Abstract
In the retina, somatostatin influences neuronal activity likely by acting at one or more somatostatin subtype (sst) receptors. Somatostatin and somatostatin-binding sites are distributed predominantly to the inner retina. The present study has investigated the cellular expression of one of the sst receptors, the sst2A receptor isoform, in the rabbit retina. These studies have used a new polyclonal antibody directed to the predicted C-terminus of mouse sst2A(361–369) receptor. Antibody specificity was tested by preadsorption of the primary antibody with a peptide corresponding to sst2A(361–369). sst2A Receptor immunoreactivity was localized mainly to the plasma membrane of rod bipolar cells and to sparsely occurring, wide-field amacrine cells. Immunostaining in rod bipolar cells was strongest in the axon and axon terminals in lamina 5 of the inner plexiform layer (IPL) and was weakest in the cell body and dendrites. Double-labeling experiments using a monoclonal antibody against protein kinase C (PKC; α and β), a rod bipolar cell-selective marker, showed complete colocalization. In horizontal sections of retina, immunostained bipolar cell bodies had a dense distribution, which is in agreement with the reported distribution of rod bipolar cell bodies. Immunoreactive amacrine cell bodies were located at the border of the inner nuclear layer and the IPL, and thin varicose processes ramified mainly in laminae 2 and 4 of the IPL. These observations indicate that somatostatin influences visual information processing in the retina 1) by acting presynaptically on rod bipolar cell axon terminals and b) by influencing the activity of sparsely occurring amacrine cells.
Indexing terms: rod bipolar cells, amacrine cells, immunohistochemistry, neuropeptides, visual system
Somatostatin (or somatotropin-release inhibiting factor; SRIF) a tetradecapeptide that was first isolated from the ovine hypothalamus, is widely distributed throughout the nervous system and peripheral tissues (Brazeau et al., 1973; Epelbaum, 1986). SRIF has a wide variety of biological functions, including the inhibition of endocrine and exocrine secretions and the modulation of transmitter release. SRIF is reported to alter locomotor and behavioral activity and to influence cognitive functions (Epelbaum, 1986; Haroutunian et al., 1987).
Different experimental approaches indicate that SRIF acts as a transmitter or modulator in the retina. SRIF has been detected in a variety of mammalian retinas, including rat, rabbit, cat, and human, and it has been observed in a number of cell types, including amacrine, interplexiform, and ganglion cells (Sagar and Marshall, 1988; Larsen et al., 1990; White et al., 1990; Rickman et al., 1996). Specific high-affinity SRIF receptor-binding sites have been detected in mammalian retinas, and they have a homogeneous distribution across the inner plexiform layer (IPL; Kossut et al., 1989; Liapakis and Thermos, 1992; Liapakis et al., 1993; Vasilaki et al., 1996). In rabbit retina, SRIF-immunoreactive cells have a broad, asymmetric distribution to the ventral retina and extensive arborization of their cellular processes mainly to laminae 1 and 5 in the IPL in all regions of the retina (Sagar, 1987; Rickman et al., 1996). Pharmacological and electrophysiological studies with an eye cup preparation have shown that SRIF alters the signal-to-noise discharge pattern and the center-surround balance of ganglion cells in the rabbit. Interestingly, these studies also showed that SRIF causes a slow hyperpolarization in rod bipolar cells (Zalutsky and Miller, 1990). In addition, an alteration of SRIF receptor-binding properties has been implicated in a night-blind phenotype and abnormal optokinetic nystagmus in the pearl mutant mouse retina (Balkema et al., 1981; Kossut et al., 1990). Together, these findings suggest that SRIF influences visual information processing in the retina.
Physiological effects of SRIF are diverse. This peptide blocks adenylyl cyclase activity, stimulates tyrosine phosphatase activity, and influences both K+and Ca2+ currents (Ikeda and Schofield, 1989; White et al., 1991; Reisine and Bell, 1995). These cellular actions are mediated through seven transmembrane receptors coupled to guanine nucleotide binding proteins. Five distinct somatostatin subtype (sst) receptor genes have been cloned and are designated sst1 through sst5 (Bruno et al., 1992; Kluxen et al., 1992; Li et al., 1992; Meyerhof et al., 1992; O’Carroll et al., 1992; Yamada et al., 1992; Yasuda et al., 1992; Hoyer et al., 1995). These receptor genes do not have introns in the coding segments, with the exception of sst2. The sst2A receptor has two isoforms, sst2A and sst2B, which occur by alternative splicing of the sst2A mRNA (Vanetti et al., 1992). Rodent sst2A and sst2B receptors differ in their length and have unique, predicted C-termini.
The distribution of sst2A receptor immunoreactivity in rabbit retina was determined by using a newly developed polyclonal antibody directed against the C-terminus of mouse sst2A(361–369) receptor (Sternini et al., 1997). A preliminary report of the localization of sst2A receptor immunoreactivity to rod bipolar cells and to sparsely occurring amacrine cells has been published in abstract form (Johnson et al., 1996).
MATERIALS AND METHODS
Tissue preparation
Adult New Zealand albino rabbits were used for this study. They were fed and housed under regular conditions with a 12 hour light-dark schedule. Care and handling of animals were approved by the Animal Research Committee of the VAMC–West Los Angeles in accordance with NIH guidelines.
Rabbits were deeply anesthetized with a mixture of ketamine hydrochloride (35–70 mg/kg, i.m.) and xylazine (5–10 mg/kg, i.m.) followed by sodium pentobarbital (5 ml of 50 mg/ml, i.v.). They were perfused through the heart with 0.1 M phosphate buffered-saline (PBS), pH 7.4. In some cases, the PBS was followed by 2% or 4% paraformaldehyde (PFA) or by 2% or 4% PFA with lysine and periodate (PLP) in 0.1 M phosphate buffer (PB), pH 7.4. The eyes were removed, the anterior segment was dissected, and the posterior eye cup containing the retina was immediately immersed in PFA or PLP in 0.1 M PB. The eye cup was fixed in PFA or PLP in 0.1 M PB from 30 minutes to 2 hours at room temperature, and, in some cases, the retina was subsequently isolated from the eye cup. The eye cup or isolated retina was then stored overnight in 25% sucrose in 0.1 M PB at 4°C. Sections of the retina were cut perpendicular or parallel to the vitreal surface with a cryostat or a sliding microtome. Cryostat sections were cut at 12–16 μm, mounted onto gelatin-coated slides, air dried, and stored at −20°C. Sliding microtome sections were cut at 25–30 μm and were stored free-floating in 0.1 M PB.
Antibodies
A specific polyclonal antibody (no. 9431) directed against the C-terminus of mouse sst2A(361–369) receptor was used. In most experiments, an affinity-purified antibody was used. Specificity of immunostaining was evaluated by preadsorbing the antibody with 10−5 M of the synthetic peptide, sst2A(361–369) receptor. sst2A Receptor immunoreactivity was not present in sections incubated in antibody that was previously preadsorbed with the synthetic peptide. This antibody has been characterized extensively with Western blots and with immunostaining of human embryonic kidney-293 cells transfected with mouse sst2A receptor cDNA (Sternini et al., 1997).
A mouse monoclonal antibody (clone MC5; RPN.536; Amersham, Arlington Heights, IL) directed against the α and β isoforms of protein kinase C (PKC) was used to confirm the identity of rod bipolar cells (Negishi et al., 1988; Wood et al., 1988; Greferath et al., 1990; Vaney et al., 1991; Wässle et al., 1991; Young and Vaney, 1991). A mouse monoclonal antibody directed against tyrosine hydroxylase (TH) was used to identify TH-immunoreactive amacrine cells (Casini and Brecha, 1992).
Immunohistochemistry
Cryostat and free-floating sections were washed in 0.1 M PB and then incubated for 12–36 hours in primary antibody containing 10% normal goat serum and 0.5% Triton X-100 at 4°C. Sections were washed in 0.1 M PB. Sections used for immunofluorescence studies were incubated at a 1:50 or 1:100 dilution in affinity-purified fluorescein isothiocyanate (FITC)-conjugated or tetramethylrhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit or goat anti-mouse immunoglobulins (IgGs; American Qualex, La Mirada, CA, or Jackson Immunoresearch Laboratories, West Grove, PA) for 2 hours at room temperature or overnight at 4°C. Sections used for avidin-biotin peroxidase studies were incubated at a dilution of 1:100 in affinity-purified biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) for 2 hours at room temperature or at 4°C overnight, washed in 0.1 M PB, and then incubated in avidin-biotin-peroxidase complex (ABC) for 2 hours at room temperature. Sections were washed and incubated in 50–100 mg 3,3′-diaminobenzidine tetra-hydrochloride (DAB; Sigma, St. Louis, MO) in 0.1 M Tris, pH 6.5, for 5–10 minutes followed by DAB with 0.03% H2O2 for 5–10 minutes. Sections were mounted if free floating and were coverslipped with a glycerol-phosphate or carbonate buffer containing 2% potassium iodide to retard fading for immunofluorescence studies or were coverslipped with Accumount 60 (Baxter, McGaw Park, IL) for ABC studies.
Confocal microscopy
sst2A Receptor immunoreactivity was evaluated in transverse or horizontal sections of retina processed by the immunofluorescence technique. Immunoreactivity was examined with a Zeiss Axiovert (Thornwood, NY) with a Plan Neofluar ×40 1.3 na objective or a PlanApo ×100 1.4 na objective and a Zeiss laser-scanning microscope 410 with a kryptonårgon laser. Optical sections were taken with a z-axis resolution of 1 μm through the immunolabeled cells. Images were collected with a magnification zoom of 1.5×. Figures 8 and 9 were produced by scanning 35-mm Ektachrome color slides with a SprintScan 35/Plus scanner (Polaroid, Cambridge, MA). Images were processed and labeled by using Adobe Photoshop (version 3.0.5 or 4.0; Adobe Systems, Inc., Mountain View, CA).
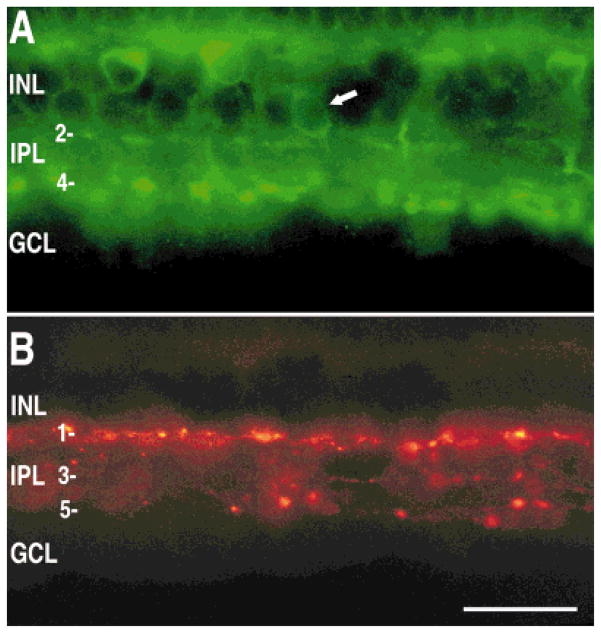
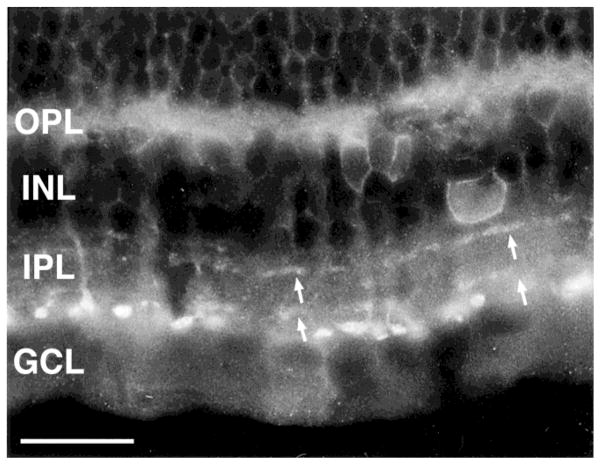
Fig. 8.
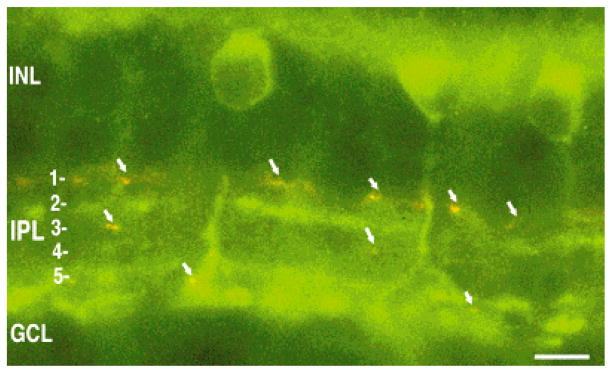
Lack of colocalization of sst2A receptor and tyrosine hydroxylase (TH) immunoreactivities. Composite image of sst2A receptor (green) and TH (red) immunoreactivities in a transverse section of retina. Arrows indicate TH-immunoreactive processes. INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 10 μm.
Fig. 9.
Localization of sst2A receptor (A) and TH (B) immunoreactivities in the same section of rabbit retina. A: The sst2A receptor containing rod bipolar cells and amacrine cells. Note the amacrine cell body (arrow) and processes in laminae 2 and 4. B: TH-immunoreactive processes were localized to laminae 1, 3, and 5 of the IPL. INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 25 μm.
In this paper, somatostatin receptor subtype 2A will be designated “sst2A,” as recommended by the IUPHAR subcommittee on somatostatin receptors (Hoyer et al., 1995). We also use the term “sst2A receptor immunoreactivity” in place of “sst2A receptor-like immunoreactivity.”
RESULTS
Specificity of the antibody
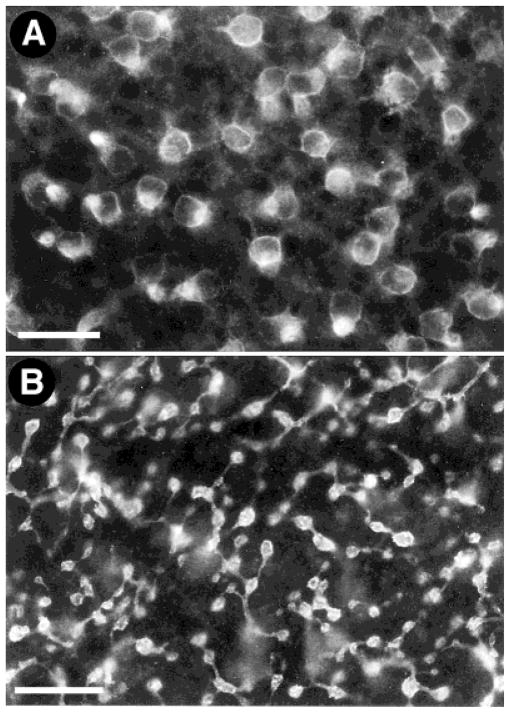
Specific immunoreactivity was eliminated completely by preadsorption of the antibody with a peptide corresponding to sst2A(361–369) receptor (Fig. 1). No differences in sst2A receptor immunoreactivity were observed in tissue that was fixed with 2–4% PFA or PLP.
Fig. 1.
Localization of somatostatin subtype 2A (sst2A) receptor immunoreactivity in the rabbit retina. A: The sst2A receptor immunostaining was prominent in rod bipolar cells. B: Lack of sst2A receptor immunostaining in a control section incubated with the sst2A receptor antibody preadsorbed with 10−5 M sst2A (361–369). OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 15 μm.
sst2A Receptor immunoreactivity in rod bipolar cells
sst2A Receptor immunoreactivity was localized mainly to cells that displayed both morphological and positional characteristics of rod bipolar cells (Figs. 1–4). Immunoreactive rod bipolar cells were distinguished from cone bipolar cells by a number of characteristics, including large axonal terminals that only arborized in lamina 5 of the IPL and cell bodies located in the outer part of the inner nuclear layer (INL; Raviola and Raviola, 1967; Famiglietti, 1981; Dacheux and Raviola, 1986; Greferath et al., 1990; Vaney et al., 1991; Wässle and Boycott, 1991; Wässle et al., 1991; Young and Vaney, 1991; Euler and Wässle, 1995).
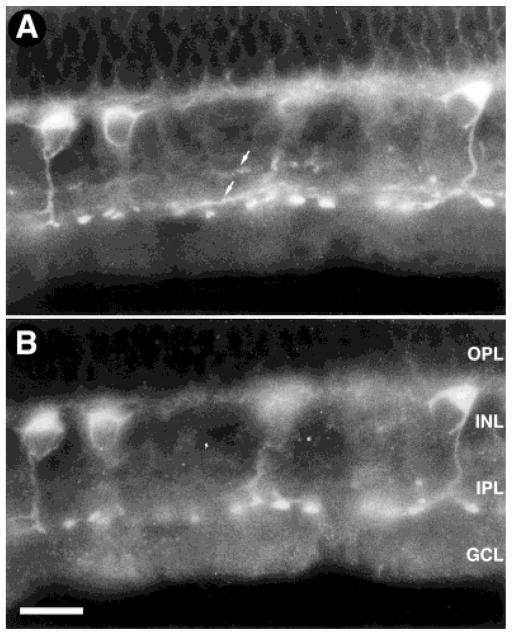
Fig. 4.
Coexpression of sst2A receptor (A) and protein kinase C (PKC; B) immunoreactivities in rod bipolar cells. The sst2A receptor showed complete coexpression with the rod bipolar, cell-selective marker PKC, further confirming that the sst2A receptor is expressed by rod bipolar cells. OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bar = 15 μm.
sst2A Receptor immunoreactivity was strongest in rod bipolar cell axons and terminals, and it was weakest in cell bodies and dendrites (Figs. 1, 2, 4). In transverse sections of retina, the cell body was usually found in the outer portion of the INL, near the outer plexiform layer (OPL; Figs. 1, 2, 4). The weakly stained dendrites formed a very fine plexus of processes in the OPL. The axon descended through the INL and the distal IPL, where it terminated in the IPL, adjacent to the ganglion cell layer (GCL), as club-or knob-like processes. The predominant localization of sst2A receptor immunoreactivity at or near the cell surface was observed in 1-μm optical sections through the cell body, axon, and axon terminal (Fig. 2).
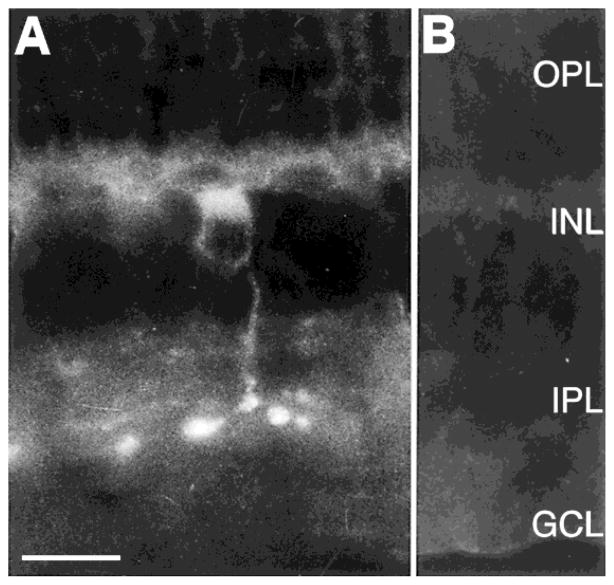
Fig. 2.
Confocal image of sst2A receptor-immunoreactive rod bipolar cell in a transverse section of retina. sst2A Receptor immunoreactivity was closely associated with the plasma membrane of rod bipolar cells. The axon descended into the inner plexiform layer (IPL), where it terminated with a few large, club-like processes in lamina 5 of the IPL. sst2A Receptor immunoreactivity was also associated with thin processes that ramified in laminae 2 and 4 of the IPL. These processes originated from sparsely occurring amacrine cells. OPL, outer plexiform layer; INL, inner nuclear layer; GCL, ganglion cell layer. Z axis = 1 μm. Scale bar = 7.5 μm.
In horizontal sections of retina, sst2A receptor-immunostained cell bodies were found to have a dense distribution, which is in agreement with the reported distribution of PKC-immunoreactive cells (Fig. 3; Vaney et al., 1991). Cell bodies measured 6 ± 0.5 μm (mean ± S.D.; n = 8) in diameter. sst2A Receptor-immunostained axon terminals in the IPL measured 2 ± 0.41 μm (mean ± S.D.; n = 13) in diameter, which is similar to the size of these terminals as reported on the basis of Golgi and intracellular labeling preparations (Dacheux and Raviola, 1986).
Fig. 3.
The sst2A receptor-immunostained cell bodies and axon terminals were found in the distal inner nuclear layer (INL) and proximal INL, respectively. Horizontal sections through the INL (A) showing sst2A receptor-immunostained cell bodies and through the inner plexiform layer (IPL; B) showing sst2A receptor-immunostained axon terminals. Scale bars = 25 μm in A, 15 μm in B.
Double-labeling experiments with PKC antibodies were used to further characterize the sst2A receptor-immunoreactive pattern. A monoclonal antibody against PKC specifically labels rod bipolar cells (Negishi et al., 1988; Wood et al., 1988; Greferath et al., 1990; Vaney et al., 1991; Wässle et al., 1991; Young and Vaney, 1991). sst2A Receptor and PKC immunoreactivities were expressed in the same bipolar cell bodies, axons, and axonal terminals (Fig. 4). Interestingly, rarely occurring PKC-immunoreactive amacrine cells (Negishi et al., 1988) did not express sst2A receptor immunoreactivity.
sst2A Receptor immunoreactivity in amacrine cells
sst2A Receptor immunostaining was also localized to infrequently occurring amacrine cell bodies located in the proximal INL (Figs. 5–9). Immunostained cell bodies were always located at the border of the INL and the IPL. In addition, there were thin, varicose processes in laminae 2 and 4 of the IPL in all retinal regions (Figs. 5, 8, 9). In transverse sections, these processes were not continuous in these laminae. In horizontal sections, the processes were wide spread and were observed at different levels in the IPL, corresponding best to laminae 2 and 4. We did not attempt to evaluate the extent of these processes to determine the overall size of individual sst2A receptor-immunoreactive amacrine cells. In horizontal sections of retina, sst2A receptor-immunoreactive amacrine cell bodies had a diameter of 19 ± 1.5 μm (mean ± S.D.; n = 3). Strong immunostaining outlined these cell bodies. The close association of sst2A receptor immunoreactivity with amacrine cell plasma membrane was further established in 1-μm optical sections by using confocal microscopy (Fig. 7).
Fig. 5.
The sst2A receptor-immunoreactive amacrine cells were sparsely occurring. Immunoreactive cell bodies were located at the border of the inner nuclear layer (INL) and the inner plexiform layer (IPL). They were likely to be multistratified and gave rise to the processes in laminae 2 and 4 of the IPL. In transverse sections, these processes in laminae 2 and 4 were not continuous. OPL, outer plexiform layer; GCL, ganglion cell layer. Scale bar = 25 μm.
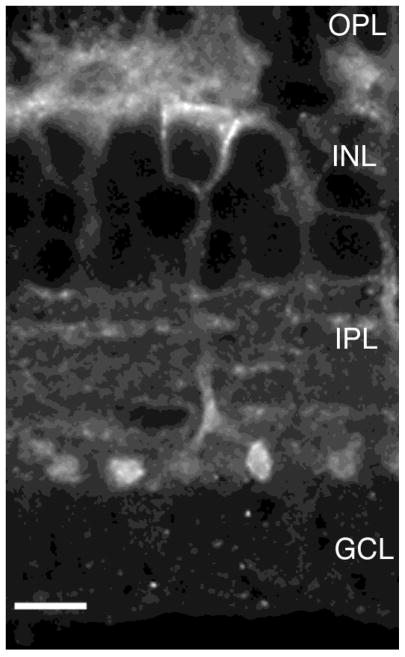
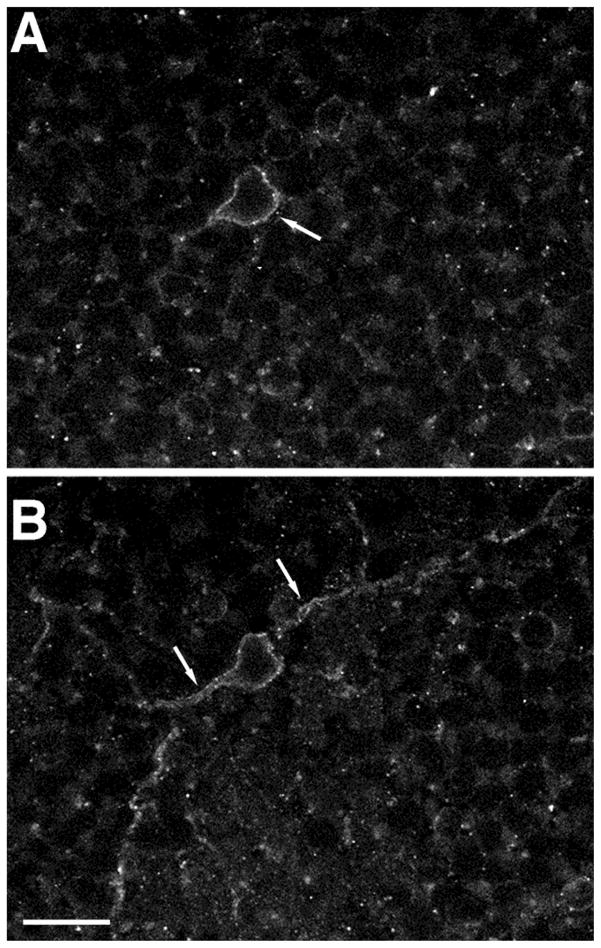
Fig. 7.
Confocal image showing the localization of sst2A receptor immunoreactivity closely associated with the plasma membrane of amacrine cells. Two different planes of focus through the same cell. Arrows indicate sstza immunoreactivity associated with the plasma membrane in the cell body (A) and the cell body and processes (B). Z axis = 1 μm. Scale bar = 12.5 μm.
Double-labeling experiments with sst2A receptor and TH antibodies were performed to further characterize the distribution of sst2A receptor-immunoreactive processes in the IPL. TH has been localized to wide-field amacrine cells in the rabbit retina, which give rise to processes that ramify mainly in lamina 1 as well as in lamina 3 and 5 of the IPL (Tauchi et al., 1990; Brecha et al., 1991; Casini and Brecha, 1992). There was no colocalization of TH and sst2A receptor immunostaining (Figs. 8, 9). The TH-immunoreactive processes in lamina 1 were distributed between the INL and the sst2A receptor-immunoreactive processes in lamina 2. In addition, the TH-immunoreactive processes in lamina 3 were between the sst2A receptor-immunoreactive processes in laminae 2 and 4. Finally, some sst2A receptor-immunoreactive processes were observed in laminae 1 and 3. They were visualized most clearly by using confocal microscopy in 1-μm optical sections (Fig. 2). These immunoreactive processes are likely to be from amacrine processes that transverse these laminae to ramify in laminae 2 and 4 of the IPL (Figs. 5, 7–9).
DISCUSSION
These investigations show that the immunoreactive SRIF receptor isoform, sst2A, is expressed by rod bipolar and amacrine cells of the rabbit retina (Fig. 10). sst2A Receptor immunoreactivity was determined by using a new polyclonal antibody directed against a synthetic peptide corresponding to the C-terminus of the mouse sst2A receptor. The predicted peptide sequence used for antibody production is identical to that of the C-terminus of the rat, mouse, and human sst2A receptor (Kluxen et al., 1992; Yamada et al., 1992). sst2A Receptor immunoreactivity was localized predominantly to rod bipolar cell axons and terminals, and double-labeling experiments showed complete colocalization of sst2A receptor and PKC (α and β) immunoreactivity in these cells. sst2A Receptor immunoreactivity was also observed in large, sparsely occurring, wide-field amacrine cells that were located at the border of the INL and IPL. They gave rise to thin, varicose processes that ramify predominantly in laminae 2 and 4 of the IPL. The sst2A receptor-immunoreactive amacrine cells were not PKC-immunoreactive and appeared to differ in their morphology and distribution from previously reported amacrine cell types (Vaney et al., 1988; Vaney, 1990).
Fig. 10.
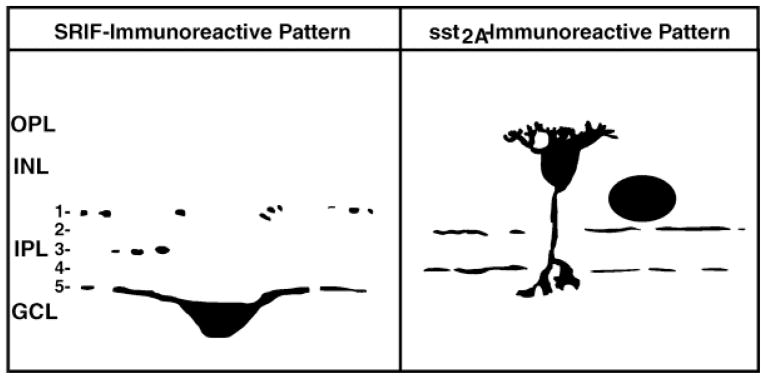
Schematic diagram illustrating the distribution of somatostatin and sst2A receptor immunoreactivities in rabbit retina. Images of the rod bipolar cell and the somatostatin-immunoreactive amacrine cell were adapted from Rickman et al. (1996) and Gillette and Dacheux (1995). SRIF, somatotropin-release inhibiting factor; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Anatomical, biochemical, and electrophysiological studies indicate a role for SRIF as a neuromodulator in the rabbit retina (Tornqvist et al., 1982; Sagar et al., 1986; Sagar, 1987; Rickman and Brecha, 1989; Zalutsky and Miller, 1990; Liapakis and Thermos, 1992; Liapakis et al., 1993; Rickman et al., 1996; Vasilaki et al., 1996). SRIF immunoreactivity has been localized to a sparse population of wide-field amacrine cells that are located predominantly in the GCL of the ventral retina (Fig. 10; Tornqvist et al., 1982; Sagar et al., 1986; Sagar, 1987; Rickman and Brecha, 1989; Rickman et al., 1996). The extensive distribution of the processes of these cells to all retinal regions indicates that these cells may have a modulatory role in the retina and may influence a number of cell types, including amacrine and bipolar cells (Masland, 1988; Zalutsky and Miller, 1990; Rickman et al., 1996). Electrophysiology studies also support a role for SRIF as a modulator in rabbit retina (Zalutsky and Miller, 1990). For example, in rabbit retinal eye-cup preparations, SRIF changes the signal-to-noise discharge pattern and the center-surround balance of ganglion cells. These changes in ganglion cell properties are thought to be due to SRIF’s action on multiple levels of the retinal circuitry, including the modulation of third-order neurons (Zalutsky and Miller, 1990). The observed expression of sst2A receptors by rod bipolar cells and amacrine cells extends these earlier studies and suggests that SRIF can influence neuronal activity in the inner retina through the sst2A receptor expressed by rod bipolar cells and amacrine cells.
sst2A Receptor is expressed by rod bipolar cells
Most sst2A receptor-immunoreactive cells are rod bipolar cells, based on their appearance, size, and distribution and on colocalization of the sst2A receptor and PKC immunoreactivities (Raviola and Raviola, 1967; Famiglietti, 1981; Dacheux and Raviola, 1986; Negishi et al., 1988; Wood et al., 1988; Greferath et al., 1990; Vaney et al., 1991; Wässle and Boycott, 1991; Wässle et al., 1991; Young and Vaney, 1991; Euler and Wässle, 1995). Cone bipolar cells did not appear to have sst2A receptor immunoreactivity. Interestingly, sst2A receptor immunoreactivity was not distributed uniformly in rod bipolar cells. Immunostaining was strongest in the axon and terminal and was weakest in the cell body and dendrites. These observations suggest that sst2A receptor activation by SRIF may influence the excitability of the rod bipolar axonal terminals. SRIF is not likely to influence glycine or γ-aminobutyric acid ionotrophic receptors in isolated rabbit rod bipolar cells (Gillette and Dacheux, 1995); however, SRIF could influence glutamate receptors (Peng et al., 1995; Lanneau et al., 1996) or directly influence ion channels.
SRIF could directly mediate presynaptic transmission by the regulation of ion channels via the sst2A receptor. Pharmacology studies report that the sst2A receptor likely regulates K+ and Ca2+ channels through interactions with G-proteins in neurons (Reisine and Bell, 1995). Furthermore, the application of selective sst2A receptor agonists to freshly dissociated amygdaloid neurons causes an inhibition of high-voltage-activated N- and P/Q-type calcium channels, probably through the sst2A receptor (Viana and Hille, 1996). Electrophysiology studies of rabbit retina report that SRIF causes a slow hyperpolarization and amplification of the light response in rod bipolar cells (Zalutsky and Miller, 1990). These authors proposed that this effect was a result of SRIF closing cation channels in other cell types (Zalutsky and Miller, 1990). Because the sst2A receptor is known to modulate cation channels (Ikeda and Schofield, 1989; White et al., 1991; Reisine and Bell, 1995), this action suggests a mechanism by which SRIF may influence one or more of the channels in rod bipolar cells. Interestingly, in rod bipolar cells isolated from goldfish retina, SRIF also causes a closure of cation channels, which results in suppression of L-type Ca2+ current (Ayoub and Matthews, 1992). Perhaps SRIF acts by similar mechanisms in both goldfish and rabbit rod bipolar cells.
These studies show a greater density of sst2A receptor immunoreactivity compared with the number and distribution of SRIF-immunoreactive processes and cells (Fig. 10; Sagar et al., 1986; Sagar, 1987; Rickman and Brecha, 1989; Rickman et al., 1996). In addition, SRIF-immunoreactive processes in lamina 5 are very sparse, with few synaptic profiles in relation to the large number of rod bipolar terminals in this lamina (Rickman et al., 1996). Assuming that the sst2A receptors are functional, then there is a mismatch (for review, see Herkenham, 1987) between the distribution of SRIF and sst2A receptor immunoreactivities in the rabbit retina. Indeed, this mismatch between the localization of peptide and receptor indicates that SRIF likely diffuses over many micrometers before interacting with the sst2A receptor on rod bipolar cell axonal terminals, thus suggesting a paracrine action for SRIF in the retina.
sst2A Receptor is expressed by amacrine cells
sst2A Receptor immunoreactivity was also observed in cells that are wide-field amacrine cells based on their appearance, size, and distribution. The immunoreactive amacrine cells appear to be unique from previously reported rabbit amacrine cells identified by histochemical and other techniques. Although they do have some resemblance to the wide-field amacrine cell types that have been identified by using reduced silver stains (Vaney et al., 1988), they are not likely to be the same cell type, that is, the reduced, silver-stained amacrine cells differ in their morphology compared with the sst2A receptor-expressing amacrine cells in the distribution of their processes in the IPL (Vaney et al., 1988).
The function of sst2A receptor amacrine cells is unknown, but the infrequently occurring cells bodies and the apparent wide arborization of their processes indicate that these cells are not likely to participate in the direct flow of information through the retina (Masland, 1988; Tauchi et al., 1990; Rickman et al., 1996). The slow onset and long-lasting effects of SRIF shown in the electrophysiology experiments with rabbit retina (Zalutsky and Miller, 1990), together with the morphology of the sst2A receptor amacrine cells, indicate that these cells are likely to have broad or global influences in the inner retina (Masland, 1988).
Multiple sst receptor subtypes may be present in the rabbit retina like in other tissue, such as rat brain (Señaris et al., 1994; Viollet et al., 1995). Assuming the presence of multiple sst receptors in the rabbit retina, these receptors may be expressed by other cell types, which suggests that SRIF may act on other cell types in the rabbit retina. Further studies investigating the sites of expression and ultrastructural organization of the sst receptors will further elucidate the role of SRIF in influencing retinal function.
In conclusion, the pattern of sst2A receptor expression suggests that SRIF modulates visual information processing in the retina both at rod bipolar cells and at sparsely occurring amacrine cells. SRIF could influence presynaptic glutamate transmission via the sst2A receptor on rod bipolar cell axonal terminals. In addition, SRIF could also influence the sparsely occurring wide-field amacrine cells, which are hypothesized to have a modulatory role in visual processing in the inner retina.
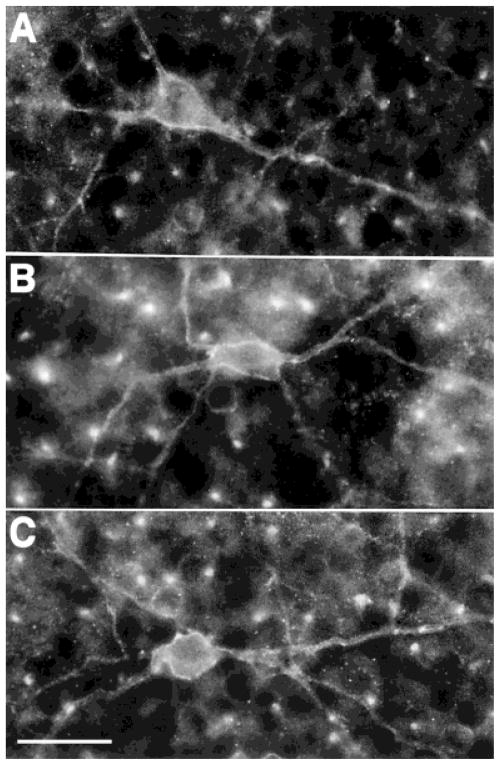
Fig. 6.
A–C: The sst2A receptor-immunoreactive amacrine cells in a horizontal section of the retina. Strong immunostaining outlined these cell bodies and their main processes. Scale bar = 25 μm.
Acknowledgments
National Institutes of Health; Grant numbers: DK 41301, DK 17294, EY 04067.
We thank Ty K. Chen for his technical assistance and Dr. C. Sternini for her helpful comments and suggestions.
LITERATURE CITED
- Ayoub GS, Matthews G. Substance P modulates calcium current in retinal bipolar neurons. Vis Neurosci. 1992;8:539–544. doi: 10.1017/s0952523800005630. [DOI] [PubMed] [Google Scholar]
- Balkema GW, Jr, Pinto LH, Drager UC, Vanable JW., Jr Characterization of abnormalities in the visual system of the mutant mouse pearl. J Neurosci. 1981;1:1320–1329. doi: 10.1523/JNEUROSCI.01-11-01320.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brecha NC, Casini G, Rickman D. Organization and development of sparsely distributed wide-field amacrine cells in the rabbit retina. In: Bagnoli P, Hodges W, editors. The Changing Visual System: Maturation and Aging in the Central Nervous System. London: Plenum Press; 1991. pp. 95–117. [Google Scholar]
- Bruno JF, Xu Y, Song J, Berelowitz M. Molecular cloning and functional expression of a brain-specific somatostatin receptor. Proc Natl Acad Sci USA. 1992;89:11151–11155. doi: 10.1073/pnas.89.23.11151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casini G, Brecha NC. Postnatal development of tyrosine hydroxylase immunoreactive amacrine cells in the rabbit retina: I. Morphological characterization. J Comp Neurol. 1992;326:283–301. doi: 10.1002/cne.903260210. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. The rod pathway in the rabbit retina: A depolarizing bipolar and amacrine cell. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum J. Somatostatin in the central nervous system: Physiology and pathological modifications. Progr Neurobiol. 1986;27:63–100. doi: 10.1016/0301-0082(86)90012-2. [DOI] [PubMed] [Google Scholar]
- Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- Famiglietti EV. Functional architecture of cone bipolar cells in mammalian retina. Vis Res. 1981;21:1559–1563. doi: 10.1016/0042-6989(81)90032-8. [DOI] [PubMed] [Google Scholar]
- Gillette MA, Dacheux RF. GABA- and glycine-activated currents in the rod bipolar cell of the rabbit retina. J Neurophysiol. 1995;74:856–875. doi: 10.1152/jn.1995.74.2.856. [DOI] [PubMed] [Google Scholar]
- Greferath U, Grunert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- Haroutunian V, Mantin R, Campbell GA, Tsuboyama GK, Davis KL. Cysteamine-induced depletion of central somatostatin-like immunoreactivity: Effects on behavior, learning, memory and brain neurochemistry. Brain Res. 1987;403:234–242. doi: 10.1016/0006-8993(87)90060-6. [DOI] [PubMed] [Google Scholar]
- Herkenham M. Mismatches between neurotransmitter and receptor localizations in brain: Observations and implications. Neuroscience. 1987;23:1–38. doi: 10.1016/0306-4522(87)90268-5. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Bell GI, Berelowitz M, Epelbaum J, Feniuk W, Humphrey PP, O’Carroll AM, Patel YC, Schonbrunn A, Taylor JE. Classification and nomenclature of somatostatin receptors. Trends Pharmacol Sci. 1995;16:86–88. doi: 10.1016/s0165-6147(00)88988-9. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Schofield GG. Somatostatin blocks a calcium current in rat sympathetic ganglion neurones. J Physiol. 1989;409:221–240. doi: 10.1113/jphysiol.1989.sp017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Wong H, Walsh JH, Brecha NC. Expression of somatostatin receptor subtype 2A (SSTR2A) in the rabbit retina. Soc Neurosci Abstr. 1996;22:881. [Google Scholar]
- Kluxen FW, Bruns C, Lubbert H. Expression cloning of a rat brain somatostatin receptor cDNA. Proc Natl Acad Sci USA. 1992;89:4618–4622. doi: 10.1073/pnas.89.10.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossut M, Yamada T, Aldrich LB, Pinto LH. Localization and characterization of somatostatin binding sites in the mouse retina. Brain Res. 1989;476:78–84. doi: 10.1016/0006-8993(89)91538-2. [DOI] [PubMed] [Google Scholar]
- Kossut M, Aldrich LB, Yamada T, Pinto LH. The binding of somatostatin to the mouse retina is altered by the pearl mutation. Brain Res. 1990;522:235–240. doi: 10.1016/0006-8993(90)91466-t. [DOI] [PubMed] [Google Scholar]
- Lanneau C, Faivre-Bauman A, Loudes C, Rivier J, Kordon C, Epelbaum J, Gardette R. Two different somatostatin receptor subtypes mediate opposite modulation of AMPA-KA responses in hypothalamic neurons. Soc Neurosci Abstr. 1996;22:80. [Google Scholar]
- Larsen JN, Bersani M, Olcese J, Holst JJ, Moller M. Somatostatin and prosomatostatin in the retina of the rat: An immunohistochemical, in-situ hybridization, and chromatographic study. Vis Neurosci. 1990;5:441–452. doi: 10.1017/s0952523800000560. [DOI] [PubMed] [Google Scholar]
- Li XJ, Forte M, North RA, Ross CA, Snyder SH. Cloning and expression of a rat somatostatin receptor enriched in brain. J Biol Chem. 1992;267:21307–21312. [PubMed] [Google Scholar]
- Liapakis G, Thermos K. Characterization of [125I]Tyr11-somatostatin binding sites in the rabbit retina. Neuropeptides. 1992;21:13–19. doi: 10.1016/0143-4179(92)90148-p. [DOI] [PubMed] [Google Scholar]
- Liapakis G, Politou E, Thermos K. Solubilization of active somatostatin receptors from rabbit retina. Biochem Pharmacol. 1993;45:1821–1828. doi: 10.1016/0006-2952(93)90439-4. [DOI] [PubMed] [Google Scholar]
- Masland RH. Amacrine cells. Trends Neurosci. 1988;11:405–410. doi: 10.1016/0166-2236(88)90078-1. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Wulfsen I, Schonrock C, Fehr S, Richter D. Molecular cloning of a somatostatin-28 receptor and comparison of its expression pattern with that of a somatostatin-14 receptor in rat brain. Proc Natl Acad Sci USA. 1992;89:10267–10271. doi: 10.1073/pnas.89.21.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi K, Kato S, Teranishi T. Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci Lett. 1988;94:247–252. doi: 10.1016/0304-3940(88)90025-0. [DOI] [PubMed] [Google Scholar]
- O’Carroll AM, Lolait SJ, Konig M, Mahan LC. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992;42:939–946. [PubMed] [Google Scholar]
- Peng YW, Blackstone CD, Huganir RL, Yau KW. Distribution of glutamate receptor subtypes in the vertebrate retina. Neuroscience. 1995;66:483–497. doi: 10.1016/0306-4522(94)00569-q. [DOI] [PubMed] [Google Scholar]
- Raviola G, Raviola E. Light and electron microscopic observations on the inner plexiform layer of the rabbit retina. Am J Anat. 1967;120:403–425. doi: 10.1002/aja.1001200303. [DOI] [PubMed] [Google Scholar]
- Reisine T, Bell GI. Molecular properties of somatostatin receptors. Neuroscience. 1995;67:777–790. doi: 10.1016/0306-4522(95)00072-q. [DOI] [PubMed] [Google Scholar]
- Rickman DW, Brecha NC. Morphologies of somatostatin-immunoreactive neurons in the rabbit retina. In: Weiler R, Osborne NN, editors. Neurobiology of the Inner Retina. Berlin: Springer-Verlag; 1989. pp. 461–468. [Google Scholar]
- Rickman DW, Blanks JC, Brecha NC. Somatostatin-immunoreactive neurons in the adult rabbit retina. J Comp Neurol. 1996;366:1–13. doi: 10.1002/(SICI)1096-9861(19960212)365:3<491::AID-CNE11>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Sagar SM. Somatostatin-like immunoreactive material in the rabbit retina: Immunohistochemical staining using monoclonal antibodies. J Comp Neurol. 1987;266:291–299. doi: 10.1002/cne.902660212. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Marshall PE. Somatostatin-like immunoreactive material in associational ganglion cells of human retina. Neuroscience. 1988;27:507–516. doi: 10.1016/0306-4522(88)90284-9. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Marshall PE, Onesti ST, Landis DM. Somatostatin immunocytochemistry in the rabbit retina. Invest Ophthalmol Vis Sci. 1986;27:316–322. [PubMed] [Google Scholar]
- Señaris RM, Humphrey PPA, Emson PC. Distribution of somatostatin receptors 1, 2 and 3 mRNA in rat brain and pituitary. Eur J Neurosci. 1994;6:1883–1896. doi: 10.1111/j.1460-9568.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Sternini C, Wong H, Wu SV, De Giorgio R, Yang M, Reeve J, Jr, Brecha NC, Walsh JH. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J Comp Neurol. 1997;386:396–408. [PubMed] [Google Scholar]
- Tauchi M, Madigan NK, Masl RH. Shapes and distributions of the catecholamine-accumulating neurons in the rabbit retina. J Comp Neurol. 1990;293:178–189. doi: 10.1002/cne.902930203. [DOI] [PubMed] [Google Scholar]
- Tornqvist K, Uddman R, Sundler F, Ehinger B. Somatostatin and VIP neurons in the retina of different species. Histochemistry. 1982;76:137–152. doi: 10.1007/BF00501917. [DOI] [PubMed] [Google Scholar]
- Vanetti M, Kouba M, Wang X, Vogt G, Hollt V. Cloning and expression of a novel mouse somatostatin receptor (SSTR2B) FEBS Lett. 1992;311:290–294. doi: 10.1016/0014-5793(92)81122-3. [DOI] [PubMed] [Google Scholar]
- Vaney DI. The mosaic of amacrine cells in the mammalian retina. Progr Ret Res. 1990;9:49–100. [Google Scholar]
- Vaney DI, Peichl L, Boycott BB. Neurofibrillar long-range amacrine cells in mammalian retinae. Proc R Soc London B Biol Sci. 1988;235:203–219. doi: 10.1098/rspb.1988.0072. [DOI] [PubMed] [Google Scholar]
- Vaney DI, Young HM, Gynther IC. The rod circuit in the rabbit retina. Vis Neurosci. 1991;7:141–154. doi: 10.1017/s0952523800011019. [DOI] [PubMed] [Google Scholar]
- Vasilaki A, Hatzilaris E, Liapakis G, Georgoussi Z, Thermos K. Somatostatin receptor subtypes (SSTR2) in the rabbit retina. Soc Neurosci Abstr. 1996;22:881. [Google Scholar]
- Viana F, Hille B. Modulation of high voltage-activated calcium channels by somatostatin in acutely isolated rat amygdaloid neurons. J Neurosci. 1996;16:6000–6011. doi: 10.1523/JNEUROSCI.16-19-06000.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet C, Faivre-Bauman A, Zhang J, Llorens-Cortes C, Loudes C, Kordan C, Epelbaum J. Differential expression of somatostatin receptors by quantitative PCR in the rat brain. Comptes Rendus de L Academie des Sciences, Serie III, Sciences de la Vie. 1995;318:851–857. [PubMed] [Google Scholar]
- Wässle H, Boycott BB. Functional architecture of the mammalian retina. Physiol Rev. 1991;71:447–480. doi: 10.1152/physrev.1991.71.2.447. [DOI] [PubMed] [Google Scholar]
- Wässle H, Yamashita M, Greferath U, Grunert U, Muller F. The rod bipolar cell of the mammalian retina. Vis Neurosci. 1991;7:99–112. doi: 10.1017/s095252380001097x. [DOI] [PubMed] [Google Scholar]
- White CA, Chalupa LM, Johnson D, Brecha NC. Somatostatin-immunoreactive cells in the adult cat retina. J Comp Neurol. 1990;293:134–150. doi: 10.1002/cne.902930111. [DOI] [PubMed] [Google Scholar]
- White RE, Schonbrunn A, Armstrong DL. Somatostatin stimulates Ca(2+)-activated K+ channels through protein dephosphorylation. Nature. 1991;351:570–573. doi: 10.1038/351570a0. [DOI] [PubMed] [Google Scholar]
- Wood JG, Hart CE, Mazzei GJ, Girard PR, Kuo JF. Distribution of protein kinase C immunoreactivity in rat retina. Histochem J. 1988;20:63–68. doi: 10.1007/BF01746605. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci USA. 1992;89:251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda K, Rens-Domiano S, Breder CD, Law SF, Saper CB, Reisine T, Bell GI. Cloning of a novel somatostatin receptor, SSTR3, coupled to adenylylcyclase. J Biol Chem. 1992;267:20422–20428. [PubMed] [Google Scholar]
- Young HM, Vaney DI. Rod-signal interneurons in the rabbit retina: 1. Rod bipolar cells J Comp Neurol. 1991;310:139–153. doi: 10.1002/cne.903100202. [DOI] [PubMed] [Google Scholar]
- Zalutsky RA, Miller RF. The physiology of somatostatin in the rabbit retina. J Neurosci. 1990;10:383–393. doi: 10.1523/JNEUROSCI.10-02-00383.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]