Abstract
Tachykinin (TK) peptides influence neuronal activity in the inner retina of mammals. The aim of this investigation was to determine the cellular localization of the neurokinin 1 receptor (NK1), whose preferred ligand is the TK peptide substance P (SP), in the rat retina. These studies used a polyclonal antiserum directed to the C-terminus of rat NK1. The majority of NK1-immunoreactive (IR) cells were located in the proximal inner nuclear layer (INL), and very rarely they were found in the distal INL. Some small and large NK1-IR somata were present in the ganglion cell layer. NK1-IR processes were densely distributed across the inner plexiform layer (IPL) with a maximum density over lamina 2 of the IPL. Immunoreactive processes also crossed the INL and ramified in the outer plexiform layer where they formed a sparse meshwork. NK1-IR processes were rarely observed in the optic nerve fiber layer. Double-label immunofluorescence studies with different histochemical markers for bipolar cells indicated that NK1 immunoreactivity was not present in bipolar cells. Together, these observations indicate that NK1 immunoreactivity is predominantly expressed by amacrine, displaced amacrine, interplexiform, and some ganglion cells. Double-label immunofluorescence experiments were also performed to characterize NK1-containing amacrine cells. Sixty-one percent of the γ-aminobutyric acid (GABA)-IR cells, 71% of the large tyrosine hydroxylase (TH)-IR cells, and 100% of the small TH-IR cells contained NK1 immunoreactivity. In addition, most (91%) of the NK1-IR cells had GABA immunoreactivity. In contrast, vasoactive intestinal polypeptide-, TK-, choline acetyltransferase-, and parvalbumin-IR amacrine cells did not express NK1 immunoreactivity. Overall, the present findings suggest that SP acts directly upon several cell populations, including GABA-containing amacrine cells and ganglion cells, to influence visual information processing in the inner retina. J. Comp. Neurol. 389:496–507, 1997.
Indexing terms: substance P, amacrine cells, bipolar cells, GABA, dopamine
The tachykinins (TKs) are bioactive peptides that are widely distributed throughout the peripheral and central nervous systems (see Maggio, 1988; Otsuka and Yoshioka, 1993, for reviews). They have a variety of biological roles, including neurotransmission, neuromodulation, and growth regulation (Pernow, 1983; Nilsson et al., 1985; Henry, 1987; Maggio, 1988; Otsuka and Yoshioka, 1993). In mammals, the TKs constitute a family of structurally related peptides that share a common COOH-terminal sequence of five amino acids (Maggio, 1988; Otsuka and Yoshioka, 1993). Substance P (SP), neurokinin A (NKA), and the NKA-related peptides, neuropeptide K and neuropeptide γ, are encoded by the preprotachykinin I gene. Neurokinin B (NKB) is encoded by the preprotachykinin II gene (Maggio, 1988; Otsuka and Yoshioka, 1993).
Bioassay and pharmacological investigations have indicated that the TK peptides act at multiple receptor sites (Otsuka and Yoshioka, 1993). Three TK peptide or neurokinin receptor (NK) genes that encode NK1, NK2, and NK3 have been identified. These receptors are characterized by seven transmembrane domains, and they belong to the superfamily of G protein-coupled receptors (Masu et al., 1987; Hersey and Krause, 1990; Tsuchida et al., 1990; Otsuka and Yoshioka, 1993). SP is the preferred ligand for NK1, whereas NKA and NKB are the preferred ligands for NK2 and NK3, respectively (Otsuka and Yoshioka, 1993).
Many investigations have reported the presence of SP in the mammalian retina by using radioimmunoassay and immunohistochemistry (Fukuda et al., 1981; Osborne et al., 1982; Brecha et al., 1982, 1984, 1987, 1989; Sakiyama et al., 1984; Pourcho and Goebel, 1988a,b; Vaney et al., 1989; Caruso et al. 1990; Li and Lam, 1990; Takatsuji et al., 1991; Yew et al., 1991; Zhang and Yeh, 1992; Cuenca et al., 1995; Lee et al., 1995), and TK mRNAs have been detected in the rat retina by using RNA blot and in situ hybridization techniques (Brecha et al., 1989). In the rat retina, the majority of cells expressing SP immunoreactivity and/or TK mRNAs are amacrine and displaced amacrine cells (Fukuda et al., 1981; Brecha et al., 1984, 1989; Sakiyama et al., 1984; Zhang and Yeh, 1992). These cells are sparsely distributed in all retinal regions, and their processes arborize at three levels in the inner plexiform layer (IPL). In addition, the presence of SP immunoreactivity in ganglion cells of the rat and rabbit retina has been reported (Brecha et al., 1987; Caruso et al., 1990; Takatsuji et al., 1991). Finally, SP-immunoreactive (IR) amacrine cells of the cat retina and ganglion cells of the rat retina also contain γ-aminobutyric acid (GABA; Pourcho and Goebel, 1988b; Vaney et al., 1989; Caruso et al., 1990).
Electrophysiological and pharmacological studies provide evidence that TK peptides influence the activity of retinal neurons. For instance, an excitatory action of SP on “ON” and “ON-OFF” ganglion cells has been reported for mudpuppy, carp, and rabbit retina (Dick and Miller, 1981; Glickman et al., 1982; Zalutsky and Miller, 1990). The excitatory action of SP is characterized by a long duration, but this peptide does not influence ganglion cell receptive field properties. In addition, in rabbit retina, SP excites some amacrine cells that are likely to be GABAergic (Zalutsky and Miller, 1990). This peptide also elicits [3H]dopamine release in the rat retina (Tsang, 1986). Together, these observations indicate that SP has modulatory influences on the excitatory activity of neurons in the inner retina.
Specific, high-affinity NK1 binding sites are present in rat retinal homogenates (Lee and Cheung, 1988). In the rat, rabbit, and bovine retina, tissue section autoradiographic studies show that specific high-affinity SP binding sites are evenly distributed across the IPL (Osborne, 1984; Mantyh et al., 1989; Denis et al., 1991). In the rat retina, NK2 and NK3 binding sites are also distributed across the IPL (Mantyh et al., 1989). In addition, NK1, NK2, and NK3 mRNAs have been detected in extracts of the rat eye by using Northern blots (Tsuchida et al., 1990). These observations support the presence of TK receptors in the retina; however, there is no information available concerning the cellular localization of these receptors that would indicate sites of TK action.
By using an antiserum directed against NK1 (Vigna et al., 1994), we determined the cellular expression pattern of this receptor in the rat retina. In addition, with double-label experiments, specific populations of retinal neurons possessing the NK1 receptor were identified. Preliminary results have been presented in abstract form (Casini et al., 1993, 1994).
MATERIALS AND METHODS
Tissue preparation
Sprague-Dawley albino rats obtained from Harlan (San Diego, CA) were used in this study. They were fed and housed under regular conditions with a 12-hour light-dark schedule. Care and handling of the animals were approved by the Animal Research Committee of the West Los Angeles VA Medical Center in accordance with all NIH animal guidelines. The animals were deeply anesthetized with 30% chloral hydrate and then perfused through the left cardiac ventricle with phosphate-buffered saline, pH 7.4. The eyes were removed, the anterior segments were cut away, and the posterior eyecups containing the retinas were immersion fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4) for 2 hours. After fixation, the retinas were stored overnight in 25% sucrose in 0.1 M PB at 4°C. Dissected retinas were cut perpendicular to the vitreal surface at 10–15 µm with a cryostat, mounted onto gelatin-coated slides, and stored at −20°C until immunohistochemical staining. Alternatively, retinal sections were cut at 30 µm parallel to the vitreal surface with a freezing microtome and free-floating sections were stored in 0.1 M PB at 4°C until processing.
Antibodies
The NK1 antibody (SPR11884-5) is a rabbit polyclonal antiserum directed to a peptide sequence (393–407) corresponding to the predicted C-terminus of rat NK1 (Vigna et al., 1994).
Colocalization of NK1 immunoreactivity with other neurochemical markers for amacrine and bipolar cells was evaluated by using double-label immunofluorescence immunohistochemistry. Amacrine cells were identified by using well-characterized polyclonal and monoclonal antibodies directed to GABA, parvalbumin (PV), vasoactive intestinal polypeptide (VIP), tyrosine hydroxylase (TH), choline acetyltransferase (ChAT), and the TK peptides. Bipolar cells were identified by using monoclonal antibodies directed to protein kinase C (PKC) and MAb 115A10, and a polyclonal antibody directed to recoverin (Table 1).
TABLE 1.
Primary Antibodies
| Antibody | Immunogen | Source | Dilution | References |
|---|---|---|---|---|
| NK-11 | C-terminus of rat NK-1 receptor | Vigna (Duke University) | 1:2,000–1:5,000 | Vigna et al., 1994 |
| GABA22 | GABA-BSA complex | Eugene Tech | 1:1,000–1:2,000 | — |
| PV 2353 | Carp parvalbumin | Sigma | 1:2,000 | Celio et al., 1988 |
| VIP 553 | Vasoactive intestinal polypeptide | Wong and Walsh (UCLA) | 1:2,000–1:5,000 | Wong et al., 1996 |
| TH 2/40/153 | Tyrosine hydroxylase | Boehringer-Mannheim | 1:200 | Rohrer et al., 1986 |
| ChAT IE63 | Choline acetyltransferase | Chemicon | 1:20 | — |
| SP 8K2 | Tachykinin peptides | Sternini (UCLA) | 1:200 | Sternini et al., 1995 |
| PKC MC53 | Protein kinase C | Amersham | 1:50 | — |
| Recoverin1 | 23-kD calcium binding protein | Dizhoor (U of Washington) | 1:1,000 | Dizhoor et al., 1991 |
| MAb 115A103 | Bipolar cell marker | Fujita (Mitsubishi Kasei Institute of Life Sciences) | 1:2,000–1:5,000 | Onoda and Fujita, 1987 |
Rabbit polyclonal.
Guinea pig polyclonal.
Mouse monoclonal.
Immunohistochemistry
Cryostat sections were washed in 0.1 M PB and incubated in NK1 antiserum diluted in 0.1 M PB containing 5% normal goat serum or normal donkey serum and 0.5% Triton X-100 overnight at 4°C. Optimal dilutions for this antiserum were 1:2,000–1:5,000 (Table 1). Sections were washed in 0.1 M PB and incubated in fluorescein isothiocyanate (FITC)- or carboxymethylindocyanine (Cy3)-conjugated goat or donkey anti-rabbit IgGs (1:50–1:100; Jackson ImmunoResearch, West Grove, PA or American Qualex, La Mirada, CA) in 0.1 M PB containing 0.5% Triton X-100 for 2 hours at room temperature. Subsequently, the sections were washed in 0.1 M PB and coverslipped in a 0.1 M carbonate buffer (pH 9.5)-glycerin mixture.
Retinal sections cut parallel to the vitreal surface were washed in 0.25 M Tris buffer and incubated in NK1 antiserum in 0.25 M Tris buffer containing 5% normal goat serum and 0.5% Triton X-100 overnight at 4°C. Following washes in 0.25 M Tris buffer, free-floating sections were incubated in biotinylated goat anti-rabbit IgG (Sigma, St. Louis, MO) for 2 hours at room temperature. After washing in 0.25 M Tris buffer, the sections were incubated in an avidin-biotin-peroxidase mixture (Vectastain ABC Kit, Vector Laboratories, Belmont, CA) for 2 hours at room temperature. The sections were subsequently washed, incubated in 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma) for 5 minutes and then in DAB with 0.03% hydrogen peroxide for 5 minutes. Sections were then mounted onto gelatin-coated slides, treated with 0.05% osmium tetroxide to intensify staining, dehydrated, and coverslipped with Krystalon.
Specificity of the immune reactions was assessed by adsorbing the primary antiserum with 10 µM synthetic NK1 peptide overnight at 4°C. No immunostaining was observed in control sections.
Double-label immunohistochemical studies
Transverse sections and free-floating sections were processed for double-label immunofluorescence as previously described (Casini and Brecha, 1992). Sections were washed in 0.1 M PB and incubated in a mixture of the NK1 antiserum and one of the antibodies (except for the recoverin antiserum, see below) listed in Table 1 overnight at 4°C. These antibodies were diluted in 0.1 M PB with 0.5% Triton X-100 containing 5% normal goat or donkey serum. Sections were then washed in 0.1 M PB and incubated in the presence of the appropriate affinity-purified secondary IgGs conjugated with FITC, tetrametylrhodamine isothiocyanate (TRITC), Cy3 or Texas Red (1:50–1:200) in 0.1 M PB containing 0.5% Triton X-100 for 2 hours at room temperature. The sections were washed in 0.1 M PB; the free-floating sections were mounted onto gelatin-coated slides and coverslipped with 0.1 M carbonate bufferglycerin. Controls were performed to ensure that the primary antibodies did not cross-react when mixed together and that secondary antibodies react only with the appropriate antigen-antibody complex (Goehler et al., 1988).
In the double-label experiments with NK1 and recoverin antisera a sequential staining procedure was used. The NK1 antiserum was initially used to immunostain a retinal section as described above. After evaluation of the immunostaining pattern, the coverslips were removed and the sections were washed in 0.1 M PB. The recoverin antiserum was then used as described above with a second fluorescent IgG.
Specificity of the immune reactions was assessed by adsorbing the NK1 or the GABA primary antiserum with 10 µM synthetic NK1 COOH peptide or with 30 µg/µl of a GABA-bovine serum albumin (BSA) conjugate, respectively. Control experiments for NK1 and TH immunostaining were performed by using preadsorbed NK1 antiserum with normal mouse serum in place of the TH antibody. Immunofluorescence staining was examined with a Leitz Orthoplan fluorescence microscope equipped with epiillumination. FITC fluorescence was visualized with a Leitz L2 or I2 filter cube, TRITC or Cy3 fluorescence was observed with a Leitz N2 cube, and Texas Red fluorescence was observed with a Chroma Texas Red cube. FITC and TRITC were also simultaneously visualized with a Chroma FITC/TRITC dual-band filter. The distribution of NK1 immunoreactivity and its colocalization with GABA or TH immunoreactivity were also examined with a Zeiss Laser Scanning Microscope 410 equipped with a krypton-argon laser (Carl Zeiss, Inc., Thornwood, New York, NY) and attached to a Zeiss Axiovert 100 microscope with PlanApo ×100 1.4 na objective. Images were collected at a magnification of ×2.5. Typically, 10–20 optical sections were taken with a z-axis of 0.5 mm. Images were processed and labeled by using Adobe Photoshop 4.0 (Adobe Systems, Mountain View, CA).
RESULTS
NK1 immunostaining patterns
Amacrine cells were preferentially labeled, even though other types of cells could be identified. Specifically, NK1 immunoreactivity was localized to amacrine cell bodies in the inner nuclear layer (INL) and ganglion cell layer (GCL) and to processes in the IPL and in the outer plexiform layer (OPL). In addition, a few immunostained fibers were observed in the optic nerve fiber layer. NK1 staining was primarily confined to the plasma membrane.
NK1-immunoreactive (IR) amacrine cells were abundant in the INL (Fig. 1 and Fig. 2). Their somata were characterized by small to medium size and a round or pyriform shape. NK1-IR somata were located in the first two or three cellular rows of the INL adjacent to the IPL. These cells usually gave rise to a single process that descended vertically or obliquely toward the IPL and ramified in the IPL. Immunoreactive fibers were densely distributed in all IPL laminae, and processes originating from amacrine cell bodies could only be followed for short distances.
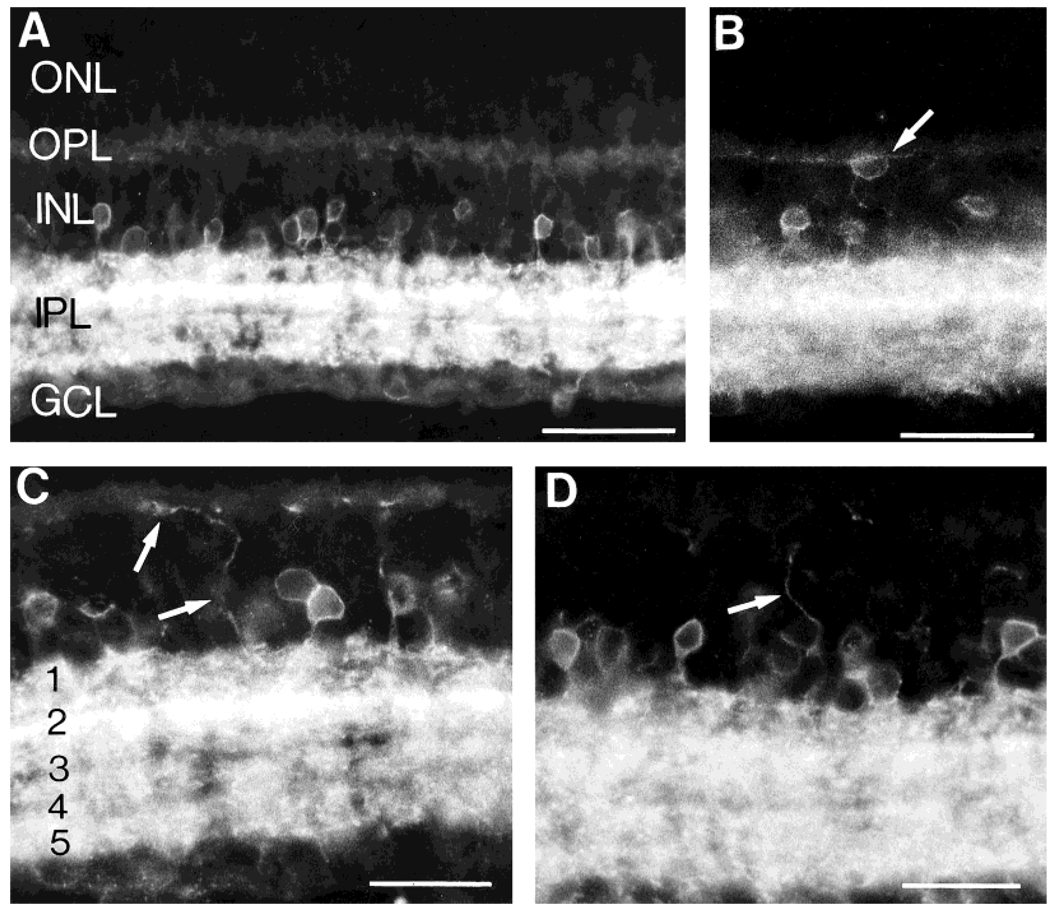
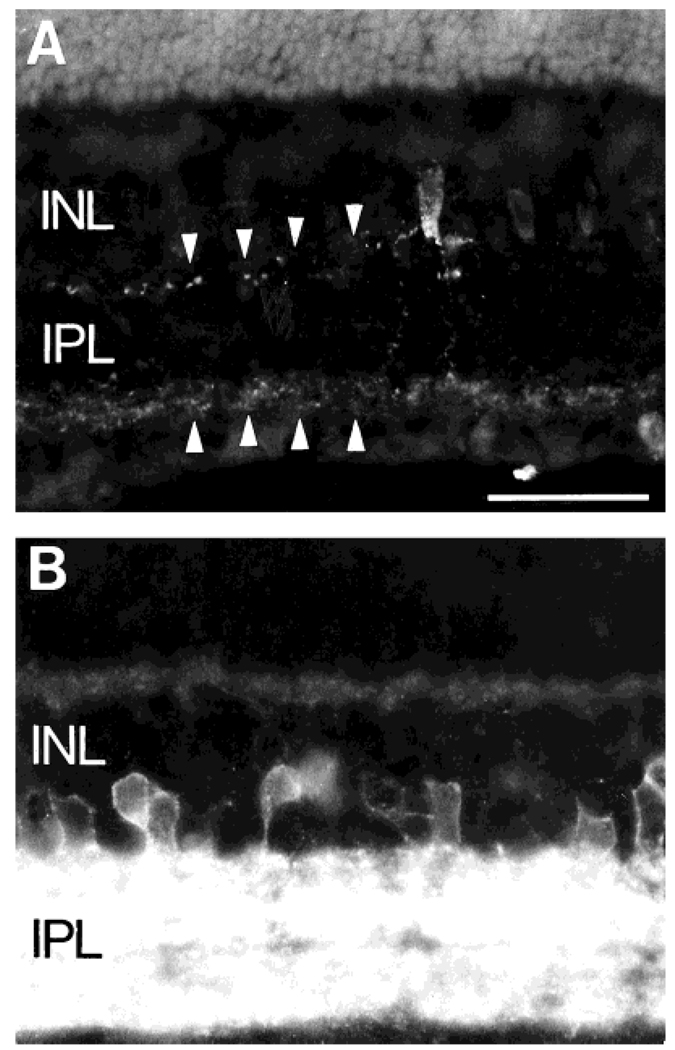
Fig. 1.
Neurokinin 1 (NK1) immunofluorescence in retinal sections cut perpendicular to the vitreal surface. A: NK1-immunoreactive (IR) cells are mainly located in the proximal inner nuclear layer (INL), whereas their processes are densely distributed to all laminae of the inner plexiform layer (IPL). B: Rare NK1-IR cells (arrow) are observed in the outer INL. C,D: NK1-IR processes (arrows) running in the distal INL and arborizing in the outer plexiform layer (OPL). In C, the IPL laminae are numbered (1–5): the highest density of NK1-IR processes is in lamina 2. GCL, ganglion cell layer; ONL, outer nuclear layer. Scale bars = 50 µm in A and B, 30 µm in C and D.
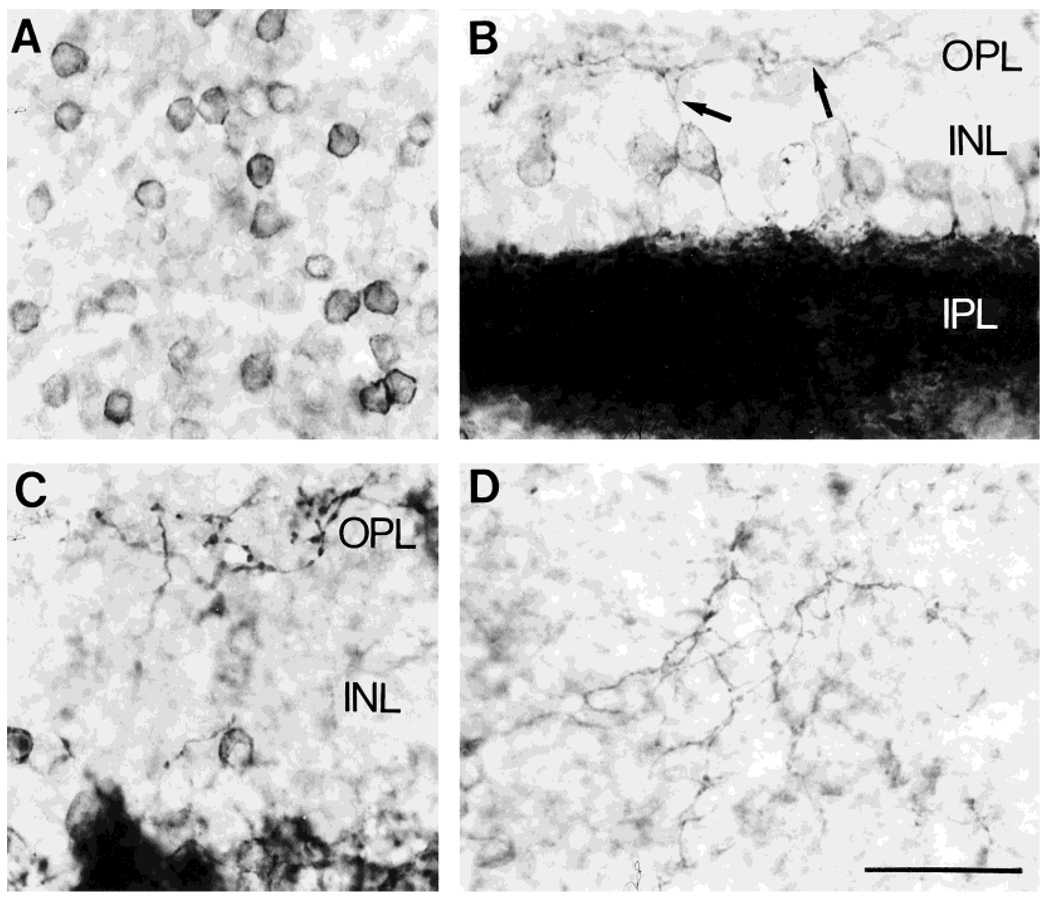
Fig. 2.
NK1 immunoreactivity in retinal sections cut parallel to the vitreal surface. Avidin-biotin-peroxidase technique. A: NK1-IR cell bodies in the INL. B,C: Photomicrographs taken from regions where the section was cut in an oblique plane, so that different retinal layers are present in the same section. Note NK1-IR processes (arrows) innervating and arborizing in the OPL. D: Section through the OPL showing the NK1-IR plexus. Scale bar = 30 µm.
A few, NK1-IR somata were observed in the distal INL adjacent to the OPL (Fig. 1B). These cells occurred rarely, and they only gave rise to processes that arborized in the OPL. Their rare occurrence, their small cell bodies, and the distribution of their processes suggest that they are similar to the type 3 indolamine-accumulating cells described in the rabbit retina (Sandell and Masland, 1989; Masland et al., 1993). In addition, there were some other rarely occurring cells in the distal INL which were very lightly immunostained. They were characterized by a long process descending to the IPL and by shorter processes entering the OPL (Fig. 3B). These cells resembled bipolar cells based on their position in the INL and their morphological features.
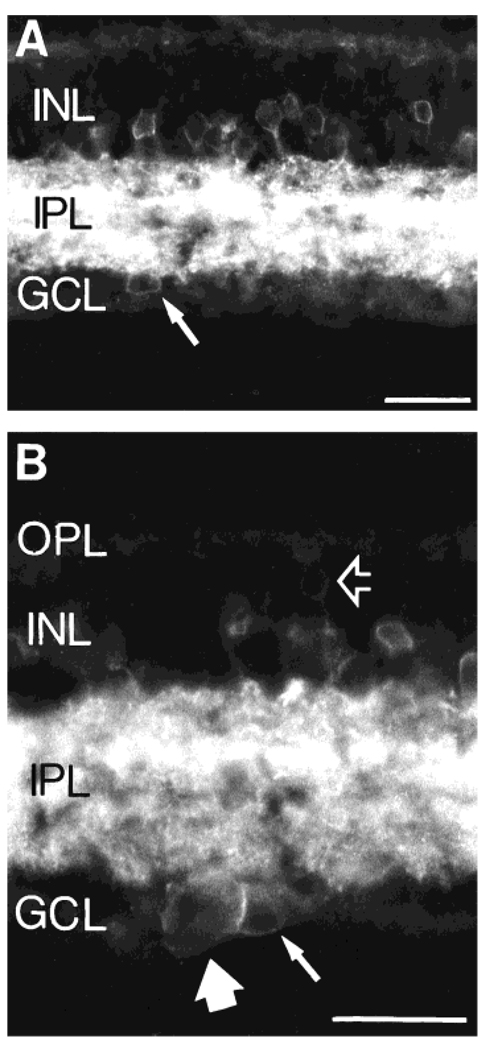
Fig. 3.
A: An NK1-IR cell (arrow) located in the GCL. The soma size of this cell is similar to that of NK1-IR cells in the INL, and it is likely to be a displaced amacrine cell. B: Higher magnification photomicrograph showing a putative NK1-IR displaced amacrine cell (small arrow) together with a larger NK1-IR cell in the GCL (large arrow), which is likely to be a ganglion cell. The open arrow points to a very faint NK1-IR cell in the distal INL. The location and morphology of this latter cell resemble those of a bipolar cell. Scale bars = 30 µm.
NK1-IR processes were more densely distributed in the distal half of the IPL than in the proximal half of the IPL. In particular, a band of very intense immunoreactivity was observed in a subdivision of the IPL best corresponding to lamina 2 (Fig. 1C) (Cajal, 1893).
Some NK1-IR cell bodies in the INL, in addition to giving rise to processes to the IPL, gave rise to another process oriented toward the OPL (Fig. 1C,D and Fig. 2). These processes were usually associated with NK1-IR cells located in the middle of the INL, although they were sometimes associated with IR cells in the INL adjacent to the IPL. These processes could be often traced to the OPL, where they formed a sparse plexus. The plexus of NK1-IR fibers in the OPL was better observed in sections cut parallel to the vitreal surface. The fine, varicose NK1-IR processes that originated from cell bodies in the INL arborized extensively in the OPL, where they ran for long distances and formed a sparse meshwork (Fig. 2).
A few NK1-IR somata were detected in the GCL. These cells were usually characterized by small to medium size, similar to immunolabeled cell bodies in the INL (Fig. 3A). In addition, some large immunolabeled cells were observed in the GCL (Fig. 3B). We were unable to visualize the processes of these cells, because they were lost in the very dense network of NK1-IR fibers in the IPL. The large immunostained cells are likely to be ganglion cells on the basis of their cell body size.
Double-labeling experiments
The pattern of NK1 immunoreactivity is consistent with the expression of this receptor by many types of amacrine cells. Because several populations of amacrine cells have been identified on the basis of their transmitter content and morphology, colocalization studies were performed to determine if NK1 was expressed by different types of amacrine cells. Furthermore, because NK1-immunolabeled processes were present in the OPL, double-label immunofluorescence experiments were performed to determine the expression of NK1 by interplexiform and bipolar cells. An evaluation of the expression of NK1 immunoreactivity in putative ganglion cells was not performed in this investigation.
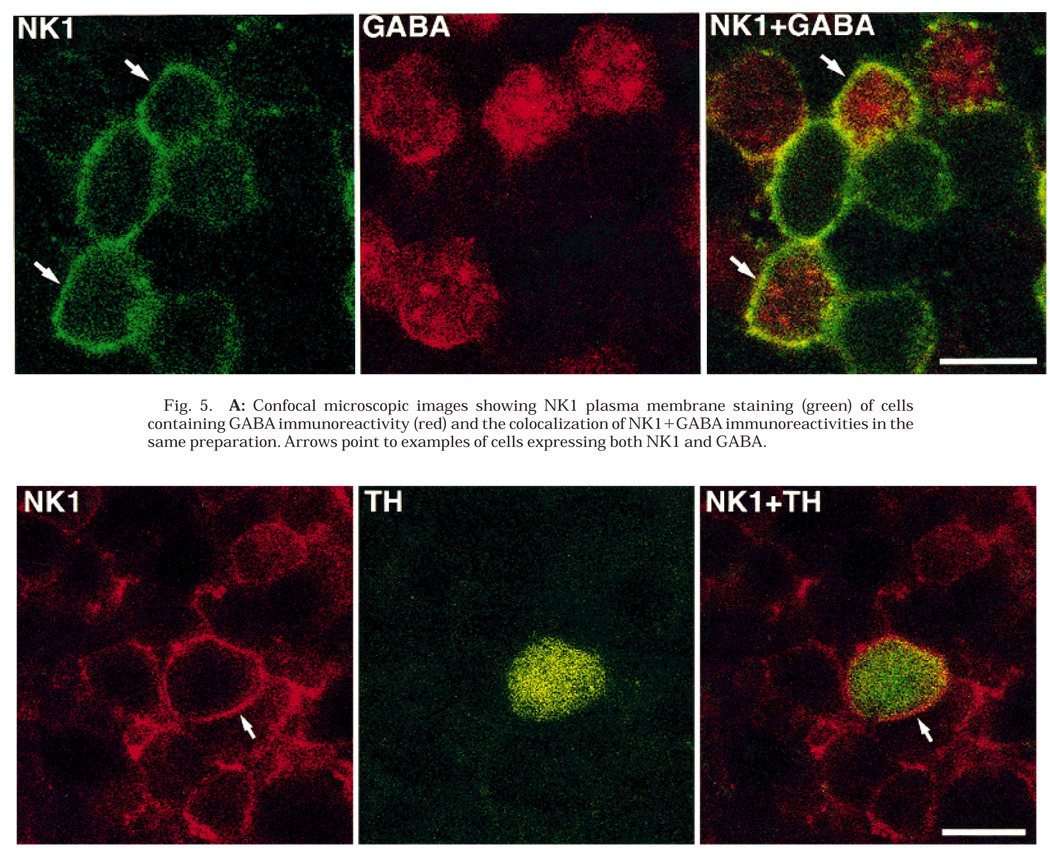
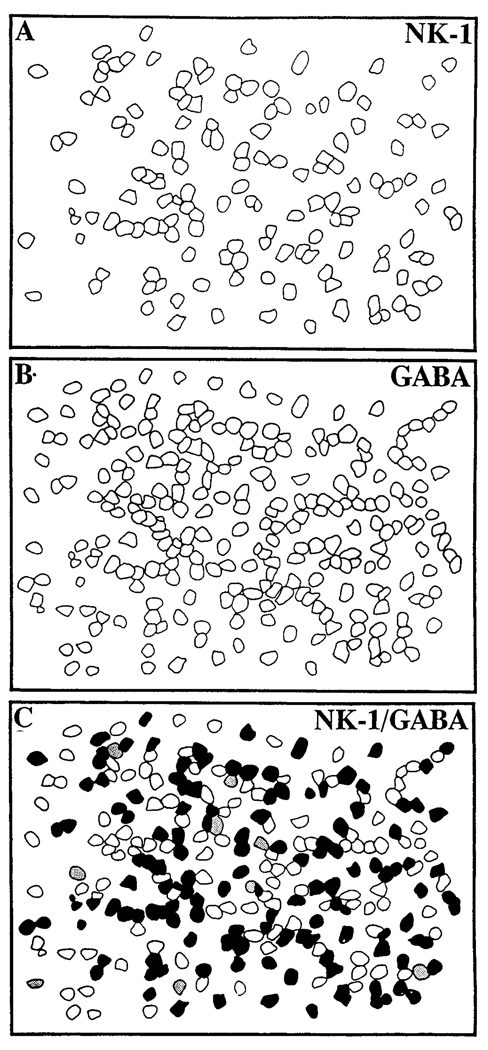
Many NK1-IR cells in the proximal INL contained GABA immunoreactivity (Fig. 4, Fig. 5A, and Fig. 6). The NK1 staining was restricted largely to the cell surface, whereas the GABA immunostaining was uniformly distributed within the cell body. In several retinal sections from different animals, cut parallel to the vitreal surface, 91% (N = 715) of the NK1-IR cells were found to contain GABA immunoreactivity. In addition, 61% (N = 1,080) of the GABAergic cells also contained NK1 immunoreactivity. These observations show that NK1 is expressed by a subset of GABA-IR amacrine cells. Control experiments confirmed the specificity of the immunolabeling.
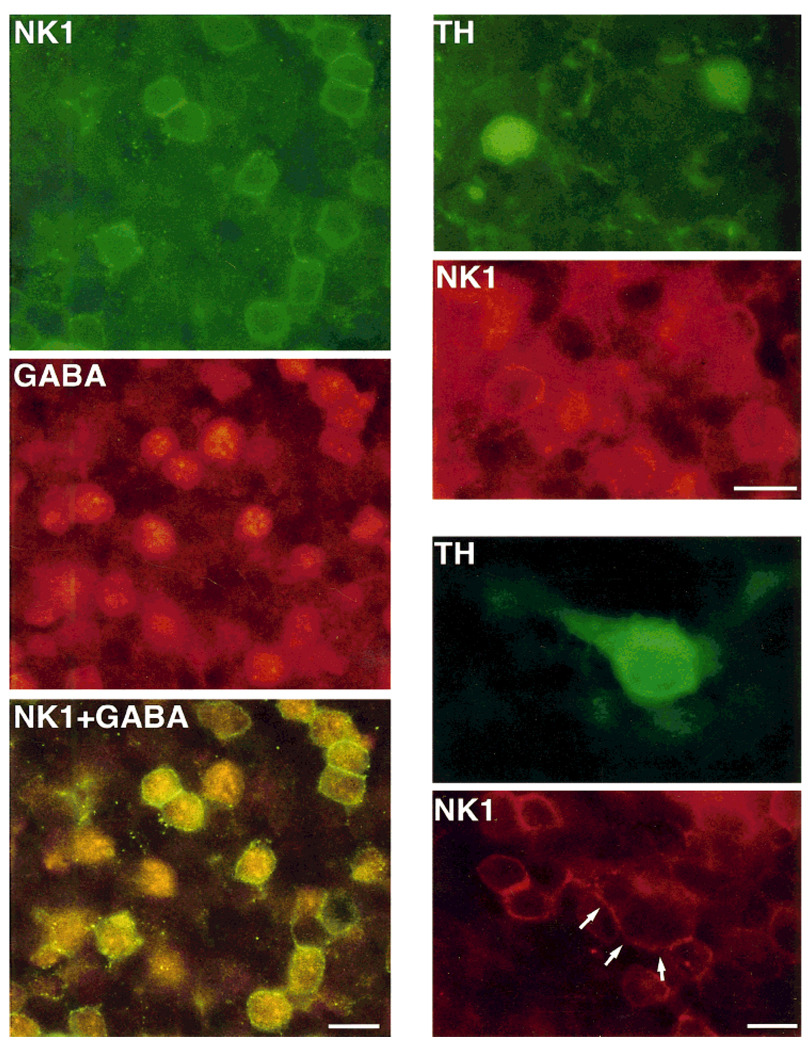
Fig. 4.
Retinal sections cut parallel to the vitreal surface and processed by double-label immunohistochemistry by using antibodies directed against NK1 and γ-aminobutyric acid (GABA), and NK1 and tyrosine hydroxylase (TH). Left: NK1 immunoreactivity is shown in green; GABA immunoreactivity is shown in red; NK1+GABA shows the simultaneous visualization of NK1 (green) and GABA (red-orange) immunoreactivities in the same preparation with a dual-band filter. Note that most GABA-IR cell bodies also display NK1 immunoreactivity, which is confined to their plasma membrane. In addition, some NK1-IR cell bodies do not contain GABA immunoreactivity, and some GABA-IR cells do not express NK1 immunoreactivity. Right: TH (green) and NK1 (red) immunoreactivities visualized with fluorescein and rhodamine filters, respectively. Top two figures show two small TH-IR cell bodies displaying NK1 immunoreactivity at their cell surface; bottom two figures illustrate the colocalization of TH and NK1 immunoreactivities in a large cell (arrows point to the NK1 cell surface staining of such cell). Scale bars = 10 µm.
Fig. 5.
A: Confocal microscopic images showing NK1 plasma membrane staining (green) of cells containing GABA immunoreactivity (red) and the colocalization of NK1 + GABA immunoreactivities in the same preparation. Arrows point to examples of cells expressing both NK1 and GABA.
B: Confocal microscopic images showing NK1 cell surface staining (red) of a cell containing TH immunoreactivity (green) and the colocalization of NK1+TH immunoreactivities in the same preparation. Arrows point to the NK1 cell surface staining. Scale bars = 10 µm.
Fig. 6.
Schematic reconstruction made from photographic negatives (T-MAX 400) of NK1- and GABA-IR profiles, respectively. C was obtained by superimposing (A) and (B); filled profiles represent cells displaying both NK1 and GABA immunoreactivities, stippled profiles represent cells only having NK1 immunoreactivity, and open profiles represent cells displaying only GABA immunoreactivity.
TH-IR amacrine cells were also found to express NK1-IR. Two different groups of TH-IR amacrine cells are present in the rat retina, and they can be differentiated on the basis of the size of their somata and distribution of their processes (Nguyen-Legros et al., 1983; Versaux-Botteri et al., 1986). In retinal sections cut parallel to the vitreal surface 71% (N = 114) of the large TH-IR cells and 100% (N = 129) of the small TH-IR cells expressed NK1 immunoreactivity (Fig. 4 and Fig. 5B).Among the large TH-IR cells, 25% displayed light NK1 immunostaining, whereas 46% contained strong NK1 immunoreactivity. Control experiments confirmed the specificity of these double-labeling immunoreactions.
NK1 immunoreactivity was not present in PV-, VIP-, ChAT-, and TK-IR amacrine cells. Interestingly, the distribution of NK1-IR processes in the IPL did not match the distribution of TK-IR processes. NK1-IR processes were densely distributed throughout the IPL with maximum density in lamina 2, whereas the TK-IR processes arborized primarily in laminae 1 and 5 of the IPL and were occasionally detected in lamina 3 (Fig. 7). In addition, although NK1-IR processes were present in the OPL, no TK-immunostained processes were observed in the OPL.
Fig. 7.
A: TK immunoreactivity in a retinal section. TK-IR cells are located in the proximal INL. Their processes arborize mainly in laminae 1 and 5 (arrowheads) of the IPL and some fine immunostained processes are also in lamina 3. B: NK1-IR processes are densely distributed throughout the IPL. Scale bar = 30 µm.
To test the possible expression of NK1 immunoreactivity by bipolar cells, double-labeling experiments were performed by using the NK1 antiserum with monoclonal antibodies to PKC and MAb 115A10, and a polyclonal antibody to recoverin, which are known to label different bipolar cell types (Negishi et al., 1988; Greferath et al., 1990; Milam et al., 1993; Euler and Wässle, 1995). Bipolar cells identified with these antibodies did not express NK1 immunoreactivity (Fig. 8). In addition, the regions of the INL where most of the NK1-IR and bipolar cell bodies were located did not overlap, with NK1-IR somata occupying a more proximal position in the INL than the bipolar cell somata.
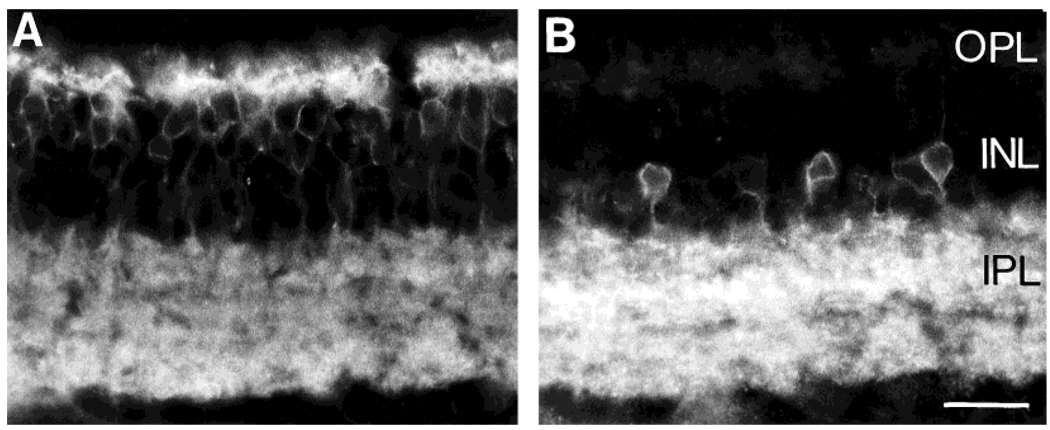
Fig. 8.
A: MAb 115A10-IR cell bodies are mainly confined to the distal INL, and their processes are densely distributed to both the OPL and IPL. B: NK1-IR somata are located in more proximal regions of the INL, and their position does not overlap with that of MAb 115A10-IR somata. Scale bar = 20 µm.
DISCUSSION
The NK1 receptor, whose preferred ligand is SP (Otsuka and Yoshioka, 1993), is expressed by GABA- and TH-IR amacrine and interplexiform cells in the rat retina. In addition, displaced amacrine and some ganglion cells are likely to express this receptor. Confirmation of the expression of NK1-IR by ganglion cells would require double-label immunohistochemical and retrograde transport studies to identify ganglion cells. NK1 immunoreactivity is also present in a few cells located in the distal INL. These cells are likely to be similar to the type 3 displaced indolamine-accumulating cells described in the rabbit retina (Sandell and Masland, 1989; Masland et al., 1993).
Amacrine and interplexiform cells expressing NK1
GABA-containing cells account for 30–40% of the total amacrine cell population in the cat and rabbit retina (Mosinger and Yazulla, 1987; Wässle and Chun, 1988). The proportion of GABAergic amacrine cells in the rat retina has not been established, but it is likely that in rats GABA-IR cells also make up a substantial percentage of the total number of amacrine cells. NK1 receptors are mainly expressed by amacrine cells and approximately 60% of the GABA-IR cells contain NK1 immunoreactivity. Conversely, most (91%) of the NK1-IR cells are GABA-IR. Assuming that GABAergic cells in the rat retina, as in cat and rabbit, make up 30–40% of the total number of amacrine cells, we therefore speculate that NK1 receptors are expressed by 20–30% of the total number of amacrine cells.
NK1-IR amacrine cells were further characterized with antibodies to VIP, ChAT, TK, and TH, reliable markers for the identification of distinct groups of GABA-IR amacrine cells in rat as well as other mammalian retinas (Osborne and Beaton, 1986; Kosaka et al., 1987, 1988; Versaux-Botteri et al., 1987; Brecha et al., 1988; Pourcho and Goebel, 1988b; Vaney and Young, 1988a; Wässle and Chun, 1988; Vaney et al., 1989; Casini and Brecha, 1991, 1992). In addition, in the rat retina TH-IR amacrine cells that contain GABA immunoreactivity (Versaux-Botteri et al., 1987) are composed of two groups, small and large, based on their cell body size and distribution of processes (Nguyen-Legros et al., 1983; Versaux-Botteri et al., 1986). NK1 immunoreactivity is only present in the TH-IR amacrine cells, and it is not expressed by those GABA-IR amacrines that contain VIP, TK, or ChAT immunoreactivity. In addition, NK1 immunoreactivity is absent in PV-IR amacrine cells, indicating that NK1 receptors are not expressed by AII amacrine cells or other amacrine cells that express PV immunoreactivity (Wässle et al., 1993).
The loose meshwork of NK1-IR fibers in the OPL is derived from interplexiform cells. This conclusion is based on the lack of colocalization of NK1 immunoreactivity with immunoreactivities for three different bipolar cell markers, PKC, MAb 115A10, and recoverin (Negishi et al., 1988; Greferath et al., 1990; Milam et al., 1993; Euler and Wässle, 1995). However, based on the morphology of the rare light immunostained NK1-IR cells resembling bipolar cells, we cannot exclude the possibility that NK1 receptors are expressed by a few bipolar cells that do not display PKC, recoverin, or MAb 115A10 immunoreactivity. The presence of NK1 immunoreactivity in interplexiform cell processes is also consistent with the predominant localization of NK1 receptors to TH-IR cells, because many TH-IR cells in the rat retina give rise to processes to the OPL (Nguyen-Legros et al., 1981; Versaux-Botteri et al., 1986).
SP is likely to influence many GABA-IR amacrine and interplexiform cells and to modulate multiple retinal circuits both directly and indirectly. For instance, in the rat retina, the large TH-IR amacrines (Nguyen-Legros et al., 1983; Versaux-Botteri et al., 1986) provide extensive inputs to the narrow-field AII amacrine cells (Voigt and Wässle, 1987), which are important interneurons of the rod pathway (Wässle and Boycott, 1991). Although NK1 receptors are not expressed by AII amacrine cells, SP may indirectly modulate the rod pathway by having a direct action on TH-IR cells. This action may involve changes in the excitability of TH-IR cells, and a concomitant increase in the amount of dopamine released from these cells (Tsang, 1986).
Distribution of NK1 and TK immunoreactivities
The pattern of TK immunoreactivity observed in the present study is consistent with previous reports of the localization of TK peptides and mRNAs in the rat retina (Fukuda et al., 1981; Osborne et al., 1982; Brecha et al., 1984, 1989; Sakiyama et al., 1984; Caruso et al., 1990; Takatsuji et al., 1991; Zhang and Yeh, 1992). In addition, NK1 immunoreactivity is not present in TK-IR cells.
Interestingly, there is a significant mismatch between the location of NK1- and TK-IR processes in the retina. NK1-IR processes are distributed to all IPL laminae, with a maximum density in lamina 2, whereas the processes of TK-IR cells are mainly distributed to laminae 1, 3, and 5 of the IPL and are characterized by varicosities. NK1 immunostaining is strong in all IPL laminae, whereas TK immunostaining is much weaker than NK1 staining. In addition, NK1-IR processes, but no TK-IR processes, are present in the OPL. There are many examples of mismatches between the distribution of neurotransmitters and their receptors in the central nervous system (Shultzberg and Hökfelt, 1986; Herkenham, 1987; Kruger et al., 1988), which suggest that a transmitter can act in a paracrine fashion at some distance from its release site. For instance, there is a mismatch in the distribution of NK1 immunoreactivity and SP-IR synaptic input in the rat cortex, striatum, and spinal cord (Liu et al., 1994). In these regions, NK1 immunoreactivity is expressed extensively on the cell body and dendrites at both synaptic and extrasynaptic sites. This pattern of immunostaining indicates that SP released from nerve terminals may act at receptors at both the synapse and at more distant sites (Liu et al., 1994). The mismatch between the distribution of NK1- and TK-IR processes in the rat retina thus suggests both a synaptic and paracrine action of SP. This peptide, released by terminals at specific IPL laminae, presumably acts synaptically at NK1-IR receptors in the same IPL laminae, and it is likely to diffuse throughout the retina and to act at receptors that are localized to processes in other IPL laminae and in the OPL.
Functional correlates
The expression of NK1 receptors by amacrine, displaced amacrine, interplexiform, and ganglion cells is consistent with pharmacological and physiological investigations reporting excitatory actions of SP in both nonmammalian and mammalian retinas. For instance, exogenously applied SP depolarizes amacrine cells that are likely to be GABAergic in the rabbit retina (Zalutsky and Miller, 1990), and it elicits the release of dopamine from amacrine cells in the rat retina (Tsang, 1986). In addition, our observation of some ganglion cells expressing NK1 immunoreactivity is congruent with the reported direct and long-lasting effects of SP in modulating the excitability of ganglion cells in the rabbit retina (Zalutsky and Miller, 1990). Interestingly, SP also has a long-lasting excitatory effect on most ganglion cells in the mudpuppy and fish retina (Dick and Miller, 1981; Glickman et al., 1982). The action of SP on amacrine cells, as well as on ganglion cells, is also in agreement with the report of SP-IR synaptic input from amacrine cells to amacrine and ganglion cell bodies and processes in the guinea pig retina (Lee et al., 1995). Overall, these investigations suggest that SP has an excitatory and long-lasting influence on multiple retinal cell populations in the inner retina.
The presence of NK1-IR processes in the OPL indicates that NK1 receptors also are expressed by interplexiform cells. The absence of NK1 immunostaining in both cone and rod bipolar cells suggests that SP does not directly influence bipolar cell responsiveness. In contrast, the expression of NK1 receptors by interplexiform cells whose processes form a loose meshwork throughout the OPL indicates that SP may directly influence “horizontal” pathways in this plexiform layer.
CONCLUSIONS
The TK receptor NK1, whose preferred ligand is SP, is expressed by amacrine, displaced amacrine, interplexiform, and some ganglion cells in the rat retina. The majority of NK1-expressing amacrine cells contain GABA immunoreactivity. Among the various populations of GABA-IR amacrine cells, NK1 is expressed by those cells having TH immunoreactivity and not by those cells having VIP, TK, or ChAT immunoreactivity. In addition, NK1 is not expressed by narrow-field AII amacrine cells. The preferential distribution of NK1 immunoreactivity to the inner retina is consistent with the reported direct and long-lasting excitatory influence of SP on many amacrine and ganglion cells. Together, these investigations suggest that SP and NK1 are involved in modulating retinal sensitivity and thus have a role in long-lasting adaptive mechanisms.
ACKNOWLEDGMENTS
We thank Robert Kui, Alan Bryce, and Katherine Wen for their valuable assistance with some of these studies. We also thank Dr. S. Vigna for providing the NK1 antibody, and Dr. J.H. Walsh and Ms. H. Wong for the VIP mouse monoclonal antibody.
Grant sponsor: NIH; Grant number: EY 04067; Grant sponsor: VA Medical Research Funds; Grant number: DK 41301.
LITERATURE CITED
- Brecha NC, Hendrickson A, Floren I, Karten HJ. Localization of substance P-like immunoreactivity within the monkey retina. Invest. Ophthalmol. Vis. Sci. 1982;23:147–153. [PubMed] [Google Scholar]
- Brecha NC, Eldred W, Kuljis RO, Karten HJ. Identification and localization of biologically active peptides in the vertebrate retina. In: Osborne NN, Chader GJ, editors. Progress in Retinal Research. Vol. 3. Oxford: Pergamon Press; 1984. pp. 185–226. [Google Scholar]
- Brecha NC, Johnson D, Bolz J, Sharma S, Parnavelas JG, Lieberman AR. Substance P-immunoreactive retinal ganglion cells and their central axon terminals in the rabbit. Nature. 1987;327:155–158. doi: 10.1038/327155a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecha NC, Johnson D, Peichl L, Wässle H. Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase and γ-aminobutyrate immunoreactivity. Proc. Natl. Acad. Sci. USA. 1988;85:6187–6191. doi: 10.1073/pnas.85.16.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecha NC, Sternini C, Anderson K, Krause JE. Expression and cellular localization of substance P/neurokinin A and neurokinin B mRNAs in the rat retina. Visual Neurosci. 1989;3:527–535. doi: 10.1017/s095252380000986x. [DOI] [PubMed] [Google Scholar]
- Cajal SR. La rétine des vértebrés. La Cellule. 1893;9:119–257. [Google Scholar]
- Caruso DM, Owczarzak MT, Pourcho RG. Colocalization of substance P and GABA in retinal ganglion cells: A computer-assisted visualization. Visual Neurosci. 1990;5:389–394. doi: 10.1017/s095252380000047x. [DOI] [PubMed] [Google Scholar]
- Casini G, Brecha NC. Co-expression of vasoactive intestinal polypeptide (VIP) and GABA in amacrine cells of rat and rabbit retinas. Invest. Ophthalmol. Vis. Sci. 1991;32:993. [Google Scholar]
- Casini G, Brecha NC. Colocalization of vasoactive intestinal polypeptide and GABA immunoreactivities in a population of wide-field amacrine cells in the rabbit retina. Visual Neurosci. 1992;8:373–378. doi: 10.1017/s0952523800005113. [DOI] [PubMed] [Google Scholar]
- Casini G, Rickman DW, Brecha NC. Localization of substance P receptor immunoreactivity in the rat retina. Soc. Neurosci. Abstr. 1993;19:748. [Google Scholar]
- Casini G, Rickman DW, Brecha NC. Cellular localization of the tachykinin receptor NK1 in the rat retina. Invest. Ophthalmol. Vis. Sci. 1994;35:1365. [Google Scholar]
- Celio MR, Baier W, Scahrer L, DeViragh PA, Gerday Ch. Monoclonal antibodies directed against the calcium binding protein parvalbumin. Cell Calcium. 1988;9:81–86. doi: 10.1016/0143-4160(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Cuenca N, De Juan J, Kolb H. Substance P-immunoreactive neurons in the human retina. J. Comp. Neurol. 1995;356:491–504. doi: 10.1002/cne.903560402. [DOI] [PubMed] [Google Scholar]
- Denis P, Fardin V, Nordmann J-P, Elena P-P, Laroche L, Saraux H, Rostene W. Localization and characterization of substance P binding sites in rat and rabbit eyes. Invest. Ophthalmol. Vis. Sci. 1991;32:1894–1902. [PubMed] [Google Scholar]
- Dick E, Miller RF. Peptides influence retinal ganglion cells. Neurosci. Lett. 1981;26:131–135. doi: 10.1016/0304-3940(81)90338-4. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolly D, Walsh KA, Philipov PP, Hurley JB, Stryer L. Recoverin: A calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991;251:915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J. Comp. Neurol. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kuwayama Y, Shiosaka S, Ishimito I, Shimizu Y, Takagi H, Inagaki S, Sakanaka M, Semba E, Takatsuki K, Toyama M. Demonstration of a substance P-like immunoreactivity in retinal cells of the rat. Neurosci. Lett. 1981;23:239–242. doi: 10.1016/0304-3940(81)90004-5. [DOI] [PubMed] [Google Scholar]
- Glickman RD, Adolph AR, Dowling JE. Inner plexiform circuits in the carp retina: Effects of cholinergic agonists, GABA, and substance P on the ganglion cells. Brain Res. 1982;234:81–99. doi: 10.1016/0006-8993(82)90474-7. [DOI] [PubMed] [Google Scholar]
- Goehler LE, Sternini C, Brecha NC. Calcitonin gene-related peptide immunoreactivity in the biliary pathway and liver of the guinea pig: Distribution and colocalization with substance P. Cell Tissue Res. 1988;253:145–150. doi: 10.1007/BF00221749. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grünert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J. Comp. Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Henry JL. Substance P and neurokinins. New York: Springer-Verlag; 1987. [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: Observations and implications. Neuroscience. 1987;23:1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Hersey AD, Krause JE. Molecular characterization of a functional cDNAencoding the rat substance P receptor. Science. 1990;247:958–962. doi: 10.1126/science.2154852. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu J-Y, Ottersen OP, Storm-Mathisen J, Hama K. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp. Brain Res. 1987;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Tauchi M, Dahl JL. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp. Brain Res. 1988;70:605–617. doi: 10.1007/BF00247609. [DOI] [PubMed] [Google Scholar]
- Kruger L, Mantyh PW, Sternini C, Brecha N, Mantyh CR. Calcitonin gene-related peptide (CGRP) in the rat central nervous system: Patterns of immunoreactivity and receptor binding sites. Brain Res. 1988;463:223–244. doi: 10.1016/0006-8993(88)90395-2. [DOI] [PubMed] [Google Scholar]
- Lee C-M, Cheung W-T. Effects of neonatal monosodium glutamate treatment on substance P binding sites in the rat retina. Neurosci. Lett. 1988;92:310–314. doi: 10.1016/0304-3940(88)90608-8. [DOI] [PubMed] [Google Scholar]
- Lee M-Y, Chun M-H, Han S-H, Oh S-J, Chung J-W. Lightand electron-microscopic study of substance P-immunoreactive neurons in the guinea pig retina. Cell Tissue Res. 1995;281:261–271. doi: 10.1007/BF00583395. [DOI] [PubMed] [Google Scholar]
- Li HB, Lam DMK. Localization of neuropeptide-immunoreactive neurons in the human retina. Brain Res. 1990;522:30–36. doi: 10.1016/0006-8993(90)91573-y. [DOI] [PubMed] [Google Scholar]
- Liu H, Brown JL, Jasmin L, Maggio JE, Vigna SR, Mantyh PW, Bausbaum AI. Synaptic relationship between substance P and the substance P receptor: Light and electron microscopic characterization of the mismatch between neuropeptides and their receptors. Proc. Natl. Acad. Sci. USA. 1994;91:1009–1013. doi: 10.1073/pnas.91.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio JE. Tachykinins. Annu. Rev. Neurosci. 1988;11:13–28. doi: 10.1146/annurev.ne.11.030188.000305. [DOI] [PubMed] [Google Scholar]
- Mantyh PW, Gates T, Mantyh CR, Maggio JE. Autoradiographic localization and characterization of tachykinin receptor binding sites in the rat brain and peripheral tissues. J. Neurosci. 1989;9:258–279. doi: 10.1523/JNEUROSCI.09-01-00258.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH, Rizzo JF, Sandell JH. Developmental variation in the structure of the retina. J. Neurosci. 1993;13:5194–5202. doi: 10.1523/JNEUROSCI.13-12-05194.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masu Y, Nakayama K, Tamaki H, Harada Y, Kuno M, Nakanishi S. cDNA cloning of bovine substance-K receptor through oocyte expression system. Nature. 1987;329:836–838. doi: 10.1038/329836a0. [DOI] [PubMed] [Google Scholar]
- Milam AH, Dacey DM, Dizhoor AM. Recoverin immunoreactivity in mammalian cone bipolar cells. Visual Neurosci. 1993;10:1–12. doi: 10.1017/s0952523800003175. [DOI] [PubMed] [Google Scholar]
- Mosinger JL, Yazulla S. Double-label analysis of GAD- and GABA-like immunoreactivity in the rabbit retina. Visual Res. 1987;27:23–30. doi: 10.1016/0042-6989(87)90139-8. [DOI] [PubMed] [Google Scholar]
- Müller F, Wässle H, Brecha NC. NADPH-diaphorase-positive amacrine cells show GABA-like immunoreactivity in cat retina. Eur. J. Neurosci. Suppl. 1988;1:153. [Google Scholar]
- Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci. Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Berger B, Vigny A, Alvarez C. TH-like immunoreactive interplexiform cells in the rat retina. Neurosci. Lett. 1981;27:255–259. doi: 10.1016/0304-3940(81)90439-0. [DOI] [PubMed] [Google Scholar]
- Nguyen-Legros J, Vigny A, Gay M. Postnatal development of TH-like immunoreactivity in the rat retina. Exp. Eye Res. 1983;37:23–32. doi: 10.1016/0014-4835(83)90146-x. [DOI] [PubMed] [Google Scholar]
- Nilsson J, von Euler AM, Dalsgaard C-J. Stimulation of connective tissue growth by substance P and substance K. Nature. 1985;315:61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Onoda N, Fujita SC. A monoclonal antibody specific for a subpopulation of retinal bipolar cells in the frog and other vertebrates. Brain Res. 1987;416:359–363. doi: 10.1016/0006-8993(87)90919-x. [DOI] [PubMed] [Google Scholar]
- Osborne NN. Substance P in the bovine retina: Localization, identification, release, uptake, and receptor analysis. J. Physiol. (Lond.) 1984;349:83–93. doi: 10.1113/jphysiol.1984.sp015144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne NN, Beaton DW. Direct histochemical localization of 5,7-dihydroxytryptamine and the uptake of serotonin by a subpopulation of GABA neurones in the rabbit retina. Brain Res. 1986;382:158–162. doi: 10.1016/0006-8993(86)90125-3. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Nicholas DA, Dockray GJ, Cuello AC. Cholecystokinin and substance P immunoreactivity in retinas of rats, frogs, lizards, and chicks. Exp. Eye Res. 1982;34:639–649. doi: 10.1016/0014-4835(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Yoshioka K. Neurotransmitter functions of mammalian tachykinins. Physiol. Rev. 1993;73:229–308. doi: 10.1152/physrev.1993.73.2.229. [DOI] [PubMed] [Google Scholar]
- Pernow B. Substance P. Pharmacol. Rev. 1983;35:85–141. [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. Substance P-like immunoreactive amacrine cells in the cat retina. J. Comp. Neurol. 1988a;275:542–552. doi: 10.1002/cne.902750405. [DOI] [PubMed] [Google Scholar]
- Pourcho RG, Goebel DJ. Co-localization of substance P and GABAin amacrine cells of the cat retina. Brain Res. 1988b;447:164–168. doi: 10.1016/0006-8993(88)90979-1. [DOI] [PubMed] [Google Scholar]
- Rohrer H, Acheson AL, Thibault J, Thoenen H. Developmental potential of quail dorsal root ganglion cells analyzed in vitro and in vivo. J. Neurosci. 1986;6:2616–2624. doi: 10.1523/JNEUROSCI.06-09-02616.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakyiama T, Kuwayama Y, Ishimoto I, Sasaoka A, Shiosaka S, Tohyama M, Manabe R, Shiotani Y. Ontogeny of substance P-containing structures in the ocular tissue of the rat:An immunohistochemical analysis. Dev. Brain Res. 1984;13:275–281. doi: 10.1016/0165-3806(84)90162-7. [DOI] [PubMed] [Google Scholar]
- Sandell JH, Masland RH. Shape and distribution of an unusual retinal neuron. J. Comp. Neurol. 1989;280:489–497. doi: 10.1002/cne.902800312. [DOI] [PubMed] [Google Scholar]
- Shultzberg M, Hökfelt T. The mismatch problem in receptor autoradiography and the coexistence of multiple messengers. Trends Neurosci. 1986;9:109–110. [Google Scholar]
- Sternini C, Su D, Gamp PD, Bunnett NW. Cellular sites of expression of the neurokinin-1 receptor in the rat gastrointestinal tract. J. Comp. Neurol. 1995;358:531–540. doi: 10.1002/cne.903580406. [DOI] [PubMed] [Google Scholar]
- Takatsuji K, Miguel-Hidalgo JJ, Tohyama M. Substance P-immunoreactive innervation from the retina to the suprachiasmatic nucleus in the rat. Brain Res. 1991;568:223–229. doi: 10.1016/0006-8993(91)91401-l. [DOI] [PubMed] [Google Scholar]
- Tsang D. Effect of substance P on dopamine release in rat retina. In: Kon OL, Chung MCM, Hwang PLH, Leong SF, Loke KH, Thiayagarajah P, Wong PHT, editors. Contemporary Themes in Biochemistry. Cambridge: Cambridge University Press; 1986. pp. 588–589. [Google Scholar]
- Tsuchida K, Shigemoto R, Yokota Y, Nakanishi S. Tissue distribution and quantitation of the mRNAs for three tachykinins receptors. J. Biochem. 1990;193:751–757. doi: 10.1111/j.1432-1033.1990.tb19396.x. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Young HM. GABA-like immunoreactivity in cholinergic amacrine cells of the rabbit retina. Brain Res. 1988a;438:369–373. doi: 10.1016/0006-8993(88)91366-2. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Whitington GE, Young HM. The morphology and topographic distribution of substance P-like immunoreactive amacrine cells in the cat retina. Proc. R. Soc. Lond. [Biol.] 1989;237:471–488. doi: 10.1098/rspb.1989.0060. [DOI] [PubMed] [Google Scholar]
- Versaux-Botteri C, Martin-Martinelli E, Nguyen-Legros J, Geffard M, Vigny A, Denoroy L. Regional specialization of the rat retina: Catecholamine-containing amacrine cell characterization and distribution. J. Comp. Neurol. 1986;243:422–433. doi: 10.1002/cne.902430311. [DOI] [PubMed] [Google Scholar]
- Versaux-Botteri C, Simon A, Vigny A, Nguyen-Legros J. Existence d’ une immunoréactivité au GABA dans les cellules amacrines dopaminergiques de la rétine de rat. Comptes Rendus des Séances de l’Académie des Sciences, Paris, Série III. 1987;305:381–386. [PubMed] [Google Scholar]
- Vigna SR, Bowden JJ, McDonald DM, Fisher J, Okamoto A, McVey DC, Payan DJ, Bunnett NW. Characterization of antibodies to the rat substance P (NK-1) receptor and to a chimeric substance P receptor expressed in mammalian cells. J. Neurosci. 1994;14:834–845. doi: 10.1523/JNEUROSCI.14-02-00834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt T, Wässle H. Dopaminergic innervation of AII amacrine cells in mammalian retina. J. Neurosci. 1987;7:4115–4128. doi: 10.1523/JNEUROSCI.07-12-04115.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Chun MH. Dopaminergic and indolamine-accumulating amacrine cells express GABA-like immunoreactivity in the cat retina. J. Neurosci. 1988;8:3383–3394. doi: 10.1523/JNEUROSCI.08-09-03383.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol. Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wässle H, Grünert U, Röhrenbeck J. Immunocytochemical staining of AII amacrine cells in the rat retina with antibodies against parvalbumin. J. Comp. Neurol. 1993;332:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- Wong HC, Sternini C, Lloyd K, De Giorgio R, Walsh JH. Monoclonal antibody to VIP: Production, characterization, immunoneutralizing activity, and usefulness in cytochemical staining. Hybridoma. 1996;15:133–139. doi: 10.1089/hyb.1996.15.133. [DOI] [PubMed] [Google Scholar]
- Yew DT, Luo CB, Zheng DR, Guan YL, Tsang D, Stadlin A. Immunohistochemical localization of substance P, enkephalin and serotonin in the developing human retina. J. Hirnforsch. 1991;32:61–67. [PubMed] [Google Scholar]
- Zalutsky RA, Miller RF. The physiology of substance P in the rabbit retina. J. Neurosci. 1990;10:394–402. doi: 10.1523/JNEUROSCI.10-02-00394.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DR, Yeh HH. Substance P-like immunoreactive amacrine cells in the adult and the developing rat retina. Dev. Brain Res. 1992;68:55–65. doi: 10.1016/0165-3806(92)90247-t. [DOI] [PubMed] [Google Scholar]