Abstract
Objectives
Sleep disturbances in pregnancy may impair glucose mechanism. This study aimed to examine associations of sleep-disordered breathing, sleep and nap-duration with one-hour glucose challenge test (GCT) levels in pregnant women after controlling for known risk factors for gestational diabetes.
Methods
This is a case-control study of 104 pregnant women. All women underwent full polysomnography and a glucose challenge test (GCT), and completed the Multivariable Apnoea Prediction and Pittsburgh Sleep Quality Indexes. The primary outcome was maternal hyperglycaemia measured by GCT. Bivariate and multivariable logistic regression analyses were performed.
Results
Over 13% subjects reported habitual snoring in the first trimester. Only 9.3% women with normoglycaemia (GCT<135) were habitual snorers, whereas 45.5% women with hyperglycaemia-(GCT≥135) had habitual snoring (p<0.001). Sleep-disordered breathing symptoms (loud snoring, snorting/gasping and apnoeas), (odds ratio (OR) 2.85; 95% confidence interval (CI) 1.50–5.41; p=0.001) and total nap-duration (OR 1.48; 95% CI 0.96–2.28; p=0.08) were associated with hyperglycaemia. After adjusting for confounders, sleep-disordered breathing symptoms (OR 3.37; 95% CI, 1.44–8.32; p=0.005) and nap-duration (OR 1.64; 95% CI, 1.00–2.681.02; p=0.05) continued to be associated with hyperglycaemia. However, the primary exposure measure, the apnea/hypopnea index in the first-trimester was not significantly associated with hyperglycaemia (OR 1.03; 95% CI 0.83–1.28; p=0.77).
Conclusions
Sleep-disordered breathing symptoms and nap duration are associated with hyperglycaemia. Sleep-duration was not associated with hyperglycaemia. Research is needed concerning whether women with sleep-disordered breathing and/or daytime napping are at risk for gestational diabetes.
Keywords: Apnoea symptom score, gestational diabetes, glucose dysregulation, sleep duration, snoring, hyperglycaemia
Introduction
Sleep-disordered breathing (SDB) and abnormal sleep duration (both short and long) have been recognized as independent risk factors for impaired glucose tolerance and type 2 diabetes. Most of these studies have been performed in middle-aged women [1–8] and men [9,2,6,5]. SDB is not prevalent in premenopausal women [10,8,11], but snoring and changes in both sleep pattern and duration are common sleep complaints of pregnant women [12–14,10,15–20]. There is some evidence that maternal snoring and short sleep duration are prognostic factors for poor pregnancy outcomes such as gestational diabetes, hypertension, pre-eclampsia, and fetal growth retardation [21,22,16,23,24,18,17,25,26]. Thus, a significant proportion of pregnant women may be at risk of hyperglycaemia and gestational diabetes mellitus (GDM), analogous to the increased risk of glucose intolerance and diabetes among those with SDB and sleep disturbances in the non-pregnant population [1–8].
GDM and even mild hyperglycaemia without overt GDM are associated with increased risk for maternal and foetal complications, i.e. pre-eclampsia and foetal growth retardation, and future development of chronic diseases (type 2 diabetes, obesity and cardiovascular disease in mothers and children) [27–31]. Although adverse outcomes of GDM are clearly recognized, the exact cause is unknown in most cases. There has been little research studying the effects of SDB and sleep disturbances on the development of GDM [18,17,24,25]. Furthermore, these observational studies led to conflicting results, with some studies reporting that glucose metabolism may be adversely affected by symptoms of SDB and both long and short sleep duration [18,17,25], whereas others did not find any independent role for pregnancy-onset habitual snoring in gestational diabetes [24].
We conducted a prospective study which measured objective sleep parameters such as SDB and sleep duration (recorded by polysomnography (PSG)) as well as subjective sleep parameters (self-reported by questionnaire) in the first and third trimesters of pregnancy. We also examined and adjusted for potential confounding factors such as neck circumference in early pregnancy and shift work [32]. Our primary hypothesis was to test whether SDB is associated with increased 1-hour glucose challenge test (GCT) levels. We also sought to investigate the association of high GCT levels with both nocturnal sleep duration and daytime nap duration.
Methods
Design
This is a case control study. Data were examined from a previously completed cohort study of SDB in pregnancy.
Subjects
Subjects were recruited from obstetric clinics at the Helen O. Dickens Center for Women’s Health at the Hospital of the University of Pennsylvania (HUP). Eligible mothers were ≤14 weeks of gestation at enrolment. The recruitment methods and study design for this study have been described in detail elsewhere [10]. Exclusion criteria included inability to communicate, cognitive or behavioural impairments potentially interfering with informed consent; the lack of a telephone; self-reported illicit drug use or alcoholism; serious pre-existing chronic medical conditions; sedative/hypnotic medications-use (≥3 times/week); current treatment for SDB. This study was approved by the Institutional Review Board (IRB) of the University of Pennsylvania School of Medicine and all subjects provided informed consent.
In the original study, 108 women underwent overnight laboratory-based PSG in the first trimester, had a GCT performed at any point during pregnancy. Four women with previous GDM were excluded because a history of GDM is a strong predictor of GDM in future pregnancies [31,33,27]. Six women required follow-up blood tests (OGTT) and were diagnosed with GDM according to chart abstraction following delivery.
All subjects completed a demographic questionnaire, the apnoea-symptom score from the Multivariable Apnoea Prediction Index (MAPI) [34] and the Pittsburgh Sleep Quality Index (PSQI) [35]. Demographic variables assessed in the first trimester included age, race, ethnicity, marital status, education, parity and whether they were working steady or rotating shift-work. Baseline data on nap duration and frequency and nighttime sleep duration was also collected. Napping frequency was rated during the last month on a five-point Likert scale, corresponding to never (including < 1 time per week), 1–2 times per week, 3 times or more per week. Total nap duration was obtained by averaging total time in hours spent napping on the days the participants napped. Total nocturnal sleep duration in hours was computed by subtracting total wake time from time in bed. Subjects had height, weight and neck circumferences recorded in the first trimester [10].
The apnoea-symptom score
SDB symptoms were assessed using apnoea-symptom score of the Multivariable Apnea Prediction Index (MAPI) to assess the frequency of sleep symptoms using a Likert response format [34]. This index evaluates the presence and frequency of the following symptoms during the previous month: loud snoring, snorting/gasping and apnoeas. Each question score ranges from 0 to 4 (0=never, 1=rarely, 2=sometimes, 3=frequently, 4=always) evaluated on a per-week basis. The total is computed by averaging non-missing responses [10]. Higher apnoea-symptom scores indicate increased likelihood of having OSA.
Pittsburgh Sleep Quality Index (PSQI)
The PSQI is a well-validated 19-item self-report measure assessing sleep quality and severity of specific sleep-related complaints over the previous month. The PSQI provides a total score of sleep quality and has seven subscales including sleep duration, latency, efficiency and daytime functioning. The total PSQI score ranges from 0–21 and subscale scores ranges from 0–3. Higher scores reflect poorer sleep [35]. The PSQI has good internal consistency, convergent and divergent reliability in pregnant population [35].
Polysomnography
In the first trimester 104 pregnant women underwent PSG. Eighty-three of them also had a third-trimester PSG. The primary exposure of interest in this study was the apnea/hypopnea index (AHI) and is defined as the frequency of apneas plus hypopneas per hour of sleep, using PSG. First and third trimester PSGs (Sandman, Melville Diagnostics) were performed including electroencephalography, electrocardiography, pulse-oximetry (Nellcor), chin and tibialis electromyograms, chest and abdominal excursion (Protech piezo belts), airflow by nasal pressure and oral thermistor. An obstructive apnoea was defined as the absence of airflow for 10 seconds or longer despite ongoing respiratory effort. A hypopnoea was defined as a 30–90% decrease in airflow associated with a reduction of oxygen saturation by at least 3% and/or an EEG arousal. Sleep and respiratory events were scored according to established criteria [26]. Objective total sleep duration was assessed from this one night sleep study in the lab. Other sleep parameters included rapid eye movement (REM) sleep time, non REM sleep time, sleep efficiency, AHI (apneas+hypopneas/hour of sleep) and arousal index (arousals/hour). The scorer who performed PSG was blind to the glycaemia category of subjects. Additionally, the researcher who performed chart review was blind to the severity of SDB of subjects.
Glucose challenge test (GCT)
The primary outcome was maternal hyperglycaemia measured by glucose challenge test (GCT). GCT results were collected from prenatal medical records. At the HUP, the GCT is performed routinely for GDM screening between 24–28 weeks’ gestation measuring plasma/serum glucose one hour after administering a 50-gram oral glucose load in a non-fasting state. Hyperglycemia was defined as a categorical variable (GCT<135 vs GCT≥135 mg/dL). Results may differ among certain ethnic groups. Setting a goal at 135 mg/dl for African American has been shown to decrease the false positive rate to less than 10% and increase the sensitivity to 95% [33].
Statistical Analysis
Subjects were categorized into two groups, high GCT (≥135 mg/dL) and normal GCT (<135 mg/dL). Differences between the groups were assessed with t-tests or Mann-Whitney test for continuous data and chi-square or Fisher’s exact test for categorical data.
Logistic regression models were created to analyze the relationship between GCT and exposure variables. The following steps were performed: (1) unadjusted analysis of the association of each exposure variable with GCT variable; (2) variables were chosen as candidates for the multivariable analysis based on the level of significance of the unadjusted association with the GCT variable (p< 0.2); (3) multivariable logistic regression analyses were performed and (4) confounders were retained in the final models based upon a >10% change in the effect size of the primary predictor. The models were examined for collinearity using Spearman correlation. Multi-collinear variables were excluded from the final model. We fitted two separate multiple logistic regression models (models 1 for SDB and 2 for nap duration). The models were adjusted for established confounding factors including age, race/ethnicity, neck circumference and night-shift work. We used neck circumference as a surrogate marker of obesity, because the correlation of BMI with GCT in the bivariate model was p≥0.2. Parity was not included in the model for SDB or nap duration since adding it into the models did not change the OR of the apnoea-symptoms (Model 1) or nap duration (Model 2). Nocturnal sleep duration was not included in model 2 as a confounder, because adding sleep duration did not have any effect on the OR of nap duration. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to estimate the strength of the association and the precision of the estimates using the SPSS statistical software package, version 17. The area under the receiver operating characteristic (ROC) curve was used as an overall measure of the ability of the models to predict hyperglycemia.
Results
The subjects’ characteristics are presented in Table 1. Anthropometric and demographic data and objective sleep parameters from PSG were collected at 12±2.1 (SD) weeks of pregnancy. GCTs were performed at 24.0±6.5 weeks of pregnancy. Individuals of other racial identity (n=3) were combined with Caucasians (n=18) to compare with African-Americans (n=73). Anthropometric and demographic characteristics were similar between hyperglycaemia (GCT≥135) and normoglycaemia (GCT<135) groups (Table 1). Of 104 pregnant women, 13% reported habitual snoring in the first trimester. Only 9.3% women with normoglycaemia snored habitually, whereas 45.5% of those with hyperglycaemia were habitual snorers (p<0.001). The MAPI apnoea-symptom score was significantly higher in the hyperglycaemic group compared to the normoglycaemic group (p<0.01). First and third trimester AHI were not significantly different between the two groups (Table 1). Overall, 8 subjects in the first trimester and 14 subjects in the third had AHI≥5 events/hour. Two subjects in the first trimester and 7 subjects in the third had AHI>10. Total nap duration (hours) tended to be longer in women with GCT≥135 than those with GCT<135 but this difference was not significant (p=0.07). Nocturnal sleep duration either from objective or subjective measures were not significantly different between the two groups (Table 1). Only 1(1%) subject reported sleeping <4 hours/night, 19(18.3%) <6 hours/night and 34(33%) <7 hours/night.
Table 1.
Characteristics of Study Population (N=104)
| GCT < 135 mg/dl (n=93) | GCT ≥ 135 mg/dl (n=11) | P value | |
|---|---|---|---|
| Variable | % | % | * |
| Caucasian | 21.5 | 9.0 | 0.5 |
| African American, % | 78.50 | 91.0 | |
| Nulliparous, % | 50.5 | 54.5 | 0.8 |
| Multiparous, % | 49.5 | 45.5 | |
| Multiple pregnancy, % | 6.0 | 0 | 1.0 |
| Married, % | 31.2 | 36.4 | 0.7 |
| Single/separated, % | 68.8 | 63.6 | |
| ≤ High school education, % | 63.4 | 72.7 | 0.7 |
| Higher education, % | 36.6 | 27.3 | |
| Steady & rotating night-shift, % | 10.9 | 27.3 | 0.1 |
| Habitual snorers, % | 9.3 | 45.5 | <0.01 |
| Mean±SD | Mean±SD | ** | |
| Age, yrs | 26.37 ± 7.2 | 28.59 ± 7.6 | 0.4 |
| Body mass index, kg/m2 | 30.16 ± 7.4 | 32.74 ± 5.7 | 0.3 |
| Pre-pregnancy BMI, kg/m2 | 29.52 ± 0.74 | 30.73 ± 1.38 | 0.6 |
| GCT level mg/dl | 94.70 ± 1.80 | 154.91 ± 5.68 | <0.001 |
| Neck circumference, cm | 35.57 ± 3.0 | 36.86 ± 2.5 | 0.2 |
| Apnoea-symptom score | 0.52 ± 0.8 | 1.53 ± 1.1 | <0.01 |
| Self-reported nocturnal sleep duration, hr | 7.71 ± 2.1 | 7.45 ± 2.7 | 0.7 |
| Self-reported total nap duration, hr | 1.49 ± 1.3 | 2.27 ± 1.4 | 0.07 |
| 1st trimester PSG | |||
| AHI/hr | 1.79 ± 2.39 | 2.04 ± 4.62 | 0.22 |
| Nadir O2 | 90.62 ± 6.4 | 90.27 ± 3.6 | 0.23 |
| Total sleep duration, hr | 6.31 ± 0.9 | 6.34 ± 1.2 | 0.92 |
| Sleep Efficiency (%) | 79.26 ± 1.14 | 76.11 ± 4.07 | 0.47 |
| Arousal index/hr | 17.74 ± 0.93 | 14.98 ± 2.48 | 0.22 |
| 3rd trimester PSG | (n=75) | (n=8) | |
| AHI/hr | 3.36 ± 5.61 | 3.16 ± 2.52 | 0.33 |
| Nadir O2 | 90.71 ± 0.66 | 89.71 ± 1.79 | 0.39 |
| Total sleep duration, hr | 5.83 ± 0.14 | 5.58 ±0.28 | 0.57 |
| Sleep Efficiency (%) | 72.45 ± 1.67 | 70.82 ± 3.95 | 0.76 |
| Arousal index/hr | 19.92 ± 1.16 | 15.08 ± 1.88 | 0.16 |
Definition of abbreviations: BMI=body mass index; GCT=Glucose Challenge Test; AHI=Apnoea Hypopnoea Index; SD=standard deviation; PSG=polysomnography;
p-value from Chi-Square test or Fisher’s exact test for categorical variables and
p-value from t tests or Mann-whitney test for continuous variable
From the 11 subjects who had a post-50g oral glucose load over 135mg/dl, six women were diagnosed with gestational diabetes. The GCT values of women with diabetes was 165.33(+/− 8.04) mg/dl and of women without diabetes was 142.40(+/−6.66).
Relationship between SDB and GCT levels
In bivariate analyses, the primary exposure variable (first trimester AHI) was not significantly associated with high GCT (≥135 mg/dl) values (odds ratio(OR) 1.03; 95% confidence interval(CI) 0.83–1.28; p=0.77) (Table 2). Nor were there significant associations between increased GCT and average oxyhaemoglobin saturation (OR 1.01; 95% CI 0.92–1.11; p=0.84) or minimum oxyhaemoglobin saturation during sleep (OR 0.99; 95% CI 0.90–1.09; p=0.86). However, 2 other measures of SDB, habitual snoring and the MAPI apnoea-symptom score, were significantly associated with high GCT in bivariate analyses (p≤0.05, Table 2). Other variables associated with high GCT at p<0.2 in bivariate analyses included neck circumference, steady night-shift work or the combination of steady and rotating night-shift work. Age, race, parity, education level, pre-pregnancy BMI, first-trimester BMI as well as arousal index did not show significant associations with high GCT (Table 2). PSG was also repeated in the third trimester in a smaller sample (n=83). The third trimester AHI was not significantly associated with high GCT (≥135 mg/dl) (OR 0.99; 95% CI 0.86–1.15; p=0.92).
Table 2.
Bivariate Associations between Sleep Variables, Anthropometric Variables and Hyperglycemia
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Maternal age, years | 1.04 | 0.96 – 1.13 | 0.34 |
| Parity | 0.85 | 0.24 – 2.99 | 0.80 |
| Race | 0.37 | 0.04 – 3.02 | 0.35 |
| Education | 1.54 | 0.38 – 6.18 | 0.55 |
| Pre-pregnancy BMI, kg/m2 | 1.03 | 0.94 – 1.12 | 0.58 |
| BMI, kg/m2 | 1.05 | 0.97 – 1.14 | 0.27 |
| Neck circumference, cm | 1.15 | 0.42 – 1.42 | 0.18 |
| Habitual snoring – MAPI | 8.13 | 2.02 – 32.69 | 0.003 |
| Apnoea-symptom score – MAPI | 2.85 | 1.50 – 5.41 | 0.001 |
| Self-reported nocturnal sleep duration, hr | 0.95 | 0.70 – 1.27 | 0.71 |
| Self-reported total nap duration, hr | 1.48 | 0.96 – 2.28 | 0.08 |
| Sleep duration – PSQI | 1.37 | 0.79 – 2.38 | 0.27 |
| Global PSQI score | 1.02 | 0.86 – 1.22 | 0.81 |
| Steady night-shift | 5.44 | 1.14 – 25.97 | 0.03 |
| Steady or rotating night-shift | 3.07 | 0.70 – 13.51 | 0.14 |
| 1st trimester PSG (n=104) | |||
| AHI/hr- PSG in 1st trimester, n=104 | 1.03 | 0.83 – 1.28 | 0.77 |
| Total sleep time, hr | 1.04 | 0.53 – 2.04 | 0.91 |
| Total arousal Index/hr | 0.96 | 0.88 – 1.05 | 0.33 |
| Sleep Efficiency | 0.98 | 0.93 – 1.03 | 0.38 |
| 3rd trimester PSG (n=83) | |||
| AHI/hr- PSG in 3rd trimester, | 0.99 | 0.86 – 1.15 | 0.92 |
| Total sleep time, hr | 0.84 | 0.46 – 1.53 | 0.57 |
| Sleep Efficiency | 0.99 | 0.94 – 1.04 | 0.76 |
Definition of abbreviations: BMI =body mass index; MAPI=Multivariable Apnoea Prediction Index; AHI=Apnoea Hypopnoea Index; PSG=polysomnography; PSQI=Pittsburgh Sleep Quality Index; OR=odd ratio; CI=confidence interval
For the multivariable model, we evaluated only subjective SDB symptoms, i.e. apnoea-symptom scores and habitual snoring (at least 3 days/week), since AHI was not associated with increased GCT in bivariate analysis. The final model (Model 1) for SDB included the apnoea-symptom score, age, race/ethnicity, neck circumference and night-shift work (Table 3). The apnoea-symptom score was used because it provided a more accurate estimate of GCT (OR=2.85; 95% CI, 1.50–5.41) and explained a greater proportion of variance than snoring (OR=8.13; 95% CI, 2.02–32.69).
Table 3.
Final multiple logistic regression for sleep-disordered breathing
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Apnoea-symptom score subscale – MAPI | 3.47 | 1.44 – 8.32 | 0.005 |
| Maternal age, years | 0.98 | 0.88 – 1.09 | 0.69 |
| Race (Caucasian reference group) | 1.40 | 0.12 – 16.27 | 0.79 |
| Neck circumference, cm | 1.03 | 0.79 – 1.32 | 0.85 |
| Steady & rotating night-shift | 4.72 | 0.83 – 26.77 | 0.08 |
Definition of abbreviations: OR=odd ratio; CI=confidence interval; MAPI=Multivariable Apnoea Prediction Index
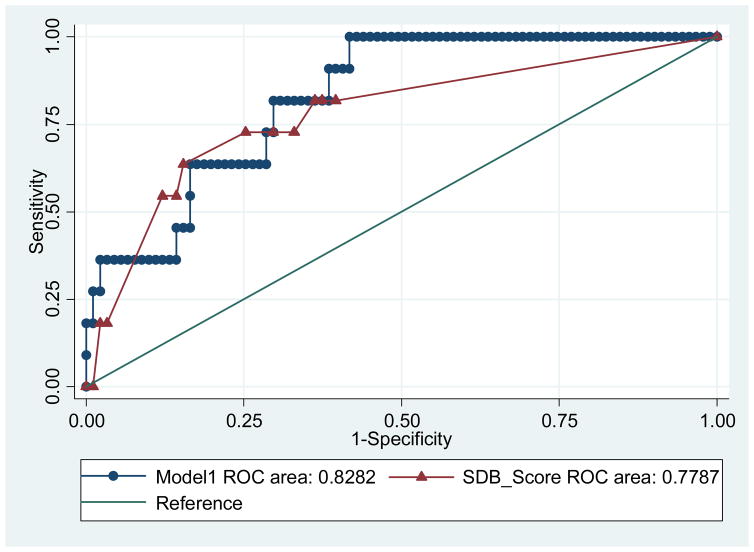
The AUC for MAPI apnoea-symptom score was not significantly different from the AUC of the model 1 (p=0.33), but the ROC area was slightly better in the model. We conclude that the model predicts hyperglycemia better than apnoea-symptom score alone (figure 1).
Figure 1.
Receiver operating characteristic (ROC) curves of Model 1.
Relationship between sleep duration, nap duration and GCT
In bivariate analyses, neither objective total sleep time nor subjective sleep duration were significantly associated with high GCT values (Table 2). Furthermore, other sleep variables including sleep stage amounts (either REM or non-REM) and sleep efficiency did not show significant associations with high GCT (Table 2). Therefore, a multivariable model for sleep duration was not generated. However, the relationship between nap duration and GCT value was of borderline significance in the bivariate analysis. Therefore, we created a multivariable model investigating the relationship between nap duration and GCT values (Model 2). We adjusted for age, race/ethnicity and neck circumference (Table 4). In this adjusted model, we found that longer total nap duration in early pregnancy was significantly associated with high GCT values (Table 4).
Table 4.
Final Multiple logistic regression Model for nap duration
| Variables | OR | 95% CI | p value |
|---|---|---|---|
| Total nap duration, hr | 1.64 | 1.00 – 2.68 | 0.05 |
| Maternal age, years | 1.08 | 0.98 – 1.18 | 0.12 |
| Race (Caucasian reference group) | 0.70 | 0.07 – 6.83 | 0.76 |
| Neck circumference, cm | 1.14 | 0.91 – 1.42 | 0.27 |
For abbreviations, please see Table 3
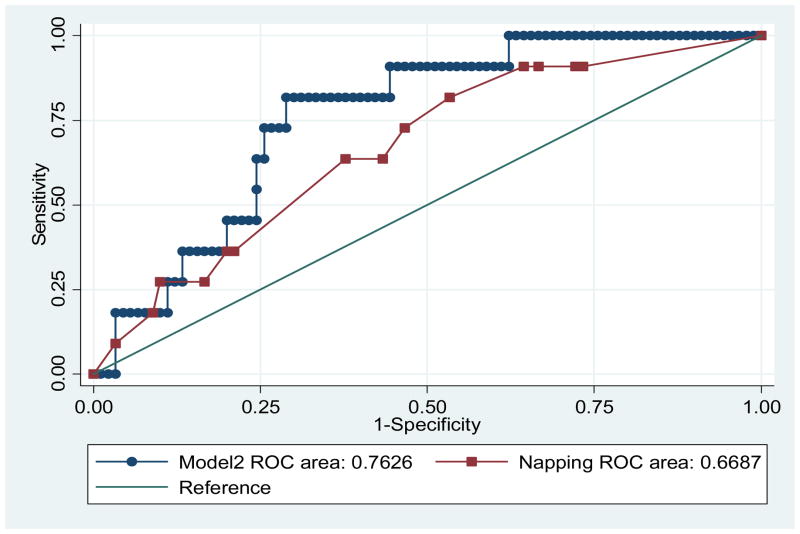
The AUC for napping is not significantly different from the AUC of model 2 (p=0.24), but the ROC area is relatively higher in the model (Figure 2). Thus, the model predicts hyperglycemia better than napping alone.
Figure 2.
Receiver operating characteristic (ROC) curves of Model 2.
Discussion
The results of this study showed that symptoms of SDB in early pregnancy were associated with hyperglycaemia as measured by the 1-hour 50-gram oral glucose challenge test, independent of age, race/ethnicity, neck circumference and night-shift work. Symptoms of SDB include snoring, gasping, choking, difficulty breathing and apnoeic events during sleep. However, AHI in early or late pregnancy was not significantly associated with maternal hyperglycaemia. Although women with hyperglycaemia had higher AHI than those with normoglycaemia, this was not statistically significant. Both hyperglycaemic and normoglycaemic groups had low frequencies of respiratory events during sleep. While self-reported total nap duration was significantly associated with high GCT in final multivariate models, neither objective nor subjective sleep duration were related to hyperglycaemia.
Our study suggests that SDB symptoms in early pregnancy are a risk factor for hyperglycaemia, in addition to well-known risk factors like obesity and family history of diabetes. This is consistent with the findings of prior studies. A large cohort study of 1290 pregnant women reported that snoring in early pregnancy was associated with a 1.86-fold increased risk of GDM (RR = 1.86; 95% CI 0.88–3.94) [25]. Similarly, a cross-sectional survey of 1000 pregnant women has shown a independent association of symptoms of SDB with GDM (2.1, 95% CI 1.3–3.4) [18]. A prospective cohort study of nulliparous women (n=189) also found a higher incidence of GDM in habitual snorers [17]. Another large population-based study of 759 Chinese women diagnosed with OSA using PSG reported that OSA is associated with GDM (OR 1.6, 95% CI 1.07–2.8) after adjusting for mother and infant characteristics [36]. Our results are also compatible with several non-pregnant studies on the association between self-reported symptoms of SDB, glucose tolerance, and insulin resistance [2,9,8]. These studies, using self-reported snoring and witnessed-apnoeas as markers for SDB, revealed a significant association between habitual snoring, elevated fasting-insulin levels and glucose intolerance [9,8,2]. However, a recent study investigating the impact of chronic vs pregnancy-onset habitual snoring on gestational diabetes did not find an association between snoring and gestational diabetes [24].
Intermittent hypoxia, arousals from sleep, and decreased slow wave sleep that occur in SDB may promote a cascade of mechanisms, including increased oxidative stress, sympathetic overactivity, elevations of pro-inflammatory response, alterations in adipokine (leptin and adiponectin) profiles and dysregulation of the hypothalamic-pituitary-adrenal axis [37–41]. These are involved in the pathophysiologic relationship between SDB and glucose dysregulation in non-pregnant population. It is probable that similar mechanisms have potential roles in the development of insulin resistance and glucose impairment during pregnancy, which may result in gestational diabetes mellitus [39].
Self-reported SDB symptoms, which were reported commonly in our study even in the absence of frank apneas and hypopneas, are likely a sign of inspiratory flow limitation. Pregnancy studies using objective measures reported that most women have subtle non-apnoeic respiratory events in the form of inspiratory flow limitation rather than having apnoeas [42,43]. Studies of women with severe pre-eclampsia also show that even pre-eclamptic women with massive oedema causing upper airway narrowing did not have AHI>10 [43,42]. Instead they had repetitive episodes of prolonged flow limitation without obvious apnoea or oxygen desaturations. Each episode was, however, associated with a surge in blood pressure [43,42]. Flow limitation is proposed as a risk factor for pre-eclampsia [44]. This is supported by studies showing that short-term relief of flow limitation by continuous positive airway pressure (CPAP) in pre-eclamptic women resulted in significant reductions in blood pressure during sleep [43–45]. It is possible, therefore, that flow limitation and snoring without obvious apnoea may also have a negative impact on glucose metabolism.
Unlike studies in non-pregnant subjects, our study did not find any association between AHI and hyperglycaemia. In studies demonstrating an independent association between SDB assessed by PSG and impaired glucose metabolism and insulin resistance, the study populations were mostly obese men and older than our subjects [5,46,38]. They had more severe SDB with higher AHIs than our subjects, which may explain why we did not observe a significant association between AHI and hyperglycaemia. However, two case-control studies of non-pregnant populations did not find an independent association between AHI and insulin resistance [9]. Another study reported that fasting-insulin levels of patients with SDB were not significantly different from those of matched-controls for age, BMI, smoking and alcohol use [9]. The absence of association in these studies may be related to small sample sizes (15–40 subjects with SDB compared with 15–41 control subjects) as is the case with our study.
Although women with hyperglycaemia had a higher BMI than those with normoglycaemia, we found no significant association between BMI in the first trimester and GCT values, and between pre-pregnancy BMI and GCT values. This is in contrast to other studies which found association between BMI and hyperglycaemia [27–30,33]. It is surprising that there was not a bivariate correlation between BMIs and GCT, as one would expect that more obese women would have higher glucose levels since obesity is in itself a major risk factor for diabetes and an important etiologic factor for SDB. Our findings are similar to a prospective cohort study of 2000 women that examined the relationship between gestational weight gain and glucose intolerance [30]. The authors did not observe an association between weight gain and frank GDM. Similarly, another prospective observational study designed to screen for OSA and describe outcomes of OSA among obese pregnant women did not find an association between OSA and increased prevalence of gestational diabetes [47]. However, the lack of association in our study is likely related to sample size and the fact that the majority of women included were overweight or obese, limiting the range of BMI.
We also observed a modest association between high GCT and longer self-reported nap duration in early pregnancy. Our study is the first to report an association between napping during pregnancy and hyperglycaemia. Our findings are compatible with results from recent studies of older adults, among whom daytime napping was associated with a higher risk of diabetes [48,49]. In our study, this association with napping cannot be explained by short nocturnal sleep duration, apnoea or obesity, as nap duration remained significant when we controlled for neck circumference, apnoea symptoms or severity, and objective/subjective sleep duration. Nap duration did not display collinearity with nocturnal sleep duration, sleep quality or SDB measures. Thus, nap duration conveyed different information. Hypothetically, disturbed sleep/wake cycles due to napping/sleep at unusual times may cause hormones that regulate glucose metabolism and appetite to fluctuate excessively, disrupting the sympathetic-parasympathetic balance [48,6]. This could conceivably impair glucose tolerance. Long naps also increase the duration of physical inactivity, which is associated with an increased risk for developing GDM [31]. To elucidate whether this association with napping has clinical implication for the treatment and prevention of GDM, further studies are required.
We did not observe an association between objective or subjective sleep duration and hyperglycaemia. However, objective sleep duration measured by PSG is not a good measure of habitual sleep duration which includes data only from a single night. Regarding subjective sleep duration, our findings differ from two pregnancy studies with larger sample sizes (n=189–1290), which reported short sleep duration is associated with increased risk for GDM [17,25]. Epidemiological data suggest that short sleep duration or chronic partial sleep deprivation may impair glucose metabolism [17,32]. However epidemiologic data regarding the role of gender is conflicting. A large cohort study of 70,000 nurses revealed that in an age-adjusted model both self-reported short (≤ 5 hours) and long duration of sleep (≥9 hours) increased the risk of incident diabetes diagnosis, but the significance for short sleep was lost when known risk factors such as BMI were included in multivariable models [3]. In a longitudinal study with more than 2663 subjects followed for 12 years, short duration of sleep (≤5 hours) and difficulty initiating and maintaining sleep were associated with higher incidence of diabetes in only men but not in women, after adjusting for confounding factors [50]. Further studies with large sample sizes are needed to clarify the conflict regarding the association of sleep duration with diabetes in women, particularly in pregnant women.
Our subjects were predominantly African American. Thus, we used 135 mg/dl as a threshold. This goal was found to be 140 mg/dl for white women. Previous pregnancy studies, predominantly recruited white women and used different criteria. Bourjeily et al. found that snoring and gasping were associated with gestational diabetes [18]. Similarly, Qui et al showed associations of snoring and short sleep duration with glucose intolerance and GDM [25]. In both studies, screening and diagnostic criteria for gestational diabetes was based on the American Diabetes Association criteria (50-g load of GCT≥140 mg/dl and at least two of the following plasma glucose values must be found: 100 OGTT, fasting: ≥95 mg/dl, 1 h: ≥180 mg/dl, 2 h: ≥155 mg/dl and 3 h: ≥140 mg/dl). Facco et al used a hyperglycemic threshold of 130 mg/dl and reported that short sleepers (< 7 hrs during pregnancy) were 2.6 times more likely to have hyperglycemia (95% CIs, 1.3–5.7) [17], but did not find significant differences between snorers and non-snorers.
There are several limitations in our study that warrant mention. Because napping-time was self-reported, misclassification bias may occur, leading to either attenuation or amplification of any true association between napping and hyperglycaemia. Additionally given the small sample size, this finding may be due to chance. The small number of subjects with multiple pregnancy (n=6) limited us from including this as a confounding factor in our multivariable models. The relatively small sample size with abnormal GCT has made the risk estimates less stable (especially around shift-work), as reflected by the large confidence intervals around the point estimates.
Our initial assessment of subjective and objective SDB and sleep duration was at 12±2.1 (SD) weeks of pregnancy. While GCT was assessed later at 24.0±6.5 weeks of pregnancy, it is conceivable that the women studied would have had higher AHI, more sleep disturbances and shorter sleep duration at the time of GCT assessment. Thus, the assessment of sleep parameters in early pregnancy may falsely attenuate any true association with GCT due to underestimation of AHI≥5, sleep disturbances and shortened sleep duration in later pregnancy. However, there was also no association between GCT and AHI (p=0.92), sleep efficiency (p=0.76) and nocturnal sleep duration (p=0.57) in the third trimester. Finally, as a large proportion of our subjects were African-American and single, our results may not be generalisable to other populations.
Conclusions
We found that symptoms of SDB (but not AHI) and self-reported longer nap duration in early gestation are independently associated with high GCT values during pregnancy. The clinical relevance of elevated GCT is supported by reports of increased adverse outcomes in mothers and infants born to women with hyperglycaemia or with GDM [27–30,33]. Future research is needed to determine what aspect of SDB confers risk for hyperglycaemia and whether treatment of SDB in early pregnancy may reduce the incidence of glucose intolerance during pregnancy.
Acknowledgments
This study was supported by grants from the National Institutes of Health (T32 HL07713 and 5K23HD041465).
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
References
- 1.Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, Nieto FJ. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165 (8):863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 2.Elmasry A, Janson C, Lindberg E, Gislason T, Tageldin MA, Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. J Int Med. 2000;248 (1):13–20. doi: 10.1046/j.1365-2796.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 3.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, Patel S, Hu FB. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26 (2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 4.Al-Delaimy WK, Manson JE, Willett WC, Stampfer MJ, Hu FB. Snoring as a risk factor for type II diabetes mellitus: a prospective study. Am J Epidemiol. 2002;155 (5):387–393. doi: 10.1093/aje/155.5.387. [DOI] [PubMed] [Google Scholar]
- 5.Botros N, Concato J, Mohsenin V, Selim B, Doctor K, Yaggi HK. Obstructive sleep apnea as a risk factor for type 2 diabetes. Am J Med. 2009;122 (12):1122–1127. doi: 10.1016/j.amjmed.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punjabi NM, Shahar E, Redline S, Gottlieb DJ, Givelber R, Resnick HE Sleep Heart Health Study I. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol. 2004;160 (6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 7.Tasali E, Van Cauter E, Hoffman L, Ehrmann DA. Impact of obstructive sleep apnea on insulin resistance and glucose tolerance in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93 (10):3878–3884. doi: 10.1210/jc.2008-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valham F, Stegmayr B, Eriksson M, Hagg E, Lindberg E, Franklin KA. Snoring and witnessed sleep apnea is related to diabetes mellitus in women. Sleep Med. 2009;10 (1):112–117. doi: 10.1016/j.sleep.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133 (2):496–506. doi: 10.1378/chest.07-0828. [DOI] [PubMed] [Google Scholar]
- 10.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005;28 (10):1299–1305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 11.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163 (3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 12.Okun ML, Hall M, Coussons-Read ME. Sleep disturbances increase interleukin-6 production during pregnancy: implications for pregnancy complications. Reprod Sci. 2007;14 (6):560–567. doi: 10.1177/1933719107307647. [DOI] [PubMed] [Google Scholar]
- 13.Izci-Balserak B. Sleep-Disordered Breathing in Pregnancy. Int J Sleep Wakefulness The International Journal of Sleep and Wakefulness. 2008;1 (3):98–108. [Google Scholar]
- 14.Lee KA, Zaffke ME, McEnany G. Parity and sleep patterns during and after pregnancy. Obstet Gynecol. 2000;95 (1):14–18. doi: 10.1016/s0029-7844(99)00486-x. [DOI] [PubMed] [Google Scholar]
- 15.Izci B, Vennelle M, Liston WA, Dundas KC, Calder AA, Douglas NJ. Sleep-disordered breathing and upper airway size in pregnancy and post-partum. Eur Respir J. 2006;27 (2):321–327. doi: 10.1183/09031936.06.00148204. [DOI] [PubMed] [Google Scholar]
- 16.Izci-Balserak B, Lee KA. Sleep Disturbances and Sleep-Related Disorders in Pregnancy. In: Kryger M, Roth T, Dement W, editors. Principles and Practice of Sleep Medicine. Vol. 5. Saunders; Philadelphia: 2010. pp. 1572–1586. [Google Scholar]
- 17.Facco FL, Grobman WA, Kramer J, Ho KH, Zee PC. Self-reported short sleep duration and frequent snoring in pregnancy: impact on glucose metabolism. Am J Obstet Gynecol. 2010;203(2):142, e141–145. doi: 10.1016/j.ajog.2010.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. 2010;36 (4):849–855. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 19.Cai XH, Xie YP, Li XC, Qu WL, Li T, Wang HX, Lv JQ, Wang LX. The prevalence and associated risk factors of sleep disorder-related symptoms in pregnant women in China. Sleep Breath. 2012 doi: 10.1007/s11325-012-0783-2. [DOI] [PubMed] [Google Scholar]
- 20.Frederick IO, Qiu C, Sorensen TK, Enquobahrie DA, Williams MA. The prevalence and correlates of habitual snoring during pregnancy. Sleep Breath. 2012 doi: 10.1007/s11325-012-0717-z. [DOI] [PubMed] [Google Scholar]
- 21.Williams MA, Miller RS, Qiu C, Cripe SM, Gelaye B, Enquobahrie D. Associations of early pregnancy sleep duration with trimester-specific blood pressures and hypertensive disorders in pregnancy. Sleep. 2010;33 (10):1363–1371. doi: 10.1093/sleep/33.10.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izci B, Riha RL, Martin SE, Vennelle M, Liston WA, Dundas KC, Calder AA, Douglas NJ. The upper airway in pregnancy and pre-eclampsia. Am J Respir Crit Care Med. 2003;167 (2):137–140. doi: 10.1164/rccm.200206-590OC. [DOI] [PubMed] [Google Scholar]
- 23.Sahin FK, Koken G, Cosar E, Saylan F, Fidan F, Yilmazer M, Unlu M. Obstructive sleep apnea in pregnancy and fetal outcome. Int J Obstet Gynecol. 2008;100 (2):141–146. doi: 10.1016/j.ijgo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien LM, Bullough AS, Owusu JT, Tremblay KA, Brincat CA, Chames MC, Kalbfleisch JD, Chervin RD. Pregnancy-onset habitual snoring, gestational hypertension, and preeclampsia: prospective cohort study. Am J Obstet Gynecol. 2012;207(6):487, e481–489. doi: 10.1016/j.ajog.2012.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu C, Enquobahrie D, Frederick IO, Abetew D, Williams MA. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: a pilot study. BMC women’s health. 2010;10:17. doi: 10.1186/1472-6874-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Champagne K, Schwartzman K, Opatrny L, Barriga P, Morin L, Mallozzi A, Benjamin A, Kimoff RJ. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009;33 (3):559–565. doi: 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 27.Moore RT. Diabetes in Pregnancy. In: Creasy RK, Resnik R, Lams JD, editors. Maternal-fetal medicine: principles and practice. 5. W. B. Saunders; Philadelphia, PA; [London]: 2004. pp. 1023–1062. [Google Scholar]
- 28.Group HSCR, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, Oats JJ, Persson B, Rogers MS, Sacks DA. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358 (19):1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 29.Simeoni U, Barker DJ. Offspring of diabetic pregnancy: long-term outcomes. Semin Fetal Neonatal Med. 2009;14 (2):119–124. doi: 10.1016/j.siny.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Herring SJ, Oken E, Rifas-Shiman SL, Rich-Edwards JW, Stuebe AM, Kleinman KP, Gillman MW. Weight gain in pregnancy and risk of maternal hyperglycemia. Am J Obstet Gynecol. 2009;201(1):61.e61–61.e67. doi: 10.1016/j.ajog.2009.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, Hod M, Kitzmiller JL, Kjos SL, Oats JN, Pettitt DJ, Sacks DA, Zoupas C. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. 2007;30(Suppl 2):S251–260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 32.Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Progress in cardiovascular diseases. 2009;51 (5):381–391. doi: 10.1016/j.pcad.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Codario RA. Current clinical practice. Humana Press; Totowa, N.J: 2005. Type 2 diabetes, pre-diabetes, and the metabolic syndrome: the primary care guide to diagnosis and management. [Google Scholar]
- 34.Maislin G, Pack AI, Kribbs NB, Smith PL, Schwartz AR, Kline LR, Schwab RJ, Dinges DF. A survey screen for prediction of apnea. Sleep. 1995;18 (3):158–166. doi: 10.1093/sleep/18.3.158. [DOI] [PubMed] [Google Scholar]
- 35.Jomeen J, Martin CR. Assessment and relationship of sleep quality to depression in early pregnancy. Journal of psychosomatic research. 2007;25 :87–99. [Google Scholar]
- 36.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2012;206(2):136 e131–135. doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Micheli K, Komninos I, Bagkeris E, Roumeliotaki T, Koutis A, Kogevinas M, Chatzi L. Sleep patterns in late pregnancy and risk of preterm birth and fetal growth restriction. Epidemiology. 2011;22 (5):738–744. doi: 10.1097/EDE.0b013e31822546fd. [DOI] [PubMed] [Google Scholar]
- 38.Aronsohn RS, Whitmore H, Van Cauter E, Tasali E. Impact of untreated obstructive sleep apnea on glucose control in type 2 diabetes. Am J Respir Crit Care Med. 2010;181 (5):507–513. doi: 10.1164/rccm.200909-1423OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Izci-Balserak B, Pien GW. Sleep-disordered breathing and pregnancy: potential mechanisms and evidence for maternal and fetal morbidity. Curr Opin Pulm Med. 2010;16 (6):574–582. doi: 10.1097/MCP.0b013e32833f0d55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tasali E, Leproult R, Spiegel K. Reduced sleep duration or quality: relationships with insulin resistance and type 2 diabetes. Prog Cardiovasc Dis. 2009;51 (5):381–391. doi: 10.1016/j.pcad.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 41.Punjabi NM, Beamer BA. Alterations in Glucose Disposal in Sleep-disordered Breathing. Am J Respir Crit Care Med. 2009;179 (3):235–240. doi: 10.1164/rccm.200809-1392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connolly G, Razak AR, Hayanga A, Russell A, McKenna P, McNicholas WT. Inspiratory flow limitation during sleep in pre-eclampsia: comparison with normal pregnant and nonpregnant women. Eur Respir J. 2001;18 (4):672–676. doi: 10.1183/09031936.01.00053501. [DOI] [PubMed] [Google Scholar]
- 43.Edwards N, Blyton DM, Kirjavainen T, Kesby GJ, Sullivan CE. Nasal continuous positive airway pressure reduces sleep-induced blood pressure increments in preeclampsia. Am J Respir Crit Care Med. 2000;162 (1):252–257. doi: 10.1164/ajrccm.162.1.9905006. [DOI] [PubMed] [Google Scholar]
- 44.Guilleminault C, Palombini L, Poyares D, Takaoka S, Huynh NTL, El-Sayed Y. Pre-eclampsia and nasal CPAP: part 1. Early intervention with nasal CPAP in pregnant women with risk-factors for pre-eclampsia: preliminary findings. Sleep Med. 2007;9 (1):9–14. doi: 10.1016/j.sleep.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 45.Poyares D, Guilleminault C, Hachul H, Fujita L, Takaoka S, Tufik S, Sass N. Pre-eclampsia and nasal CPAP: part 2. Hypertension during pregnancy, chronic snoring, and early nasal CPAP intervention. Sleep medicine. 2007;9 (1):15–21. doi: 10.1016/j.sleep.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 46.Foster GD, Sanders MH, Millman R, Zammit G, Borradaile KE, Newman AB, Wadden TA, Kelley D, Wing RR, Sunyer FX, Darcey V, Kuna ST, Sleep ARG. Obstructive sleep apnea among obese patients with type 2 diabetes. Diabetes Care. 2009;32 (6):1017–1019. doi: 10.2337/dc08-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Louis J, Auckley D, Miladinovic B, Shepherd A, Mencin P, Kumar D, Mercer B, Redline S. Perinatal Outcomes Associated With Obstructive Sleep Apnea in Obese Pregnant Women. Obstet Gynecol. 2012;120:1–9. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lam KH, Jiang CQ, Thomas GN, Lao XQ, McGhee SM, Zhang WS, Schooling CM, Adab P, Cheng KK, Arora T, Taheri S, Lam TH. Napping Is Associated with Increased Risk of Type 2 Diabetes: The Guangzhou Biobank Cohort Study. Sleep. 2010;33 (3):402–407. doi: 10.1093/sleep/33.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu Q, Song Y, Hollenbeck A, Blair A, Schatzkin A, Chen H. Day napping and short night sleeping are associated with higher risk of diabetes in older adults. Diabetes Care. 2010;33 (1):78–83. doi: 10.2337/dc09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005;28 (11):2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]