 10.1016/0896-6273(92)90172-a. [DOI] [PubMed] [Google Scholar]
- 16.López-Corcuera B, Liu QR, Mandiyan S, Nelson H, Nelson N. Expression of a mouse brain cDNA encoding novel γ-aminobutyric acid transporter. J Biol Chem. 1992;267:17491–17493. [PubMed] [Google Scholar]
- 17.Yamauchi A, Uchida S, Kwon HM, et al. Cloning of a Na+ and Cl− dependent betaine transporter that is regulated by hypertonicity. J Biol Chem. 1992;267:649–652. [PubMed] [Google Scholar]
- 18.Liu Q-R, Lopez-Corcuera B, Mandiyan S, Nelson H, Nelson N. Molecular characterization of four pharmacologically distinct γ-aminobutyric acid transporters in mouse brain. J Biol Chem. 1993;268:2106–2112. [PubMed] [Google Scholar]
- 19.Minelli A, Brecha NC, Karschin C, De Biasi S, Conti F. GAT-1 a high-affinity GABA plasma membrane transporter is localized to neurons and astroglia in cerebral cortex. J Neurosci. 1995;15:7734–7746. doi: 10.1523/JNEUROSCI.15-11-07734.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minelli A, De Biasi S, Brecha NC, Conti F. GAT-3 a high-affinity GABA plasma membrane transporter is localized to astrocytic processes and it is not confined to the vicinity of GABAergic synapses in the cerebral cortex. J Neurosci. 1996;16:6255–6264. doi: 10.1523/JNEUROSCI.16-19-06255.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ribak CE, Tong WMY, Brecha NC. GABA plasma membrane transporters GAT-1 and GAT-3 display different distributions in the rat hippocampus. J Comp Neurol. 1996;367:595–606. doi: 10.1002/(SICI)1096-9861(19960415)367:4<595::AID-CNE9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 22.Conti F, Melone M, De Biasi S, Minelli A, Brecha NC, Ducati A. Neuronal and glial localization of GAT-1 a high-affinity gamma-aminobutyric acid plasma membrane transporter in human cerebral cortex with a note on its distribution in monkey cortex. J Comp Neurol. 1998;396:51–63. doi: 10.1002/(sici)1096-9861(19980622)396:1<51::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Augood SJ, Waldvogel HJ, Munkle MC, Faull RL, Emson PC. Localization of calcium-binding proteins and GABA transporter GAT-1 messenger RNA in the human subthalamic nucleus. Neuroscience. 1999;88:521–534. doi: 10.1016/s0306-4522(98)00226-7. [DOI] [PubMed] [Google Scholar]
- 24.Ikegaki N, Saito N, Hashima M, Tanaka C. Production of specific antibodies against GABA transporter subtypes GAT1, GAT2, GAT3 and their application to immunocytochemistry. Mol Brain Res. 1994;26:47–54. doi: 10.1016/0169-328x(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 25.Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TE, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Mol Brain Res. 1995;33:7–21. doi: 10.1016/0169-328x(95)00101-w. [DOI] [PubMed] [Google Scholar]
- 26.Conti F, Zuccarello LV, Barbaresi P, Minelli A, Brecha NC, Melone M. Neuronal glial and epithelial localization of gamma-aminobutyric acid transporter 2, a high-affinity gamma-aminobutyric acid plasma membrane transporter in the cerebral cortex and neighboring structures. J Comp Neurol. 1999;409:482–494. [PubMed] [Google Scholar]
- 27.Conti F, Minelli A, Melone M. GABA transporters in the mammalian cerebral cortex: localization development and pathological implications. Brain Res Rev. 2004;45:196–212. doi: 10.1016/j.brainresrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Dingledine R, Korn SJ. Gamma-aminobutyric acid uptake and the termination of inhibitory synaptic potentials in the rat hippocampal slice. J Physiol. 1985;366:387–409. doi: 10.1113/jphysiol.1985.sp015804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solis JM, Nicoll RA. Postsynaptic action of endogenous GABA released by nipecotic acid in the hippocampus. Neurosci Lett. 1992;147:16–20. doi: 10.1016/0304-3940(92)90764-x. [DOI] [PubMed] [Google Scholar]
- 30.Thompson SM, Gahwiler BH. Effects of the GABA uptake inhibitor tiagabine on inhibitory synaptic potentials in rat hippocampal slice cultures. J Neurophysiol. 1992;67:1698–1701. doi: 10.1152/jn.1992.67.6.1698. [DOI] [PubMed] [Google Scholar]
- 31.Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- 32.Engel D, Schmitz D, Gloveli T, Frahm C, Heinemann U, Draguhn A. Laminar difference in GABA uptake and GAT-1 expression in rat CA1. J Physiol. 1998;512:643–649. doi: 10.1111/j.1469-7793.1998.643bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ichinose T, Lukasiewicz PD. GABA transporters regulate inhibition in the retina by limiting GABA(C) receptor activation. J Neurosci. 2002;22:3285–3292. doi: 10.1523/JNEUROSCI.22-08-03285.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vivar C, Gutierrez R. Blockade of the membranal GABA transporter potentiates GABAergic responses evoked in pyramidal cells by mossy fiber activation after seizures. Hippocampus. 2005;15:281–284. doi: 10.1002/hipo.20063. [DOI] [PubMed] [Google Scholar]
- 36.Frahm C, Enge D, Draguhn A. Efficacy of background GABA uptake in rat hippocampal slices. Neuroreport. 2001;12:1593–1596. doi: 10.1097/00001756-200106130-00016. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell SJ, Silver RA. GABA spillover from single inhibitory axons suppresses low-frequency excitatory transmission at the cerebellar glomerulus. J Neurosci. 2000;20:8651–8658. doi: 10.1523/JNEUROSCI.20-23-08651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brecha NC, Weigmann C. Expression of GAT-1, a high affinity gamma-aminobutyric acid plasma membrane transporter, in the rat retina. J Comp Neurol. 1994;354:602–611. doi: 10.1002/cne.903450410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howd AG, Rattray M, Butt AM. Expression of GABA transporter mRNAs in the developing and adult rat optic nerve. Neurosci Lett. 1997;235:98–100. doi: 10.1016/s0304-3940(97)00699-x. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz M, Egal H, Sarthy V, Qian X, Sarkar HK. Cloning expression and localization of a mouse retinal γ-aminobutyric acid transporter. Invest Ophthalmol Vis Sci. 1994;35:4039–4048. [PubMed] [Google Scholar]
- 41.Honda S, Yamamoto M, Saito N. Immunocytochemical localization of three subtypes of GABA transporter in rat retina. Mol Brain Res. 1995;33:319–325. doi: 10.1016/0169-328x(95)00150-q. [DOI] [PubMed] [Google Scholar]
- 42.Johnson J, Chen TK, Rickman DW, Evans C, Brecha NC. Multiple γ-aminobutyric acid plasma membrane transporters GAT-1, GAT-2, GAT-3 in the rat retina. J Comp Neurol. 1996;375:212–224. doi: 10.1002/(SICI)1096-9861(19961111)375:2<212::AID-CNE3>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu M, Bruun A, Ehinger B. Expression of GABA transporter subtypes GAT-1, GAT-3 in the adult rabbit retina. Acta Ophthalmol Scand. 1999;77:255–260. doi: 10.1034/j.1600-0420.1999.770302.x. [DOI] [PubMed] [Google Scholar]
- 44.Trasarti L, Casini G, Sabatini A, Bosco L, Brecha NC. GABA plasma membrane transporter-1 GAT-1 expression in the rabbit retina. Soc Neurosci Abs. 1999;25:166. [Google Scholar]
- 45.Dmitrieva NA, Lindstrom JM, Keyser KT. The relationship between GABA-containing cells and the cholinergic circuitry in the rabbit retina. Vis Neurosci. 2001;18:93–100. doi: 10.1017/s0952523801181083. [DOI] [PubMed] [Google Scholar]
- 46.Biedermann B, Bringmann A, Reichenbach A. High-affinity GABA uptake in retinal glial Müller cells of the guinea pig: electrophysiological characterization immunohistochemical localization and modeling of efficiency. Glia. 2002;39:217–228. doi: 10.1002/glia.10097. [DOI] [PubMed] [Google Scholar]
- 47.Sivakami S, Ganapathy V, Leibach FH, Miyamoto Y. The gamma-aminobutyric acid transporter and its interaction with taurine in the apical membrane of the bovine retinal pigment epithelium. Biochem J. 1992;283:391–397. doi: 10.1042/bj2830391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehinger B. Glial and neuronal uptake of GABA glutamic acid glutamine and glutathione in the rabbit retina. Exp Eye Res. 1977;25:221–234. doi: 10.1016/0014-4835(77)90089-6. [DOI] [PubMed] [Google Scholar]
- 49.Agardh E, Ehinger B. 3H-muscimol 3H-nipecotic acid and 3H-isoguvacine as autoradiographic markers for GABA neurotransmission. J Neural Transm. 1982;54:1–18. doi: 10.1007/BF01249274. [DOI] [PubMed] [Google Scholar]
- 50.Hendrickson A, Ryan M, Noble B, Wu JY. Colocalization of [3H]muscimol and antisera to GABA and glutamic acid decarboxylase within the same neurons in monkey retina. Brain Res. 1985;348:391–396. doi: 10.1016/0006-8993(85)90464-0. [DOI] [PubMed] [Google Scholar]
- 51.Mosinger JL, Yazulla S. Colocalization of GAD-like immunoreactivity and 3H-GABA uptake in amacrine cells of rabbit retina. J Comp Neurol. 1985;240:396–406. doi: 10.1002/cne.902400407. [DOI] [PubMed] [Google Scholar]
- 52.Pow DW, Baldridge W, Crook DK. Activity-dependent transport of GABA analogues into specific cell types demonstrated at high resolution using a novel immunocytochemical strategy. Neuroscience. 1996;73:1129–1143. doi: 10.1016/0306-4522(96)00097-8. [DOI] [PubMed] [Google Scholar]
- 53.Pow DV, Sullivan RK, Williams SM, Scott HL, Dodd PR, Finkelstein D. Differential expression of the GABA transporters GAT-1 and GAT-3 in brains of rats cats monkeys and humans. Cell Tissue Res. 2005;320:379–392. doi: 10.1007/s00441-004-0928-0. [DOI] [PubMed] [Google Scholar]
- 54.Erdo SL, Wolff JR. Gamma-aminobutyric acid outside the mammalian brain. J Neurochem. 1990;54:363–372. doi: 10.1111/j.1471-4159.1990.tb01882.x. [DOI] [PubMed] [Google Scholar]
- 55.Wong HC, Sternini C, Lloyd K, De Giorgio R, Walsh JH. Monoclonal antibody to VIP: production characterization immunoneutralizing activity and usefulness in cytochemical staining. Hybridoma. 1996;15:133–139. doi: 10.1089/hyb.1996.15.133. [DOI] [PubMed] [Google Scholar]
- 56.Rohrer H, Acheson AL, Thibault J, Thoenen H. Developmental potential of quail dorsal root ganglion cells analyzed in vitro and in vivo. J Neurosci. 1986;6:2616–2624. doi: 10.1523/JNEUROSCI.06-09-02616.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Onoda N, Fujita SC. A monoclonal antibody specific for a subpopulation of retinal bipolar cells in the frog and other vertebrates. Brain Res. 1987;416:359–363. doi: 10.1016/0006-8993(87)90919-x. [DOI] [PubMed] [Google Scholar]
- 58.Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- 59.Woo T-U, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal γ-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Biasi S, Vitellaro-Zuccarello L, Brecha NC. Immunoreactivity for the GABA transporter-1 and GABA transporter-3 is restricted to astrocytes in the rat thalamus: a light and electron-microscopic immunolocalization. Neuroscience. 1998;83:815–828. doi: 10.1016/s0306-4522(97)00414-4. [DOI] [PubMed] [Google Scholar]
- 61.Cajal SR. La rétine des vertèbres. Cellule. 1893;9:119–255. [Google Scholar]
- 62.Casini G, Brecha NC. Colocalization of vasoactive intestinal polypeptide and GABA immunoreactivities in a population of wide-field amacrine cells in the rabbit retina. Vis Neurosci. 1992;8:373–378. doi: 10.1017/s0952523800005113. [DOI] [PubMed] [Google Scholar]
- 63.Nguyen-Legros J, Versaux-Botteri C, Savy C. Aminergic and GABAergic retinal cell populations in mammals. Microsc Res Tech. 1997;36:26–42. doi: 10.1002/(SICI)1097-0029(19970101)36:1<26::AID-JEMT3>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 64.Agardh E, Ehinger B, Wu JY. GABA and GAD-like immunoreactivity in the primate retina. Histochem. 1987;86:485–490. doi: 10.1007/BF00500621. [DOI] [PubMed] [Google Scholar]
- 65.Grünert U, Wässle H. GABA-like immunoreactivity in the macaque monkey retina: a light and electron microscopic study. J Comp Neurol. 1990;297:509–524. doi: 10.1002/cne.902970405. [DOI] [PubMed] [Google Scholar]
- 66.Martin PR, Grünert U. Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Comp Neurol. 1992;323:269–287. doi: 10.1002/cne.903230210. [DOI] [PubMed] [Google Scholar]
- 67.Van Haesendonck E, Missotten L. A subgroup of bipolar cells in human retina is GABA-immunoreactive. Neurosci Lett. 1993;161:187–190. doi: 10.1016/0304-3940(93)90290-2. [DOI] [PubMed] [Google Scholar]
- 68.Kalloniatis M, Marc RE, Murry RF. Amino acid signatures in the primate retina. J Neurosci. 1996;16:6807–6829. doi: 10.1523/JNEUROSCI.16-21-06807.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.da Costa BL, Hokoç JN, Pinaud RR, Gattass R. GABAergic retino-collicular projection in the New World monkey Cebus apella. Neuroreport. 1997;8:1797–1802. doi: 10.1097/00001756-199705260-00001. [DOI] [PubMed] [Google Scholar]
- 70.Jones EM. Na+- and Cl−-dependent neurotransmitter transporters in bovine retina: identification and localization by in situ hybridization histochemistry. Vis Neurosci. 1995;12:1135–1142. doi: 10.1017/s0952523800006775. [DOI] [PubMed] [Google Scholar]
- 71.Agardh E, Ehinger B. Retinal GABA neuron labelling with [3H]isoguvacine in different species. Exp Eye Res. 1983;36:215–229. doi: 10.1016/0014-4835(83)90007-6. [DOI] [PubMed] [Google Scholar]
- 72.Ryan MK, Hendrickson AE. Interplexiform cells in macaque monkey retina. Exp Eye Res. 1987;45:57–66. doi: 10.1016/s0014-4835(87)80078-7. [DOI] [PubMed] [Google Scholar]
- 73.Sarthy PV, Fu M. Localization of L-glutamic acid decarboxylase mRNA in monkey and human retina by in situ hybridization. J Comp Neurol. 1989;288:691–697. doi: 10.1002/cne.902880413. [DOI] [PubMed] [Google Scholar]
- 74.Crooks J, Kolb H. Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. J Comp Neurol. 1992;315:287–302. doi: 10.1002/cne.903150305. [DOI] [PubMed] [Google Scholar]
- 75.Koontz MA, Hendrickson LE, Brace ST, Hendrickson AE. Immunocytochemical localization of GABA and glycine in amacrine and displaced amacrine cells of macaque monkey retina. Vis Res. 1993;33:2617–2628. doi: 10.1016/0042-6989(93)90220-q. [DOI] [PubMed] [Google Scholar]
- 76.Nag TC, Wadhwa S. Expression of GABA in the fetal postnatal and adult human retinas: an immunohistochemical study. Vis Neurosci. 1997;14:425–432. doi: 10.1017/s0952523800012104. [DOI] [PubMed] [Google Scholar]
- 77.Andrade da Costa BL, de Mello FG, Hokoç JN. Transporter-mediated GABA release induced by excitatory amino acid agonist is associated with GAD67 but not GAD65 immunoreactive cells of the primate retina. Brain Res. 2000;863:132–142. doi: 10.1016/s0006-8993(00)02111-9. [DOI] [PubMed] [Google Scholar]
- 78.Vardi N, Kaufman DL, Sterling P. Horizontal cells in cat and monkey retina express different isoforms of glutamic acid decarboxylase. Vis Neurosci. 1994;11:135–142. doi: 10.1017/s0952523800011172. [DOI] [PubMed] [Google Scholar]
- 79.Sarthy PV, Fu M. Localization of L-glutamic acid decarboxylase mRNA in cat retinal horizontal cells by in situ hybridization. J Comp Neurol. 1989;288:593–600. doi: 10.1002/cne.902880406. [DOI] [PubMed] [Google Scholar]
- 80.Hökfelt T, Ljungdahl A. Autoradiographic identification of cerebral and cerebellar cortical neurons accumulating labeled gamma-aminobutyric acid 3H-GABA. Exp Brain Res. 1972;14:354–362. doi: 10.1007/BF00235032. [DOI] [PubMed] [Google Scholar]
- 81.Sotelo C, Privat A, Drian MJ. Localization of [3H]GABA in tissue culture of rat cerebellum using electron microscope radioautography. Brain Res. 1972;45:302–308. doi: 10.1016/0006-8993(72)90242-9. [DOI] [PubMed] [Google Scholar]
- 82.Rattray M, Priestley JV. Differential expression of GABA transporter-1 messenger RNA in subpopulations of GABA neurones. Neurosci Lett. 1993;156:163–166. doi: 10.1016/0304-3940(93)90463-u. [DOI] [PubMed] [Google Scholar]
- 83.Esclapez M, Tillakaratne NJ, Kaufman DL, Tobin AJ, Houser CR. Comparative localization of two forms of glutamic acid decarboxylase and their mRNAs in rat brain supports the concept of functional differences between the forms. J Neurosci. 1994;14:1834–1855. doi: 10.1523/JNEUROSCI.14-03-01834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ribak CE, Tong WMY, Brecha NC. Astrocytic processes compensate for the apparent lack of GABA transporters in the axon terminals of cerebellar Purkinje cells. Anat Embryol. 1996;194:379–390. doi: 10.1007/BF00198540. [DOI] [PubMed] [Google Scholar]
- 85.Bruun A, Ehinger B. Uptake of certain possible neurotransmitters into retinal neurons of some mammals. Exp Eye Res. 1974;19:435–447. doi: 10.1016/0014-4835(74)90052-9. [DOI] [PubMed] [Google Scholar]
- 86.Koontz MA, Hendrickson AE, Ryan MK. GABA-immunoreactive synaptic plexus in the nerve fiber layer of primate retina. Vis Neurosci. 1989;2:19–25. doi: 10.1017/s0952523800004284. [DOI] [PubMed] [Google Scholar]
- 87.Davanger S, Ottersen OP, Storm-Mathisen J. Glutamate GABA and glycine in the human retina: an immunocytochemical investigation. J Comp Neurol. 1991;311:483–494. doi: 10.1002/cne.903110404. [DOI] [PubMed] [Google Scholar]
- 88.Wilson JR, Cowey A, Somogy P. GABA immunopositive axons in the optic nerve and optic tract of macaque monkeys. Vis Res. 1996;10:1357–1363. doi: 10.1016/0042-6989(95)00235-9. [DOI] [PubMed] [Google Scholar]



