Abstract
Technical failure (TF) continues to have a significant impact on the success of pancreas transplantation. We assessed risk factors for TF in 1,115 pancreas transplants performed at a single center between 1998–2011. The relationships of donor and recipient factors, surgical approach, allo-sensitization and matching, and immunosuppressive agents were correlated with risk of TF. In a multivariable model, donor BMI ≥30, donor Cr ≥2.5, donor age >50, and preservation time >20 hours were associated with TF. Bladder drainage of exocrine secretions was protective. We incorporated these factors in a Composite Risk Model and tested its ability to predict TF in comparison to existing models (pDRI). In the Composite Risk Model, the presence of one risk factor did not significantly increase risk of TF, but two or more risk factors in combination were predictive. The analysis also identifies many factors that were not predictive of TF, including previous transplants, immunosuppressive agent selection, and almost all recipient demographic parameters. While the model suggests that two or more risk factors predict TF, strategies to reduce preservation time may mitigate some of this risk.
Keywords: Pancreas, Transplant, Technical Failure, Risk Factors, Cost, Survival
Introduction
Pancreas transplantation increases longevity and improves quality of life in patients with diabetes (1–5). However, pancreas transplants are associated with a high incidence of surgical complication leading to significantly greater technical failure (TF) and graft loss rates than seen with other solid organ transplants. TF has been reported in 7% to 22% of recipients (6–11), with the most common causes being thrombosis, intra-abdominal infections, leaks, bleeding, and allograft pancreatitis.
Advances in patient management, operative technique, and immunosuppression over the early decades of pancreas transplantation have resulted in significant improvements in both patient and graft survival (12). In contrast, despite reductions in surgical complications through earlier eras of pancreas transplant, the TF rate through recent eras has remained stagnant and surgical complications leading to TF remain a significant underlying cause of morbidity and mortality in pancreas recipients.
In order to define the basis for the continued high incidence of technical failure in pancreas transplantation we reviewed transplants performed in the modern era. This study focuses on early graft loss (within 90 days) due to technical failure. Other models have been developed for modeling longer term graft function (13). Pancreas utilization rates are lower than other organs (14). We hypothesized that we would find a limited set of risk factors for early TF. By extension, identifying factors that do predict TF also identifies factors that do not increase risk, or that could be modified to reduce their impact (i.e., reducing preservation time). Careful consideration of such donors that might have previously been discarded may thereby increase utilization.
Methods
Study Population
We performed a retrospective review of all pancreas transplants performed at the University of Minnesota between June 1998 and July 2011. Data from paper and electronic medical records were incorporated in a prospectively assembled transplant database. Exclusions included living donor pancreas transplants, pediatric recipients (age < 18 years), segmental/partial pancreas transplants, and simultaneous live-donor kidney/deceased-donor pancreas (PwK) transplants, and patients with incomplete medical records (n = 9). The Institutional Review Board approved the study.
Surgical Procedure
Pancreas transplants were performed as simultaneous pancreas-kidney transplants (SPK), pancreas after kidney transplants (PAK), or pancreas transplants alone (PTA). The donor allograft recovery, back table preparation, and implant technique were described previously (15). Donor perfusion was routinely performed with University of Wisconsin (UW) solution. The grafts were examined after surgical exposure in the donor, on the back table before packaging, and once more upon arrival at our institution. Organs that had fatty infiltration, edematous appearance, were firm or nodular on palpation, or had vascular injury or aberrant anatomy making reconstruction or repair complicated were discarded. Surgery was via midline laparotomy. Transplants were performed in the head-down orientation with systemic venous drainage via donor portal vein anastomosis to recipient iliac vein or vena cava. Splenic and superior mesenteric arteries were anastomosed to the recipient iliac artery or aorta after construction of a Y-graft conduit with donor iliac artery. Exocrine drainage was via duodenocystosomy or duodenojejunostomy. Patients were anticoagulated with bolus heparin (70 Units/kg) prior to vascular clamping. Heparin infusion was started four hours postoperatively (3 Units/kg/hr), increased eight hours postoperatively (7 Units/kg/hr) if patient was stable, and continued for five days. Aspirin was started within 48 hours at 81 mg/day and increased to 325 mg/day when heparin was discontinued. Perioperative antibiotic prophylaxis consisted of broad-spectrum antibiotics (ampicillin/sublactam or piperacillin/tazobactam) for three days and fluconazole for seven days.
Immunosuppression
Three immunosuppression protocols were utilized during the course of these transplants. The first protocol (7/1998 – 12/2002) consisted of an induction with five to seven doses of an anti-T cell antibody [equine antithymocyte globulin (ATGAM), Pfizer, New York, NY; or rabbit antithymocyte globulin (Thymoglobulin), Genzyme Corporation, Boston, MA] and pulse steroids, followed by maintenance therapy with tacrolimus and mycophenolate mofetil (MMF), and low dose prednisone. The second protocol (1/2003 – 8/2005) consisted of induction with alemtuzumab (Campath, Sanofi, Paris, France), MMF maintenance, and calcineurin inhibitor (CNI) and steroid avoidance. The final protocol (9/2005 – 6/2011) consisted of induction with rabbit antithymocyte globulin, maintenance with tacrolimus and MMF, and rapid steroid discontinuation. Anti-IL-2R antibody was used in addition to other induction agents 1998 to 1999, and then in selected cases. Cyclosporine was used in rare patients with tacrolimus-associated symptoms or intolerance.
Statistical Analysis
We analyzed the association of pancreas technical failure with donor, recipient, immunologic, immunosuppression, and surgical risk factors. Technical failure was defined as graft loss within the first 90 days following transplant due to thrombosis, bleeding, pancreatitis, or intra-abdominal infections including anastomotic or pancreatic leak. Risk factors were analyzed for their relationship with technical failure using univariable and multivariable analyses.
Recipient factors included gender, age, BMI, re-transplant, history of pre-transplant vascular disease (including myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, stroke, transient ischemic attack, claudication, amputation, or peripheral bypass grafting/intervention), pre-transplant dialysis history (any time prior to pancreas transplant), and smoking at time of transplant. BMI was categorized according to CDC criteria for normal (<25), overweight (25–30), and obese (>30). Donor factors were gender, age, race, cause of death, history of drug or alcohol abuse, history of pancreatitis (as reported in the donor information packet), BMI, donation after cardiac death, creatinine (terminal Cr ≥ 2.5 mg/dL), serum amylase (terminal amylase > 500 U/L), interval from donor admission to declaration of death, cold preservation time, and pDRI (13). Surgical factors were type of exocrine drainage (bladder v. enteric drainage). Immune factors included PRA, and number of HLA-A, -B, and – DR mismatches. Individual immunosuppression agents are described above.
Statistical analyses were performed using version 9.2 of SAS™ (SAS Institute Inc., Cary, NC) and SPSS version 21 (IBM, Armonk, New York). Univariable statistics were summarized as a count and percentage or a mean and standard error of the mean (SEM). For bivariate associations between risk factors and outcomes, categorical variables were tested using the Chi-square statistic. The t-test was applied to continuous variables. Graft and patient survival was assessed by the Kaplan-Meier life table method using log-rank and Wilcoxon test statistics (16, 17). Cases with missing data points were censored at the point of inclusion in each model, except for in the calculation of the pDRI where the mean value of appropriate similar cases were used in the calculation of the eSUM (e.g., cases with missing preservation time were assigned the average time for import or local organs, accordingly). The dataset was extensively vetted and all attempts were made to obtain missing data points. The pDRI data set was >99% complete.
Confounding risk factors statistically related to technical failure were incorporated into the multivariable analysis by Cox proportional hazards regression. Factors achieving a P value less than 0.2 in the unadjusted univariable hazard model analysis were selected for inclusion in the full Cox model. Factors with P in excess of 0.2 in the full model were excluded in a reduced multivariable model. The validity of this model was verified by bootstrap resampling (1,000 times). The final model included the entire sample set. Log-log curves were used to test the proportionality (18). The independent association of risk factors and outcomes were adjusted for confounding factors.
A Composite Risk Model was created using factors associated with TF in the multivariable model. Transplants with no risk factors were compared to those with one or more risk factors and adjusted for confounding characteristics. Pancreas Donor Risk Index (pDRI) was calculated as described (13). The pDRI model was compared with the simpler risk factor model under various scenarios. For the pDRI, the highest 5%, 10% and 20% risk values were used. What has been described earlier in reports as the “highest risk group” was equivalent to our 20% level. For the risk factor approach, we used 1 or more, 2 or more and 3 or more risk factors. Each modeling approach was compared for the percent correct, sensitivity, specificity, and the c-statistic. Each of the models devised for predicting technical failure was compared with the covariates only approach. To test whether the models were a statistical improvement over and above covariates alone, we used the difference in the log-likelihood values for the covariate alone model and each alternative model (19). Finally, we compared the pDRI for 20% with the 3 or more risk factors also using the difference in log-likelihood method.
Results
In order to define risk factors for technical failure we examined 1,115 transplants performed between June 1998 and July 2011. Of these 306 (27.4%) were simultaneous pancreas-kidney transplants (SPK), 321 (28.8%) were pancreas transplant alone (PTA), and 488 (43.8%) were pancreas after kidney transplants (PAK). Patient demographics, donor characteristics, immunosuppression, immune parameters, and surgical details are summarized in Table 1.
Table 1. Patient demographics in the current era (1998–2011).
| PAK n = 488 |
PTA n = 321 |
SPK n = 306 |
Total n = 1115 |
|||||
|---|---|---|---|---|---|---|---|---|
| Recipient Factors | ||||||||
| Female | 227 | 46.5% | 202 | 62.9% | 125 | 40.8% | 554 | 49.6% |
| Age | 43.8 | ± 0.4 | 42.0 | ± 0.6 | 44.7 | ± 0.5 | 43.5 | ± 0.3 |
| BMI | 25.4 | ± 0.2 | 25.5 | ± 0.2 | 25.8 | ± 0.3 | 25.5 | ± 0.1 |
| <25 | 236 | 49.8% | 154 | 50.0% | 142 | 47.8% | 532 | 49.3% |
| 25–30 | 172 | 36.3% | 114 | 37.0% | 99 | 33.3% | 385 | 35.7% |
| >30 | 66 | 13.9% | 40 | 13.0% | 56 | 18.9% | 162 | 15.0% |
| Retransplant | 152 | 31.1% | 67 | 20.9% | 39 | 12.7% | 258 | 23.1% |
| History of Vascular Diseasea | 237 | 48.6% | 81 | 25.2% | 147 | 48.0% | 465 | 41.7% |
| Dialysisb | 260 | 57.5% | 40 | 13.4% | 201 | 76.1% | 501 | 49.4% |
| Smokerc | 56 | 12.1% | 36 | 13.8% | 34 | 11.1% | 126 | 12.2% |
| Donor Factors | ||||||||
| Female | 180 | 36.9% | 131 | 41.1% | 131 | 42.8% | 442 | 39.7% |
| Age | 29.1 | ± 0.6 | 27.7 | ± 0.7 | 33.1 | ± 0.8 | 29.8 | ± 0.4 |
| Race | ||||||||
| White | 414 | 93.2% | 271 | 92.2% | 250 | 95.1% | 935 | 93.4% |
| African American | 29 | 6.0% | 14 | 4.4% | 12 | 3.9% | 55 | 4.9% |
| Asian | 3 | 0.6% | 4 | 1.3% | 3 | 1.0% | 10 | 0.9% |
| Cause of Death | ||||||||
| Cerebrovascular Accident (Stroke) | 153 | 31.5% | 92 | 28.7% | 103 | 33.9% | 348 | 31.3% |
| Head Trauma or Gunshot Wound | 251 | 51.6% | 185 | 57.6% | 155 | 51.0% | 591 | 53.2% |
| Other | 84 | 17.3% | 44 | 13.7% | 63 | 20.6% | 176 | 15.8% |
| Social History | ||||||||
| Alcohol Abuse | 37 | 7.8% | 32 | 10.3% | 26 | 8.9% | 95 | 8.8% |
| Drug Abuse | 21 | 5.1% | 20 | 7.4% | 17 | 6.6% | 58 | 6.2% |
| Pancreatitis Donor | 2 | 0.8% | 2 | 0.7% | 1 | 0.3% | 5 | 0.5% |
| Donation After Cardiac Death | 7 | 1.4% | 12 | 3.8% | 13 | 4.2% | 32 | 2.9% |
| Body Mass Index | 24.8 | ± 0.2 | 24.3 | ± 0.3 | 25.1 | ± 0.3 | 24.7 | ± 0.1 |
| Elevated Recent Creatinine | 19 | 4.2% | 8 | 2.8% | 2 | 0.7% | 29 | 2.6% |
| Elevated Serum Amylase (>500) | 20 | 4.6% | 9 | 3.4% | 8 | 2.9% | 37 | 3.8% |
| Preservation Time in Hours | 17.3 | ± 0.2 | 17.3 | ± 0.3 | 17.4 | ± 0.3 | 17.3 | ± 0.1 |
| Interval Admission to Brain Death (Days) | 2.2 | ± 0.3 | 3.4 | ± 1.2 | 2.0 | ± 0.3 | 2.5 | ± 0.4 |
| Pancreas Donor Risk Index (pDRI) | 1.25 | ± 0.02 | 1.33 | ± 0.03 | 1.56 | ± 0.04 | 1.36 | ± 0.02 |
| Surgical Factors | ||||||||
| Bladder Drainage | 373 | 76.6% | 254 | 79.1% | 83 | 27.1% | 710 | 63.7% |
| Allosensitization | ||||||||
| Peak PRA Class I | 8.62 | ± 1.06 | 11.10 | ± 1.48 | 9.23 | ± 1.63 | 9.52 | ± 0.77 |
| Peak PRA Class II | 7.30 | ± 1.06 | 11.36 | ± 1.63 | 8.42 | ± 1.54 | 8.80 | ± 0.79 |
| Peak_Overall_PRA | 17.64 | ± 1.41 | 22.06 | ± 1.98 | 20.24 | ± 2.02 | 19.63 | ± 1.01 |
| Zero Mismatches | 8 | 1.7% | 12 | 3.8% | 45 | 14.8% | 65 | 5.9% |
| Peak PRA Greater than 80 | 47 | 9.7% | 46 | 14.4% | 50 | 16.5% | 143 | 12.9% |
| Immunosuppression | ||||||||
| Thymoglobulin or Atgam | 432 | 88.9% | 290 | 91.5% | 271 | 88.9% | 993 | 89.6% |
| Campath | 92 | 18.9% | 78 | 24.6% | 44 | 14.4% | 214 | 19.3% |
| IL-2R Monoclonal Antibody | 282 | 58.0% | 167 | 52.7% | 156 | 51.1% | 605 | 54.6% |
| Tacrolimus | 407 | 83.7% | 258 | 81.4% | 262 | 85.9% | 927 | 83.7% |
| Cyclosporine | 32 | 6.6% | 6 | 1.9% | 4 | 1.3% | 42 | 3.8% |
| MMF | 430 | 88.8% | 305 | 96.8% | 264 | 86.8% | 988 | 89.6% |
| Prednisone | 298 | 61.4% | 155 | 49.4% | 163 | 53.6% | 616 | 55.8% |
Count and Percent; or Mean ± S.E.M.
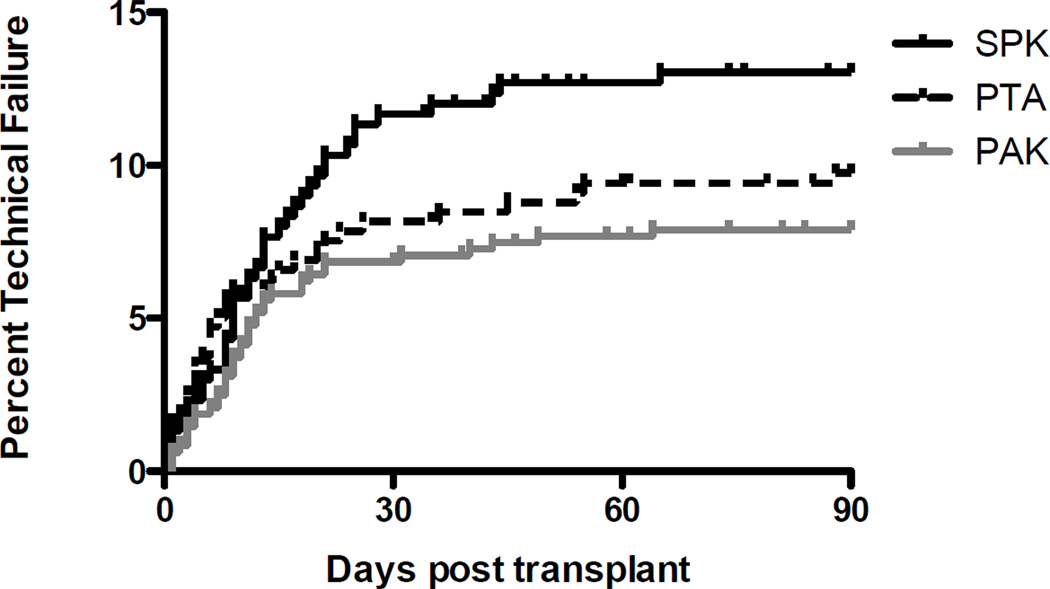
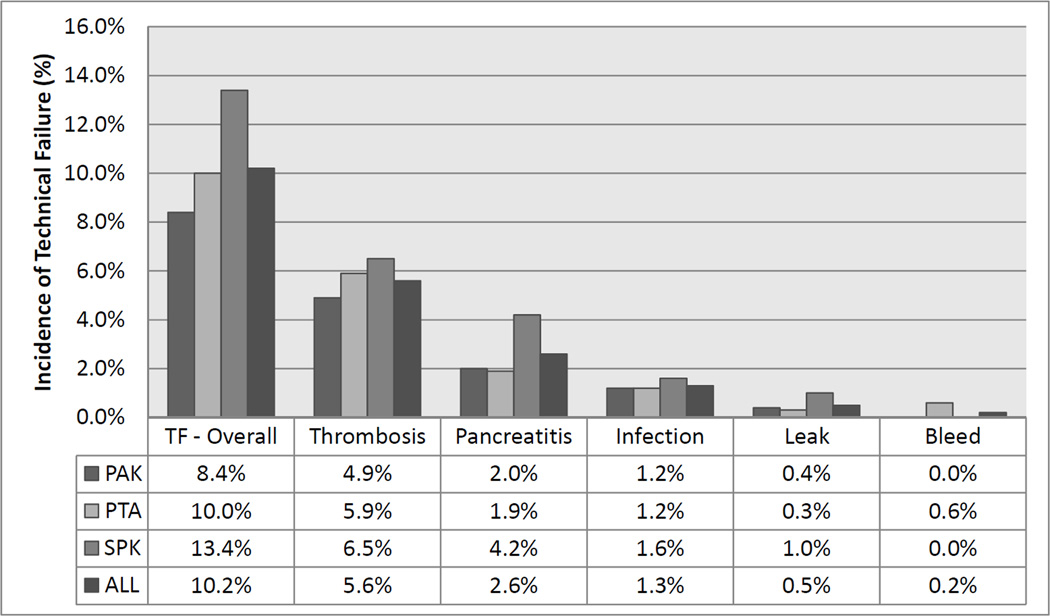
The overall technical failure rate for pancreas transplants in this modern cohort was 10.2%. TF rate was 13.4% for SPK transplants, 8.4% for PAK, and 10.0% for PTA (Fig. 1). Allograft thrombosis was the most common cause of technical failure in this analysis, occurring in 5.6% of transplants and representing 54.9% of TFs (Fig. 2). Thrombosis was slightly less frequent in solitary pancreas transplants occurring in 4.9% and 5.9% of PAK and PTA recipients, respectively. Pancreatitis was the second most common cause of TF, again occurring more frequently in SPK transplants (4.2% of transplants). Overall intra-abdominal infection resulted in TF in 1.8% of patients, encompassing both anastomotic leaks (0.5% of cases) and other abdominal/peripancreatic infections (1.3% of cases).
Figure 1. Technical failure by transplant type.
The incidence of technical failure for each transplant category (PTA, PAK, SPK) in the current era.
Figure 2. Distribution of causes of technical failure for each transplant category.
For each category all p values > 0.05 for pairwise comparison between transplant types and overall Chi-Square comparison.
Factors that achieved statistical significance (p < 0.05) as risk factors for TF in unadjusted univariable analysis were SPK transplants, pancreatitis in the donor, donor Cr ≥ 2.5, preservation (cold ischemia) time > 20 hours, donor age > 50, donor BMI ≥ 30, pDRI, and donor cause of death other than head trauma (Table 2, 3). Factors that trended towards significance included donor history of alcohol abuse, recipient BMI >30, recipient smoking history, and elevated donor terminal amylase. Bladder drained transplants were protective from TF. Notable factors not increasing risk for TF included pancreas re-transplant, pre-transplant vascular disease in the recipient, or recipient dialysis history. Alemtuzumab (Campath)-based immunosuppression was borderline protective (p = 0.093), but other immunosuppressive agents including thymoglobulin, steroids, and IL-2R antibodies were not related to TF. Similarly, HLA-matching, recipient sensitization (PRA values), and donor race were not statistically related to TF.
Table 2. Patient demographics with and without technical failure.
| Technical Failure n = 114 |
No Technical Failure n = 1001 |
P value | ||||
|---|---|---|---|---|---|---|
| Recipient Factors | ||||||
| Female | 50 | 43.9% | 504 | 50.3% | 0.189 | |
| Age | 43.18 | ± 0.80 | 43.57 | ± 0.28 | 0.653 | |
| BMI | ||||||
| <25 | 46 | 42.6% | 486 | 50.1% | 0.232 | |
| 25–30 | 41 | 38.0% | 344 | 35.4% | 0.269 | |
| >30 | 21 | 19.4% | 141 | 14.5% | 0.103 | |
| Retransplant | 27 | 23.7% | 231 | 23.1% | 0.884 | |
| History of Vascular Disease | 44 | 38.6% | 421 | 42.1% | 0.478 | |
| Dialysis | 54 | 49.1% | 447 | 49.4% | 0.944 | |
| Smoking History | 10 | 10.3% | 116 | 12.4% | 0.530 | |
| Donor Factors | ||||||
| Female | 42 | 36.8% | 400 | 40.0% | 0.509 | |
| Age | 34.22 | ± 1.18 | 29.31 | ± 0.40 | < 0.001 | |
| Race | ||||||
| White | 99 | 92.5% | 836 | 93.5% | 0.697 | |
| African American | 6 | 5.3% | 49 | 4.9% | 0.867 | |
| Asian | 1 | 0.9% | 9 | 0.9% | 0.980 | |
| Cerebrovascular Accident (Stroke) | 45 | 39.8% | 303 | 30.4% | 0.040 | |
| Alcohol Abuse | 15 | 13.6% | 80 | 8.3% | 0.060 | |
| Head Trauma or Gunshot Wound | 50 | 44.2% | 541 | 54.2% | 0.044 | |
| Pancreatitis Donor | 3 | 2.9% | 2 | 0.2% | < 0.001 | |
| Drug Abuse | 5 | 5.3% | 53 | 6.3% | 0.718 | |
| Donation After Cardiac Death | 1 | 0.9% | 31 | 3.1% | 0.178 | |
| BMI | 26.38 | ± 0.43 | 24.53 | ± 0.15 | < 0.001 | |
| Elevated Recent Creatinine | 8 | 7.5% | 21 | 2.3% | 0.002 | |
| Elevated Recent Serum Amylase | 6 | 5.7% | 31 | 3.6% | 0.209 | |
| Preservation Time in Hours | 18.52 | ± 0.45 | 17.19 | ± 0.15 | 0.004 | |
| Interval Admit to Brain Death (d) | 1.67 | ± 0.42 | 2.61 | ± 0.44 | 0.126 | |
| Pancreas Donor Risk Index | 1.61 | ± 0.06 | 1.33 | ± 0.02 | < 0.001 | |
| Surgical Factors | ||||||
| Bladder Drainage | 63 | 55.8% | 647 | 64.6% | 0.063 | |
| Allosensitization | ||||||
| Peak PRA Class I | 10.81 | ± 2.74 | 9.38 | ± 0.80 | 0.581 | |
| Peak PRA Class II | 8.79 | ± 2.43 | 8.80 | ± 0.83 | 0.995 | |
| Peak_Overall_PRA | 18.40 | ± 3.15 | 19.77 | ± 1.06 | 0.682 | |
| Zero Mismatches | 8 | 7.1% | 57 | 5.8% | 0.591 | |
| Peak PRA Greater than 80 | 13 | 11.6% | 130 | 13.1% | 0.663 | |
| Immunosuppression | ||||||
| Thymoglobulin or Atgam | 99 | 88.4% | 894 | 89.8% | 0.653 | |
| Campath | 15 | 13.4% | 199 | 20.0% | 0.094 | |
| IL-2R Monoclonal Antibody | 62 | 55.4% | 543 | 54.5% | 0.866 | |
| Tacrolimus | 92 | 82.1% | 825 | 82.8% | 0.646 | |
| Cyclosporine | 4 | 3.6% | 38 | 3.8% | 0.898 | |
| MMF | 99 | 90.8% | 900 | 90.5% | 0.968 | |
| Prednisone | 56 | 50.5% | 560 | 56.5% | 0.227 | |
Count and Percent; or Mean ± S.E.M.
Table 3. Univariable association of donor, recipient, surgical, allo-sensitization, and immunosuppressive agents with risk of technical failure.
The univariable unadjusted association hazard ratio (HR), confidence interval [lower confidence level (LCL) and upper confidence level (UCL)], and p values.
| Hazard Ratio | LCL | UCL | p value | |
|---|---|---|---|---|
| Transplant Type | ||||
| PTA | 0.96 | 0.64 | 1.45 | 0.855 |
| PAK | 0.71 | 0.48 | 1.04 | 0.078 |
| SPK | 1.52 | 1.04 | 2.23 | 0.031 |
| Recipient Characteristics | ||||
| Female | 0.78 | 0.54 | 1.13 | 0.196 |
| Age | 0.95 | 0.77 | 1.17 | 0.621 |
| BMI | ||||
| <25 | - | - | - | 0.232 |
| 25–30 | 1.27 | 0.83 | 1.93 | 0.269 |
| >30 | 1.54 | 0.92 | 2.57 | 0.103 |
| Retransplant | 1.06 | 0.69 | 1.63 | 0.781 |
| Pretransplant Vascular Disease | 0.86 | 0.59 | 1.26 | 0.440 |
| Pretransplant Dialysis | 0.99 | 0.68 | 1.44 | 0.974 |
| Smoker | 0.75 | 0.51 | 1.09 | 0.135 |
| Donor Characteristics | ||||
| Female | 0.89 | 0.61 | 1.31 | 0.558 |
| Age | 1.03 | 1.01 | 1.04 | < 0.001 |
| Age 50 or Older | 2.08 | 1.19 | 3.64 | 0.010 |
| Race: White | 0.86 | 0.42 | 1.77 | 0.687 |
| Race: African American | 1.07 | 0.47 | 2.44 | 0.866 |
| Race: Asian | 0.97 | 0.14 | 6.93 | 0.974 |
| Body Mass Index | 1.08 | 1.04 | 1.12 | < 0.001 |
| BMI >30 | 1.75 | 1.16 | 2.63 | 0.007 |
| Cause of Death - Stroke | 1.45 | 1.00 | 2.12 | 0.052 |
| Cause of Death - Head Trauma | 0.69 | 0.48 | 1.00 | 0.049 |
| Social History - Alcohol Abuse | 1.69 | 0.98 | 2.92 | 0.058 |
| Social History - Drug Abuse | 0.86 | 0.35 | 2.12 | 0.742 |
| Past Medical History - Pancreatitis | 7.39 | 2.34 | 23.29 | < 0.001 |
| Donation After Cardiac Death | 0.30 | 0.04 | 2.15 | 0.231 |
| Elevated Creatinine | 3.06 | 1.49 | 6.30 | 0.002 |
| Elevated Amylase | 1.62 | 0.79 | 3.33 | 0.191 |
| Preservation Time (Hours) | 1.06 | 1.02 | 1.10 | 0.004 |
| Preservation Time 20 Hours or Greater | 1.82 | 1.25 | 2.65 | 0.002 |
| Interval Admit to Brain Death (Days) | 0.89 | 0.69 | 1.16 | 0.386 |
| Pancreas Donor Risk Index (pDRI) | 1.89 | 1.48 | 2.40 | < 0.001 |
| Surgical Factors | ||||
| Bladder Drainage | 0.69 | 0.48 | 1.00 | 0.052 |
| Allosensitization | ||||
| Peak PRA Class I | 1.00 | 1.00 | 1.01 | 0.544 |
| Peak PRA Class II | 1.00 | 0.99 | 1.01 | 0.946 |
| Peak PRA Overall | 1.00 | 0.99 | 1.01 | 0.691 |
| Zero Mismatches | 1.24 | 0.60 | 2.54 | 0.560 |
| Peak PRA Greater than 80% | 0.89 | 0.50 | 1.58 | 0.679 |
| Immunosuppression | ||||
| Thymoglobulin or Atgam | 0.87 | 0.49 | 1.56 | 0.645 |
| Campath | 0.63 | 0.36 | 1.08 | 0.093 |
| IL-2R Monoclonal Antibody | 1.03 | 0.71 | 1.49 | 0.884 |
| Tacrolimus | 0.87 | 0.54 | 1.42 | 0.582 |
| Cyclosporine | 0.94 | 0.35 | 2.56 | 0.909 |
| MMF | 1.01 | 0.53 | 1.94 | 0.968 |
| Prednisone | 0.79 | 0.55 | 1.15 | 0.223 |
Parameters identified as potential predictors of TF risk in the univariable analysis (P < 0.2) were selected for inclusion in the multivariable Cox model (Table 4). Donor history of pancreatitis (history identified in the donor chart) was an independent risk factor for TF, but because of low numbers these cases were excluded from subsequent multivariable models. Factors that persisted in demonstrating increased independent risk included donor BMI ≥ 30, donor age > 50, donor Cr ≥ 2.5, and preservation time > 20 hours. Bladder drainage remained protective. Other factors identified in the univariable models failed to have statistical significance as independent risk factors in the multivariable model. These factors included donor alcohol abuse, donor mechanism of death, elevated donor amylase, Campath-based immunosuppression, recipient BMI, recipient smoking history, and SPK transplants. A reduced Cox model included donor age > 50 and other independent risk factors identified in the full model (Table 4). In the reduced model, donor BMI, creatinine, and cold preservation time > 20 hours statistically increased risk whereas bladder drainage decreased risk. Donor age > 50 trended towards significance (p = 0.082).
Table 4. Multivariable Cox Model of risk factors for technical failure.
Factors identified in the univariable analysis were incorporated in a full multivariable Cox Model and verified in a reduced model. The predictive factors were assembled in a Composite Risk Model incorporating donor age, donor BMI, donor Cr, and preservation time and adjusted for surgical approach. Transplants with no risk factors were compared to those with one, two, or three risk factors. For comparison the pDRI risk categories were similarly modeled.
| Full Model | ||||
| Hazard Ratio | LCL | UCL | p value | |
| SPK Transplant | 1.12 | 0.65 | 1.94 | 0.683 |
| Bladder Drainage | 0.52 | 0.30 | 0.88 | 0.015 |
| Donor Age >50 | 2.05 | 1.01 | 4.18 | 0.047 |
| Preservation Time >20 hours | 2.07 | 1.31 | 3.29 | 0.002 |
| Donor BMI ≥30 | 1.60 | 0.95 | 2.68 | 0.076 |
| Cause of Death - Stroke | 0.78 | 0.43 | 1.41 | 0.415 |
| Cause of Death - Head Trauma | 0.76 | 0.43 | 1.34 | 0.344 |
| Social History - Alcohol Abuse | 1.41 | 0.67 | 2.96 | 0.365 |
| Elevated Creatinine (≥ 2.5) | 3.10 | 1.21 | 7.93 | 0.018 |
| Elevated Amylase (≥ 500) | 1.50 | 0.54 | 4.16 | 0.438 |
| Recipient BMI >30 | 1.40 | 0.79 | 2.49 | 0.251 |
| Recpient Smoking History | 0.97 | 0.48 | 1.97 | 0.940 |
| Campath Induction | 0.88 | 0.47 | 1.66 | 0.692 |
| Reduced Model | ||||
| Hazard Ratio | LCL | UCL | p value | |
| Bladder Drainage | 0.54 | 0.36 | 0.80 | 0.002 |
| Donor Age >50 | 1.73 | 0.93 | 3.19 | 0.082 |
| Preservation Time >20 hours | 2.17 | 1.45 | 3.23 | <0.001 |
| Donor BMI ≥30 | 1.87 | 1.21 | 2.88 | 0.005 |
| Elevated Creatinine (≥ 2.5) | 3.16 | 1.37 | 7.26 | 0.007 |
| Composite Model | ||||
| Hazard Ratio | LCL | UCL | p value | |
| Bladder Drainage | 0.61 | 0.42 | 0.88 | 0.009 |
| Risk Factors | ||||
| No Risk Factors (n = 519) | 1.00 | |||
| 1 Risk Factor (n = 377) | 1.35 | 0.80 | 1.91 | 0.346 |
| 2 Risk Factors (n = 87) | 3.65 | 2.20 | 6.06 | < 0.001 |
| 3 Risk Factors (n= 14) | 7.66 | 3.26 | 18.04 | < 0.001 |
| pDRI Model | ||||
| Hazard Ratio | LCL | UCL | p value | |
| Bladder Drainage | 0.67 | 0.46 | 0.97 | 0.036 |
| pDRI Quintiles | ||||
| First | 1.00 | |||
| Second | 1.01 | 0.46 | 2.23 | 0.980 |
| Third | 1.56 | 0.70 | 3.50 | 0.276 |
| Fourth | 2.53 | 1.16 | 5.48 | 0.019 |
| Fifth | 3.15 | 1.39 | 7.11 | 0.006 |
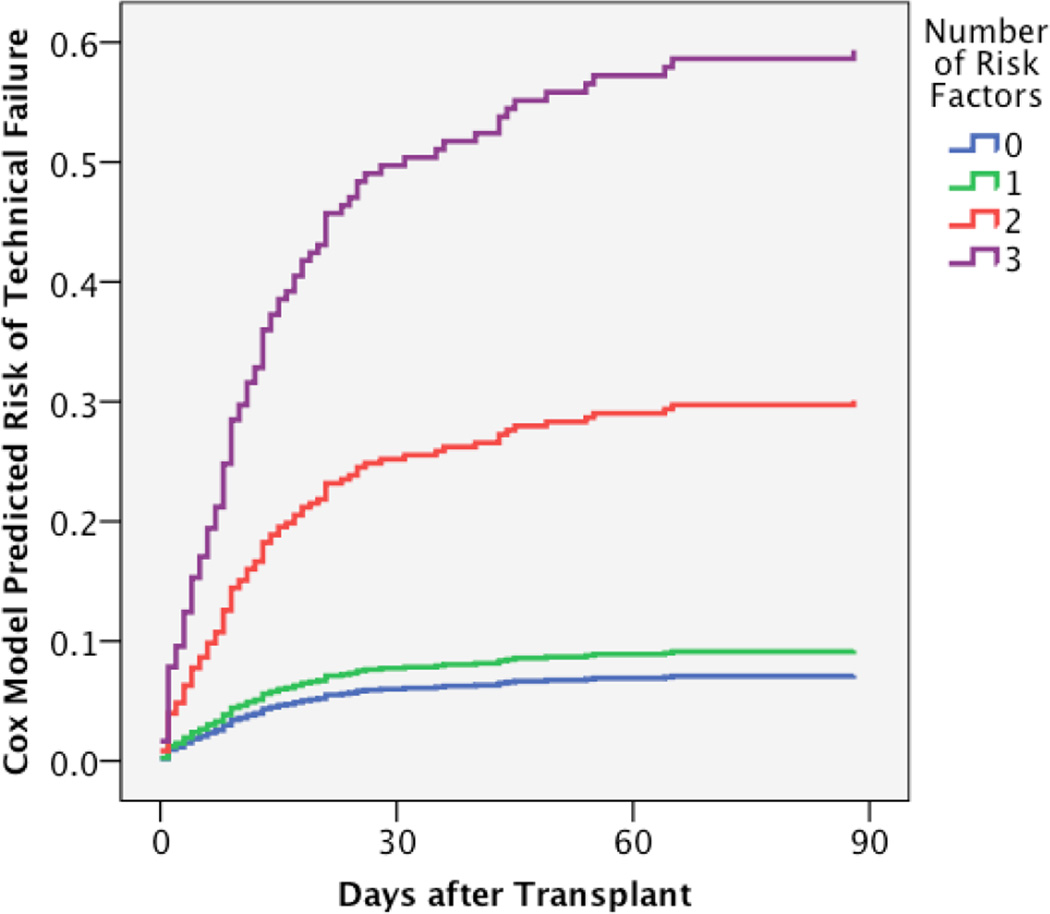
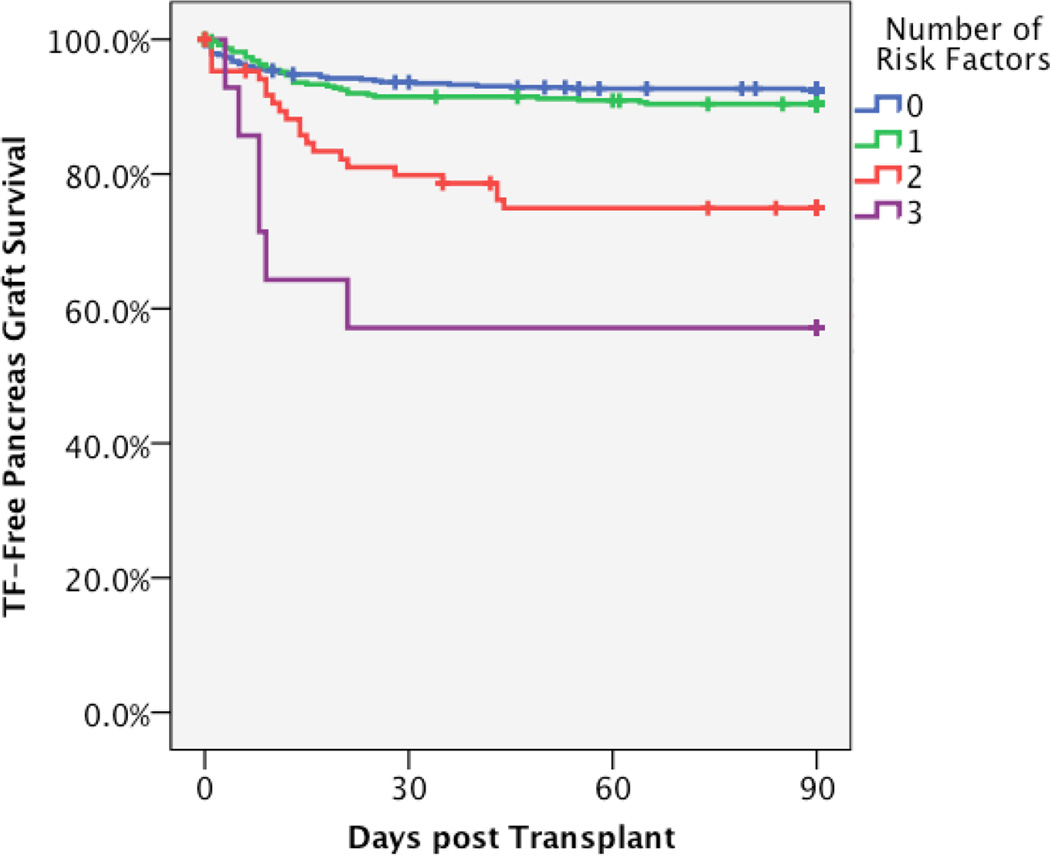
A Composite Risk Model was compiled for transplants with one or more of the following risk factor: donor age > 50, donor BMI ≥ 30, donor creatinine ≥ 2.5, and preservation time > 20 hours (Table 4). Transplants with none of these risk factors were compared to those with one, two, or three risk factors. No transplants had all four factors. The composite score was analyzed in a multivariable model that included other confounding factors (bladder drainage). Transplants with no risk factors had a TF rate of 7.3%; the presence of one risk factor did not significantly increase the risk of TF. Two risk factors resulted in a 3.6-fold increased risk of TF, whereas three risk factors increased the rate greater than 7-fold (Table 5, Fig. 3A, Fig. 3B). The impact of more than one risk factor was present for SPK and solitary transplants, with SPK transplants demonstrating an increased risk at each level (Table 6).
Table 5. Comparison of Composite Risk Model and pDRI.
Predictive outcomes of each model (Incidence of TF by category) and test validation parameters (Sensitivity, Specificity, C-statistic).
| Incidence of Technical Failure |
Sensitivity | Specificity | C-Statistic | |
|---|---|---|---|---|
| pDRI | ||||
| Best 80% | 8.1% | |||
| Worst 20% | 17.6% | 35.1% | 81.7% | 0.58 |
| Worst 10% | 18.9% | 18.9% | 91.0% | 0.55 |
| Worst 5% | 26.8% | 13.5% | 95.9% | 0.55 |
| Composite Risk Model | ||||
| No Risk Factors | 7.3% | |||
| One or More Risk Factors | 12.8% | 61.6% | 53.6% | 0.60 |
| Two or More Risk Factors | 26.7% | 27.3% | 91.8% | 0.59 |
| Three or More Risk Factors | 42.9% | 6.1% | 99.1% | 0.52 |
Figure 3. Composite Risk Model prediction of technical failure risk and observed pancreas graft survival.
A. The final Cox analysis was used to generate predictive model of technical failure risk according to the number of risk factors present. The risk model is adjusted for bladder/enteric drainage and history of pancreatitis in the donor. B. The observed technical failure-free pancreas graft survival in this cohort is depicted according to number of risk factors by univariable Kaplan Meier survival.
Table 6. Predictive Performance of Composite Risk Model for SPK and Solitary Pancreas Transplants.
| Solitary Pancreas | SPK | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | LCL | UCL | P value | Incidence | Hazard Ratio | LCL | UCL | P value | Incidence | |
| Bladder_Drainage | 0.48 | 0.28 | 0.83 | 0.008 | 0.67 | 0.31 | 1.44 | 0.303 | ||
| No Risk Factors | 1.00 | - | - | 0.000 | 7.1% | 1.00 | - | - | 0.000 | 8.0% |
| One Risk Factor | 1.23 | 0.69 | 2.17 | 0.483 | 7.9% | 1.58 | 0.69 | 3.59 | 0.277 | 12.0% |
| Two Risk Factors | 3.98 | 1.95 | 8.15 | 0.000 | 21.6% | 4.23 | 1.76 | 10.16 | 0.001 | 27.8% |
| Three Risk Factors | 5.11 | 1.21 | 21.54 | 0.026 | 28.6% | 12.52 | 3.73 | 42.08 | 0.000 | 57.1% |
Examination of the impact of combinations of individual risk factors was consistent with the hazard ratio values observed in the multivariable models. The predicted order of importance was as follows: elevated Cr > prolonged preservation time > BMI above 30 > age over 50. Although infrequent, the combination of Cr ≥ 2.5 and preservation time > 20 hours was the highest risk of any of the two-risk factor combinations (Table 5). Donor age is the weakest predictor of TF and combinations with longer preservation time or donor BMI resulted in the lowest increase risk of TF.
Finally, we investigated the previously established pancreas Donor Risk Index (pDRI) for its ability to predict TF (Table 4). The pDRI incorporates a range of donor related risk factors in a multivariable model that predict pancreas allograft failure (13). This model incorporates donor gender, age, race, BMI, height, cause of death, donation after cardiac death, Cr, and preservation time. We calculated the pDRI for each transplant in our series and then categorized the transplants by the quintile categories of risk described in the development of the pDRI. Increasing pDRI correlated with increased risk for TF. The highest quintile of pDRI transplants (highest risk group) had a TF incidence of 17.6% compared a TF rate of 6.2% in the lowest risk category. The highest risk category had a relative risk of TF of 2.8. The Composite Risk Model performed slightly better that the pDRI at predicting risk of TF. The composite model had a greater hazard ratio (3.65 and 7.66 for two or three risk factors, respectively). Table 7 summarizes the comparative sensitivities and specificities of each model for predicting TF. Direct comparison of the Composite Risk Model and the pDRI showed similar performance. Results from this analysis support the advantages of using either the pDRI or the risk factor approach over a set of basic covariates. However, using 3 or more risk factors was shown to be statistical improvement over using pDRI highest risk group for technical failure (p value = 0.047).
Table 7. Predictive Performance of Combinations of Individual Risk Factors.
| N | Incidence Hazard | Ratio* | LCL | UCL | P value | |
|---|---|---|---|---|---|---|
| 0–1 Risk Factors | 999 | 8% | 1.000 | - | - | <0.001 |
| Elevated Cr + CIT > 20 hr | 5 | 60% | 10.903 | 3.420 | 34.763 | <0.001 |
| Elevated Cr + BMI > 30 | 2 | 50% | 6.233 | 0.867 | 44.828 | 0.069 |
| CIT >20 hr + Donor Age > 50 | 20 | 20% | 2.773 | 1.015 | 7.575 | 0.047 |
| Donor Age > 50 + BMI > 30 | 60 | 23% | 3.299 | 1.869 | 5.824 | <0.001 |
, Adjusted for Bladder Drainage
Discussion
In the present study we have found a 10.2% risk of technical failure in pancreas transplantation. This rate has not changed significantly over several decades. The lack of improvement in recent eras is in contrast to other longer-term measures of transplant outcome such as overall graft and patient survival, where outcomes continue to improve (12). TF results in longer hospital stays, increased costs, increased loss of secondary transplants, and increased mortality (data not shown).
In univariable analysis, we identified risk factors for TF in the current era and then verified them in a multivariable Cox model. Significant risk factors for TF included donor history of pancreatitis, enteric drainage of exocrine secretions, donor age > 50, donor Cr ≥ 2.5, donor BMI ≥ 30, and cold preservation time > 20 hours. A composite model was created that incorporated the latter four factors. Presence of one risk factor had little impact on the TF rate, but two or more had a significantly increased risk for TF.
Comparison to Other Reports
We have previously examined surgical complications and technical failures in earlier eras of our transplant experience. In a report of the first 1,000 pancreas transplants performed at our institution, TF rates were as high as 22–33% in select subgroups of the earliest transplant eras (10). TF rates improved to between 2% and 18% in different transplant categories in subsequent eras, with the average TF rate for SPK transplants being 13% in the second half of the series. In a thorough analysis of 441 bladder-drained pancreas transplants performed during that era (20), we found a 32% relaparotomy rate. Relaparotomy was associated with a 57% transplant pancreatectomy rate and overall perioperative mortality rate of 9%. In 937 transplants performed between 1994 and 2003, we found a 13.1% technical failure rate (8). The reasons for TF in this previous era were similar to those in the current analysis with 52% of TF caused by thrombosis, 20.3% pancreatitis, 6.5% leaks, and 2.4% bleeding.
Studies from other centers have reported similar rates of TF. In Sollinger’s report of 1,000 SPK transplants at the University of Wisconsin, TF from thrombosis was seen in 3.1% of cases, with 2.1% graft loss from leaks, 1.2% from infection, 0.8% from bleeding, and 0.8% from pancreatitis (11). Fellmer reported on 210 SPK transplants and found a 4.9% thrombosis rate, 3.3% leak rate, 30.2% pancreatitis rate, and 12.1% bleeding rate, although not all leading to graft loss (21). Several studies have correlated surgical complications to the Pre-procurement Pancreas Allocation Suitability Score (P-PASS), a composite risk measure used in Europe. In 46 SPK transplants, Ziaja showed a 10% early graft loss rate from TF and a 10.9% death rate during transplant admission (6). In univariableunivariable analysis, a P-PASS score > 16 predicted graft loss due to infection or thrombosis, and early death. However, the statistical significance did not hold up in multivariable analysis. Similarly, Schenker examined 405 pancreas transplants and compared transplants with a P-PASS score <17 to those ≥ 17 (22). They found a greater rate of venous thrombosis, increased rate of relaparotomy, and longer hospital stay in the higher-risk category, but the P-PASS score was not associated with long-term outcomes.
Risk Factors
Several risk factors for surgical complications and technical failures have previously been identified. These include donor age (8, 20, 21), prolonged cold preservation time (8), donor BMI (8), donor cause of death other than head trauma (8), enteric drained pancreas transplants (8), pancreas retransplant (20, 23), use of HTK preservative solution (21, 24), recipient cardiac disease (21), and certain individual immunosuppressive agents (21).
Our analysis confirmed that donor age, donor Cr, donor BMI, donor history of pancreatitis, cold preservation time, and enteric drainage (compared to bladder drainage) were risk factors for technical failure. In our final multivariable analysis, SPK transplants, re-transplant, recipient history of cardiovascular disease, immunosuppressive agents, and donor cause of death were not significant risk factors. We did not include preservative solution as a variable in our study, due to insufficient numbers in the non-UW-solution group. Other notable factors that were not significant in the final analyses included the following: recipient age, gender, BMI, smoking history, vascular disease, history of dialysis transplant, or category of transplant (i.e., SPK, PTA, or PAK); donor history of drug or alcohol abuse, cause of death, interval from admission death, race, DCD recovery, or elevated amylase; and immune factors including donor/recipient HLA matching or recipient HLA-sensitization.
Bladder Drainage of pancreatic exocrine secretions was protective against TF, as has been previously reported by others (11, 25) and us (8). We hypothesize that this is due to two reasons, reduced graft torsion and reduced impact of exocrine anastomotic leak. We position the graft in a head-down orientation. By fixing the pancreas to the bladder there is reduced rotational freedom. This fixation may lessen the chance of twisting or kinking of the venous outflow. Likewise, tension resulting from the enteric drainage limb may lead to twisting and predispose to venous thrombosis. In addition, bladder leaks may be controlled more easily due to reduced bacterial contamination in comparison to enteric leaks. The difference between bladder and enteric drainage is not universal, as other groups have reported similar outcomes with both methods (26–28).
Composite Model
We sought to incorporate those factors identified in the multivariable analysis in a Composite Risk Model that might be useful for the evaluation of potential pancreas donors. Donor history of pancreatitis portended increased risk of technical failure, but was rare and therefore excluded in the final analysis. A single risk factor only resulted in a relative risk of 1.35 and a decrease in 90 day TF-free graft survival from 92.5% to 90.5% (not statistically significant). Two risk factors resulted in a 3-fold risk of TF and further decreased graft survival to 75.9%. Three risk factors resulted in a 7-fold risk of TF and decreased survival to only 57.1%. These factors suggest that transplants with one donor risk factor could be safely performed, but those with two or more should be avoided under most circumstances.
We compared the performance of our Composite Risk Model to the previously described pancreas donor risk index (pDRI). The pDRI predicts allograft failure based on a number of factors that were incorporated in our risk assessment. These factors included donor age, donor BMI, donor Cr, and preservation time; all of which were confirmed as significant in our analysis. The pDRI also incorporates a number of factors that were not significant in our model (donor race, cause of death, gender, and donation after cardiac death). Inclusion of these extra parameters did not improve the model’s ability to predict TF. The models were similar in statistical accuracy, with a slight improvement with the Composite Risk Model compared to the pDRI model. The highest risk pDRI category (pDRI ≥ 2.12) had a hazard ratio of 3.15 compared to the 7-fold increase in relative risk predicted by three our more risk factors in our model. Overall sensitivity of the Composite Risk Model with two or more factors in predicting TF was only 27%, but with a specificity of 92%.
Our model may be more predictive of early graft loss (<90 days) whereas the pDRI may be better at looking at intermediate term function (< 1 year). The Composite Risk Model may better discretely identify transplants with high TF risk, whereas the pDRI is a continuous predictive scale of overall graft survival. Each scale may be useful in different, but complementary, risk stratification. The Composite Risk Model is useful in as a initial screening for donor avoidance whereas the pDRI may more valuable for assessing individual organ offers based on recipient need and overall donor quality. The Composite Risk Model has the particular advantage of ease of use for rapid evaluation of potential donors based on a very limited set of screening parameters (donor age, BMI, Cr, and preservation time).
The simplicity of our model brings an inherent limitation in its statistical performance. The individual factors could be differentially weighted and modeled as was done for the pDRI. This would improve the model over the individual binary components and improve sensitivity and specificity. However, such complexity would make it less useful for a rapid screening of individual donor offers.
Implications for selecting donors
The use of the Composite Risk Model to predict risk of TF may assist in optimal donor selection for future pancreas transplants. This model aids in maximizing the number of organs utilized by including donors with a single risk factor that have similar outcomes to those with no risk factors, and that might otherwise have been discarded. It also minimizes adverse outcomes by avoiding transplants with two or more risk factors. In addition to identifying a limited set of risk factors for TF, this analysis shows that many factors that may make a donor seem less desirable do not actually predict the incidence of TF. Several reports have documented the successful use of donors that have risk factors here identified (29–33). However, careful consideration should be employed when considering donors with combinations of these risk factors.
To summarize, neither the Composite Risk Model nor the pDRI are especially good tests to predict TF. However, the Composite Risk Model identifies a limited number of factors that when combined predict adverse outcomes. By inference, donors with less than two of these risk factors, or those that have any number of the factors found not to be significant should be considered for transplant.
For any donor/recipient offer the only modifiable risk factor is preservation time. We have previously shown similar results with local versus imported pancreas donors, with the only impact of importation being longer preservation time (34). Strategies that reduce preservation time such as charter flights for imported organs could effectively reduce the number of risk factors from two down to one, thereby increasing the number of organs utilized and improving overall outcomes. This approach has been used successfully by a large center to effectively convert imported organs to local ones (32). Added costs associated with charter flights may be offset by the reduced hospital costs associated with TF. Consideration of risk prediction for donor/recipient selection and risk-factor modulation continues to be especially relevant as alternative options for minimally invasive beta cell replacement become available. As we move towards new pancreas allocation practices that favor organ sharing, we must consider options to reduce shipping times. Reduced preservation time may reduce risk of TF and increase utilization of organs that might otherwise be discarded.
Abbreviations
- TF
Technical Failure
- pDRI
pancreas Donor Risk Index
- HLA
Human Leukocyte Antigen
- PRA
Panel of Reactive Antibodies
- SPK
Simultaneous Pancreas and Kidney transplant
- PTA
Pancreas Transplant Alone
- PAK
Pancreas After Kidney transplant
- PwK
Pancreas with live-donor Kidney transplant
- CNI
Calcinurin Inhibitor
- MMF
Mycophenolate Mofetil
- Cr
Creatinine
- CIT
Cold Ischemia Time
- SD
Standard Deviation
- SEM
Standard Error of Mean
Footnotes
Disclosure:
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Becker BN, Brazy PC, Becker YT, Odorico JS, Pintar TJ, Collins BH, et al. Simultaneous pancreas-kidney transplantation reduces excess mortality in type 1 diabetic patients with end-stage renal disease. Kidney International. 2000;57(5):2129–2135. doi: 10.1046/j.1523-1755.2000.00064.x. Epub 2000/05/03. [DOI] [PubMed] [Google Scholar]
- 2.Gross CR, Limwattananon C, Matthees B, Zehrer JL, Savik K. Impact of transplantation on quality of life in patients with diabetes and renal dysfunction. Transplantation. 2000;70(12):1736–1746. doi: 10.1097/00007890-200012270-00013. Epub 2001/01/11. [DOI] [PubMed] [Google Scholar]
- 3.Morath C, Zeier M, Dohler B, Schmidt J, Nawroth PP, Opelz G. Metabolic control improves long-term renal allograft and patient survival in type 1 diabetes. J Am Soc Nephrol. 2008;19(8):1557–1563. doi: 10.1681/ASN.2007070804. Epub 2008/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan DM, Fogel H, Norman D, Russell PS, Tolkoff-Rubin N, Delmonico FL, et al. Long-term metabolic and quality of life results with pancreatic/renal transplantation in insulin-dependent diabetes mellitus. Transplantation. 1991;52(1):85–91. doi: 10.1097/00007890-199107000-00018. Epub 1991/07/11. [DOI] [PubMed] [Google Scholar]
- 5.White SA, Shaw JA, Sutherland DE. Pancreas transplantation. Lancet. 2009;373(9677):1808–1817. doi: 10.1016/S0140-6736(09)60609-7. Epub 2009/05/26. [DOI] [PubMed] [Google Scholar]
- 6.Ziaja J, Krol R, Pawlicki J, Heitzman M, Wilk J, Kowalik A, et al. Donor-dependent risk factors for early surgical complications after simultaneous pancreas-kidney transplantation. Transplant Proc. 2011;43(8):3092–3096. doi: 10.1016/j.transproceed.2011.08.072. Epub 2011/10/15. [DOI] [PubMed] [Google Scholar]
- 7.Ziaja J, Krol R, Chudek J, Pawlicki J, Kolonko A, Heitzman M, et al. Intra-abdominal infections after simultaneous pancreas - kidney transplantation. Ann Transplant. 2011;16(3):36–43. doi: 10.12659/aot.881993. Epub 2011/10/01. [DOI] [PubMed] [Google Scholar]
- 8.Humar A, Ramcharan T, Kandaswamy R, Gruessner RW, Gruessner AC, Sutherland DE. Technical failures after pancreas transplants: why grafts fail and the risk factors--a multivariate analysis. Transplantation. 2004;78(8):1188–1192. doi: 10.1097/01.tp.0000137198.09182.a2. Epub 2004/10/27. [DOI] [PubMed] [Google Scholar]
- 9.Norman SP, Kommareddi M, Ojo AO, Luan FL. Early pancreas graft failure is associated with inferior late clinical outcomes after simultaneous kidney-pancreas transplantation. Transplantation. 2011;92(7):796–7801. doi: 10.1097/TP.0b013e31822dc36b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland DE, Gruessner RW, Dunn DL, Matas AJ, Humar A, Kandaswamy R, et al. Lessons learned from more than 1,000 pancreas transplants at a single institution. Annals of Surgery. 2001;233(4):463–501. doi: 10.1097/00000658-200104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sollinger HW, Odorico JS, Becker YT, D'Alessandro AM, Pirsch JD. One thousand simultaneous pancreas-kidney transplants at a single center with 22-year follow-up. Annals of Surgery. 2009;250(4):618–630. doi: 10.1097/SLA.0b013e3181b76d2b. [DOI] [PubMed] [Google Scholar]
- 12.Gruessner AC. 2011 update on pancreas transplantation: comprehensive trend analysis of 25,000 cases followed up over the course of twenty-four years at the International Pancreas Transplant Registry (IPTR) Rev Diabet Stud. 2011;8(1):6–16. doi: 10.1900/RDS.2011.8.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelrod DA, Sung RS, Meyer KH, Wolfe RA, Kaufman DB. Systematic evaluation of pancreas allograft quality, outcomes and geographic variation in utilization. Am J Transplant. 2010;10(4):837–845. doi: 10.1111/j.1600-6143.2009.02996.x. Epub 2010/02/04. [DOI] [PubMed] [Google Scholar]
- 14.Tuttle-Newhall JE, Krishnan SM, Levy MF, McBride V, Orlowski JP, Sung RS. Organ donation and utilization in the United States: 1998–2007. American Journal of Transplantation. 2009;9(4 Pt 2):879–893. doi: 10.1111/j.1600-6143.2009.02565.x. [DOI] [PubMed] [Google Scholar]
- 15.Humar A, Khwaja K, Sutherland DR. Pancreas Transplantation. Springer London: Atlas of Organ Transplantation; 2006. pp. 133–195. %8 2006-01-01. [Google Scholar]
- 16.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: Wiley; 1980. [Google Scholar]
- 17.Lee ET. Statistical Methods for Survival Data Analysis. Belmont, CA: Lifetime Learning Publications; 1980. [Google Scholar]
- 18.Allison PD. Institute SAS. Survival Analysis Using the SAS System: A Practical Guide. Cary, NC: SAS Institute; 1995. [Google Scholar]
- 19.Kleinbaum D. Survival Analysis: A Self-Learning Text. New York: Springer-Verlag; 1996. 1996. [Google Scholar]
- 20.Troppmann C, Gruessner AC, Dunn DL, Sutherland DE, Gruessner RW. Surgical complications requiring early relaparotomy after pancreas transplantation: a multivariate risk factor and economic impact analysis of the cyclosporine era. Annals of Surgery. 1998;227(2):255–268. doi: 10.1097/00000658-199802000-00016. Epub 1998/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fellmer PT, Pascher A, Kahl A, Ulrich F, Lanzenberger K, Schnell K, et al. Influence of donor- and recipient-specific factors on the postoperative course after combined pancreas-kidney transplantation. Langenbecks Arch Surg. 2010;395(1):19–25. doi: 10.1007/s00423-009-0552-2. Epub 2009/09/05. [DOI] [PubMed] [Google Scholar]
- 22.Schenker P, Vonend O, Ertas N, Wunsch A, Viebahn R. Preprocurement pancreas allocation suitability score does not correlate with long-term pancreas graft survival. Transplantation Proceedings. 2010;42(1):178–180. doi: 10.1016/j.transproceed.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 23.Humar A, Kandaswamy R, Drangstveit MB, Parr E, Gruessner AG, Sutherland DE. Surgical risks and outcome of pancreas retransplants. Surgery. 2000;127(6):634–640. doi: 10.1067/msy.2000.105034. Epub 2000/06/07. [DOI] [PubMed] [Google Scholar]
- 24.Alonso D, Dunn TB, Rigley T, Skorupa JY, Schriner ME, Wrenshall LE, et al. Increased pancreatitis in allografts flushed with histidine-tryptophan-ketoglutarate solution: a cautionary tale. Am J Transplant. 2008;8(9):1942–1945. doi: 10.1111/j.1600-6143.2008.02312.x. Epub 2008/09/13. [DOI] [PubMed] [Google Scholar]
- 25.Marang-van de Mheen PJ, Nijhof HW, Khairoun M, Haasnoot A, van der Boog PJ, Baranski AG. Pancreas-kidney transplantations with primary bladder drainage followed by enteric conversion: graft survival and outcomes. Transplantation. 2008;85(4):517–523. doi: 10.1097/TP.0b013e31816361f7. Epub 2008/03/19. [DOI] [PubMed] [Google Scholar]
- 26.Cattral MS, Bigam DL, Hemming AW, Carpentier A, Greig PD, Wright E, et al. Portal venous and enteric exocrine drainage versus systemic venous and bladder exocrine drainage of pancreas grafts: clinical outcome of 40 consecutive transplant recipients. Ann Surg. 2000;232(5):688–695. doi: 10.1097/00000658-200011000-00011. Epub 2000/11/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stratta RJ, Shokouh-Amiri MH, Egidi MF, Grewal HP, Kizilisik AT, Nezakatgoo N, et al. A prospective comparison of simultaneous kidney-pancreas transplantation with systemic-enteric versus portal-enteric drainage. Ann Surg. 2001;233(6):740–751. doi: 10.1097/00000658-200106000-00003. Epub 2001/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coosemans W, Metsemakers WJ, Aerts R, Monbaliu D, Pirenne J. Pancreas transplantation: towards minimization of technical graft loss. Acta Chir Belg. 2008;108(1):58–65. Epub 2008/04/17. [PubMed] [Google Scholar]
- 29.Singh RP, Rogers J, Farney AC, Moore PS, Hartmann EL, Reeves-Daniel A, et al. Outcomes of extended donors in pancreatic transplantation with portal-enteric drainage. Transplant Proc. 2008;40(2):502–505. doi: 10.1016/j.transproceed.2008.02.014. Epub 2008/04/01. [DOI] [PubMed] [Google Scholar]
- 30.Stratta RJ. Donor age, organ import, and cold ischemia: effect on early outcomes after simultaneous kidney-pancreas transplantation. Transplantation Proceedings. 1997;29(8):3291–3292. doi: 10.1016/s0041-1345(97)82925-9. [DOI] [PubMed] [Google Scholar]
- 31.Stratta RJ, Sundberg AK, Farney AC, Rohr MS, Hartmann EL, Adams PL. Successful simultaneous kidney-pancreas transplantation from extreme donors. Transplantation Proceedings. 2005;37(8):3535–3537. doi: 10.1016/j.transproceed.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 32.Fridell JA, Mangus RS, Hollinger EF, Milgrom ML, Taber TE, Mohler E, et al. No difference in transplant outcomes for local and import pancreas allografts. Transplantation. 2009;88(5):723–728. doi: 10.1097/TP.0b013e3181b2a01b. [DOI] [PubMed] [Google Scholar]
- 33.Fridell JA, Rogers J, Stratta RJ. The pancreas allograft donor: current status, controversies, and challenges for the future. Clin Transplant. 2010;24(4):433–449. doi: 10.1111/j.1399-0012.2010.01253.x. Epub 2010/04/14. [DOI] [PubMed] [Google Scholar]
- 34.Finger EB, Radosevich DM, Bland BJ, Dunn TB, Chinnakotla S, Sutherland DE, et al. Comparison of recipient outcomes following transplant from local versus imported pancreas donors. Am J Transplant. 2012;12(2):447–457. doi: 10.1111/j.1600-6143.2011.03828.x. Epub 2011/11/11. [DOI] [PubMed] [Google Scholar]