Abstract
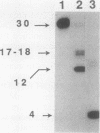
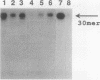
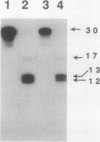
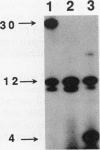
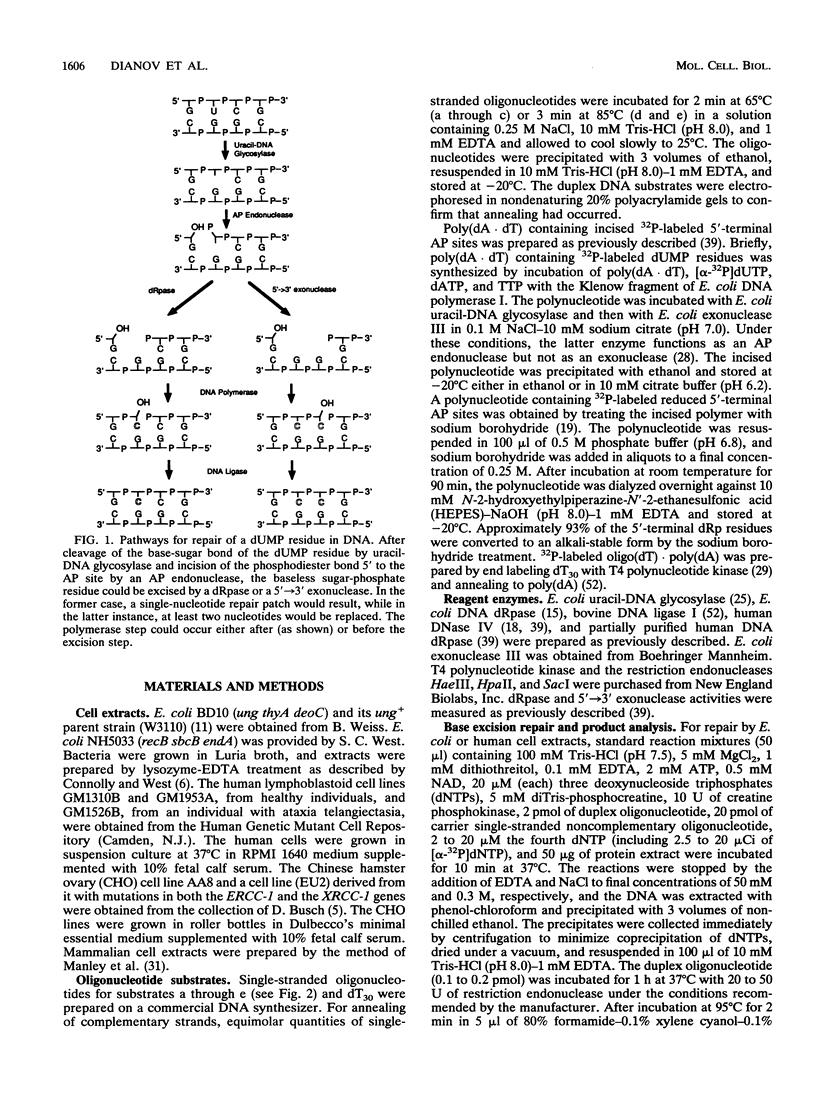
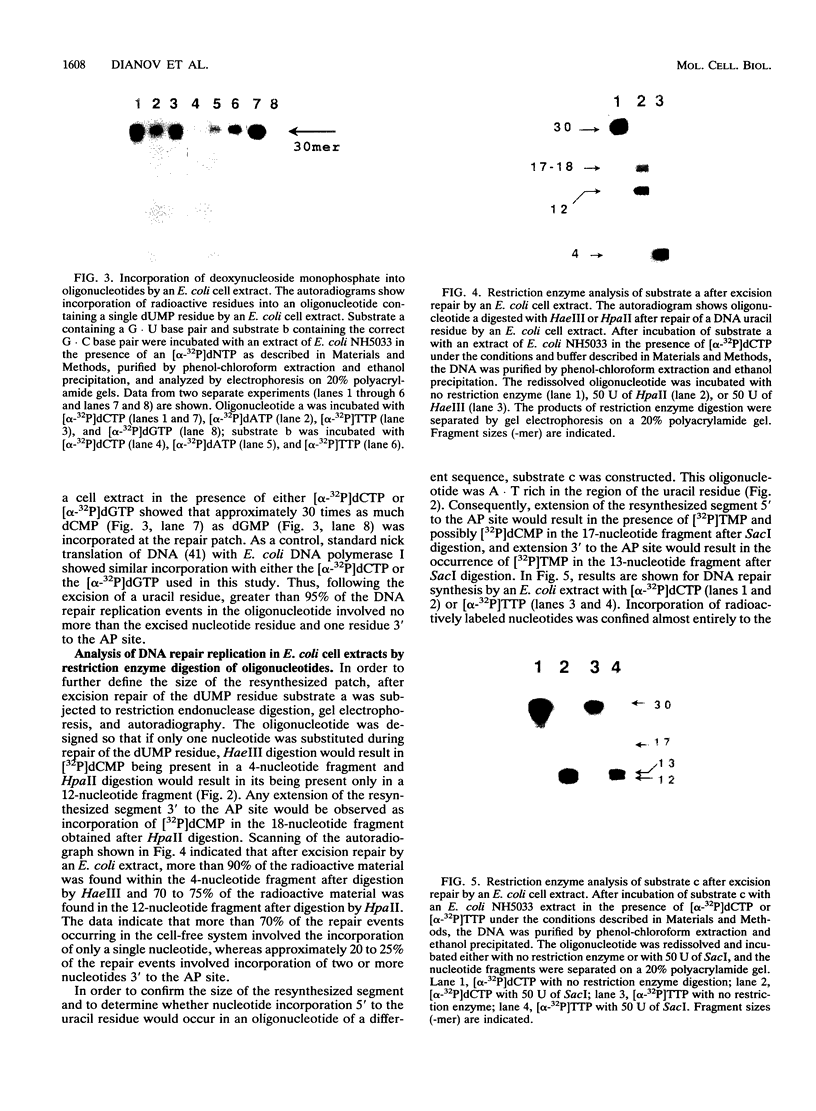
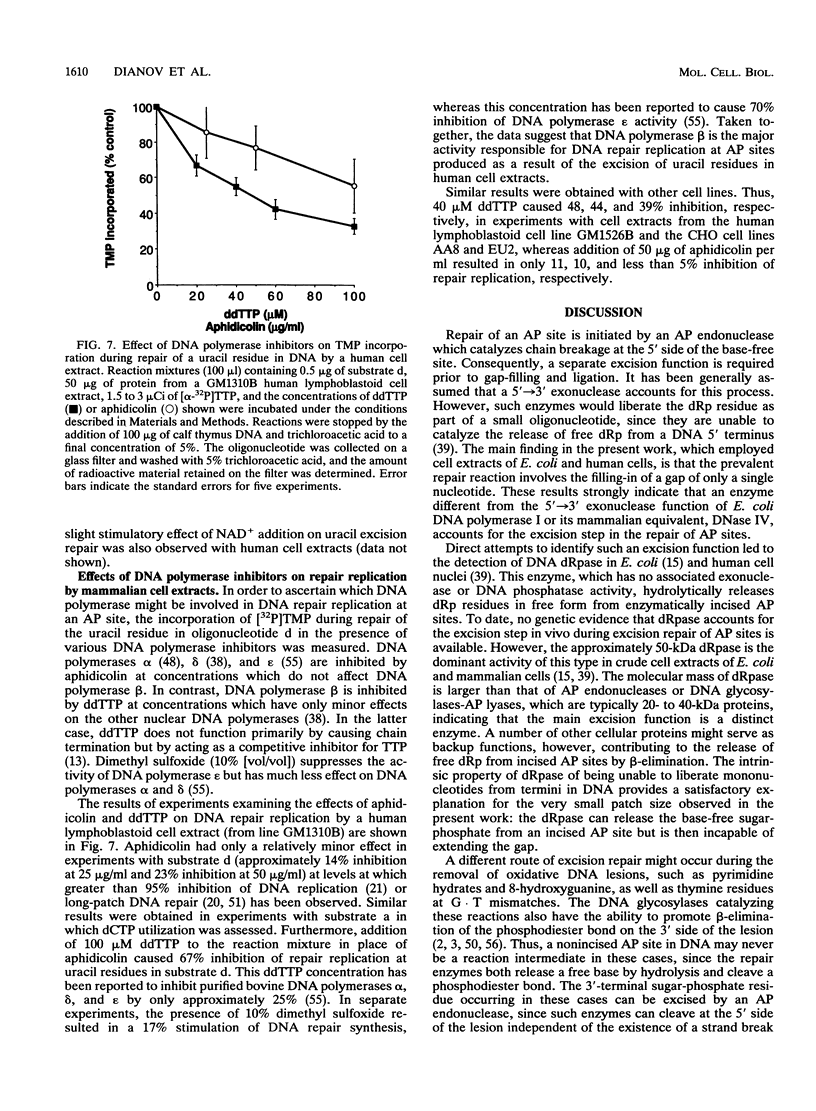
The extent and location of DNA repair synthesis in a double-stranded oligonucleotide containing a single dUMP residue have been determined. Gently prepared Escherichia coli and mammalian cell extracts were employed for excision repair in vitro. The size of the resynthesized patch was estimated by restriction enzyme analysis of the repaired oligonucleotide. Following enzymatic digestion and denaturing gel electrophoresis, the extent of incorporation of radioactively labeled nucleotides in the vicinity of the lesion was determined by autoradiography. Cell extracts of E. coli and of human cell lines were shown to carry out repair mainly by replacing a single nucleotide. No significant repair replication on the 5' side of the lesion was observed. The data indicate that, after cleavage of the dUMP residue by uracil-DNA glycosylase and incision of the resultant apurinic-apyrimidinic site by an apurinic-apyrimidinic endonuclease activity, the excision step is catalyzed usually by a DNA deoxyribophosphodiesterase rather than by an exonuclease. Gap-filling and ligation complete the repair reaction. Experiments with enzyme inhibitors in mammalian cell extracts suggest that the repair replication step is catalyzed by DNA polymerase beta.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au K. G., Clark S., Miller J. H., Modrich P. Escherichia coli mutY gene encodes an adenine glycosylase active on G-A mispairs. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8877–8881. doi: 10.1073/pnas.86.22.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Verly W. G. Escherichia coli endonuclease III is not an endonuclease but a beta-elimination catalyst. Biochem J. 1987 Mar 1;242(2):565–572. doi: 10.1042/bj2420565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailly V., Verly W. G., O'Connor T., Laval J. Mechanism of DNA strand nicking at apurinic/apyrimidinic sites by Escherichia coli [formamidopyrimidine]DNA glycosylase. Biochem J. 1989 Sep 1;262(2):581–589. doi: 10.1042/bj2620581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggerstaff M., Robins P., Coverley D., Wood R. D. Effect of exogenous DNA fragments on human cell extract-mediated DNA repair synthesis. Mutat Res. 1991 May;254(3):217–224. doi: 10.1016/0921-8777(91)90059-x. [DOI] [PubMed] [Google Scholar]
- Busch D. B., Cleaver J. E., Glaser D. A. Large-scale isolation of UV-sensitive clones of CHO cells. Somatic Cell Genet. 1980 May;6(3):407–418. doi: 10.1007/BF01542792. [DOI] [PubMed] [Google Scholar]
- Connolly B., West S. C. Genetic recombination in Escherichia coli: Holliday junctions made by RecA protein are resolved by fractionated cell-free extracts. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8476–8480. doi: 10.1073/pnas.87.21.8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham R. P., Saporito S. M., Spitzer S. G., Weiss B. Endonuclease IV (nfo) mutant of Escherichia coli. J Bacteriol. 1986 Dec;168(3):1120–1127. doi: 10.1128/jb.168.3.1120-1127.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGiuseppe J. A., Dresler S. L. Bleomycin-induced DNA repair synthesis in permeable human fibroblasts: mediation of long-patch and short-patch repair by distinct DNA polymerases. Biochemistry. 1989 Nov 28;28(24):9515–9520. doi: 10.1021/bi00450a040. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Cunningham R. P. The enzymology of apurinic/apyrimidinic endonucleases. Mutat Res. 1990 Sep-Nov;236(2-3):173–201. doi: 10.1016/0921-8777(90)90004-o. [DOI] [PubMed] [Google Scholar]
- Doetsch P. W., Helland D. E., Haseltine W. A. Mechanism of action of a mammalian DNA repair endonuclease. Biochemistry. 1986 Apr 22;25(8):2212–2220. doi: 10.1021/bi00356a054. [DOI] [PubMed] [Google Scholar]
- Duncan B. K., Weiss B. Specific mutator effects of ung (uracil-DNA glycosylase) mutations in Escherichia coli. J Bacteriol. 1982 Aug;151(2):750–755. doi: 10.1128/jb.151.2.750-755.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap B., Cerutti P. Apyrimidinic sites in gamma-irradiated DNA. FEBS Lett. 1975 Mar 1;51(1):188–190. doi: 10.1016/0014-5793(75)80884-2. [DOI] [PubMed] [Google Scholar]
- Fisher P. A., Wang T. S., Korn D. Enzymological characterization of DNA polymerase alpha. Basic catalytic properties processivity, and gap utilization of the homogeneous enzyme from human KB cells. J Biol Chem. 1979 Jul 10;254(13):6128–6137. [PubMed] [Google Scholar]
- Franklin W. A., Lindahl T. DNA deoxyribophosphodiesterase. EMBO J. 1988 Nov;7(11):3617–3622. doi: 10.1002/j.1460-2075.1988.tb03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard F., Verly W. G. Properties of the main endonuclease specific for apurinic sites of Escherichia coli (endonuclease VI). Mechanism of apurinic site excision from DNA. Eur J Biochem. 1978 Jan 16;82(2):321–332. doi: 10.1111/j.1432-1033.1978.tb12026.x. [DOI] [PubMed] [Google Scholar]
- Goulian M., Richards S. H., Heard C. J., Bigsby B. M. Discontinuous DNA synthesis by purified mammalian proteins. J Biol Chem. 1990 Oct 25;265(30):18461–18471. [PubMed] [Google Scholar]
- Guggenheimer R. A., Nagata K., Kenny M., Hurwitz J. Protein-primed replication of plasmids containing the terminus of the adenovirus genome. II. Purification and characterization of a host protein required for the replication of DNA templates devoid of the terminal protein. J Biol Chem. 1984 Jun 25;259(12):7815–7825. [PubMed] [Google Scholar]
- Hadi S. M., Goldthwait D. A. Endonuclease II of Escherichia coli. Degradation of partially depurinated deoxyribonucleic acid. Biochemistry. 1971 Dec 21;10(26):4986–4993. doi: 10.1021/bi00802a024. [DOI] [PubMed] [Google Scholar]
- Holmes J., Jr, Clark S., Modrich P. Strand-specific mismatch correction in nuclear extracts of human and Drosophila melanogaster cell lines. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5837–5841. doi: 10.1073/pnas.87.15.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y., Claude A., Bullock P., Hurwitz J. Complete enzymatic synthesis of DNA containing the SV40 origin of replication. J Biol Chem. 1988 Dec 25;263(36):19723–19733. [PubMed] [Google Scholar]
- Kataoka H., Sekiguchi M. Are purine bases enzymatically inserted into depurinated DNA in Escherichia coli? J Biochem. 1982 Sep;92(3):971–973. doi: 10.1093/oxfordjournals.jbchem.a134014. [DOI] [PubMed] [Google Scholar]
- Levin J. D., Demple B. Analysis of class II (hydrolytic) and class I (beta-lyase) apurinic/apyrimidinic endonucleases with a synthetic DNA substrate. Nucleic Acids Res. 1990 Sep 11;18(17):5069–5075. doi: 10.1093/nar/18.17.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Ljungquist S., Siegert W., Nyberg B., Sperens B. DNA N-glycosidases: properties of uracil-DNA glycosidase from Escherichia coli. J Biol Chem. 1977 May 25;252(10):3286–3294. [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Ljungquist S., Andersson A., Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. II. Further studies on the substrate specificity. J Biol Chem. 1974 Mar 10;249(5):1536–1540. [PubMed] [Google Scholar]
- Ljungquist S., Lindahl T. Relation between Escherichia coli endonucleases specific for apurinic sites in DNA and exonuclease III. Nucleic Acids Res. 1977 Aug;4(8):2871–2879. doi: 10.1093/nar/4.8.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Samuels M., Sharp P. A. In vitro transcription: whole-cell extract. Methods Enzymol. 1983;101:568–582. doi: 10.1016/0076-6879(83)01038-1. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Bogenhagen D. F. Repair of a synthetic abasic site in DNA in a Xenopus laevis oocyte extract. Mol Cell Biol. 1989 Sep;9(9):3750–3757. doi: 10.1128/mcb.9.9.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Bogenhagen D. F. Repair of a synthetic abasic site involves concerted reactions of DNA synthesis followed by excision and ligation. Mol Cell Biol. 1991 Sep;11(9):4441–4447. doi: 10.1128/mcb.11.9.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M. F., Ebisuzaki K. Base excision repair of DNA in gamma-irradiated human cells. Carcinogenesis. 1987 Apr;8(4):607–609. doi: 10.1093/carcin/8.4.607. [DOI] [PubMed] [Google Scholar]
- Mosbaugh D. W., Linn S. Characterization of the action of Escherichia coli DNA polymerase I at incisions produced by repair endodeoxyribonucleases. J Biol Chem. 1982 Jan 10;257(1):575–583. [PubMed] [Google Scholar]
- Padgett R. A., Hardy S. F., Sharp P. A. Splicing of adenovirus RNA in a cell-free transcription system. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5230–5234. doi: 10.1073/pnas.80.17.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter R. B., Young B. R. Repair replication in mammalian cells after x-irradiation. Mutat Res. 1972 Feb;14(2):225–235. doi: 10.1016/0027-5107(72)90049-8. [DOI] [PubMed] [Google Scholar]
- Perrino F. W., Loeb L. A. Animal cell DNA polymerases in DNA repair. Mutat Res. 1990 Sep-Nov;236(2-3):289–300. doi: 10.1016/0921-8777(90)90012-t. [DOI] [PubMed] [Google Scholar]
- Price A., Lindahl T. Enzymatic release of 5'-terminal deoxyribose phosphate residues from damaged DNA in human cells. Biochemistry. 1991 Sep 3;30(35):8631–8637. doi: 10.1021/bi00099a020. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B. Two forms of repair in the DNA of human cells damaged by chemical carcinogens and mutagens. Cancer Res. 1974 Dec;34(12):3318–3325. [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robins P., Jones C. J., Biggerstaff M., Lindahl T., Wood R. D. Complementation of DNA repair in xeroderma pigmentosum group A cell extracts by a protein with affinity for damaged DNA. EMBO J. 1991 Dec;10(12):3913–3921. doi: 10.1002/j.1460-2075.1991.tb04961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson C. N., Milne A. M., Pappin D. J., Hickson I. D. Isolation of cDNA clones encoding an enzyme from bovine cells that repairs oxidative DNA damage in vitro: homology with bacterial repair enzymes. Nucleic Acids Res. 1991 Mar 11;19(5):1087–1092. doi: 10.1093/nar/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakumi K., Sekiguchi M. Structures and functions of DNA glycosylases. Mutat Res. 1990 Sep-Nov;236(2-3):161–172. doi: 10.1016/0921-8777(90)90003-n. [DOI] [PubMed] [Google Scholar]
- Sanderson B. J., Chang C. N., Grollman A. P., Henner W. D. Mechanism of DNA cleavage and substrate recognition by a bovine apurinic endonuclease. Biochemistry. 1989 May 2;28(9):3894–3901. doi: 10.1021/bi00435a040. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S., Mori S., Nakashima A., Oda T. Effects of ATP and other nucleotides on DNA repair synthesis in bleomycin-pretreated permeable mouse sarcoma cells. Carcinogenesis. 1987 Oct;8(10):1391–1394. doi: 10.1093/carcin/8.10.1391. [DOI] [PubMed] [Google Scholar]
- Sheaff R., Ilsley D., Kuchta R. Mechanism of DNA polymerase alpha inhibition by aphidicolin. Biochemistry. 1991 Sep 3;30(35):8590–8597. doi: 10.1021/bi00099a014. [DOI] [PubMed] [Google Scholar]
- Takeshita M., Chang C. N., Johnson F., Will S., Grollman A. P. Oligodeoxynucleotides containing synthetic abasic sites. Model substrates for DNA polymerases and apurinic/apyrimidinic endonucleases. J Biol Chem. 1987 Jul 25;262(21):10171–10179. [PubMed] [Google Scholar]
- Tchou J., Kasai H., Shibutani S., Chung M. H., Laval J., Grollman A. P., Nishimura S. 8-oxoguanine (8-hydroxyguanine) DNA glycosylase and its substrate specificity. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4690–4694. doi: 10.1073/pnas.88.11.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Roberts J. D., Kunkel T. A. Heteroduplex repair in extracts of human HeLa cells. J Biol Chem. 1991 Feb 25;266(6):3744–3751. [PubMed] [Google Scholar]
- Tomkinson A. E., Lasko D. D., Daly G., Lindahl T. Mammalian DNA ligases. Catalytic domain and size of DNA ligase I. J Biol Chem. 1990 Jul 25;265(21):12611–12617. [PubMed] [Google Scholar]
- Tsurimoto T., Melendy T., Stillman B. Sequential initiation of lagging and leading strand synthesis by two different polymerase complexes at the SV40 DNA replication origin. Nature. 1990 Aug 9;346(6284):534–539. doi: 10.1038/346534a0. [DOI] [PubMed] [Google Scholar]
- Wallace S. S. AP endonucleases and DNA glycosylases that recognize oxidative DNA damage. Environ Mol Mutagen. 1988;12(4):431–477. doi: 10.1002/em.2860120411. [DOI] [PubMed] [Google Scholar]
- Weiser T., Gassmann M., Thömmes P., Ferrari E., Hafkemeyer P., Hübscher U. Biochemical and functional comparison of DNA polymerases alpha, delta, and epsilon from calf thymus. J Biol Chem. 1991 Jun 5;266(16):10420–10428. [PubMed] [Google Scholar]
- Wiebauer K., Jiricny J. In vitro correction of G.T mispairs to G.C pairs in nuclear extracts from human cells. Nature. 1989 May 18;339(6221):234–236. doi: 10.1038/339234a0. [DOI] [PubMed] [Google Scholar]
- Wiebauer K., Jiricny J. Mismatch-specific thymine DNA glycosylase and DNA polymerase beta mediate the correction of G.T mispairs in nuclear extracts from human cells. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5842–5845. doi: 10.1073/pnas.87.15.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withka J. M., Wilde J. A., Bolton P. H., Mazumder A., Gerlt J. A. Characterization of conformational features of DNA heteroduplexes containing aldehydic abasic sites. Biochemistry. 1991 Oct 15;30(41):9931–9940. doi: 10.1021/bi00105a017. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Robins P., Lindahl T. Complementation of the xeroderma pigmentosum DNA repair defect in cell-free extracts. Cell. 1988 Apr 8;53(1):97–106. doi: 10.1016/0092-8674(88)90491-6. [DOI] [PubMed] [Google Scholar]