Abstract
We investigated the cellular localization in the salamander retina of one of the somatostatin [or somatotropin release-inhibiting factor (SRIF)] receptors, sst2A, and studied the modulatory action of SRIF on voltage-gated K+ and Ca2+ currents in rod and cone photoreceptors. SRIF immunostaining was observed in widely spaced amacrine cells, whose perikarya are at the border of the inner nuclear layer and inner plexiform layer. sst2A immunostaining was seen in the inner segments and terminals of rod and cone photoreceptors. Additional sst2A immunoreactivity was expressed by presumed bipolar and amacrine cells. SRIF, at concentrations of 100–500 nm, enhanced a delayed outwardly rectifying K+ current (IK) in both rod and cone photoreceptors. SRIF action was blocked in cells pretreated with pertussis toxin (PTX) and was substantially reduced by intracellular GDPβS. Voltage-gated L-type Ca2+currents in rods and cones were differently modulated by SRIF. SRIF reduced Ca2+ current in rods by 33% but increased it in cones by 40%, on average. Both effects were mediated via G-protein activation and blocked by PTX. Ca2+-imaging experiments supported these results by showing that 500 nm SRIF reduced a K+-induced increase in intracellular Ca2+ in rod photoreceptor terminals but increased it in those of cones. Our results suggest that SRIF may play a role in the regulation of glutamate transmitter release from photoreceptors via modulation of voltage-gated K+ and Ca2+ currents.
Keywords: somatostatin, retina, Ca2+ channel, K+ channel, G-protein, patch clamp
Somatostatin, also called somatotropin release-inhibiting factor (SRIF), initially was identified as a hypothalamic peptide but subsequently has been shown to be widely distributed in the nervous system and in peripheral endocrine organs (Delfs and Dichter, 1985). The cellular actions of SRIF are mediated via five distinct G-protein-coupled receptors, sst1–5 (Hoyer et al., 1995). In addition, there are two sst2 isoforms resulting from alternative mRNA splicing (Vanetti et al., 1992). SRIF has been shown to modulate K+ and Ca2+currents in neurons, endocrine cells, and some cell lines. Many classes of K+ current are reported to be increased by SRIF, including a K+ leak current (Schweitzer et al., 1998), an inward rectifier (Takano et al., 1997), a delayed rectifier K+current (Wang et al., 1989), and a Ca-activated K+ current (White et al., 1991). The effect of SRIF on Ca current is inhibitory, and it acts on high voltage-activated Ca2+ currents of the L-type (Rosenthal et al., 1988) and N-type (Shapiro and Hille, 1993). In axonal terminals, the combined action of SRIF on K+ and Ca2+currents has been reported to reduce transmitter release (Katayama and Hirai, 1989; Boehm and Betz, 1997).
In the vertebrate retina, SRIF-containing neurons typically are amacrine (Yamada et al., 1980; Li and Lam, 1990; Rickman et al., 1996) or interplexiform cells (Smiley and Basinger, 1988). SRIF-immunoreactive fibers are predominantly distributed to selected laminae of the inner plexiform layer (IPL). Physiological data on the role of SRIF in retinal function, however, are scant. Zalutsky and Miller (1990) reported that SRIF was excitatory to most ganglion cells tested in rabbit retina, but whether the peptide acted directly on ganglion cells or via circuitry that is presynaptic to ganglion cells was not determined. More recently, sst2Aimmunostaining was localized to a variety of rat and rabbit retinal neurons, including bipolar cells and cone photoreceptors (Johnson et al., 1998, 2000). The location of sst2A receptors on photoreceptor terminals suggested a possible role of SRIF in regulating the release of glutamate, the identified neurotransmitter of photoreceptors (Marc et al., 1990; for review, see Thoreson and Witkovsky, 1999).
In the present study we examined the action of SRIF on rod and cone photoreceptor terminals in the salamander retina. We showed, by immunocytochemistry, that in the salamander retina, both rods and cones express sst2A receptors. Using the whole-cell patch-clamp technique, we found that SRIF had a differential action on the high voltage-activated L-type Ca2+currents of rods and cone inner segments; it reduced the Ca2+ current of rods but increased that of cones. In addition, SRIF increased the delayed rectifier K+ current of rods and cones. These data suggest that, like dopamine (for review, see Witkovsky and Dearry, 1991), SRIF may play a role in governing the balance of information flow through rod and cone circuits.
MATERIALS AND METHODS
Animals. Salamanders (Ambystoma tigrinum) were obtained from a commercial supplier (Charles Sullivan, Nashville, TN) and kept at 4°C until used. The handling and maintenance of animals met the National Institutes of Health guidelines and were approved by the animal research committees of New York University School of Medicine and University of California, Los Angeles, School of Medicine (UCLA).
Tissue preparation for immunohistochemical experiments.After decapitation, the eyes were removed, the anterior segment was dissected away, and the posterior eyecup containing the retina was immediately immersed in 4% paraformaldehyde (PFA) in 0.1 mphosphate buffer (PB). The eyecup was fixed for 1 hr at room temperature and then stored in 25% sucrose in 0.1 m PB at 4°C. Vertical sections of the retina were cut perpendicular to the vitreal surface with a cryostat at 12–16 μm, mounted onto gelatin-coated slides, and then air-dried and stored at −20°C.
Cell isolation procedure. The retinas were removed from the salamander eyecups, exposed to 20 U/mg papain (Worthington, Freehold, NJ) for 30 min, and then washed several times with Ringer's solution [composition (in mm), NaCl 100; KCl 2.5; CaCl2 1.8; MgCl2 1.0; and NaHCO3 25, pH 7.4]. The remaining procedures for cell isolation are identical to those reported previously (Akopian and Witkovsky, 1996). Dissociated cells were plated onto concanavalin A (Sigma, St. Louis, MO)-coated coverslips. Isolated cells were used in immunocytochemical and Ca2+-imaging experiments. For immunocytochemical experiments, cells were fixed in 4% PFA for 10 min followed by three washes with 0.1 mPB.
Antibodies. The SRIF antibody and the tyrosine hydroxylase monoclonal mouse antibody were obtained from Chemicon (Temecula, CA). A rabbit affinity-purified polyclonal antibody (#9431) directed against the C terminus of mouse sst2A 361–369 was a generous gift of Drs. J. Walsh and Helen Wong of UCLA. After a blocking step in an antibody diluent solution, the primary antibodies were placed on the tissues or isolated cells for 12–36 hr at 4°C and then washed in 0.1 m PB. The immunoreaction was visualized with fluorescein-isothiocyanate-coupled goat anti-rabbit antibodies (American Qualex, La Mirada, CA) or rhodamine donkey anti-mouse (Jackson ImmunoResearch, West Grove, PA) for 1–2 hr at room temperature. Sections were coverslipped with a glycerol phosphate or carbonate buffer containing 2% potassium iodide to retard fading.
The specificity of the sst2A antibody was evaluated by preadsorbing it with 10−5m synthetic sst2A peptide, which completely abolished labeling. In addition, the sst2A antiserum has been characterized extensively in a previous study using transfected cell lines, Western blotting, and immunohistochemistry on tissue sections (Sternini et al., 1997).
Image processing. Images were photographed using T-Max 400 or Ektachrome 1600 film. The photographic images were scanned at 2700 dpi with a SprintScan 35/Plus scanner (Polaroid, Cambridge, MA) and saved as TIFF files. Images were adjusted to the final size, corrected for contrast and brightness, and labeled using Adobe Photoshop 5.0 (Adobe Systems, Mountain View, CA). Images were saved at 320 dpi.
Ca2+ imaging. Retinal cells were isolated, incubated for 10–15 min at room temperature in darkness with the membrane-permeable fluorescent dye fura-2 AM (Molecular Probes, Eugene, OR) at a concentration of 5–10 μm, and then washed in Ringer's solution. Rods and cones were identified by their characteristic morphology. A coverslip with the cells was transferred to a stage mounted on a Zeiss 135 Axiovert inverted microscope on which the cells were superfused with Ringer's solution containing either 50 or 100 mm K+ alone or with 0.2–0.5 μm SRIF. Fluorescence measurements were performed on photoreceptor inner segments with a 40× 1.3 numerical aperture fluar objective, using an Attofluor-imaging system (Atto Instruments, Rockville, MD). Optical excitation was accomplished using 340 and 380 nm wavelengths with an emission at 510 nm. The intensity of the fluorescence was minimized to prevent dye bleaching during experiments. Fluorescence measurements were acquired using Attofluor Ratiovision software and graphed with Attograph and Sigma Plot. The Attofluor system was calibrated using high (1 μm) and low (Ca-free solution containing 1 mmEGTA), and the gray scale intensities were adjusted to avoid saturation. The concentration of fura-2 in the calibration tests was 8.8 μm, similar to the 5–10 μmconcentration of fura-2 AM used in the experiments.
Electrophysiology: slice preparation. The procedures for obtaining retinal slices were similar to those described by Lukasiewicz et al. (1994). Briefly, the eyes were enucleated and hemisected, and the cornea, lens, and iris were removed. The retina was transferred, vitreal side down, to a Millipore filter paper (0.22 μm pore) and then sectioned manually into 150- to 200-μm-thick slices, which were mounted in a chamber and superfused at 2 ml/min during the experiment.
Solutions. Whole-cell voltage-clamp recordings of K+ currents were made using patch electrodes containing (in mm): K gluconate 100, MgCl2 2, HEPES 10, ATP 2, and GTP 0.1, adjusted to pH 7.3 with KOH. The bath solution contained (in mm): NaCl 100, KCl 3, CaCl2 2, MgCl2 2, and HEPES 10, adjusted to pH 7.6 with NaOH. To isolate Ca2+ currents, the K gluconate of the intracellular solution was replaced by equimolar CsCl, while tetraethylammonium (TEA, 20 mm) was added to the bath solution, replacing an equimolar amount of NaCl. No Ca2+ chelator was included in the intracellular solution used to record either K+ or Ca2+currents. Somatostatin-14 (Bachem Bioscience, King of Prussia, PA) was applied to the bathing solution through the superfusion system at concentrations of 0.1–0.5 μm. Rods and cones were identified by their shape and location and by their characteristic hyperpolarization-activated Ih current. In some experiments 3 mm CsCl was included in the bath solution to block Ih. To test for a possible involvement of Gi/Go proteins in the somatostatin-induced response, the eyecups were incubated 16–20 hr in Ringer's solution containing 400 ng/ml pertussis toxin (PTX) at 7°C in darkness.
Recording procedures. Whole-cell voltage- and current-clamp recordings were obtained in a conventional way (Hamill et al., 1981) using an Axopatch 200B amplifier. Recording pipettes were made from borosilicate glass tubing (1.2 mm outer diameter; 0.6 mm inner diameter). Electrode resistance was typically 5–8 MΩ in the bath solution. After seal rupture the series resistance (10–15 MΩ) was compensated (70–80%) by a standard circuit. Whole-cell K+ and Ca2+currents were typically <1 nA, and the voltage errors resulting from inadequate compensation were estimated to be at most 3–5 mV. The average input resistance for rods, estimated from the steady-state current induced by a 10 mV voltage step from −60 mV, was 1.0 ± 0.1 GΩ (n = 10). Currents were filtered at 1 kHz by a low-pass Bessel filter and were sampled at 5–10 kHz. The pClamp software package (Axon Instruments) was used for data acquisition and analysis. Summary data are presented as means ± SE. The statistical comparison between groups was made with paired ttests; the corresponding p values are given in the text. In voltage-clamp experiments the membrane potential usually was held at −70 mV.
RESULTS
Localization of SRIF and the sst2A receptor
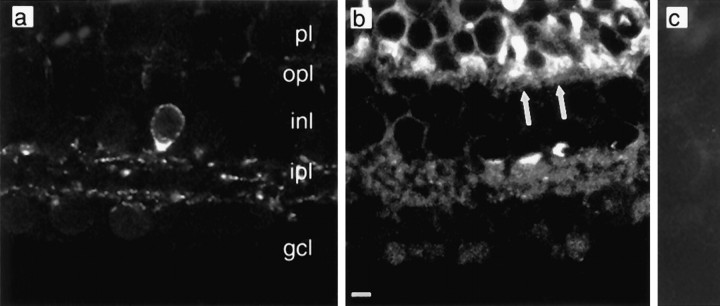
SRIF immunostaining was observed in widely spaced amacrine cells, whose perikarya are at the border of the inner nuclear layer (INL) and the IPL. Immunolabeled amacrine cell processes were distributed within two narrow strata: sublamina 1 at the border of the INL and the IPL and sublamina 5 at the border of the IPL and the ganglion cell layer (GCL) (Fig. 1a). Because the morphology of these cells resembled that of the dopaminergic amacrine cells in tiger salamander (Watt et al., 1988), we conducted double-labeling experiments and determined that SRIF and tyrosine hydroxylase were found within two distinct cell populations (data not shown).
Fig. 1.
Retinal distribution of SRIF and somatostatin sst2A immunoreactivity. a, A SRIF-immunoreactive amacrine cell body whose processes extend into both distal and proximal portions of the IPL. b,sst2A receptor immunoreactivity that is located in both plexiform layers. Arrows indicate positive staining of photoreceptor bases. c, Absence of sst2Aimmunoreactivity when antibody was preabsorbed with a blocking peptide.Labels indicate retinal layers: gcl, ganglion cell layer; inl, inner nuclear layer;ipl, inner plexiform layer; opl, outer plexiform layer; and pl, photoreceptor layer. Scale bar:a–c, 5 μm.
sst2A receptor immunoreactivity was localized to both the inner and outer retina, including cell bodies in the photoreceptor layer, and to processes in both plexiform layers (Fig.1b). sst2A-immunoreactive photoreceptors with prominent staining throughout the inner segment and synaptic terminals were observed (Fig. 1b,arrows). sst2A receptor immunoreactivity also was expressed by isolated rod and cone photoreceptors, with strong staining of the inner segments and synaptic terminals, similar to the staining pattern observed in retinal sections (data not shown).
sst2A-immunoreactive bipolar and amacrine cell bodies also were noted in which immunostaining was characterized by a thin rim of immunoreactivity at, or adjacent to, the plasma membrane. A dense network of immunostained processes was present in the outer plexiform layer and in all laminae of the IPL (Fig.1b). Diffuse immunostaining was observed in the GCL. Immunostaining was completely eliminated in sections incubated in antibody that was preadsorbed with 10−5m sst2A 361–369 (Fig.1c)
Effect of SRIF on voltage-gated currents in photoreceptors
Characteristics of outward K+ current in photoreceptors
In the retinal slice preparation, rods were stepped for 50 msec to depolarizing voltages between −60 and +40 mV in 20 mV increments from a holding potential of −70 mV (Fig.2A, left). Outward current was reliably observed for voltage steps positive to −40 mV. The tail current reversal potential for the outward current recorded was near −75 mV (data not shown), which is positive to the equilibrium potential of K+ of −88 mV, assuming that the intracellular concentration of K+ was equal to that in the pipette. The current was blocked in the presence of 20 mm TEA (data not shown).
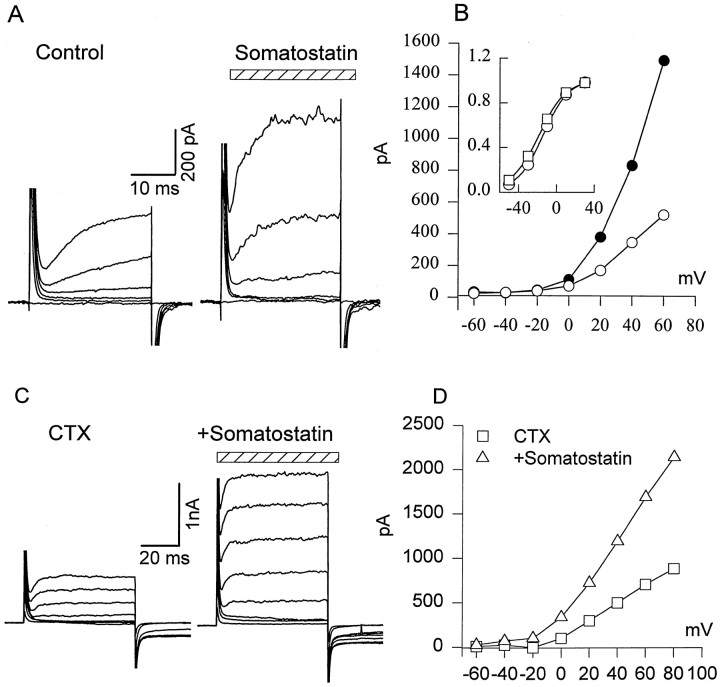
Fig. 2.
Effect of SRIF on delayed outward K+ current (IK) in rods. A, SRIF increased voltage-activated K+ current. IK was evoked by holding cells at −70 mV and applying depolarizing steps from −60 to +40 mV in 20 mV increments in control external solution (left) and after a 4 min exposure to 0.5 μm SRIF (stripedhorizontalbar; right). B,I–V relationship of IKobtained from the experiment described in A is shown. Inset, Somatostatin increasedIK amplitude without substantially changing the current–voltage relation. ■, Control; ○, somatostatin. They-axis of the inset is normalized current. C, CTX reduced outward currents (left) but did not prevent a somatostatin-induced increase in IK. D, I–Vrelationship of outward current in the presence of CTX (■), or CTX + somatostatin (▵); n = 3.
Effect of SRIF on IK
To examine the effect of SRIF receptor activation onIK, various concentrations of SRIF from 0.1 to 1 μm were applied to rods. Even at the holding potential of −70 mV, 0.5 μm SRIF induced a steady outward current of 16 ± 3 pA (n= 3). This steady current is not reflected in the current–voltage plots of IK (Fig. 2), because it was subtracted as a baseline current. Exposure to SRIF progressively increased the IK evoked by depolarizing pulses (Fig. 2A, right) compared with those recorded in control Ringer's solution (Fig. 2A, left). Partial recovery was observed after a 10–15 min wash in SRIF-free Ringer's solution (data not shown). The correspondingI–V relationships are illustrated in Figure2B, showing that an increase inIK amplitude is not accompanied by a shift of the current–voltage relation along the voltage axis (Fig.2B, inset). The threshold dose at which SRIF increased IK was near 0.1 μm, and the maximum effect was obtained at ∼1 μm. In subsequent experiments, we used concentrations of 0.1–0.5 μm. The mean increase of IK (at +30 mV voltage step) induced by 0.5 μm SRIF was 58 ± 13% (n = 20; p < 0.001). The effect of SRIF was observed after ∼1 min of application and reached a maximum in 2–4 min. A dose–response function for SRIF could not be obtained on a single cell because of incomplete recovery ofIK, even after a 10–15 min wash in normal Ringer's solution. A similar enhancement ofIK by SRIF was observed in cones (0.5 μm SRIF; mean increase, 37 ± 8%;n = 5; p < 0.001). In separate experiments (n = 3) done in the absence of Cs+ in the bath solution, we found that the hyperpolarization-activated current (Ih) was not affected by SRIF in the same cells that showed a substantial increase inIK current (data not shown).
Previous studies indicate that, in salamander photoreceptors, depolarization activates at least two types of K+ current: a delayed rectifier and a Ca2+-dependent K+ current IK–Ca) (Barnes and Hille, 1989). We used charybdotoxin (CTX; 20 nm) to block IK–Ca(Knaus et al., 1994). This reduced the outward current evoked by a depolarizing step from −70 to 0 mV (Fig. 2C, mean reduction, 36 ± 17%; n = 3). Thereafter the slice was superfused with a mixture of charybdotoxin and SRIF (500 nm), resulting in a 49 ± 19% increase in outward current (Fig. 2C,D), which is the same degree of enhancement noted without charybdotoxin treatment. Thus, on the basis of its kinetic and pharmacological characteristics we identify the SRIF-sensitive outward current (Fig. 2A) as a delayed rectifier K+ current (IK).
Previous studies in other preparations have demonstrated that the action of SRIF on voltage-gated K+ and Ca2+ currents is sensitive to PTX, indicating the participation of a PTX-sensitive G-protein (White et al., 1991; Ishibashi and Akaike, 1995; Delmas et al., 1998). We found that in slices obtained from eyecups after a 16–20 hr incubation with PTX (400 ng/ml), IK of either rods or cones was not augmented by SRIF (Fig.3a). The mean change of peakIK by SRIF in PTX-pretreated cells was −8 ± 7% (n = 3; p > 0.1). There were no significant differences in the I–Vcharacteristics of the IK current recorded in control Ringer's solution or in the presence of SRIF (Fig.3b). The histogram of Figure 3c summarizes the data obtained in control and PTX-treated slices.
Fig. 3.
Sensitivity to PTX of SRIF effect onIK. By the use of a voltage protocol similar to that in Figure 2, a family of IK currents was recorded in rods pretreated for 16–20 hr with PTX (400 ng/ml).a, Exposure to 0.5 μm SRIF for 5 min failed to enhance IK. b, There was no significant difference in the I–Vrelationship of IK recorded in control solution and in the presence of SRIF. c, The histogram summarizes the effects of SRIF on IK in control and PTX-pretreated rods. Numbers inparentheses in this and subsequent figures are the sample size.
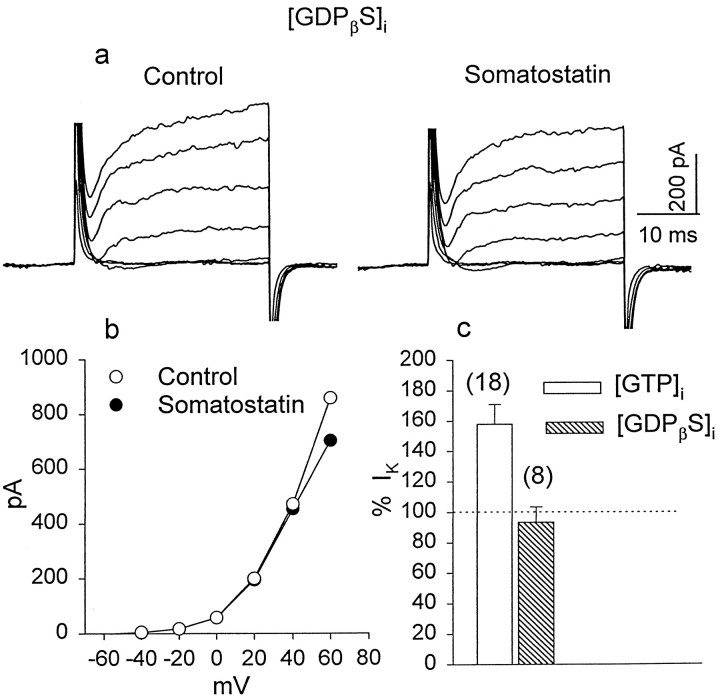
Another test of G-protein involvement was the addition to the pipette solution of GDPβS (500 μm), a compound that blocks the G-protein-mediated effects of neurotransmitters on neuronal Ca2+currents (Holtz et al., 1986). After rupturing the cell membrane, a period of 4–5 min was allowed to ensure adequate dialysis with GDPβS. Inclusion of GDPβS in the patch pipette substantially abolished the SRIF-induced enhancement ofIK. In many cases we observed even a slight reduction of IK after exposure to SRIF. In the experiment illustrated in Figure4a,IK was first recorded in control Ringer's solution and then after a 4 min exposure to 0.5 μm SRIF. The corresponding I–Vrelationships are illustrated in Figure 4b. The mean change of IK induced by exposure to SRIF in the presence of GDPβS was −6 ± 10% (n = 8; p > 0.1). Figure 4csummarizes these results, which indicate that a G-protein is implicated in the cascade underlying a SRIF-induced enhancement ofIK in retinal photoreceptors.
Fig. 4.
The effect of GDPβS on SRIF-induced changes in IK. a, K+ currents were recorded with a patch pipette containing 500 μm GDPβS in control solution and after a 5 min exposure to 0.5 μm SRIF.b, The I–V relationship ofIK obtained from the experiments described in a indicates that SRIF failed to alterIK in the presence of GDPβS.c, The histogram summarizes SRIF-induced changes inIK when the internal solution contained GTP (openbar) and when GTP was replaced by GDPβS (hatchedbar) to block G-protein activation.
SRIF reduces Ca2+ current in rods
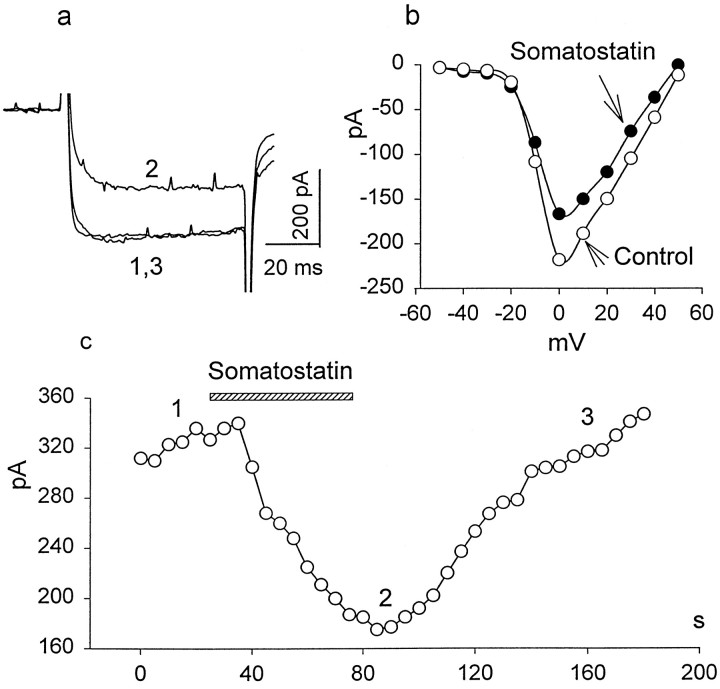
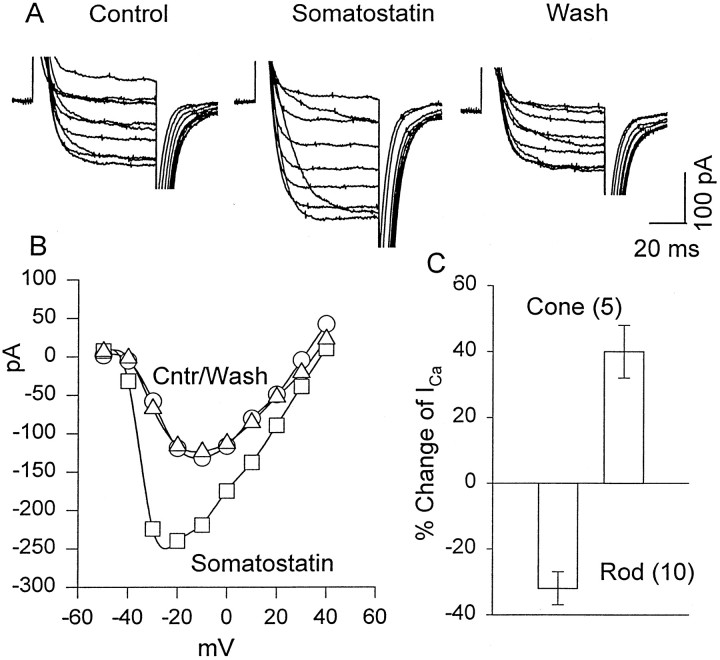
Ca2+ currents were isolated from other voltage-gated currents using ion substitution and channel blockers and were recorded in rods and cones under whole-cell voltage clamp. From a holding potential of −70 mV, depolarizing pulses of 70 msec duration were applied from −60 to +60 mV, in 10 mV increments, using 20 mm Ba2+ as the current carrier. For depolarizing steps positive to −40 mV, rods responded with a sustained inward current, which was completely blocked by application of 100 μm Cd2+, increased by 10 μm BAY K 8644, and reduced in the presence of 50 μmnifedipine (data not shown), indicating that the Ca2+ current is mediated by dihydropyridine-sensitive, L-type Ca channels. In general, the kinetic and voltage-dependent characteristics of the Ca2+ current were similar to those described previously for salamander photoreceptors (Bader et al., 1982;Barnes and Hille, 1989). Figure5a illustrates a representative experiment in which Ca2+current was evoked by a depolarizing step from −70 to 0 mV in control external solution (1), after a 1 min exposure to 0.2 μm SRIF (2), and 2 min after the washout of drug (3). The SRIF-induced reduction of Ca2+ current was accompanied by a slowing of its activation kinetics. In control solution the time constant of activation was 2.2 ± 0.3 msec (n = 6), increasing significantly (p < 0.05; n = 6) to 3.4 ± 0.3 msec in the presence of SRIF. SRIF (0.2 μm) reversibly reduced peak Ca2+ current in rods by 33 ± 3% (n = 10; p < 0.05). Figure5b illustrates the steady-state I–V relationship of Ca2+ current recorded in control external solution (open circles), and in the presence of SRIF (closed circles). It shows that the reduction of peak current is not accompanied by a significant shift of the voltage dependence of the calcium current.
Fig. 5.
The inhibitory effect of SRIF on high voltage-activated Ca2+ currents in rods. The test solution contained 0 CaCl2 and 20 mmBaCl2 substituted for equimolar NaCl. a, A depolarizing step to 0 mV from a holding potential of −70 mV was applied to record whole-cell Ca2+ current in control external solution (1), after a 1 min exposure to 0.2 μm SRIF (2), and after a wash (3).b, The I–V relationship of Ca2+ currents evoked by depolarizing voltage steps from −50 to +50 mV in 10 mV increments in the absence (opencircles) and the presence of 0.2 μm SRIF (closedcircles) is shown. c, The time course of SRIF blockade was evaluated by applying 70 msec depolarizing pulses to a test potential of 0 mV from a holding potential of −70 mV each 5 sec. The time of SRIF application is shown by the hatchedhorizontalbar. Thenumbers correspond to the traces ina.
The time course of SRIF blockade was evaluated, using the following protocol. We applied 70 msec depolarizing pulses to a test potential of 0 mV from a holding potential of −70 mV each 5 sec (Fig.5c). In other experiments a voltage ramp (from −70 to +50 mV) was applied every 10 sec. After obtaining five to six stable sequential responses, SRIF-containing solution was perfused into the bath, and the recordings continued another 2–3 min. Figure5c illustrates that the actions of SRIF on Ca2+ current developed relatively rapidly (<1 min) and complete recovery was observed within a 1–2 min wash in control external solution, unlike the incomplete recovery ofIK even after a 10–15 min wash.
SRIF enhances Ca2+ current in cones
Surprisingly, we found that in contrast to its inhibitory action on Ca2+ current in rods, SRIF enhancedICa in cones. Figure6A illustrates an experiment in which a cone was held at a membrane potential of −70 mV, and depolarizing pulses were applied from −40 to +40 mV in 10 mV increments. A family of whole-cell Ca2+currents was recorded in control Ringer's solution, in the presence of 0.5 μm SRIF and after a wash in control Ringer's solution. In the presence of SRIF, the peak Ca2+ current was increased by 40 ± 8% (n = 5; p < 0.005). Figure6B illustrates the I–V characteristics that show that SRIF enhanced Ca2+ current more dramatically at negative voltage steps (e.g., at −30 mV compared with 0 mV), resulting in a shift of peak Ca2+ current toward negative potentials. The data of Figures 5 and 6 are summarized in the histogram of Figure6C that illustrates that SRIF enhancesICa in cones but decreasesICa in rods.
Fig. 6.
Excitatory effects of SRIF on the Ca2+ current in cones. A, Cones were held at −70 mV, and depolarizing pulses were applied from −40 to +40 mV in 10 mV steps. Responses were recorded in 20 mm Ba solution as described for Figure 5. Left, In control Ba Ringer's solution. Middle, In 0.5 μmSRIF. Right, After a wash in Ba Ringer's solution.B, Current–voltage plot of the data in Ais shown. C, A summary of the changes induced in the peak Ca current of rods and cones by SRIF is shown.Cntr, Control (○), wash (▵).
The SRIF-induced inhibition of ICa is G-protein coupled
sst2A receptors are coupled to Gi or Go proteins in different systems (Law et al., 1991, 1993; Gu and Schonbrunn, 1997). Some forms of G-protein-mediated inhibition of Ca2+ currents by neuromodulators are voltage dependent, being relieved by strong depolarizations (Bean, 1989; Hille, 1994). For example, in hippocampal neurons, the SRIF-induced inhibition of an N-type Ca2+current has been shown to be highly sensitive to PTX and to depolarizing prepulses (Ishibashi and Akaike, 1995). To test for the possible involvement of a G-protein in the SRIF-induced inhibition ofICa, we performed experiments on eyecups pretreated with 400 ng/ml PTX. We found that PTX attenuated the inhibitory action of SRIF on ICa. The mean inhibition of ICa by somatostatin in PTX-pretreated rods was 8 ± 2% (n = 3;p > 0.1), compared with the 33% reduction observed in untreated rods.
A G-protein-dependent inhibition ofICa by SRIF may be mediated via an intracellular second messenger system or by a direct membrane-delimited mechanism. G-protein-dependent inhibition ofICa has been found to use either pathway, depending on the neurotransmitter involved (Beech et al., 1991; Shapiro and Hille, 1993). We tested whether intracellular Ca2+ might serve as a second messenger by changing the pipette solution to one containing 0 Ca2+/10 mm BAPTA. The mean reduction of ICa by SRIF in the presence of BAPTA was ∼37% (n = 3), the same degree of inhibition observed in rods not treated with BAPTA (data not shown). We concluded that SRIF-induced inhibition ofICa in rods is not mediated via changes in intracellular [Ca2+].
Effect of SRIF on intracellular Ca2+ accumulation
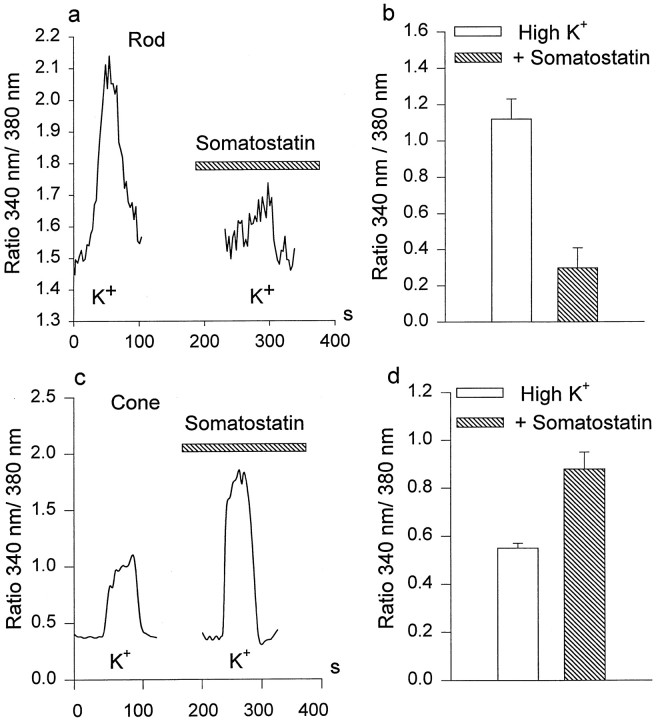
We used Ca2+-imaging techniques to examine whether SRIF altered a depolarization-induced elevation of [Ca2+]i in isolated photoreceptor synaptic endings. In these experiments, 50–100 mm K+ (substituted for an equivalent amount of NaCl) was used to depolarize the cells and to stimulate Ca2+ entry through voltage-gated Ca2+ channels. Exposure to elevated [K+]o induced a sustained increase of [Ca2+]i in the synaptic terminals of isolated rods and cones (Fig.7a,c). This effect was substantially reduced (∼80%) in the presence of nifedipine (50 μm) and completely blocked in the presence of Cd2+ (100 μm), indicating that elevation of intracellular [Ca2+] was caused by activation of voltage-gated L-type Ca2+ channels, although release of Ca2+ from intracellular stores may also have contributed to the Ca2+ signal (Krizaj and Copenhagen, 1998). We found that, in rods, SRIF (0.2–0.5 μm) significantly reduced the [Ca2+]iaccumulation induced by high K+ (Fig.7a). The mean reduction of Ca2+entry by 0.5 μm somatostatin was 55 ± 18% (n = 5; p < 0.05). In agreement with electrophysiological results, we noted that in cones, 0.5 μm SRIF enhanced intracellular Ca2+ accumulation induced by high K+ (Fig. 7c). The mean increase of [Ca2+]i by 0.5 μm SRIF in cones was 50 ± 15% (n = 5; p < 0.05). Figure 7,b and d, summarizes these data that show that SRIF differentially affects Ca2+ signals in rods and cones. In control experiments we observed that two pulses of 90 mm K+ elicited approximately equal increases in [Ca]i, when separated by the same 1–2 min used for the experiments illustrated in Figure 7 (data not shown). Second, it was found that preexposure to PTX abolished the SRIF-induced increase in [Ca2+]i in cones (−4 ± 1%; n = 4; p > 0.1; data not shown). Those cells that still possessed synaptic terminals after isolation manifested the most extensive changes in intracellular Ca2+ induced both by high K+ and SRIF, indicating that SRIF receptors and/or Ca2+ channels may be concentrated in the photoreceptor synaptic terminals (Bader et al., 1982).
Fig. 7.
Effects of somatostatin on K+-induced Ca2+ accumulation in rods and cones. a, c, KCl (100 mm) was used to stimulate Ca2+ entry in the absence and the presence (hatchedhorizontalbar) of 0.5 μm somatostatin in rods (a) and cones (c). Somatostatin reduced Ca2+ accumulation in rods but increased it in cones. b, d, Histograms summarize the somatostatin-induced inhibition of Ca2+ accumulation in rods (b; n = 5) and its enhancement in cones (d; n = 5).Verticalbars show the mean values ± 1 SE.
DISCUSSION
Cellular distribution of SRIF-containing neurons and SRIF receptors in the vertebrate retina
SRIF immunoreactivity has been described in the retinas of a variety of cold-blooded and homeotherm vertebrates (for review, seeBrecha, 1983). The SRIF-containing cells are inner retinal neurons, typically amacrine or interplexiform cells (Yamada et al., 1980; Li et al., 1986; Smiley and Basinger, 1988; Rickman et al., 1996), with perikarya located either in the GCL or at the border of the INL and IPL. SRIF-containing neurons in mammals often are displaced amacrines, i.e., with cell bodies in the GCL (Engelmann and Peichl, 1996; Rickman et al., 1996). The retinal density of SRIF neurons is low [<100 cells mm−2 (Rickman et al., 1996)] in most parts of the retina but may reach a few thousand cells per square millimeter in restricted retinal regions (Engelmann and Peichl, 1996;Rickman et al., 1996). In spite of the low cellular density, SRIF processes create a continuous network in the IPL. Within the IPL the distribution of SRIF processes varies; in chicken retina, it is diffuse (Ishimoto et al., 1986), whereas in rabbit and salamander retinas, SRIF processes extend horizontally in laminae 1 and 5 of the IPL (Rickman et al., 1996) (present report). sst2A receptors, on the other hand, are found in both the inner and outer retina (Johnson et al., 1998, 1999) (present report). The wider retinal distribution of SRIF receptors in relation to SRIF cells and processes indicates that SRIF reaches some targets by diffusion. Thus in general outline, the retinal SRIF system resembles closely that of the dopamine system (Witkovsky and Schutte, 1991), a resemblance that is heightened by the presumption that SRIF will affect synaptic transmission between rod and cone photoreceptors and second-order retinal neurons, as shown previously for dopamine (Witkovsky et al., 1988).
In the absence of antibodies against other forms of the SRIF receptors, we cannot conclude with certainty that the SRIF-induced physiological effects we have described were mediated by the sst2A receptor, although this is a reasonable supposition because of the high density of sst2Areceptors on rod and cone terminals. The finding that these effects were blocked by PTX is not diagnostic, because all forms of SRIF receptor are reported to act via subtypes of either Gi or Go proteins (Law et al., 1991; Takano et al., 1997), all of which are blocked by PTX.
SRIF-induced modulation of Ca2+ and K+ currents in photoreceptors
K+ current
Photoreceptors possess a mixture of voltage-dependent K+ currents, including a delayed rectifier, IK (Bader et al., 1982), a Ca2+-dependent K+ current, IK–Ca(Corey et al., 1984), and Ih, a mixed cation current (Fain et al., 1978; Barnes and Hille, 1989). Our data indicate that SRIF selectively augments the delayed rectifier K+ current. The enhancement of outward currents remained in the presence of charybdotoxin that blocksIK–Ca, and it increased with depolarizing steps (Fig. 2) at which potentialsIh is inactivated (Akopian and Witkovsky, 1996). Exposure to SRIF also elicited a non-inactivating outward current that was 16 pA at the holding potential of −70 mV. This steady current is not attributable to Ih that has a reversal potential near −30 mV and so would generate an inward current at −70 mV. If the steady current were a leakage current, it would add a linear component to the total outward current, whose magnitude can be estimated by a regression line passing through EK (−88 mV) and 16 pA at −70 mV. Because this putative current was not observed (Fig. 2), we conclude that the action of SRIF on K current is mediated primarily byIK in salamander rods and cones.
The SRIF-induced steady K+ current would be expected to hyperpolarize photoreceptors. At the resting potential of the photoreceptor in darkness (−40 mV) and with a value of 100 MΩ resistance to ground for rods in an intact retina (Owen and Copenhagen, 1977), photoreceptors will be hyperpolarized 1 mV for each 10 pA of steady current.
We noted that the SRIF-induced increase inIK was blocked by PTX and by GDPβS, consistent with the general finding that SRIF acts via PTX-sensitive G-proteins (Law et al., 1991; Rens-Domiano and Reisine, 1992). Our data agree with the demonstration by Wang et al. (1989) that in rat neocortical neurons, the enhancement by SRIF of a delayed rectifier current was antagonized by PTX. Takano et al. (1997) found that Kir was activated by SRIF via Gαi 1 or 2 proteins, and Gu and Schonbrunn (1997) showed that the sst2A receptor complexed specifically with Gαi 1–3 proteins, all of which are PTX-sensitive. Thus our data are consistent with the hypothesis that they were mediated by the sst2Areceptor that rod and cone photoreceptors express (Fig. 1), but a more compelling proof would require showing that SRIF receptors other than the sst2A subtype are absent on salamander photoreceptors.
Ca2+ currents
The Ca2+ currents of salamander rods and cones have been investigated intensively (Bader et al., 1982; Corey et al., 1984; Barnes and Hille, 1989; Wilkinson and Barnes, 1996). They are of the L-type (dihydropyridine-sensitive, high voltage-activated, and nondesensitizing). Studies in several systems show that L-type currents are reduced by SRIF in a rapid and reversible manner (Ikeda and Schofield, 1989; Dryer et al., 1991; Shapiro and Hille, 1993;Ishibashi and Akaike, 1995). Our data on the fast, reversible inhibition of ICa in rods by SRIF and the independence of SRIF-induced effects on changes in intracellular [Ca2+] suggest that the SRIF-induced inhibition of ICa is mediated by a membrane-delimited pathway. This possibility is consistent with the demonstration by Delmas et al. (1998) that a Gβγ unit underlies the inhibition, by noradrenaline and SRIF, of an N-type Ca2+current in rat sympathetic neurons.
The finding that the ICa of cone photoreceptors is enhanced by SRIF is novel. The enhancement consisted of a shift toward negative voltage of the activation function. This shift will be functionally important for cones, because their operating range extends for 20–30 mV negative to the membrane potential of approximately −40 mV in darkness (for review, see Attwell, 1990). The voltage-clamp records of ICa in cones are buttressed by the data from Ca2+imaging that show that SRIF augments the increase in [Ca2+]i. It is possible that the differential action of SRIF on rod and cone calcium currents indicate a difference in the underlying Ca channel. Wilkinson and Barnes (1996) classified the cone L-Ca channel as the D subtype on the basis of its pharmacological profile, but we are unaware of a comparable study on the rod Ca channel.
Significance for retinal function of SRIF-induced alterations in photoreceptor K+ and Ca2+currents
The photoreceptor synapse is unusual in that rods and cones release glutamate tonically in darkness. Light, by hyperpolarizing the photoreceptor, reduces the rate of glutamate release (for review, seeAttwell, 1990). Because glutamate release by photoreceptors is a Ca2+-dependent process, it requires a sustained, voltage-dependent Ca2+ current, in accord with the demonstration that rods and cones use an L-type Ca current to mediate transmitter release (Rieke and Schwartz, 1996;Schmitz and Witkovsky, 1997; Witkovsky et al., 1997). A growing number of studies document that transmitter release from photoreceptor terminals is subject to multiple sources of neuromodulation. These include pH (Barnes et al., 1993), possibly related to GABA release by horizontal cells (Verweij et al., 1996), dopamine (Stella and Thoreson, 1998), and Ca release from intracellular stores (Krizaj et al., 1999).
SRIF will contribute to the overall modulation by downregulating glutamate release from rods via two mechanisms. The steady outward K+ current will hyperpolarize the rod. Even a small (1–2 mV) hyperpolarization might be important, because of the change of slope of the Ca2+ current activation function that occurs near the −40 mV resting potential of the rod in darkness (Rieke and Schwartz, 1996; Witkovsky et al., 1997). Second, by further reducing ICadirectly, glutamate release by rods will be attenuated.
The situation for cones is complex in that the shift toward negative voltages of the Ca2+ current–voltage function by SRIF will tend to be counterbalanced by any hyperpolarization resulting from a SRIF-induced increase inIK. Thus a good test of the net action of SRIF on photoreceptor signaling will be to examine rod and cone inputs to the second-order retinal neurons and horizontal and bipolar cells. In amphibian retinas these second-order neurons receive a mixed input from rods and cones (Hare et al., 1986). On the basis of the findings of the present study, one would expect SRIF to reduce rod input and increase cone input, just as has been reported for the action of dopamine on amphibian horizontal cells (Witkovsky et al., 1988).
Footnotes
This work was supported by National Institutes of Health Grants EY 03570 to P.W., EY 07026 to J.J, and EY 04067 to N.B. and by a Veterans Administration Career Scientist award to N.B. Additional support came from the Hoffritz Foundation and an unrestricted grant from Research to Prevent Blindness to the Department Ophthalmology, New York University School of Medicine.
Correspondence should be addressed to Dr. Abram Akopian, New York University School of Medicine, 550 First Avenue, New York, NY 10016. E-mail: aa3@is4.nyu.edu.
REFERENCES
- 1.Akopian A, Witkovsky P. D2 dopamine receptor-mediated inhibition of a hyperpolarization-activated current in rod photoreceptors. J Neurophysiol. 1996;76:1828–1835. doi: 10.1152/jn.1996.76.3.1828. [DOI] [PubMed] [Google Scholar]
- 2.Attwell D. The photoreceptor output synapse. Prog Retin Res. 1990;9:337–362. [Google Scholar]
- 3.Bader CR, Bertrand D, Schwartz EA. Voltage-activated and Ca2+-activated currents studied in solitary rod inner segments from the salamander retina. J Physiol (Lond) 1982;331:253–284. doi: 10.1113/jphysiol.1982.sp014372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes S, Hille B. Ionic channels of the inner segment of tiger salamander photoreceptors. J Gen Physiol. 1989;94:719–743. doi: 10.1085/jgp.94.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes S, Merchant V, Mahmud F. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc Natl Acad Sci USA. 1993;90:10081–10085. doi: 10.1073/pnas.90.21.10081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bean BP. Neurotransmitter inhibition of neuronal Ca2+ currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 7.Beech D, Bernheim L, Hille B. Intracellular Ca buffers disrupt muscarinic suppression of Ca current and M current in rat sympathetic neurons. Proc Natl Acad Sci USA. 1991;88:652–656. doi: 10.1073/pnas.88.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehm S, Betz H. Somatostatin inhibits excitatory transmission at rat hippocampal synapses via presynaptic receptors. J Neurosci. 1997;17:4066–4075. doi: 10.1523/JNEUROSCI.17-11-04066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brecha N. Retinal neurotransmitters: histochemical and biochemical studies. In: Emson PC, editor. Chemical neuroanatomy. Raven; New York: 1983. pp. 85–129. [Google Scholar]
- 10.Corey DP, Dubinsky JM, Schwartz EA. The calcium current in inner segments of rods from the salamander (Ambystoma tigrinum) retina. J Physiol (Lond) 1984;354:557–575. doi: 10.1113/jphysiol.1984.sp015393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delfs JR, Dichter MA. Somatostatin. In: Rogawski MA, Barker JL, editors. Neurotransmitter actions in the vertebrate nervous system. Plenum; New York: 1985. pp. 411–437. [Google Scholar]
- 12.Delmas P, Brown DA, Dayrell M, Abogadie FC, Caufield MP, Buckley NJ. On the role of endogenous G-protein beta gamma subunits in N-type Ca current inhibition by neurotransmitters in rat sympathetic neurons. J Physiol (Lond) 1998;506:319–329. doi: 10.1111/j.1469-7793.1998.319bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dryer SE, Dourado MM, Wisgirda ME. Properties of Ca currents in acutely dissociated neurons of the chick ciliary ganglion: inhibition by somatostatin-14 and somatostatin-28. Neuroscience. 1991;44:663–672. doi: 10.1016/0306-4522(91)90086-4. [DOI] [PubMed] [Google Scholar]
- 14.Engelmann R, Peichl L. Unique distribution of somatostatin-immunoreactive cells in the retina of the tree shrew (Tupaia belangeri). Eur J Neurosci. 1996;8:220–228. doi: 10.1111/j.1460-9568.1996.tb01183.x. [DOI] [PubMed] [Google Scholar]
- 15.Fain GL, Quandt FN, Bastian BL, Gerschenfeld HM. Contribution of a caesium sensitive conductance increase to the rod photoreceptors. Nature. 1978;272:467–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- 16.Gu YZ, Schonbrunn A. Coupling specificity between somatostatin receptor sst2A and G proteins: isolation of the receptor-G protein complex with a receptor antibody. Mol Endocrinol. 1997;11:527–537. doi: 10.1210/mend.11.5.9926. [DOI] [PubMed] [Google Scholar]
- 17.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp technique for high resolution current recording from cell and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 18.Hare WA, Lowe JS, Owen WG. Morphology of physiologically identified bipolar cells in the retina of the tiger salamander, Ambystoma tigrinum. J Comp Neurol. 1986;252:130–138. doi: 10.1002/cne.902520108. [DOI] [PubMed] [Google Scholar]
- 19.Hille B. Modulation of ion-channel function by G-protein coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 20.Holtz GG, Rane SG, Dunlap K. GTP-binding proteins mediate transmitter inhibition of voltage-dependent calcium channels. Nature. 1986;319:670–672. doi: 10.1038/319670a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O'Carroll AM, Patel YC, Schonbrunn A, Taylor JE. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda SR, Schofield GG. Somatostatin blocks a calcium current in rat sympathetic ganglion neurons. J Physiol (Lond) 1989;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishibashi H, Akaike N. Somatostatin modulates high-voltage-activated Ca channels in freshly dissociated rat hippocampal neurons. J Neurophysiol. 1995;74:1028–1036. doi: 10.1152/jn.1995.74.3.1028. [DOI] [PubMed] [Google Scholar]
- 24.Ishimoto I, Millar T, Chubb IW, Morgan IG. Somatostatin-immunoreactive amacrine cells of chicken retina: retinal mosaic, ultrastructural features, and light-driven variations in peptide metabolism. Neuroscience. 1986;17:1217–1233. doi: 10.1016/0306-4522(86)90089-8. [DOI] [PubMed] [Google Scholar]
- 25.Johnson J, Wong H, Walsh J, Brecha N. Expression of the somatostatin subtype receptor in the rabbit retina. J Comp Neurol. 1998;392:1–9. doi: 10.1002/(sici)1096-9861(19980330)393:1<93::aid-cne9>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson J, Wu V, Wong H, Walsh JH, Brecha N. Somatostatin receptor subtype 2A in the rat retina. Neuroscience. 1999;94:675–683. doi: 10.1016/s0306-4522(99)00170-0. [DOI] [PubMed] [Google Scholar]
- 27.Katayama Y, Hirai K. Somatostatin presynaptically inhibits transmitter release in feline parasympathetic ganglion. Brain Res. 1989;487:62–68. doi: 10.1016/0006-8993(89)90940-2. [DOI] [PubMed] [Google Scholar]
- 28.Knaus H-G, Eberhart A, Kaczorowski GJ, Garcia ML. Covalent attachment of charybdotoxin to the 2A β-subunit of the high conductance Ca2+-activated K+ channel. J Biol Chem. 1994;269:23336–23341. [PubMed] [Google Scholar]
- 29.Krizaj D, Copenhagen DR. Compartmentalization of calcium extrusion mechanisms in the outer and inner segments of photoreceptors. Neuron. 1998;21:249–256. doi: 10.1016/s0896-6273(00)80531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krizaj D, Bao JX, Schmitz Y, Witkovsky P, Copenhagen DR. Caffeine-sensitive calcium stores regulate synaptic transmission from retinal rod photoreceptors. J Neurosci. 1999;19:7249–7261. doi: 10.1523/JNEUROSCI.19-17-07249.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law S, Manning D, Reisine T. Identification of the subunits of GTP-binding proteins coupled to somatostatin receptors. J Biol Chem. 1991;266:17885–17897. [PubMed] [Google Scholar]
- 32.Law SF, Yasuda K, Bell GI, Reisine T. Gi alpha 3 and G (o) alpha selectively associate with the cloned somatostatin receptor subtype SSTR2. J Biol Chem. 1993;268:10721–10727. [PubMed] [Google Scholar]
- 33.Li HB, Lam DMK. Localization of neuropeptide-immunoreactive neurons in the human retina. Brain Res. 1990;522:30–36. doi: 10.1016/0006-8993(90)91573-y. [DOI] [PubMed] [Google Scholar]
- 34.Li HB, Chen NX, Watt CB, Lam DMK. The light microscopic localization of substance P and somatostatin-like immunoreactive cells in the larval tiger salamander retina. Exp Brain Res. 1986;63:93–102. doi: 10.1007/BF00235650. [DOI] [PubMed] [Google Scholar]
- 35.Lukasiewicz P, Maple B, Werblin FS. A novel GABA receptor on bipolar cell terminals in the tiger salamander retina. J Neurosci. 1994;14:1202–1212. doi: 10.1523/JNEUROSCI.14-03-01202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marc RE, Liu WL, Kalloniatis M, Raiguel SF, Van Haesendonck E. Patterns of glutamate immunoreactivity in the goldfish retina. J Neurosci. 1990;10:4006–4034. doi: 10.1523/JNEUROSCI.10-12-04006.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owen WG, Copenhagen DR. Characteristics of the electrical coupling between rods in the turtle retina. In: Barlow HB, Fatt P, editors. Vertebrate photoreception. Academic; 1977. pp. 169–192. [Google Scholar]
- 38.Rens-Domiano S, Reisine T. Biochemical and functional properties of somatostatin receptors. J Neurochem. 1992;58:1987–1996. doi: 10.1111/j.1471-4159.1992.tb10938.x. [DOI] [PubMed] [Google Scholar]
- 39.Rickman DW, Blanks JC, Brecha NC. Somatostatin-immunoreactive neurons in the adult rabbit retina. J Comp Neurol. 1996;365:491–503. doi: 10.1002/(SICI)1096-9861(19960212)365:3<491::AID-CNE11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 40.Rieke F, Schwartz E. Asynchronous transmitter release: control of exocytosis and endocytosis at the salamander rod synapse. J Physiol (Lond) 1996;493:1–8. doi: 10.1113/jphysiol.1996.sp021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenthal W, Hescheler J, Klaus-Dieter H, Spicher K, Trautwei W, Schultz G. Cyclic AMP-independent, dual regulation of voltage-dependent Ca currents by LHRH and somatostatin in pituitary cell line. EMBO J. 1988;7:1627–1633. doi: 10.1002/j.1460-2075.1988.tb02989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz Y, Witkovsky P. Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. Neuroscience. 1997;78:1209–1216. doi: 10.1016/s0306-4522(96)00678-1. [DOI] [PubMed] [Google Scholar]
- 43.Schweitzer P, Madamba SG, Siggins GR. Somatostatin increases a voltage-insensitive K+ conductance in rat CA1 hippocampal neurons. J Neurophysiol. 1998;79:1230–1238. doi: 10.1152/jn.1998.79.3.1230. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro MS, Hille B. Substance P and somatostatin inhibit calcium channels in rat sympathetic neurons via different G protein pathways. Neuron. 1993;10:11–20. doi: 10.1016/0896-6273(93)90237-l. [DOI] [PubMed] [Google Scholar]
- 45.Smiley JF, Basinger F. Somatostatin-like immunoreactivity and glycine high-affinity uptake colocalize to an interplexiform cell of the Xenopus laevis retina. J Comp Neurol. 1988;274:608–616. doi: 10.1002/cne.902740409. [DOI] [PubMed] [Google Scholar]
- 46.Stella S, Thoreson WB. Differential modulation of rod and cone calcium currents by cAMP and a D2 dopamine receptor agonist. Invest Ophthalmol Vis Sci. 1998;39:S983. [Google Scholar]
- 47.Sternini CH, Wong SV, Wu R, De Giorgio M, Yang J, Reeve NC, Jr, Brecha N, Walsh J. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J Comp Neurol. 1997;386:396–408. [PubMed] [Google Scholar]
- 48.Takano K, Yasufuku-Takano J, Kozasa T, Nakajima S, Nakajima Y. Different G proteins mediate somatostatin-induced inward rectifier K+ currents in murine brain and endocrine cells. J Physiol (Lond) 1997;502:559–567. doi: 10.1111/j.1469-7793.1997.559bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thoreson WB, Witkovsky P. Glutamate receptors and circuits in the vertebrate retina. Prog Retin Eye Res. 1999;18:765–810. doi: 10.1016/s1350-9462(98)00031-7. [DOI] [PubMed] [Google Scholar]
- 50.Vanetti M, Kouba M, Wang X, Vogt G, Hollt V. Cloning and expression of a novel mouse somatostatin receptor (SSTR2B). FEBS Lett. 1992;311:290–294. doi: 10.1016/0014-5793(92)81122-3. [DOI] [PubMed] [Google Scholar]
- 51.Verweij J, Kamermans M, Spekreijse H. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 1996;36:3943–3953. doi: 10.1016/s0042-6989(96)00142-3. [DOI] [PubMed] [Google Scholar]
- 52.Wang HL, Bogen G, Reisine T, Dichter M. Somatostatin-14 and somatostatin-28 induce opposite effects on K+ currents in rat neocortical neurons. Proc Natl Acad Sci USA. 1989;86:9616–9620. doi: 10.1073/pnas.86.23.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watt CB, Yang SZ, Lam DMK, Wu SM. Localization of tyrosine-hydroxylase-like-immunoreactive amacrine cells in the larval tiger salamander retina. J Comp Neurol. 1988;272:114–126. doi: 10.1002/cne.902720108. [DOI] [PubMed] [Google Scholar]
- 54.White RE, Schonbrunn A, Armstrong D. Somatostatin stimulates Ca-activated K channels through protein dephosphorylation. Nature. 1991;351:570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- 55.Wilkinson MF, Barnes S. The dihydropyridine-sensitive calcium channel subtype in cone photoreceptors. J Gen Physiol. 1996;107:621–630. doi: 10.1085/jgp.107.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Witkovsky P, Dearry A. A functional role of dopamine in the vertebrate retina. In: Osborne NN, Chader C, editors. Progress in retinal research. Pergamon; Oxford: 1991. pp. 247–292. [Google Scholar]
- 57.Witkovsky P, Schutte M. The organization of dopaminergic neurons in vertebrate retinas. Vis Neurosci. 1991;7:113–124. doi: 10.1017/s0952523800010981. [DOI] [PubMed] [Google Scholar]
- 58.Witkovsky P, Stone S, Besharse J. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res. 1988;449:332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- 59.Witkovsky P, Schmitz Y, Akopian A, Krizaj D, Tranchina D. Gain of rod to horizontal cell synaptic transfer: relation to glutamate release and a dihydropyridine-sensitive calcium current. J Neurosci. 1997;17:7297–7306. doi: 10.1523/JNEUROSCI.17-19-07297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamada T, Marshak D, Basinger S, Walsh J, Moreley J, Stell W. Somatostatin-like immunoreactivity in the retina. Proc Natl Acad Sci USA. 1980;77:1691–1695. doi: 10.1073/pnas.77.3.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zalutsky RA, Miller RF. The physiology of somatostatin in the rabbit retina. J Neurosci. 1990;10:383–393. doi: 10.1523/JNEUROSCI.10-02-00383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]