Abstract
The advent of technologies that allow tissue specific expression or ablation of genes has contributed enormously to our knowledge of the mechanism regulating organ development and maintenance in mice. The tetracycline inducible system allows reversible regulation of gene products upon administration of Doxycycline. Here we describe the generation and activity of two transgenic lines expressing the cDNAs for the Tet responsive transcription factors rtTA and tTA (Tet-on and off) respectively under the control of an element that drives expression in the epithelium of the developing and adult kidney. Both lines show inducible and reversible activity in the embryonic and adult organ.
Keywords: Kidney development, kidney epithelium, kidney tubule, Tet-on, Tet-off
Results and Discussion
Due to the many similarities in their anatomy, physiology and pathology, the mouse kidney is an excellent system to model human kidney development, function and disease. Technological advances made over the last several years have allowed researchers to genetically engineer increasingly sophisticated mouse strains that have facilitated studies on the cellular and molecular processes regulating normal development and physiology as well as pathological conditions.
The generation and proliferation of transgenic lines expressing tissue specific Cre recombinases in concert with the engineering of strains carrying floxed alleles of various genes has provided mouse geneticists with the ability to spatially and temporally regulate gene expression. Although incredibly powerful, this system does have one weakness in that Cre mediated excision is an irreversible process. This greatly complicates any attempts to reversibly alter gene expression.
The tetracycline inducible system has been developed as an alternative to recombinase based technology (Bockamp et al., 2002; Gossen and Bujard, 1992; Gossen et al., 1995; Rossi and Blau, 1998). Briefly, in this system, tissue specific expression of a tet-activator (tTA) or reverse-tet-activator (rtTA), in combination with the administration of Doxycycline (Dox), represses or activates gene expression (respectively) downstream of a tet operator (TetO) promoter element. Withdrawal of Dox reverses the effect.
Cadherin 16 (Ksp cadherin) mRNA is expressed primarily in the developing and adult kidney epithelium (Bai et al., 2002; Thomson and Aronson, 1999). A 1.6 kb promoter element has been characterized that drives kidney specific gene expression (referred to as the Ksp promoter), primarily in the distal segments of the nephron and collecting duct(Shao et al., 2002a). This element has successfully been used to drive constitutive and tamoxifen inducible forms of Cre recombinase in the mouse kidney(Patel et al., 2008; Shao et al., 2002b). Here, we have characterized two new lines of mice expressing tTA and rtTA cDNAs under the control of the Ksp regulatory element.
KsprtTA
An rtTA (Tet-on) cDNA was cloned into the Ksp-BGH plasmid that contains the Ksp promoter, a BGH poly-adenylation site(Shao et al., 2002a; Shao et al., 2002b). The resulting plasmid KsprtTA, was injected into the nucleus of 1 cell embryos that were subsequently transferred to the ovaries of pseudo-pregnant females. G0 founders were PCR genotyped using primers specific to the KsprtTA transgene. 12 animals (7 females and 5 males) were scored as positive for the transgene. Each founder strain was crossed to a TetO-Histone-H2B-GFP (TetOH2B-GFP) (Tumbar et al., 2004) breeder to assess activity of the transgene. Females were separated on the day of vaginal plug and provided either with normal drinking water or a 5% sucrose solution containing 0.5 mg Dox/ml. None of the KsprtTA; TetO-H2B-GFP pups that were born to mothers that had not been administered Dox had detectable GFP in the kidneys although all of the Dox treated pups carrying the TetO-H2B-GFP allele had faint nuclear GFP in the spinal cord, even in the absence of the rtTA transgene (not shown). 4 of the 12 KsprtTA lines generated pups with GFP expression in the newborn kidney after being exposed to Dox in utero.
F2 males were generated from each of the KsprtTA lines that showed activity in the kidney and bred to TetO-H2B-GFP females. Plugged females were given ad libidum access to normal drinking water or drinking water containing 0.5 mg Dox/ml. Offspring from both treatments were collected at P1 and P30, fixed, sectioned and co-stained with an antibody to GFP, lectins that mark the collecting duct (DBA) or the proximal tubule (LTA) and the nuclear marker TO-PRO-3 iodide (Topro3). The percentage of GFP positive collecting duct and proximal tubule cells was calculated for both treatments. Of the 4 lines tested, KsprtTA line # 3733 had the highest percentage of labeled nuclei in both segments (88% of proximal tubule and 92% of collecting duct cells) after Dox administration.
The Ksp promoter element is reported to drive Cre expression specifically in the Wolffian duct and the distal portion of the ureteric bud (UB) from early embryonic stages (Karner et al., 2009). H2B-GFP expression was visible in the Wolffian duct of KsprtTA;TetO-H2B-GFP as early as E10.5 (data not shown). At E11.5, expression in the Wolffian duct was maintained and expression in the distal (stalk) portion of the UB was also observed (Figure 1a). No GFP positive nuclei were visible in the UB tips or the cap mesenchyme (Figure 1a). Kidney expression remained specific to the distal bud until around E15.5 at which point expression was also observed in non-UB derived epithelia. Expression continued in the collecting ducts and distal nephron segments through birth into adult stages (Figure 1 and data not shown).
Figure1. Characterization of KsprtTA activity.
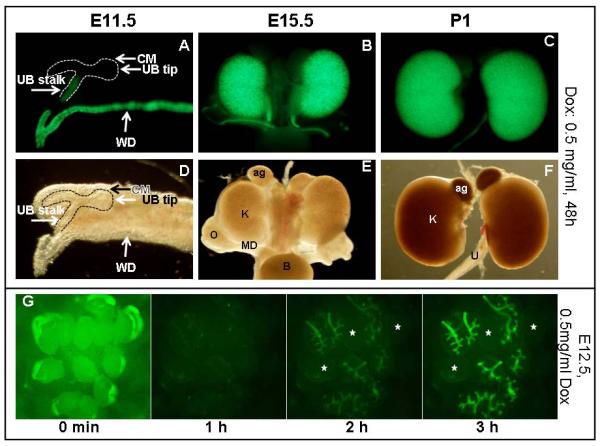
Fluorescent (A–C) and bright field (D–F) images of urogenital systems removed from embryos/pups at E11.5 (A,D), 15.5 (B, E) and P1(C,F). Pregnant females were provided with drinking water containing 0.5 mg/mL Dox for 48h prior to dissection. (G) A time course analysis shows rapid activation of KsprtTA. 12 kidneys were dissected out from E12.5 embryos and cultured in media containing 0.5 mg/mL Dox. Images were captured every 20 min. The first image is an overexposure to show all 12 dissected kidneys at time 0. After 60 minutes, 9 of the 12 kidneys showed weak TetO-H2B-GFP expression. Expression was significantly more robust after 2 hours. Expression peaked at 3 hours. The exposure times at 1, 2 and 3 hours are identical. The three non-fluorescent kidneys are indicated by stars. CM= condensed mesenchyme, UB= ureteric bud, WD=Wolffian duct.
In addition to expression in the developing urogenital system, line 3733 also showed Dox-dependent nuclear GFP in the tip of the tail at both E11.5 and 15.5. This expression appeared to be the result of enhancer piracy, as it was not observed in any of the other KsprtTA lines or in the KsptTA line (data not shown).
To test the kinetics of KsprtTA activity in the developing urogenital system, we utilized embryonic organ culture. E12.5 embryos were collected from pregnant TetO-H2B-GFP dams that had been bred to KsprtTA sires. Dams were administered normal drinking water without Dox. Dissected embryonic kidneys were placed in media containing 0.5 mg/ml of Dox and monitored over the next 24 hours using a microscope with built in environmental chamber. Photographs were taken every 20 minutes. Nuclear GFP was weakly detectable within 40 minutes of treatment and strongly expressed within 2 hours of Dox administration ex vivo (Figure 1G and data not shown).
The kinetics of activation was also tested in adult mice that had not been exposed to Dox. As mentioned, in the absence of Dox, Ksp-rtTA;TetO-H2B-GFP showed no GFP expression in P30 kidneys. However, GFP was readily detectable 24 hours after the mice were provided with drinking water containing Dox, with 66% of proximal tubules and 87% of collecting duct cells expressing H2B-GFP (Figure 2). After 48 hours, 88 percent of proximal tubules cells and 92 percent of collecting duct cells expressed H2B-GFP (Figure 2). Thus the system is rapidly and efficiently inducible both in vitro and in vivo.
Figure 2. Quantification of KsprtTA activity in different nephron segments.

Sections of kidneys from P1 (A,B) and P30 (C,D) KsprtTA; TetO-H2B-GFP kidneys were stained with the proximal tubule marker LTA (red), GFP (green) and the nuclear marker Topro-3 (blue) in A and C or the collecting duct marker DBA (red), GFP (green) and Topro (blue) in B and D. Pregnant Dams or Kspteton;TetO-H2B-GFP mice were given water with 0.5 mg/mL Dox for 24 or 48 h before the kidneys were dissected out at P1 or P30. (E) Percentage of GFP positive nuclei in the proximal tubule and the collecting duct.
We next tested the reversibility of this system. Pregnant dams were administered 0.5, 0.1 or .01 mg/ml of Dox from the point of conception, dissected at E12.5 and grown in media without Dox. Kidneys from Dams administered 0.5 mg/ml Dox still retained high levels of GFP activity 96 hours after removal from the mother (Figure 3A–C). For 0.1 mg/ml, H2B-GFP appeared to be expressed in all newly forming collecting duct epithelia and maintained at high levels in the distal collecting ducts for the first 24 hours (Figure 3D). However, after 48 hours of culture, newly formed UB branch tips did not express GFP and the levels decreased in the distal collecting ducts (Figure 3). Expression levels continued to decline in the distal collecting ducts over the next 3 days but were still faintly detectable in the ureter 5 days after withdrawal of Dox (Figure 3F). For Dams administered .01 mg/ml Dox, H2B-GFP was readily visible at the time of dissection (not shown). After 24 hours, levels appeared decreased in the oldest ducts and ureter and undetectable in newly formed ureteric buds and collecting ducts (Figure 3G). At later time points, H2B-GFP was only detectable at low levels within the ureter (Figure 3H–I). The low rates of GFP loss in the distal ducts/ureter may be caused by early exit from the cell cycle and/or low protein turnover in these cells (Adams and Oxburgh, 2009).
Figure 3. KsprtTA activity is reversible ex vivo.
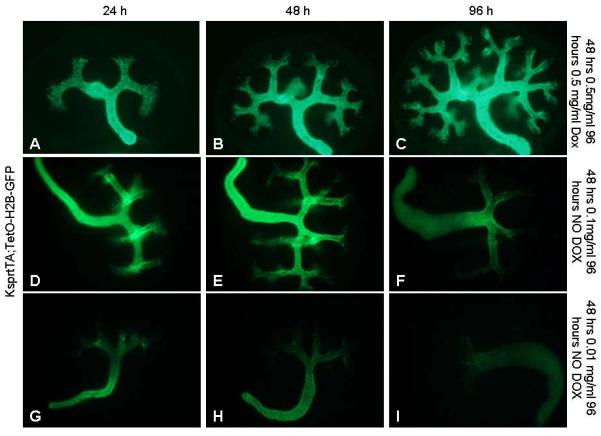
Pregnant Dams were administered 0.5 (A–C), 0.1 (D–F) or .01 (G–I) mg/mL at E9.5 then dissected out at E11.5 and cultured in media with (A–C) or without Dox (D–I). Photographs were captured after 24 (A, D, G), 48 (B, E, H) or 96 (C, F, I) hours of culture.
Similar results were obtained in vivo. Mice receiving high initial doses of Dox maintained H2B-GFP expression for extended periods of time after returning to normal drinking water. Detectable levels of GFP were still present in Ksp-rtTA; TetO-H2B-GFP adults administered 0.5 mg/ml of Dox three weeks after Dox withdrawal (Data not shown.). Retention of Dox in body tissues such as fat may explain the perdurance of H2BGFP expression in vivo. Thus although the Tet-on system is reversible, complete loss of expression of the protein(s) regulated by the Tet operator will depend on a variety of factors including protein stability, Dox dose, duration of administration and time after administration.
KsptTA
Nine independent G0 founder lines were identified for KsptTA. Each line was crossed to the TetO-H2B-GFP reporter and the pregnant dams were given normal drinking water. Only one of the nine founders gave rise to progeny that drove expression of H2B-GFP in the kidney of newborn (P1) pups. All further analysis was performed on this line.
F2 males were generated from the single active Ksp-tTA line. These males were crossed to TetO-H2B-GFP females that were provided with normal drinking water. Litters were collected at E10.5, 12.5, 15.5 and P1. Unlike the KsprtTA mice, the KsptTA;TetO-H2B-GFP embryos did not show detectable GFP expression until E15.5 (Figure 4c and data not shown). At this time, the H2B-GFP expression was primarily restricted to the renal vesicle derivatives (proximal tubule through distal tubule, Figure 5). The highest degree of activity appeared to be in the loop of Henle (Fig 5). A very low percentage of DBA positive (collecting duct) cells were stained (Figure 5). 61 % of proximal tubule nuclei from P30 KsptTA;H2B-GFP animals were GFP positive while only 25% of the collecting duct nuclei were positive (Figure 6).
Figure 4. Characterization of KsptTA activity.
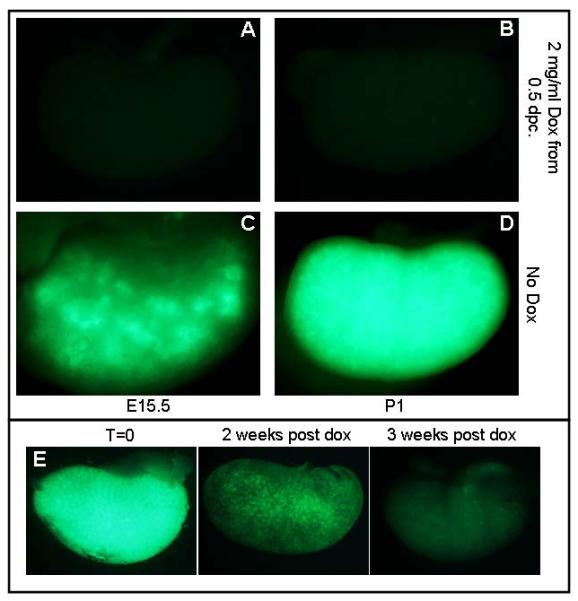
Whole mount images of E15.5 (A,C) and P1 (B,D) KsptTA;TetO-H2B-GFP kidneys from Dams who were given water with (A,B) or without (C,D) 2 mg/mL Dox. (E) Wholemount images of kidneys dissected from KsptTA;TetO-H2B-GFP adult mice that were given water containing 2 mg/mL Dox beginning at P30 and harvested after the time indicated. Exposure times for each image are the same.
Figure 5. Activity of KsptTA in different nephron segments.

E15.5 (A–D), P1 (E–H) and P30 (I–L) KsptTA;TetO-H2B-GFP positive kidneys sections stained with an antibody to GFP (green), the nuclear marker Topro (blue) and the proximal tubule marker LTA (red in A, E and I), the collecting duct marker DBA (red in B, F and J), antibody to the thick ascending limb marker Tamm-Horsfall Protein (THP. Red in C, G and K) and an antibody to the thin descending limb marker aquaporin 1 (Aqp1. Red in D, H and L). Scale bars for photographs are 200 μM and inserts are 40 μM.
Figure 6. Quantification of KsptTA activity.
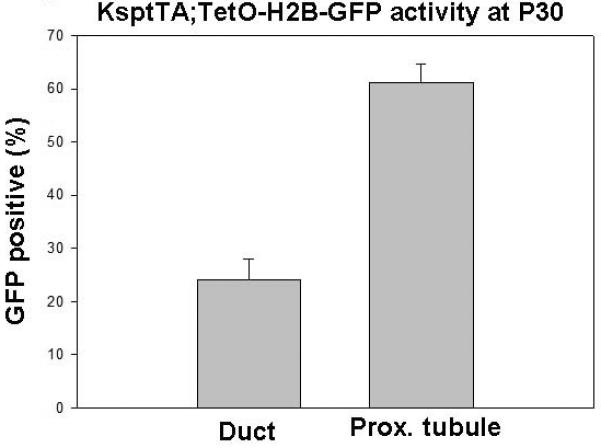
The percentage of collecting duct and proximal tubule nuclei that co-expressed H2b-GFP at P30. KsptTA;TetO-H2B-GFP mice were provided with normal drinking water from birth.
We also tested the reversibility of this system. No GFP positive cells were detectable in P1 kidneys of KsptTA;H2B-GFP pups exposed to Dox (administered to mother) from the time of conception (Figure 4). GFP expression was undetectable in one-month-old Ksp-tTA; TetO-H2BGFP mice that were administered Dox from birth (not shown). If P30 Ksp-tTA; TetO-H2B-GFP mice were administered Dox, decreased levels of GFP were evident within 72 hours. However GFP activity was not completely extinguished for nearly 2 weeks (Figure 4).
Methods
Plasmid Constructs
The KsptTA and KsprtTA expression plasmids contain the tTA or rtTA genes under the control of the kidney specific (Ksp) promoter, Cadherin 16 promoter. The pTet-on Advanced vector was purchased from Clontech. The KsprtTA plasmid was obtained by gel isolation of an EcoRI/Mfe1 digested fragment of pTet-on Advanced vector. The purified fragment was ligated into the Ksp/BGH link vector digested with EcoRI. The tTA fragment was obtained by gel isolation of an EcoRI/Mfe1 digested fragment of pUHD plasmid and ligated into the Ksp/BGH vector digested with EcoRI.
Transgenic Animals
The KsprTA and KsprtTA plasmids were linearized by KpnI and purified by the EluTip D column. Transgenic mouse lines (KsprtTA and KsptTA) were generated by pronuclear injection by the transgenic core and analyzed by the Southern blot technique. Transgenic animals were fed Doxycycline hydrochloride (Sigma) dissolved in 5% sucrose supplied as drinking water. Water was changed every 3 days.
These mice are available to the research community through the UTSW George M. O'Brien Kidney Research Core. For requests, please visit (http://www.utsouthwestern.edu/research/core-facilities/obrien-kidney/index.html) and submit a core request form found under the core A/animal models offerings pull down.
Ex vivo organ culture
Organ culture was as previously described. Briefly, E11.5 kidneys were grown on transwell filters at the air/media interface for different time points. The media was supplemented with or without Dox. The media was replaced with fresh media with the treatment every 2 days. All treatments were repeated at least three times with a minimum of six individual kidneys from six distinct embryos assayed per replicate. Embryos were cultured in the environmental chamber built into an Olympus MVX10 fluorescent macroscope.
Immunohistochemistry
Immunohistochemistry was performed as previously described. Tissues were fixed with 4% paraformaldehyde in PBS and cryoprotected in 30% sucrose. Specimens were then embedded in OCT and cryosectioned at 10 μM thickness. Sections were stained with the primary antibodies as: anti-GFP (chicken; 1:1000, AvesLabs), anti-DBA (Biotinylated; 1:500, VectorLabs), anti-LTA (Bioltinylated, 1:500, VectorLabs), anti-Tamm Horsfall protein (THP, rabbit, 1:500, Biomedical Technologies Inc.), and anti-Aqp1 (rabbit, 1:500, provided by Dr. Mark Knepper). Nuclei were counterstained using TO-PRO-3 iodide (1:1000, Invitrogen). Results shown are representative examples from one of at least three different stainings of three different kidneys. Images were captured on a Zeiss LSM 510 confocal microscope.
Statistics
All statistical analysis was performed using the Students t-test.
Acknowledgments
This work was supported by the UT Southwestern O'Brien Kidney Research Core Center (P30DK079328). Work in the Carroll lab is supported by grants from the NIH (R01DK080004, R01DK095057) and the March of Dimes. XP is supported by a fellowship from the National Kidney Foundation.
References
- Adams DC, Oxburgh L. The long-term label retaining population of the renal papilla arises through divergent regional growth of the kidney. Am J Physiol Renal Physiol. 2009;297:F809–815. doi: 10.1152/ajprenal.90650.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Pontoglio M, Hiesberger T, Sinclair AM, Igarashi P. Regulation of kidney-specific Ksp-cadherin gene promoter by hepatocyte nuclear factor-1beta. Am J Physiol Renal Physiol. 2002;283:F839–851. doi: 10.1152/ajprenal.00128.2002. [DOI] [PubMed] [Google Scholar]
- Bockamp E, Maringer M, Spangenberg C, Fees S, Fraser S, Eshkind L, Oesch F, Zabel B. Of mice and models: improved animal models for biomedical research. Physiol Genomics. 2002;11:115–132. doi: 10.1152/physiolgenomics.00067.2002. [DOI] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet. 2008;17:1578–1590. doi: 10.1093/hmg/ddn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi FM, Blau HM. Recent advances in inducible gene expression systems. Curr Opin Biotechnol. 1998;9:451–456. doi: 10.1016/s0958-1669(98)80028-1. [DOI] [PubMed] [Google Scholar]
- Shao X, Johnson JE, Richardson JA, Hiesberger T, Igarashi P. A minimal Ksp-cadherin promoter linked to a green fluorescent protein reporter gene exhibits tissue-specific expression in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002a;13:1824–1836. doi: 10.1097/01.asn.0000016443.50138.cd. [DOI] [PubMed] [Google Scholar]
- Shao X, Somlo S, Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J Am Soc Nephrol. 2002b;13:1837–1846. doi: 10.1097/01.asn.0000016444.90348.50. [DOI] [PubMed] [Google Scholar]
- Thomson RB, Aronson PS. Immunolocalization of Ksp-cadherin in the adult and developing rabbit kidney. Am J Physiol. 1999;277:F146–156. doi: 10.1152/ajprenal.1999.277.1.F146. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]


