Abstract
Chromatin remodeling proteins regulate multiple aspects of cell homeostasis, making them ideal candidates for misregulation in transformed cells. Here, we explore Sin3A, a member of the Sin3 family of proteins linked to tumorigenesis that are thought to regulate gene expression through their role as histone deacetylases (HDACs). We identified Drosophila Sin3a as an important mediator of oncogenic Ret receptor in a fly model of Multiple Endocrine Neoplasia Type 2. Reducing Drosophila Sin3a activity led to metastasis-like behavior and, in the presence of Diap1, secondary tumors distant from the site of origin. Genetic and Chip-Seq analyses identified previously undescribed Sin3a targets including genes involved in cell motility and actin dynamics, as well as signaling pathways including Src, Jnk and Rho. A key Sin3a oncogenic target, PP1B, regulates stability of β-Catenin/Armadillo: the outcome is to oppose T-cell factor (TCF) function and Wg/Wnt pathway signaling in both fly and mammalian cancer cells. Reducing Sin3A strongly increased the invasive behavior of A549 human lung adenocarcinoma cells. We show that Sin3A is downregulated in a variety of human tumors and that Src, JNK, RhoA and PP1B/β-Catenin are regulated in a manner analogous to our Drosophila models. Our data suggest that Sin3A influences a specific step of tumorigenesis by regulating a module of genes involved in cell invasion. Tumor progression may commonly rely on such ‘modules of invasion’ under the control of broad transcriptional regulators.
Keywords: Sin3a, Ret, Src, Rho, wingless, Drosophila
INTRODUCTION
Chromatin remodeling pathways have significant but complex roles in cancer progression. Both histone hypoacetylation and hyperacetylation are associated with various cancer subtypes. Histone deacetylases (HDACs) are commonly recruited by co-activators and co-repressors to provide crucial points of pathway control in both normal and cancerous tissues.1 Consequently, considerable effort has been placed on finding small molecule inhibitors that regulate HDAC function in vivo.2 Recently, two studies have shown that loss of global chromatin regulators mH2A and L(3)MBT promote melanoma progression and brain tumors, respectively,3,4 demonstrating that cancer progression can be driven by loss of genes that have a broad effect on the cellular transcription profile. This view is amplified in recent work on retinoblastoma, in which epigenetic changes are central to disease progression.5 Nevertheless, deregulation of chromatin remodeling activity and its global effect on modulation of various pathways that lead to disease progression in whole animal cancer models remain poorly understood.
In mammals, Sin3 proteins can recruit HDACs to chromatin-bound transcription factors to repress expression of target genes.6,7 Sin3A regulates processes important for development and homeostasis including mitochondrial biogenesis, cell death and neuronal fate selection.8–10 In addition, Sin3A is an obligate partner of Mad (Mxi1)-class nuclear factors and is required for Mad-mediated repression of Myc-dependent transcription.11,12 Sin3B has some overlapping functional properties with Sin3A that include regulating E2F/Rb and Myc-dependent gene transcription.13 Whole animal mouse models found that knockouts for both sin3A and sin3B led to different degrees of early embryonic lethality and differential targeting of muscle precursors.8,13
Sin3A is an important negative regulator of several cancer-related factors including p53, Rb and E2F in addition to Myc.14–16 Members of the Sin3 complex have also been linked to tumorigenesis, most notably BMRS1.17–19 However, the complex roles of chromatin-related factors in cancer are mirrored by recent Sin3 studies. For example, downregulation of Sin3A is associated with progression of non-small cell lung cancer but, conversely, reduced viability of metastatic MDA-MB-231 and MCF7 breast cancer cell lines.20 A clear mechanistic understanding has yet to emerge of how Sin3 proteins contribute to tumorigenesis in situ.
Dominant activating mutations in the receptor tyrosine kinase (RTK) Ret lead to Multiple Endocrine Neoplasia Type 2 (MEN2), a cancer syndrome characterized by Medullary Thyroid Carcinoma (MTC)—an overgrowth of parafollicular C cells—and potentially other tumors including pheochromocytoma and parathyroid adenomas. We recently established a Drosophila MEN2 model21 in which Drosophila Ret (dRet) was engineered to contain analogous MEN2-type mutations; these dRetMEN2 isoforms were then targeted to the emerging eye epithelium. Our genetic studies indicated that dRetMEN2 activates multiple signaling pathways previously implicated in tumorigenesis including Ras, Src and JNK; these pathways are also active in the presence of mammalian oncogenic Ret isoforms.22
Mutations in the sin3a locus—encoding the sole Drosophila Sin3 ortholog—proved to be especially strong genetic modifiers of dRetMEN2 activity, indicating a strong functional link between the two loci.21 Here, we demonstrate that Drosophila Sin3a regulates important aspects of tumorigenesis including altered polarity, destabilized adherens junctions, activation of metastasis markers and epithelial-to-mesenchymal transition (EMT). We find that Sin3a potently modulates dRetMEN2-, Src- and JNK-dependent invasion by regulating a specific module of genes that include both known and novel Sin3 targets. Interestingly, the majority of these novel targets are normally activated—not repressed—by Sin3a, suggesting it can act in an HDAC-independent manner. We further establish that Sin3 proteins can regulate similar sets of cell invasion genes in human thyroid and lung cancer cell lines, and show that reducing Sin3 levels enhance their invasive potential. This view is supported by our analysis of patient lung tumor samples and ONCOMINE data indicating that Sin3 mRNA is significantly downregulated in a variety of cancers. Through a variety of approaches, our studies establish for the first time a role for Sin3 proteins in regulating an important and specific aspect of tumorigenesis: invasion/metastasis.
RESULTS
Sin3a loss enhanced oncogenic dRet and led to metastasis-like behavior
To better understand the function of Sin3A, we explored an in-vivo model of tumorigenesis. Removal of one genomic copy of the sin3a locus significantly enhanced the ‘rough eye phenotype’ of targeted dRetMEN2B (gmr>dRetMEN2B; sin3a+/−), leading to a smaller eye with a greater disruption of the ommatidial array; this indicates that Sin3a normally functions to counter oncogenic dRetMEN2B activity (Figures 1a–c).21 Recently, we developed an invasion/migration assay in which transgenes are targeted to a discrete region of the developing wing epithelium using a driver based on the patched (ptc) promoter.23 Targeting oncogenic Ret to this region (ptc>RetMEN2B) showed mild overgrowth and minimal migration of cells from the ptc domain (Figure 1f). Additional RNA interference-mediated knockdown of sin3a (ptc>sin3aRNAi; dRetMEN2B) led to strong enhancement: large numbers of cells shifted basally below the epithelium and migrated significant distances from the ptc domain (Figure 1g). These data indicate that reducing Sin3a significantly increases the invasive capacity of cells transformed by dRetMEN2B.
Figure 1.
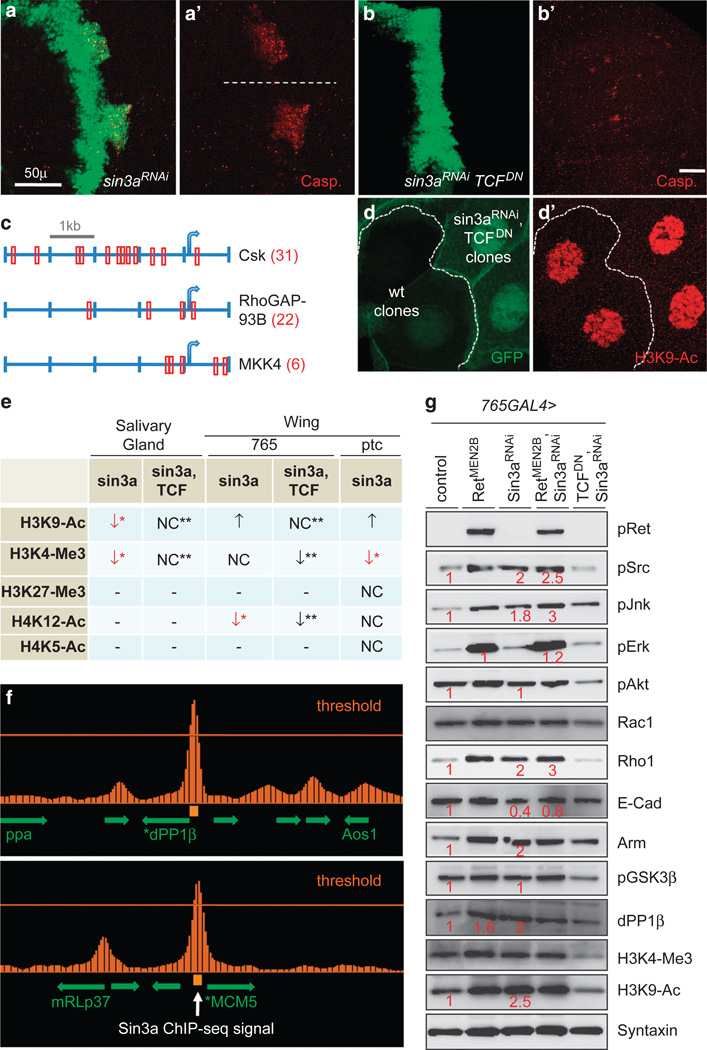
sin3a interacts genetically with dRetMEN2 and is a tumor suppressor. (a–c) Bright field images of adult eyes of the indicated genotypes. (a–c) sin3a dominantly enhanced the gmr-dRetMEN2B phenotype. (d) sin3a knockdown led to a rough-eye phenotype and outgrowths from the eye (arrow). (e–i, k–n) Invasion assays in the L3 wing disc using ptc>dicer2, eGFP. All images are composite overlays of Z-stacks spanning the full depth of the epithelia; in this and subsequent figures cells are visualized with uas-eGFP and the apical surface is visualized as part of a whole mounted tissue. Yellow brackets in (e), (h) and (i) demarcate the patched (ptc) domain; white bracket indicates region of migrating cells at base of wing disc. Expression of oncogenic Ret, ptc>dRetMEN2B (f) leads to some proliferation but little invasion compared with control (e). Additional knockdown of sin3a, ptc>sin3aRNAi dRetMEN2B (g) leads to significantly enhanced cell invasion into adjacent wild-type (WT) tissue (non-GFP). (h, i) sin3a knockdown is sufficient to induce invasion into adjacent WT tissue. (j) Co-expression of Caspase inhibitor Diap1 and sin3a knockdown transgenes in the developing eye (gmr>sin3aRNAi diap1 GFP) led to migration to distant sites including adult legs (arrows). (k, l) Expression of Drosophila Myc leads to overproliferation and some invasion (k) which is considerably enhanced when sin3a is simultaneously knocked down, ptc>sin3aRNAi dMycB (l). (m, n) Co-expression of apoptosis inhibitor p35 in sin3a knockdown cells, ptc4sin3aRNAi p35, led to expression of EMT marker N-Cadherin in individual cells (m) or in groups of cells (n).
Remarkably, reducing sin3a alone (ptc>sin3aRNAi) was sufficient to direct mild but consistent cell migration as targeted cells released basally (Figure 1h). A stronger knockdown led to more aggressive cell invasion as many migrating cells traveled a significant distance before eventually undergoing apoptosis (Figure 1i; Supplementary Figures S1A and B). Blocking apoptotic cell death with the Caspase inhibitor P35 also enhanced the sin3aRNAi migration phenotype (Supplementary Figures S1C and D). Similarly, targeted sin3a reduction led to a rough eye phenotype, often with growths emerging from the eye (Figure 1d; gmr> sin3aRNAi RetMEN2B, Supplementary Figures 1G–I); addition of the Caspase inhibitor Diap1 led to enhanced overgrowth and secondary tumors that grew stably at distant sites in approximately one-third of the adults examined. Figure 1j shows an example of secondary tumors due to migration to the adult legs. dCsk23 and sin3a are therefore the only two Drosophila loci reported to direct invasive migration when their activity alone is reduced.
Recent reports have proposed that mammalian Sin3A acts as an oncogene or as a tumor suppressor. For example, Sin3A was found to promote ERα-positive breast cancer cell survival24 or act in opposition to the Myc oncogene.11,25 Sin3a acted in opposition to Myc in our assays: expressing Drosophila Myc in the context of dCskRNAi (not shown) or sin3aRNAi (Figure 1l) led to strong enhancement of tissue overgrowth. These data are again consistent with Sin3a acting as a tumor suppressor in our assays.
Reducing sin3a promoted EMT and invasive migration
Invasive behavior of transformed cells is commonly associated with destabilization of adherens junctions, loss of cell polarity and increased motility of cells, collectively referred to as EMT. Markers of EMT include the mesenchymal fate marker N-Cadherin.26,27 We observed cell-autonomous N-Cadherin expression in a subset of ptc>sin3aRNAip35 cells within the wing pouch region; expression was most often modest but was substantial in some discrete groups (Figures 1m and n). Upregulation of N-Cadherin was not observed either by knocking down sin3a or by expressing p35 alone (not shown). Similar to knockdown of dCsk,23 ptc>sin3aRNAi cells lost their elongate morphology and shifted basally at the ‘tumor’ boundary. Cell polarity was disrupted in these cells as assessed by loss of the adherens junction markers E-Cadherin and Armadillo and the septate junction marker Discs large (Figures 2b–g; Supplementary Figures S1E and F).
Figure 2.
sin3a knockdown induces invasive migration and EMT. (a–g) Cells with reduced sin3a, ptc>sin3aRNAi showed reduced adherens junctions, altered polarity, and extruded basally and migrated away from the ptc boundary. (b’) These cells displayed reduced E-Cadherin at the adherens junctions: asterisks indicate reduced E-Cadherin levels within the ptc domain. (c) Reduced levels of adherens junction component Armadillo (Arm) by sin3a knockdown. Compare Arm signal in GFP+ cells with signal from adjacent wild-type (WT) cells. (d–g) Transverse (Z-series) reconstruction showed reduced E-Cadherin at adherens junctions (e) and the polarity marker Dlg (g) over ptc expression domains (bracket). (h–i’) In contrast to ptc>dicer2 controls (h, h’), knockdown of sin3a (ptc4sin3aRNAi) (i, i’) led to increased Mmp1 signal (arrow). (j, k) Anti-Laminin antibody staining of ptc>dicer2 (j) and ptc>sin3aRNAi (k) discs. Note reduced Laminin at the ptc domain and GFP-positive cells ‘burrowing’ through adjacent tissue (k; for example, arrows).
Invasive migration and EMT require the activity of matrix metalloproteases (MMPs). The Drosophila genome encodes two MMP orthologs, Mmp1 and Mmp2. Reducing sin3a activity by knockdown (ptc>sin3aRNAi) or in genotypically sin3a−/− mutant clones within the wing led to a strong increase in Mmp1 protein deposition at the site where transformed cells invaded basally (Figure 2i; Supplementary Figures S2A and B). Consistent with increased MMP activity, migrating sin3aRNAi cells were frequently found ‘burrowing’ through basal regions where the ECM was degraded (Figure 2k). These data indicate that Sin3a normally acts to oppose EMT.
Sin3a acts primarily as a positive regulator of genes involved in cell invasion/migration
To identify Sin3a genomic targets that, when deregulated, are candidates to promote invasion/migration, we performed a genome-wide ChIP-seq analysis. We generated and validated an antibody to the N-terminus of Drosophila Sin3a (Supplementary Figure S2D; Negre et al.28). ChIP-seq analysis of 0–16 h embryo extracts identified ~100 genes that met our threshold and reproducibility criteria (Supplementary Figures S4 and S5; Materials and methods).
A striking number of Sin3a targets that emerged from our analysis are closely linked to tumorigenesis (Figure 3a). Utilizing RT-PCR we confirmed 41/44 targets as altered in vivo in sin3aRNAi larval extracts. Of note, orthologs of several genes previously shown to be Sin3a targets—for example, MCM5, Rbf, p53 and MMS198—were indeed bound (ChIP-seq) and regulated (RT-PCR) by Drosophila Sin3a (Figure 3a). ChIP-seq analysis placed Sin3a at the promoter regions of practically all of its targets; additional low-level ChIP-seq signals extended into the adjacent transcribed regions (Figure 5f) as has been reported for mammalian Sin3 proteins.29
Figure 3.
Sin3a transcriptionally regulates genes involved in cell invasion/migration. (a) RT-PCR analysis of putative sin3a targets identified by ChIP-seq. Expression of putative sin3a targets in sin3aRNAi (blue columns) and dicer, sin3aRNAi (red columns) was normalized with respect to expression in control w− embryos. Error bars represent standard error. The majority of targets examined showed lower expression when sin3a was knocked down, suggesting that sin3a is acting as an activator on these loci. (b) H3K4Me3 staining in ptc>sin3aRNAi experiments, note significantly lower signal in tissue (green, asterisk) where sin3a is reduced. (c) H3K9Ac staining in salivary gland ‘FLP-out’ clones generated by heat shocking act>CD2>GAL4; hsFLP,eGFP;sin3aRNAi dicer2 larvae and analyzing cells containing eGFP-positive clones. Note significantly lower signal in tissue (green) where sin3a is reduced.
Figure 5.
Sin3a regulates Wg signaling by both opposing TCF and controlling β-Catenin stability. (a, b) sin3a knockdown induces invasion and Caspase activation and invasion at a distance from the D/V boundary of the wing disc (a, a’). (b, b’) Co-expression of TCFDN suppressed sin3aRNAi-mediated invasion. A few cleaved Caspase-positive cells are still visible along the ptc boundary. (c) A subset of sin3a targets including dCsk show enriched consensus TCF-binding sites AGAWAW (W =A/T). Red boxes indicate predicted binding regions (some containing multiple sites) and arrows indicate predicted translation start sites. Number in parentheses indicates total sites predicted within 5 KB of ATG site. (d) Salivary gland ‘FLP-out’ clones that express sin3aRNAi TCFDN showed equivalent H3K9Ac signal in clones and wild-type (WT) cells. Compare with Figure 4b. (e) Analysis of histone modification markers in three contexts: salivary gland FLP-out clones, expression of UAS constructs throughout the whole wing disc (765-GAL4) and expression of UAS constructs within the ptc domain (ptc-GAL4). The loss of many markers of active chromatin by sin3aRNAi was restored by co-expressing TCFDN showing that Sin3a and TCF oppose each other globally on chromatin. (f) Strong Sin3a ChIP-seq signal at the transcriptional start region of Drosophila PP1-87B, the ortholog of vertebrate PP1β. Strong signal in the upstream region of MCM5, a previously described target of Sin3a. Image generated by Integrated Genome Browser 6.5.3 software (Integrated Genome Browser, UNC, Charlotte, NC, USA). (g) Western detection of indicated proteins from indicated genotypes; syntaxin was used as loading control. sin3a knockdown resulted in increased pJnk, pSrc, Rho1, dPP1α and Arm/β-Catenin. Co-expression of TCFDN suppressed all markers that were elevated upon sin3a knockdown.
To our surprise, our ChIP-seq and RT-PCR data indicated that Sin3a acts predominantly as an activator: while 33% (11/33) of its targets were reduced in the presence of sin3aRNAi, 73% (24/33) were reduced with the stronger dicer;sin3aRNAi knockdown (Figure 3a), consistent with recent mammalian data.29 To monitor the status of active chromatin more directly, we explored the change in histone modification patterns upon sin3a knockdown. Acetylation of lysine residues on histones (for example, H3K9Ac, H4K12Ac and H4K5Ac) promotes active transcription through loosening and unfolding of local chromatin structure.30 Methylation is primarily associated with transcriptional silencing but methylation of some histones, like H3K4Me3, promote active transcription through inhibition of repressive nucleosome remodeling and histone-deacetylase complexes.31 Indeed, knockdown of sin3a led to in-vivo loss of active chromatin markers H3K4Me3 and H4K12Ac in the wing disc and H3K9Ac in the salivary glands (Figures 3b, c and 4p). Reducing Sin3a in the wing disc did not alter other markers of active chromatin including H3K22Me3 and H4K5Ac. In contrast to the salivary gland, H3K9Ac signal was increased in sin3aRNAi wing epithelia (Figure 5g), indicating that Sin3a’s role as a transcription factor is complex and context dependent.
Figure 4.
sin3a knockdown regulates Src, JNK signaling and a module of pro-invasive genes (a, b) sin3a dominantly enhanced the gmr >dCskRNAi-mediated increase in ommatidia and enlargement of eye size. This effect is quantified in Supplementary Figure S2E (c–e) While (c) dCsKRNAi and (d)sin3aRNA individually induced low-level invasion, simultaneous knockdown of both (e) dCskRNAi, sin3aRNA led to synergistic enhancement of invasion. (f–g’) Enhanced invasion by strong sin3a knockdown, ptc>sin3aRNAi-strong(Figure 1i) was suppressed by removing a genomic copy of bsk (f) or jun (g). Mmp1 signal was still visible in discs with incomplete suppression of invasion by jun (g’). (h, h’) Enhanced invasion was also suppressed by co-expression of bskDN (h); anti-Caspase staining (h’) indicated apoptosis in cells that were not invading. (i–j’) Mmp1 expression in ptc>sin3aRNAi dCskRNAi (i,i’) and ptc>sin3aRNAi dCskRNAi bskDN (j, j’) discs. bskDN suppressed the synergistic invasion induced by sin3a/dCsk double knockdown. In ptc>sin3aRNAi dCskRNAi bskDN cells, Mmp1 expression was still induced (j’) and considerable proliferation was observed (j), suggesting a non-linear effect of Jnk pathway inhibition in this triple combination. (k) Removal of a genomic copy of the Jnk-phosphatase puc (k) led to enhanced invasion compared with sin3a knockdown alone (Figure 1h). (I) Overexpression of E-cad, ptc>sin3aRNAi Ecad, increased migration. (m) Removal of a genomic copy of E-Cadherin, ptc>sin3asRNAi-strong Ecad−/+, reduced migration. (n, o) Removal of a genomic copy of polychaetoid, ptc>sin3aRNAi-strong pyd−/+ (o) did not alter sin3aRNAi -driven invasion (n). (p) Western detection of indicated proteins from indicated genotypes; tubulin was used as loading control. sin3a knockdown resulted in increased pJnk, pSrc and reduced E-Cadherin and Sin3a levels; H4K12Ac levels, indicative of actively transcribed chromatin, also decreased slightly. Stronger sin3a knockdown led to intermediate E-Cadherin levels. Relative pixel density for each marker as measured by ImageJ software is shown in red.
Sin3a opposes Src pathway activity
Western analysis of wing discs epithelia with mild and strong sin3a knockdowns confirmed that the Sin3a pathways identified through ChIP-seq were indeed deregulated. These include effectors of the Src, Jnk, pathways as well as regulators of actin dynamics (Figure 4p), creating the potential for an oncogenic ‘module’. To test this concept, we examined several of these factors to determine whether they mediated Sin3’s regulation of invasion in both flies and mammals.
Our ChIP-seq and RT–PCR data indicated that Drosophila C-terminal Src-kinase (dCsk) is a transcriptional target of the Sin3a complex (Figure 3a). Csk-class proteins actively suppress Src. activity by directly phosphorylating residues within Src’s C-terminus.32 The Src pathway is an important mediator of tumorigenesis including oncogenic Ret signaling,21,33,34 and we further explored the relationship between Csk/Src and Sin3a.
Reducing sin3a activity broadly within the wing disc led to both reduced dCsk mRNA expression and increased levels of active Src (pSrc; Figures 4p and 5g). It also led to a strong, synergistic enhancement of dCskRNAi as assessed by increased migration of cells in the wing (Figure 4e) and enhanced overgrowth and progressive tissue degeneration in the eye (Figure 4b; Supplementary Figure S2E). Lethality of hypomorphic dCsk alleles35 was enhanced by removing a genomic copy of sin3a (Supplementary Figure S2F). In contrast, knockdown of dCsk in the wing did not affect Sin3a protein levels (Supplementary Figures S3A–C).
Together, these data indicate that Sin3a normally acts to oppose Src pathway activity, at least in part through regulation of dCsk. Reduced Sin3a activity leads to increased Src function.
Jnk and E-cadherin signaling regulates sin3a-dependent invasion
The Jnk pathway is commonly mis-regulated in human tumors. It has been linked to both mouse and Drosophila models of Ret-induced tumorigenesis21,36 and to MMP1 expression in flies.37 Our ChIP-seq data indicated that the Sin3a complex regulated the Jnk pathway components Atf-2 and MKK4, which were downregulated upon sin3a knockdown (Figure 3a). MKK4 is a metastasis suppressor that is frequently lost in a number of human cancer cell lines and in a large breast tumor subgroup.38–40 Previous Drosophila studies indicated that MKK4 opposed Jnk pathway activity in a complex interaction with MKK7 (Hep) and MAPKKK (TAK1), demonstrating the careful balance of signals required for Jnk pathway activity.41 Indeed, activated phospho-Jnk (pJnk) levels were elevated in a dose-dependent manner when sin3a was reduced (Figures 4p and 5g), indicating that reduction of MKK4 may contribute to Jnk activation.
Removing one genomic copy of the Drosophila jnk ortholog bsk or its target jun significantly suppressed the EMT and invasion resulting from reduced sin3a in the wing; expressing a dominant-negative form of bsk also led to near complete loss of cell migration (Figures 4f – h). Interestingly, expression of bskDN in the context of sin3aRNAi dCskRNAi led to massive overgrowth and increased MMP1 expression but invasive migration was still absent (Figure 4i), pointing to multiple, complex interactions between Sin3a, Src and Jnk. Conversely, sin3aRNAi-induced invasion was enhanced by removing a genomic copy of the dual specificity Jnk phosphatase puckered (puc), a negative regulator of Jnk signaling (Figure 4k). We conclude that Sin3a regulates Ret-mediated invasion/migration in part through its regulation of Jnk pathway components.
Our previous work indicated that reducing E-Cadherin suppressed dCsk-mediated cell migration,23 suggesting that E-Cadherin normally provides a signal to promote dCsk-dependent invasion. Recent studies in B-raf-driven mouse cancer models and human tumor analysis have suggested that highly metastatic cells may have elevated levels of E-Cadherin to aid metastatic spread.42 sin3aRNAi-mediated migration displayed a similar requirement for E-Cadherin: ectopic E-Cadherin expression in mild sin3aRNAi cells led to increased invasion as assessed by greater distances traveled from the ptc expression domain, while removal of a genomic copy of E-Cadherin suppressed sin3aRNAi-strong-driven invasion (Figures 4I and m). By contrast, removal of a genomic copy of polychaetoid—a component of the adherens junction that provides structural support—did not affect sin3aRNAi-driven invasion (Figure 4o). Our western analysis showed that, compared with control, mild sin3aRNAi cells had the lowest levels of E-Cadherin whereas more invasive sin3aRNAi-strong cells had intermediate levels (Figure 4p). Combined with our genetic data, this suggested that although reduced E-Cadherin is linked to invasion (Figures 2e and 4p), optimal invasion may require some E-Cadherin. Finally, as neither E-Cadherin nor Dlg promoters were bound strongly by Sin3a in our ChIP-seq analysis, deregulation of these junctional components likely occurs indirectly through downstream Sin3a targets.
Sin3a regulates actin remodeling factors
Based on our ChIP-seq data, one of the largest classes of Sin3a targets are regulators of cell migration. These include Rho1, Rac1, Arf51F and members of the RhoGAP and RhoGEF families (Figure 3a). The Rho superfamily of small GTPases regulates cell migration by altering Actin cytoskeletal dynamics, and has been implicated in progression of various cancers.43 Interestingly, knockdown of sin3a led to decreased mRNA expression of the RhoGAPs RhoGAP54B and RhoGAP93B (with strong Sin3a knockdown) while expression of the RhoGEFs RhoGEF2 and rtGEF was increased, consistent with their opposing roles in regulating cytoskeletal motility and cell migration. Our western analysis revealed that levels of Rho1 were increased upon mild sin3a knockdown (Figures 3a and 5g) while RhoGEF2 mRNA was consistently increased under these conditions (Figure 3a); the resulting synergy should increase Rho1 activity and alter cell motility. Other regulators of Actin remodeling including pSrc, pJnk, and Rho1 levels were also significantly elevated (Figures 4p and 5g).
We observed similar changes in the presence of dRetMEN2B for nearly all targets we examined. Activation of Ret—alone or in the presence of sin3a knockdown—led to activated pSrc, pJnk and pErk as well as increased Rho1 expression (Figure 5g). These effects were enhanced in some targets by further knocking down Sin3a, while other targets that were already strongly activated were unaffected by further reduction of Sin3a (Figure 5g). An important exception was E-Cadherin. Although sin3a knockdown led to lower levels of E-Cadherin (Figures 2e and 4p), simultaneous activation of Ret led to intermediate levels of E-Cadherin (Figure 5g). Taken together, these findings suggest that careful titration of E-Cadherin levels might be required for aggressive invasion/migration (Figures 1g and i).
Our data emphasize the importance of Sin3a in targeting regulators of signal transduction and cell motility, two important aspects of transformation and invasion. They also point to the remarkable consistency by which loss of Sin3a upregulates activators and downregulates inhibitors to achieve these ends.
Sin3a regulates Wg signaling by both opposing TCF and controlling β-Catenin stability
In the process of scoring sin3a phenotypes in the wing we noted that a region flanking the dorso-ventral (D/V) boundary showed especially strong cell migration and Caspase activation (Figure 5a). The Drosophila Wnt ortholog Wg is expressed by a single row of cells at the D/V boundary44 and we postulated that a Wg-dependent signal was affecting invasion/migration of flanking sin3a knockdown cells. Indeed, expression of a dominant-negative isoform of the Wg effector TCF fully suppressed invasion by sin3aRNAi cells (ptc>sin3aRNAi TCFDN; Figure 5b).
Drosophila TCF can act either as an activator or as a repressor depending on its association with the β-Catenin ortholog Armadillo; the in-vivo consensus binding site AGAWAW (W=A/T) has been established for TCF.45 We identified a subset of Sin3a target genes that contained consensus TCF-binding sites within 5 kb of their transcriptional start sites. For example, and relevant to the data presented above, 6 sites were identified for Mkk4, 22 for RhoGAP93B and 31 for dCsk (Figure 5c), whereas chance would predict a single site within the 5-kb region. This suggested that TCF might oppose transcriptional regulation of a subset of Sin3a targets. We addressed this directly by a western analysis of the Sin3a targets identified through ChIP-seq and RT–PCR. While knockdown of Sin3a led to increased Src, Jnk and Rho1 activities, simultaneous expression of TCFDN strongly suppressed their activation (Figure 5g). Global histone modification changes induced by sin3a knockdown were also reversed by simultaneous expression of TCFDN, indicating that Sin3a and TCF functionally oppose each other on chromatin at a global level (Figures 5d, e and g). Other signaling pathways including PI3K (pAKT) were suppressed by TCFDN but were unaffected by sin3aRNAi alone (Figure 5g), indicating that TCF has roles beyond its interactions with Sin3a.
Knockdown of sin3a led to reduced levels of the β-Catenin ortholog Armadillo (Arm) at the adherens junctions (Figure 2c) yet, surprisingly, led to an overall increase in Arm protein levels within wing discs (Figure 5g). Activated dRetMEN2 also induced increased Arm levels, similar to a recent report regarding human MEN2 cancer cells.46 This shift of Arm away from the junctions is reminiscent of a similar phenomenon described in human tumors,47–49 though the mechanism by which β-Catenin is altered is not well understood.
We explored the possibility that Sin3a increases cytoplasmic Arm stability by targeting a component of the Arm/β-Catenin degradation complex. The complex is composed of Axin, glycogen synthase kinase 3β (GSK3β), Casein Kinase 1 and Adenomatous polyposis coli (Apc): together they promote phosphorylation and degradation of β-Catenin. Protein Phosphatase 1β (PP1) counters the activity of the degradation complex by dephosphorylating Axin, which leads to reduced phosphorylation and increased stabilization of β-Catenin.50 In our ChIP-seq analysis, Sin3a bound strongly to the promoter of PP1–87B (dPP1β), the Drosophila ortholog of PP1β (Figure 5f); as a control we found that Sin3a bound to MCM5, a known target of mammalian Sin3A.8,51 Indeed, knockdown of sin3a—or expression of dRetMEN2B—led to increased dPP1β levels; as anticipated, phosphorylation of the upstream degradation complex component GSK3β was unaffected (Figure 5g). Apc is also a target of Sin3a and knockdown of sin3a led to increased levels of Apc mRNA (Figure 3a); however, our findings indicate that, at least in the Drosophila wing disc, increased dPP1β levels have a dominant effect over increased Apc levels, allowing for increased levels of β-Catenin.
We conclude that reduction of Sin3a upregulates PP1, in turn stabilizing and therefore increasing levels of Arm protein. These results indicate a novel functional and molecular link between Sin3a and the Wg-Arm/β-Catenin axis. It supports the view that a key role for Sin3 proteins is to oppose activation of Wnt pathway signaling, adding PP1β as a component of the ‘invasion module’ regulated by the Sin3 complex.
Reduced Sin3 in human cancers
Overall, our studies demonstrate that activation of Drosophila dRetMEN2B or reduction of Sin3a leads to activation of a ‘tumor module’—including Src, Jnk, Rho1 and Arm/β-Catenin pathways— that in turn promotes cell motility and invasion. We next asked if a similar process is conserved in human cancers. Mutations in sin3 loci have not been reported for human tumors and we hypothesized that, similar to flies, partial reduction of Sin3 mRNA levels would be optimal to promote cancer progression. Of note, a recent report associated reduced sin3a expression with greater likelihood of non-small cell lung cancer tumors but did not detect significant loss of heterozygosity (LOH) at the sin3a locus.52
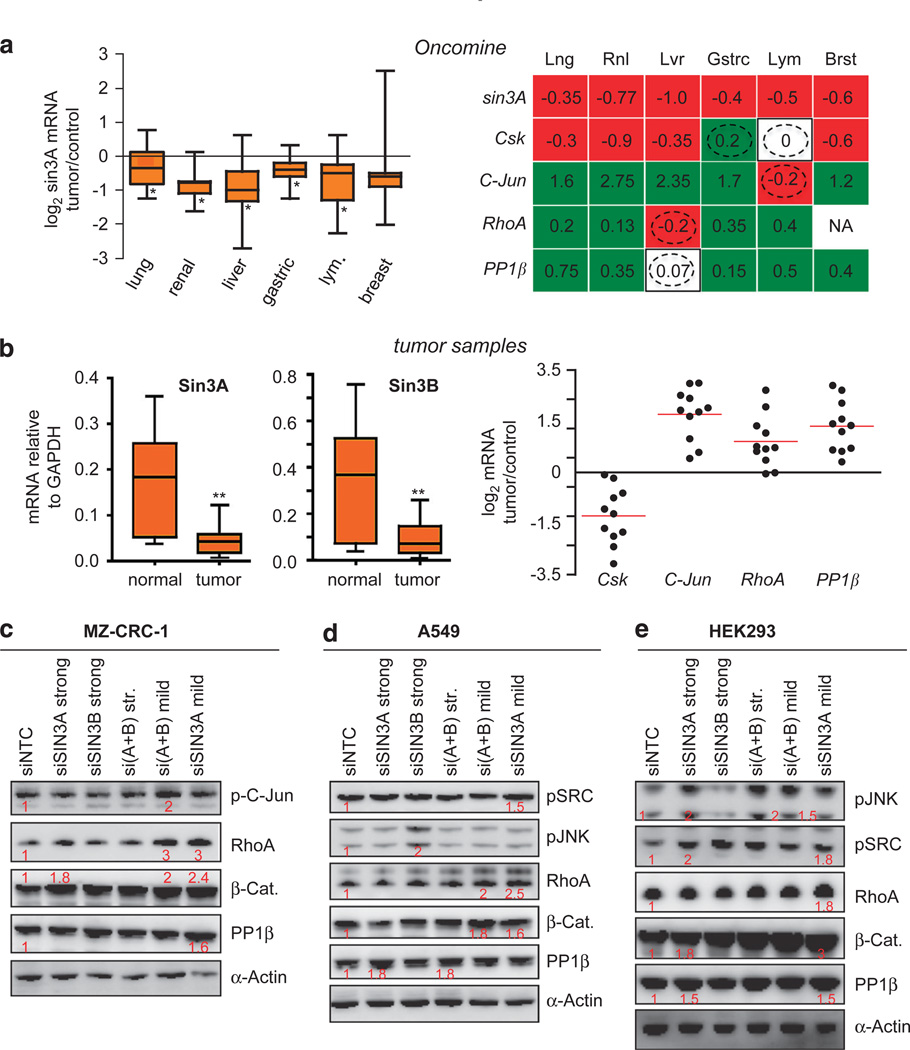
Analyzing patient tumor microarray data from the ONCOMINE database, we found that Sin3 mRNA levels were consistently and significantly reduced in most tumor types we examined including lung, kidney, liver, gastric and breast tumors, as well as in lymphoma (Figure 6a). We analyzed 12 patient lung tumor samples and found a median fourfold reduction of sin3A/B mRNA levels compared with non-diseased lung tissue (Figure 6b; Supplementary Figure S6A). In addition, we found that Sin3a targets established through our fly studies—Csk, Rho and PP1β—were regulated in an analogous manner in the human tumor cohorts from ONCOMINE (Figure 6a; Supplementary Figure 8A) as well as in the 12 patient lung tumor samples (Figure 6b). For example, in these tumor cohorts Csk mRNA was downregulated while PP1β and RhoA were upregulated. C-jun gene is a known transcriptional target of the β-Catenin axis:53 both in the Oncomine collection and in our patient tumor samples C-jun mRNA was significantly increased in tumor cohorts with reduced Sin3 mRNA levels. β-Catenin mRNA levels were not significantly altered in our patient tumor samples, indicating that changes in β-Catenin activity are not due to alterations in its own transcription (Supplementary Figure 8C). These data further suggest that Sin3 proteins act as suppressors of an ‘invasion module’ in both flies and human tumors. We next tested this model in transformed and normal human cell lines.
Figure 6.
Sin3 mRNA reduced in human tumors; leads to deregulation of pro-invasive genes. (a) Left: log2 Sin3A mRNA levels are reduced in a variety of human tumors (see Materials and methods) relative to normal tissue. Orange box encompasses 25th–75th percentile, solid black line indicates median and error bars (left) indicate range. Right: Sin3A targets Csk, c-Jun, RhoA and PP1β are deregulated in these tumors. Median log2 mRNA levels of each gene are represented inside each box. Red: downregulated, green: upregulated, white: no significant change, NA: data not available. Dotted circle signifies opposite mode of regulation to our findings. Scatter plots of individual tumor data for each gene are in Supplementary Figure 8A (b) Left: Expression levels of Sin3A and Sin3B mRNA in patient lung tumor samples (n=12) compared with normal tissue. mRNA levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels in each tissue. **P<0.002 as determined by two-tailed, Student’s T-test. Right: Sin3A targets Csk, c-Jun, RhoA and PP1β are deregulated in these patient samples. Relative log2 mRNA levels of each gene are plotted for each tumor. Median relative log2 mRNA levels are represented as red bars. Csk=−1.42, c-Jun= +2.14, RhoA= + 0.86 and PP1β=+ 1.62. (c) Western detection of indicated proteins in MZ-CRC-1 thyroid cancer lines transfected with indicated siRNAs, siNTC control (lane 1), siSin3A-40 nM (lane 2), siSin3B-40 nM (lane 3), siSin3A + B-40 nM each (lane 4), siSin3A + B-20 nM each (lane 5), siSin3A-20 nM (lane 6). Proteins with increased levels are quantified in red; actin was used as loading control. Knockdown of Sin3 induced phospho-c-Jun, RhoA, PP1β and β-Catenin. (d) Western detection of indicated proteins in A549 lung adenocarcinoma cancer lines transfected with the shown siRNAs as in (c). Knockdown of Sin3 induced JNK and Src activity as well as RhoA, PP1β and β-Catenin. (e) Western detection of indicated proteins in HEK293 cell lines transfected with Sin3 siRNAs as in (c). Again, knockdown of Sin3 induced JNK and Src activity as well as RhoA, PP1β and β-Catenin.
Reducing human Sin3 activated a similar module of pro-invasive genes
We examined a human MZ-CRC-1 thyroid cancer cell line derived from the parafollicular cells of an MEN2B patient. siRNA-mediated knockdown of Sin3A and/or Sin3B (Supplementary Figures S6B, S7C and S7D) resulted in elevated levels of pC-jun, RhoA, β-Catenin and PP1β proteins (Figure 6c; Supplementary Figure S9), mirroring our Drosophila results.
Our ONCOMINE and lung tumor data suggested that Sin3 proteins may act in tumors other than MTCs (Figures 6a and b; Supplementary Figures S6 and S8). We provided functional data to support this view by examining a human A549 lung adenocarcinoma cell line with siRNAs that showed consistent knockdown of Sin3A or Sin3B (Supplementary Figures S6B, S7C and S7D and data not shown). siRNA-mediated Sin3A, Sin3B or Sin3A + B knockdown within A549 cells resulted in elevation of pSRC, pJNK, RhoA, β-Catenin and PP1β levels (Figure 6d; Supplementary Figure S9).
Finally, we tested if Sin3 proteins controlled the same module of genes in non-transformed cells. siRNA knockdown of Sin3 proteins in HEK293 human embryonic kidney cells showed robust upregulation of pSRC, pJNK, RhoA, β-Catenin and PP1β levels, indicating that Sin3 proteins can regulate expression of these genes during normal cellular function (Figure 6e; Supplementary Figure S9).
Importantly, milder siRNA mediated knockdowns (20 nm) consistently activated Sin3 targets more potently than stronger knockdowns (40 nm; Figures 6c–e; Supplementary Figure S9). This is consistent with previous reports that full Sin3A/B knockouts exhibit significant cell lethality.8,13 The stronger effects of mild Sin3 reduction may explain why human tumors exhibited reduced levels of Sin3 mRNA but not complete loss (Figures 6a and b).
Reducing Sin3 levels increased invasiveness of human lung adenocarcinoma cells
Together, our Drosophila studies indicate that Sin3a is a tumor suppressor that regulates key pathways involved in proliferation, EMT and invasion/migration. Similar to in-vivo tumors cultured MZ-CRC-1 cells are only slowly migratory (not shown), and we therefore focused on A549 human lung adenocarcinoma cells.54 Overexpression of human Sin3A and/or Sin3B (Supplementary Figures S7A and B) reduced cell number up to ~40% (Figure 7a), indicating that Sin3 proteins can oppose tumor cell expansion. We next assessed Sin3’s role in migration of transformed mammalian cells using a wound healing ‘scratch’ assay. Importantly, siRNA-mediated knockdown of Sin3A increased cell motility/invasiveness of A549 cells almost twofold compared with control (Figures 7b and d). That is, moderate reduction of Sin3A did not make cells unhealthy but instead increased their motility and potential for invasion. FACS analysis indicated a modest shift of these cells into S phase, indicating that excess proliferation is unlikely to account for the rapid wound healing (Supplementary Figure S6C).
Figure 7.
Sin3a regulates a ‘module’ of cell invasion. (a) A549 lung adenocarcinoma cells were transiently transfected with pCINEO, pCINEO-Sin3a and pCINEO-Sin3b constructs and analyzed after 48 h for Sin3A and Sin3B protein levels (Supplementary Figures 6A and B). Cell viability decreased as assessed with an MTT assay. Data are presented as mean, error bars represent standard error of mean, and **P<0.005 as determined by two-tailed Student’s t-test. (b–d) Wound healing assay performed with confluent A549 cells that were transiently transfected with siRNAs targeting Sin3A and Sin3B. Sin3A knockdown enhanced A549 cells invasion/migration capacity by twofold or more compared with siNTC control (b), representative images after 66 h of wound healing (c, d); P-values: *P<0.005; **P<0.004; ***P<0.007 as determined by two-tailed Student’s t-test. See Materials and methods for description of imaging and analysis. Sin3B difference was not statistically significant. (e) A model for metastatic invasion at tumor boundaries. Sin3a regulates diverse signaling pathways including Src, Jnk and Actin remodeling factors including Rho and Rac. Through PP1, Sin3a also regulates β-Catenin levels. We propose that these genes act as a Sin3 ‘invasion module’ that can strongly enhance other oncogenes. Activation of these pathways leads to invasion; Sin3a normally acts to restrain cells from invasive signals.
These data are consistent with the view that Sin3 proteins normally act as negative regulators of tumor progression including migration in both lung adenocarcinoma and MEN2 thyroid cells. Our data demonstrate a marked parallel between Sin3-regulated pathways in Drosophila and vertebrates, controlling cell motility and invasion during cell transformation.
DISCUSSION
Despite growing interest, the precise in-vivo role of Sin3 proteins in tumorigenesis remains poorly defined. Our Drosophila studies define specific in-vivo target pathways and demonstrate that Sin3a promotes tumorigenesis including cell motility and invasion. Pathways include Src, Jnk, Rho-GTPases and Wnt/β-Catenin/TCF, defining a ‘module of cell invasion’ (Figure 7e). The result is emergence of invasive migration, the first step in metastasis. Our genetic studies further predicted that partial Sin3 complex reduction would strongly enhance other oncogenes. Consistent with this view, our studies in human tumors and tumor cells have confirmed that (i) Sin3 expression is commonly reduced in a broad palate of tumor types and (ii) reduction of Sin3 deregulates many of the pathways defined in our Drosophila studies as involved in cell motility and invasion. (iii) Many of these Sin3a targets are also regulated in a similar manner in the human tumors, further supporting our hypothesis that Sin3 proteins regulate a genetic ‘module of invasion’.
Novel insights into sin3a–mediated invasive migration pathways
ChIP-seq and RT–PCR analyses validated our genetic evidence that Sin3a is a significant regulator of invasion/cell migration and EMT. Several genes are particularly noteworthy. Direct targeting of dCsk by the Sin3a complex confirmed the strong synergy observed between sin3a and dCsk mutants in vivo, and is consistent with our previous work demonstrating that dCsk/Src signaling is a primary regulator of cell migration.23 Similarly, identification of Rho and Rho-GTPases as targets of Sin3a is also consistent with its role in normally opposing migration. The ability of TCFDN to suppress Sin3a–mediated (i) transcriptional regulation, (ii) invasion and (iii) histone modification suggests a globally antagonistic relationship between TCF and Sin3a.
Our studies also establish a novel link between Sin3 and β-Catenin activity. We provide evidence that Sin3 proteins control the stability of β-Catenin protein by transcriptionally regulating PP1β, an important component of the β-Catenin degradation complex. β-Catenin protein levels are sensitive to even slight changes in phosphorylation of components of the degradation complex including Axin and GSK3β.50 Increased β-Catenin levels, for example, through mutations in APC gene in colorectal tumors, result in translocation to the nucleus where it can direct various pro-invasive programs of gene expression.49,55,56 Reduction of Sin3 function represents a novel mechanism by which increased levels of nuclear β-Catenin emerge to promote tumor progression in the absence of mutations in degradation complex components such as APC.
In addition to controlling β-Catenin stability, Sin3a and Wnt signaling interact at a second level: many Sin3a target promoters also had TCF-binding sites. How these two interact at target sites is unclear. Emerging evidence in both flies and vertebrates indicates that not only is TCF converted to an activator by β-Catenin but, conversely, TCF/β-Catenin can act as a repressor complex (reviewed in Cadigan57). Add to this the ability of reduced Sin3a to increase nuclear β-Catenin, and the potential for complexity including feedback loops is substantial. These possibilities will need to be examined on a target by target basis.
Sin3a acts primarily as a transcriptional activator
More than 70% of Sin3a’s targets were under positive regulation; we also observed significant in-vivo loss of active chromatin markers H3K4Me3, H3K9Ac and H4K12Ac in cells where Sin3a levels were reduced. Previous studies have provided circumstantial evidence that Sin3A functions as an activator independent of HDACs: knockdown of HDAC and sin3A had opposite effects on H3 and H4 acetylation;51 gene expression profiles of HDAC and sin3A knockdown showed only limited overlap;58 and Sin3A positively regulated a large number of its targets.29 Perhaps these context-dependent differences in Sin3 activity reflect regulated access to multiple transcriptional complexes.29
Implications for cancer
Sin3 proteins control diverse transcriptional networks that likely make it unfeasible for tumor cells to completely lose sin3 function. Indeed, null sin3a clones failed to thrive in vertebrates or Drosophila S2 cells,8,51 a major reason for our focus on knockdown approaches. Indeed, milder knockdowns of Sin3 components in cancer cells resulted in more potent activation of Src, Jnk, Rho and β-Catenin pathways. Further, our RT–PCR analysis and a survey of a wide variety of human tumors showed consistent but partial downregulation of Sin3 mRNA.
Oncogene and tumor suppressor function have traditionally been analyzed in the context of a few targets/pathways. Our in-vivo analysis of Sin3 protein function suggests that the overall ‘network effect’ of tumor suppressors may underlie their ability to regulate tumorigenesis. Moderate regulation of multiple targets relevant for invasion/migration, as we show for Sin3a, might be an effective mechanism to promote tumorigenesis without disrupting, for example, cell viability.
Our studies further link Sin3 as a central modulator of RetMEN2 disease progression. MEN2 tumors typically require years to exhibit metastatic disease,59 providing an opportunity for secondary loci to participate in advancement to metastasis. Significant to this point, in our invasion studies expression of dRetMEN2B alone directed proliferation but was unable to induce invasion; the addition of sin3a knockdown was required to develop an aggressive metastasis-like phenotype. This highlights the potential for a sin3a-regulated ‘invasion module’ that promotes and enhances tumor progression as an adjunct to primary oncogenic mutations. Such ‘invasion modules’ might be a fairly common mechanism underlying tumor progression.
MATERIALS AND METHODS
Analysis of cell-cycle distribution
After the cells were transfected, they were stained with propidium iodide to ascertain the DNA content and determine cell-cycle distribution within the cell population as previously described.60
ChIP-Seq
Rabbit polyclonal Sin3a antibody for Chip-seq and immunohistochemistry: we fused N-terminal amino acids 500–620 of Drosophila Sin3a to Glutathione S-Transferase. Fusion protein was expressed, purified, and rabbits were immunized (ProteinTech Group Inc., Manchester, UK). ChIP-seq experiments have been performed on 0–16 h developing Drosophila embryos as previously described.28 Two replicate experiments and one reference sample (Input chromatin) were run on three different lanes of Illumina Genome Analyzer II (Illumina Genome Analyzer, San Diego, CA, USA) (1 x 36 bp). Sequences obtained were mapped on the DM3 reference genome with bowtie and signal files and peaks were generated using MACS with a 1% false discovery rate setting. The reproducibility of 4083 transcription factor-binding sites was 0.87 (recommended 0.8 per modENCODE;53,54 good signal-to-noise ratio of 114.77 (median signal-to-noise ratio for modENCODE projects is 132.22; min 33; max 540). Raw data are accessible in GEO with the following accession number: GSE23122.
Fly stocks, genetics and subcloning
Fly stocks were obtained from Bloomington and VDRC (Vienna Drosophila RNAi Center) Drosophila stock centers, R Carthew, M Mlodzik, M Vidal and E Bangi. UAS-sin3aRNAi flies obtained from VDRC were mobilized by crossing to flies expressing transposase (D2–3 Sb; TM6B). A 1-h heat shock of hs-FLPase was used to generate FLP-out clones of RNAi transgenes in 0–48 h old animals. UAS-RetMEN2B flies were generated by ligating a partial EcoR1-digested GMR-dRetMEN2B DNA fragment21 into EcoR1 site of pUAST vector. Transgenic flies were generated by standard protocol.
Histology and antibodies
Third instar salivary glands and wing discs were staged and fixed in 4% paraformaldehyde. Immunofluorescence was performed as described.61 Antibodies used were Cleaved Caspase3, pRet, pJnk, pAkt, pGSK3β, p-C-jun (Cell Signaling, Danvers, MA, USA), anti-pSrc(Y418) (Invitrogen, Grand Island, NY, USA), anti-Laminin (Abcam, Cambridge, MA, USA), anti-pERK (Sigma, St Louis, MO, USA), plus anti-Actin, anti-Arm, anti-Dlg, anti-E-Cadherin, anti-Mmp, anti-Rho1, anti-Syntaxin (Developmental Studies Hybridoma Bank). Anti-Sin3A, anti-Sin3B, anti-Actin, anti-GAPDH, anti-PP1α and anti-RhoA antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and anti-β-Catenin antibody (from BD Biosciences, San Jose, CA, USA). All histone modification antibodies were obtained from ActiveMotif (Carlsbad, CA, USA).
ONCOMINE data
Primary sources for the tumor data for the different cancers from ONCOMINE were the following: lung,62 lymphoma,63 liver,64 breast,65 kidney,66 and gastric.67
Plasmids and siRNAs
Human Sin3A (Origene, Rockville, MD, USA) was transfected into cells by Lipofectamine 2000 incubation (Invitrogen) for 24 h. Validated Sin3A and Sin3B–specific siRNA was transfected with HiPerfect (Qiagen, Valencia, CA, USA) into cell lines seeded at 60–70% confluency. Knockdown was achieved with Sin3A and Sin3B ‘ON-TARGETplus Set of 4’ siRNAs (Dharmacon, Lafayette, CO, USA). Knockdown and overexpression were assessed by western blotting and quantitative real-time PCR.
Quantitative real-time PCR and RT-PCR
RNA was isolated from cell lines and patient tumors using the RNeasy Mini Kit (Qiagen). For each PCR, 1 µg RNA was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). Each cDNA sample was subjected to PCR amplification with the SYBR green PCR Master Mix (Applied Biosystems Inc., Carlsbad, CA, USA) on an ABI PRISM 7900HT plate-reader instrument (Applied Biosystems Inc). All values were normalized to glyceraldehyde-3-phosphate dehydrogenase levels and compared with both 18S and Actin expression and additional controls.
For RT-PCR analysis of putative Sin3a targets, cDNA was made using RNA extracts from w-, sin3aRNAi and Dicer; sin3aRNAi embryos. RT-PCR with gene-specific primers was performed using standard procedures. PCR products were run on 2% agarose gels and band pixel intensity was analyzed using Quantity One (Bio-Rad) Image analysis software. Experiments were performed at least three independent times and expression of each target in sin3a knockdown conditions was normalized with respect to expression in w- samples.
Western analysis
Ten third-instar discs of each genotype were dissolved in Lysis Buffer (50 mm Tris, 150mm NaCl, 1% Triton X-100,1 mm EDTA) supplemented with protease inhibitor cocktail (Sigma) and phosphatase inhibitor cocktail (Sigma). For human cell lines, lysis was performed with RIPA buffer. Total protein in each sample was quantitated using BIORAD protein assay. Samples were boiled, resolved on SDS-PAGE and transferred by standard protocols. Membranes were stripped with SIGMA Restore stripping buffer and reprobed with other antibodies to assess signal under exactly the same loading conditions.
Wound healing assay
A549 cells were transiently transfected with siRNAs when they were fully confluent. Twenty-four hours later media was changed and a single scratch per well was inflicted with a 1-ml pipette tip. Phase-contrast images were taken every 6–8 h at fixed landmarks (3 per well) in every well until wound was almost closed in knockdown experiments. Rate of wound closing was measured as the difference between original wound width minus final width divided by the hours elapsed (Do – DF)/hours. Three measurements per image were taken giving 9 (3×3) data points per time point per treatment and the mean of these measurements was represented graphically.
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the Cagan Lab for sharing reagents, information, and helpful advice and Justin Graves for providing help mapping UAS-dRetMEN2 transgenic flies. This work was supported by NIH/NCI grants R01-CA084309 and R01-CA109730 to RC and American Cancer Society Fellowship grant 120886-PFM-11-137-01-DDC to TD.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Author contributions: TD. and R.C. designed the project. T.D. performed the in vivo Drosophila work, the transient siRNA transfection and western analysis on cell lines, and analyzed the ONCOMINE data. T.D. and J.S. performed wound healing assay. J.S. performed MTT assay. G.N. and J.S. generated and analyzed patient tumor expression data. N.N. generated the ChIP-seq data. T.D. and R.C. analyzed the data and wrote the manuscript.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
REFERENCES
- 1.Lafon-Hughes L, Di Tomaso MV, Mendez-Acuna L, Martinez-Lopez W. Chromatin-remodelling mechanisms in cancer. Mutat Res. 2008;658:191–214. doi: 10.1016/j.mrrev.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 3.Janic A, Mendizabal L, Llamazares S, Rossell D, Gonzalez C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science. 2010;330:1824–1827. doi: 10.1126/science.1195481. [DOI] [PubMed] [Google Scholar]
- 4.Kapoor A, Goldberg MS, Cumberland LK, Ratnakumar K, Segura MF, Emanuel PO, et al. The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature. 2010;468:1105–1109. doi: 10.1038/nature09590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J, Benavente CA, McEvoy J, Flores-Otero J, Ding L, Chen X, et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481:329–334. doi: 10.1038/nature10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hassig CA, Fleischer TC, Billin AN, Schreiber SL, Ayer DE. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/s0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 7.Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, et al. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 8.Dannenberg JH, David G, Zhong S, van der Torre J, Wong WH, Depinho RA. mSin3A corepressor regulates diverse transcriptional networks governing normal and neoplastic growth and survival. Genes Dev. 2005;19:1581–1595. doi: 10.1101/gad.1286905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimes JA, Nielsen SJ, Battaglioli E, Miska EA, Speh JC, Berry DL, et al. The co-repressor mSin3A is a functional component of the REST-CoREST repressor complex. J Biol Chem. 2000;275:9461–9467. doi: 10.1074/jbc.275.13.9461. [DOI] [PubMed] [Google Scholar]
- 10.Pile LA, Spellman PT, Katzenberger RJ, Wassarman DA. The SIN3 deacetylase complex represses genes encoding mitochondrial proteins: implications for the regulation of energy metabolism. J Biol Chem. 2003;278:37840–37848. doi: 10.1074/jbc.M305996200. [DOI] [PubMed] [Google Scholar]
- 11.Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, et al. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- 12.Rao G, Alland L, Guida P, Schreiber-Agus N, Chen K, Chin L, et al. Mouse Sin3A interacts with and can functionally substitute for the amino-terminal repression of the Myc antagonist Mxi1. Oncogene. 1996;12:1165–1172. [PubMed] [Google Scholar]
- 13.David G, Grandinetti KB, Finnerty PM, Simpson N, Chu GC, Depinho RA. Specific requirement of the chromatin modifier mSin3B in cell cycle exit and cellular differentiation. Proc Natl Acad Sci USA. 2008;105:4168–4172. doi: 10.1073/pnas.0710285105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai A, Kennedy BK, Barbie DA, Bertos NR, Yang XJ, Theberge MC, et al. RBP1 recruits the mSIN3-histone deacetylase complex to the pocket of retinoblastoma tumor suppressor family proteins found in limited discrete regions of the nucleus at growth arrest. Mol Cell Biol. 2001;21:2918–2932. doi: 10.1128/MCB.21.8.2918-2932.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy M, Ahn J, Walker KK, Hoffman WH, Evans RM, Levine AJ, et al. Tran-scriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev. 1999;13:2490–2501. doi: 10.1101/gad.13.19.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Akinmade D, Hamburger AW. The ErbB3 binding protein Ebp1 interacts with Sin3A to repress E2F1 and AR-mediated transcription. Nucleic Acids Res. 2005;33:6024–6033. doi: 10.1093/nar/gki903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst DR, Xie Y, Edmonds MD, Welch DR. Multiple forms of BRMS1 are differentially expressed in the MCF10 isogenic breast cancer progression model. Clin Exp Metastasis. 2009;26:89–96. doi: 10.1007/s10585-008-9216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samant RS, Debies MT, Hurst DR, Moore BP, Shevde LA, Welch DR. Suppression of murine mammary carcinoma metastasis by the murine ortholog of breast cancer metastasis suppressor 1 (Brms1) Cancer Lett. 2006;235:260–265. doi: 10.1016/j.canlet.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Meehan WJ, Samant RS, Hopper JE, Carrozza MJ, Shevde LA, Workman JL, et al. Breast cancer metastasis suppressor 1 (BRMS1) forms complexes with retino-blastoma-binding protein 1 (RBP1) and the mSin3 histone deacetylase complex and represses transcription. J Biol Chem. 2004;279:1562–1569. doi: 10.1074/jbc.M307969200. [DOI] [PubMed] [Google Scholar]
- 20.Farias EF, Petrie K, Leibovitch B, Murtagh J, Chornet MB, Schenk T, et al. Interference with Sin3 function induces epigenetic reprogramming and differentiation in breast cancer cells. Proc Natl Acad Sci USA. 2010;107:11811–11816. doi: 10.1073/pnas.1006737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Read RD, Goodfellow PJ, Mardis ER, Novak N, Armstrong JR, Cagan RLA. Drosophila model of multiple endocrine neoplasia type 2. Genetics. 2005;171:1057–1081. doi: 10.1534/genetics.104.038018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plaza-Menacho I, Burzynski GM, de Groot JW, Eggen BJ, Hofstra RM. Current concepts in RET-related genetics, signaling and therapeutics. Trends Genet. 2006;22:627–636. doi: 10.1016/j.tig.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Vidal M, Larson DE, Cagan RL. Csk-deficient boundary cells are eliminated from normal Drosophila epithelia by exclusion, migration, and apoptosis. Dev Cell. 2006;10:33–44. doi: 10.1016/j.devcel.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Ellison-Zelski SJ, Alarid ET. Maximum growth and survival of estrogen receptor-alpha positive breast cancer cells requires the Sin3A transcriptional repressor. Mol Cancer. 2010;9:263. doi: 10.1186/1476-4598-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nascimento EM, Cox CL, Macarthur S, Hussain S, Trotter M, Blanco S, et al. The opposing transcriptional functions of Sin3a and c-Myc are required to maintain tissue homeostasis. Nat Cell Biol. 2011;13:1395–1405. doi: 10.1038/ncb2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 27.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 28.Negre N, Brown CD, Ma L, Bristow CA, Miller SW, Wagner U, et al. A cis-regulatory map of the Drosophila genome. Nature. 2011;471:527–531. doi: 10.1038/nature09990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Oevelen C, Wang J, Asp P, Yan Q, Kaelin WG, Jr, Kluger Y, et al. A role for mammalian Sin3 in permanent gene silencing. Mol Cell. 2008;32:359–370. doi: 10.1016/j.molcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code? Curr Opin Genet Dev. 2005;15:163–176. doi: 10.1016/j.gde.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Cooper JA, Gould KL, Cartwright CA, Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986;231:1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- 33.Iwashita T, Asai N, Murakami H, Matsuyama M, Takahashi M. Identification of tyrosine residues that are essential for transforming activity of the ret proto-oncogene with MEN2A or MEN2B mutation. Oncogene. 1996;12:481–487. [PubMed] [Google Scholar]
- 34.Songyang Z, Carraway KL, 3rd, Eck MJ, Harrison SC, Feldman RA, Mohammadi M, et al. Catalytic specificity of protein-tyrosine kinases is critical for selective signalling. Nature. 1995;373:536–539. doi: 10.1038/373536a0. [DOI] [PubMed] [Google Scholar]
- 35.Read RD, Bach EA, Cagan RL. Drosophila C-terminal Src kinase negatively regulates organ growth and cell proliferation through inhibition of the Src, Jun N-terminal kinase, and STAT pathways. Mol Cell Biol. 2004;24:6676–6689. doi: 10.1128/MCB.24.15.6676-6689.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasegawa T, Enomoto A, Kato T, Kawai K, Miyamoto R, Jijiwa M, et al. Roles of induced expression of MAPK phosphatase-2 in tumor development in RET-MEN2A transgenic mice. Oncogene. 2008;27:5684–5695. doi: 10.1038/onc.2008.182. [DOI] [PubMed] [Google Scholar]
- 37.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. EMBO J. 2006;25:5294–5304. doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teng DH, Perry WL, 3rd, Hogan JK, Baumgard M, Bell R, Berry S, et al. Human mitogen-activated protein kinase kinase 4 as a candidate tumor suppressor. Cancer Res. 1997;57:4177–4182. [PubMed] [Google Scholar]
- 39.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahn YH, Yang Y, Gibbons DL, Creighton CJ, Yang F, Wistuba II, et al. Map2k4 functions as a tumor suppressor in lung adenocarcinoma and inhibits tumor cell invasion by decreasing peroxisome proliferator-activated receptor gamma2 expression. Mol Cell Biol. 2011;31:4270–4285. doi: 10.1128/MCB.05562-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geuking P, Narasimamurthy R, Lemaitre B, Basler K, Leulier F. A non-redundant role for Drosophila Mkk4 and hemipterous/Mkk7 in TAK1-mediated activation of JNK. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0007709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Damsky WE, Curley DP, Santhanakrishnan M, Rosenbaum LE, Platt JT, Gould Rothberg BE, et al. Beta-catenin signaling controls metastasis in Braf-activated Pten-deficient melanomas. Cancer Cell. 2011;20:741–754. doi: 10.1016/j.ccr.2011.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parri M, Chiarugi P. Rac and Rho GTPases in cancer cell motility control. Cell Commun Signal. 2010;8:23. doi: 10.1186/1478-811X-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duman-Scheel M, Johnston LA, Du W. Repression of dMyc expression by Wingless promotes Rbf-induced G1 arrest in the presumptive Drosophila wing margin. Proc Natl Acad Sci USA. 2004;101:3857–3862. doi: 10.1073/pnas.0400526101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blauwkamp TA, Chang MV, Cadigan KM. Novel TCF-binding sites specify transcriptional repression by Wnt signalling. EMBO J. 2008;27:1436–1446. doi: 10.1038/emboj.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellone MD, De Falco V, Rao DM, Bellelli R, Muthu M, Basolo F, et al. The beta-catenin axis integrates multiple signals downstream from RET/papillary thyroid carcinoma leading to cell proliferation. Cancer Res. 2009;69:1867–1876. doi: 10.1158/0008-5472.CAN-08-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart JM, et al. Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs. 2007;185:61–65. doi: 10.1159/000101304. [DOI] [PubMed] [Google Scholar]
- 48.Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer Metastasis Rev. 2009;28:151–166. doi: 10.1007/s10555-008-9179-y. [DOI] [PubMed] [Google Scholar]
- 50.Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, et al. Protein phosphatase 1 regulates assembly and function of the beta-catenin degradation complex. EMBO J. 2007;26:1511–1521. doi: 10.1038/sj.emboj.7601607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pile LA, Schlag EM, Wassarman DA. The SIN3/RPD3 deacetylase complex is essential for G(2) phase cell cycle progression and regulation of SMRTER corepressor levels. Mol Cell Biol. 2002;22:4965–4976. doi: 10.1128/MCB.22.14.4965-4976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suzuki H, Ouchida M, Yamamoto H, Yano M, Toyooka S, Aoe M, et al. Decreased expression of the SIN3A gene, a candidate tumor suppressor located at the prevalent allelic loss region 15q23 in non-small cell lung cancer. Lung Cancer. 2008;59:24–31. doi: 10.1016/j.lungcan.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Sancho R, Nateri AS, de Vinuesa AG, Aguilera C, Nye E, Spencer-Dene B, et al. JNK signalling modulates intestinal homeostasis and tumourigenesis in mice. EMBO J. 2009;28:1843–1854. doi: 10.1038/emboj.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiFeo A, Feld L, Rodriguez E, Wang C, Beer DG, Martignetti JA, et al. A functional role for KLF6-SV1 in lung adenocarcinoma prognosis and chemotherapy response. Cancer Res. 2008;68:965–970. doi: 10.1158/0008-5472.CAN-07-2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Bienz M, Clevers H. Linking colorectal cancer to Wnt signaling. Cell. 2000;103:311–320. doi: 10.1016/s0092-8674(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 57.Cadigan KM. TCFs and Wnt/beta-catenin signaling: more than one way to throw the switch. Curr Top Dev Biol. 2012;98:1–34. doi: 10.1016/B978-0-12-386499-4.00001-X. [DOI] [PubMed] [Google Scholar]
- 58.Foglietti C, Filocamo G, Cundari E, De Rinaldis E, Lahm A, Cortese R, et al. Dissecting the biological functions of Drosophila histone deacetylases by RNA interference and transcriptional profiling. J Biol Chem. 2006;281:17968–17976. doi: 10.1074/jbc.M511945200. [DOI] [PubMed] [Google Scholar]
- 59.Smith-Hicks CL, Sizer KC, Powers JF, Tischler AS, Costantini F. C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B. EMBO J. 2000;19:612–622. doi: 10.1093/emboj/19.4.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sangodkar J, Shi J, DiFeo A, Schwartz R, Bromberg R, Choudhri A, et al. Functional role of the KLF6 tumour suppressor gene in gastric cancer. Eur J Cancer. 2009;45:666–676. doi: 10.1016/j.ejca.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brachmann CB, Jassim OW, Wachsmuth BD, Cagan RL. The Drosophila bcl-2 family member dBorg-1 functions in the apoptotic response to UV-irradiation. Curr Biol. 2000;10:547–550. doi: 10.1016/s0960-9822(00)00474-7. [DOI] [PubMed] [Google Scholar]
- 62.Garber ME, Troyanskaya OG, Schluens K, Petersen S, Thaesler Z, Pacyna-Gengel-bach M, et al. Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci USA. 2001;98:13784–13789. doi: 10.1073/pnas.241500798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenwald A, Alizadeh AA, Widhopf G, Simon R, Davis RE, Yu X, et al. Relation of gene expression phenotype to immunoglobulin mutation genotype in B cell chronic lymphocytic leukemia. J Exp Med. 2001;194:1639–1647. doi: 10.1084/jem.194.11.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen X, Cheung ST, So S, Fan ST, Barry C, Higgins J, et al. Gene expression patterns in human liver cancers. Mol Biol Cell. 2002;13:1929–1939. doi: 10.1091/mbc.02-02-0023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 66.Higgins JP, Shinghal R, Gill H, Reese JH, Terris M, Cohen RJ, et al. Gene expression patterns in renal cell carcinoma assessed by complementary DNA microarray. Am J Pathol. 2003;162:925–932. doi: 10.1016/S0002-9440(10)63887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen X, Leung SY, Yuen ST, Chu KM, Ji J, Li R, et al. Variation in gene expression patterns in human gastric cancers. Mol Biol Cell. 2003;14:3208–3215. doi: 10.1091/mbc.E02-12-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.