Abstract
The development of cell-cell junctions was a fundamental step in metazoan evolution, and human health depends on the formation and function of cell junctions. Although it has long been known that actin and conventional myosin have important roles in cell junctions, research has begun to reveal the specific functions of the different forms of conventional myosin. Exciting new data also reveals that a growing number of unconventional myosins have important roles in cell junctions. Experiments showing that cell junctions act as mechanosensors have also provided new impetus to understand the functions of myosins and the forces they exert. In this review we will summarize recent developments on the roles of myosins in cell junctions.
Keywords: Myo10, Myo15a, Myo1e, Myo6, Myo7a, Myo9a, Myo9b, adherens junction, dachs, myosin, nonmuscle myosin, tight junction
Introduction
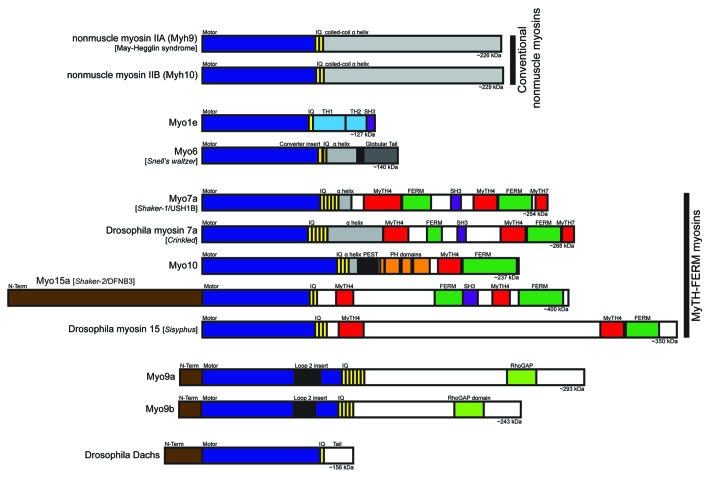
Myosins are a superfamily of molecular motors that bind to actin filaments to generate force and movement. Myosins serve a growing array of functions within the cell, including cell motility, cell shape regulation, organelle trafficking and cell signaling.1,2 Myosins have a three-part structure consisting of a head, neck and tail. They are defined by the presence of a conserved myosin head domain that can bind to actin filaments, hydrolyze ATP and generate force. The neck domain consists of one or more IQ motifs, each of which provides a binding site for calmodulin or a calmodulin-like light chain. The tail domain varies dramatically between different classes of myosins, and endows each myosin class with specific functions such as binding to membranes or forming bipolar filaments. The human genome encodes genes for 38 different myosin heavy chains (plus five named pseudogenes) that can be divided into 12 classes3 (see also the HUGO gene nomenclature website). Fourteen of these myosin heavy chain genes encode conventional (class II) myosins, the class that was first identified in studies of muscle contraction. Three of the class II myosins are broadly and abundantly expressed in nonmuscle cells, and these nonmuscle myosin-IIs function as major cellular force generators in processes ranging from cell migration to cytokinesis.4 In addition to the conventional myosins, the human genome encodes heavy chains for 24 unconventional myosins. These are divided into 11 distinct classes and have widely varying patterns of expression. In addition to two or three nonmuscle myosin-II genes, a typical cell is likely to express a dozen or more unconventional myosins. Although many different myosins are expressed in polarized epithelial cells, a metazoan-specific cell type with specialized cell junctions, only in recent times have we begun to elucidate the functions of the different myosins in polarized epithelial cells. In addition, until the past few years, most studies focused on investigating the roles of nonmuscle myosin-II. However, several recent studies have identified important functional roles for unconventional myosins in cell junctions in polarized epithelia. Here, we review the functions of myosins at epithelial cell junctions with particular emphasis on unconventional myosins (Fig. 1).
Figure 1. Bar diagrams of myosin heavy chains that have roles in cell junctions in vertebrates and/or Drosophila. See the text for descriptions of the different myosins and their key structural features.
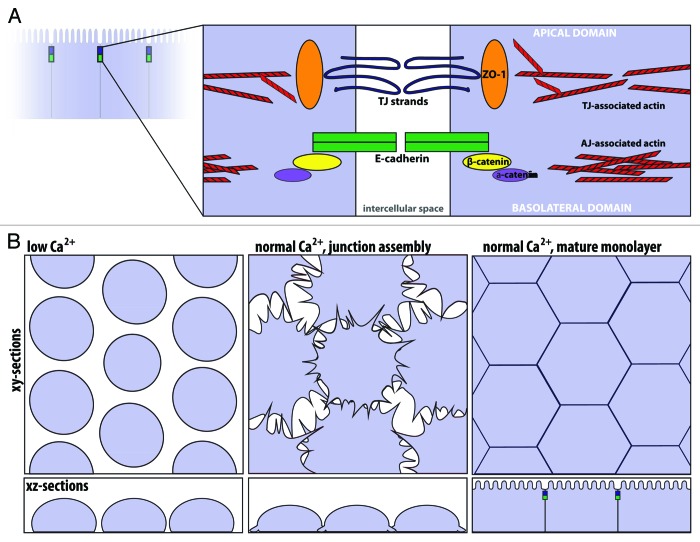
In vertebrate epithelial cells that line organs such as the gut and kidney, polarity is defined by the apical, lumen-facing domain and the underlying basolateral domain. At the intersection of these two domains, the apical junctional complex is comprised of two parts: the tight junction and the adherens junction (Fig. 2A). The tight junction (or zonula occludens) is apical-most, composed of a network of intramembranous strands that form a selective paracellular barrier between neighboring cells.5 Key components of the tight junction include the occludins, a family of transmembrane proteins responsible for forming the permeability barrier, and the ZO-1 family of proteins, large multidomain scaffolding proteins found on the cytoplasmic side of the tight junction.6 Key components of the adherens junction include the cadherins, a family of transmembrane proteins that mediate calcium dependent cell-cell adhesion.7 The cytoplasmic tail of classical cadherins binds to β-catenin, a cytoplasmic protein that in turn binds to α-catenin, a scaffolding molecule that contains binding sites for many other proteins including actin filaments and actin binding proteins.8 A dense circumferential belt of actin filaments is located on the cytoplasmic face of the adherens junction9 and a looser network of actin filaments is associated with the tight junction.10 Importantly, nonmuscle myosin-II is a well-known component of the circumferential actin belt.11,12
Figure 2. The apical junctional complex and calcium-switch model of junction assembly. (A) The tight junction and adherens junction, and their associated actin cytoskeleton, comprise the apical junctional complex. The tight junction strands form a semi-permeable barrier, and ZO-1 functions as a tight junction scaffolding protein. At the adherens junction, the cadherin-catenin complex contributes to cell adhesion. The apical junctional complex separates the apical and basolateral domains. (B) In the calcium-switch model, junctions are disassembled by removing calcium (left, low Ca2+). Upon calcium re-addition (center), the junctions begin to assemble, and radial actin cables are observed at early cell-cell contacts. Cells are fully polarized in a mature monolayer (right).
The apical junctional complex is essential to maintain the barrier function and integrity of the epithelial cell sheet. Notably, the apical junctional complex is not static, but is instead dynamic and constantly remodeling.13 In cell culture, junction assembly and disassembly are often modeled using the calcium-switch assay.14 In this assay, removal of extracellular calcium results in loss of cadherin-mediated cell-cell adhesion and disassembly of cell junctions, while subsequent re-addition of calcium triggers junction re-assembly (Fig. 2B). Importantly, junctional disassembly and assembly are accompanied by reorganization of the actin cytoskeleton. After junction disassembly, the now unpolarized cells often exhibit a ring of F-actin around their apical aspect.15 Then, during junction assembly, actin-rich protrusions that mature into radially oriented actin bundles are observed at sites of initial cell-cell contact.16 Using this model, it has been observed that nascent adherens-like junctions precede tight junction formation.12,17 As epithelial cells mature, junctional proteins and the circumferential actin belt organize at the cell junctions.
Indeed, the actin cytoskeleton is critical for the apical junctional complex, as disruption of actin dynamics affects the structural and functional integrity of both the adherens junction and the tight junction.12,15,18 Yet, how does the apical junctional complex physically interact with the actin cytoskeleton? Numerous proteins have been shown to interact with the tight and adherens junction cytoplasmic plaques, some of which are actin-binding proteins.19,20 Despite this, the identity of the linkages between the adherens junction and actin cytoskeleton, originally attributed to direct binding of α-catenin to F-actin, remains controversial.21,22 As actin-binding proteins that can generate force and movement, myosins are prime candidates to interact with the actin cytoskeleton at the apical junctional complex.
The importance of myosins and forces at cell junctions is also highlighted by growing evidence that cell junctions are mechanosensitive.23 For example, application of tugging forces to endothelial cells recruits VE-cadherin (vascular endothelial cadherin) to junctions and increases junction size.24 At the molecular level, α-catenin can act as a tension transducer that undergoes force-dependent changes in conformation.25 These changes unmask its binding site for vinculin, a cytoskeletal protein that binds to actin filaments. Another actin-binding protein that binds to α-catenin is EPLIN (epithelial protein lost in neoplasm).26 Although EPLIN localizes to linear adherens junctions (zonula adherens), it does not localize to punctate adherens junctions, which are small spot-like junctions thought to participate in the assembly of linear junctions; punctate adherens junctions are typically found at sites of cell-cell contact where actin bundles are oriented radially/perpendicular to the plasma membrane. Importantly, the localization of EPLIN to linear adherens junctions is force-dependent and requires the activity of nonmuscle myosin-II.26
Nonmuscle Myosin-II and Its Isoform Specific Roles at Cell Junctions
All metazoan cells express one or more forms of nonmuscle myosin-II, a myosin which can constitute several percent of total protein and is thus likely to be the major force generator in most cells. Mammals express three different genes for nonmuscle myosin-II,3 with most cells expressing two or more class II myosins.27,28 We will refer to these myosins here as NMII-A, NMII-B and NMII-C, but it should be noted that the official gene symbols for their heavy chains are MYH9, MYH10 and MYH14. Like all class II myosins, the tails of these heavy chains homodimerize by the formation of a parallel coiled coil, and the neck of each heavy chain binds to one essential light chain and one regulatory light chain.4 The native NMII hexamer thus consists of two heavy chains, two essential light chains and two regulatory light chains. The hexamers can, in turn, assemble into bipolar myosin filaments with each myosin minifilament containing a dozen or more myosin heads. The bipolar organization of these minifilaments endows class II myosins with their unique ability to exert contractile forces on antiparallel- and randomly-oriented arrays of actin filaments.
The ATPase and motor activity of NMII is activated by phosphorylation of its regulatory light chain. Since the regulatory light chain is only found in class II myosins, this form of regulation occurs in NMII but not in unconventional myosins. Light chain phosphorylation can be mediated by several kinases, including myosin light chain kinase (MLCK), Rho kinase (ROCK) and myotonic dystrophy kinase-related Cdc42-binding kinase (MRCK).4,29 Most research on NMII has focused on NMII-A and NMII-B (Fig. 1) rather than the more recently discovered NMII-C. NMII-A and NMII-B differ in their duty ratio, the proportion of time the motor spends binding actin during its ATPase cycle. Since NMII-B has a higher duty ratio, it remains bound to actin for a greater fraction of its ATPase cycle than NMII-A.30,31 Finally, all three NMII isoforms can be inhibited by blebbistatin, an ATPase inhibitor that targets class II myosins, trapping them in a state that only binds weakly to actin.32
Since the circumferential actin belt forms a ring at the cytoplasmic face of the adherens junction that contains NMII and actin filaments,9,11 it has long been thought that minifilaments of NMII pull on antiparallel actin filaments in the actin belt to generate contractile forces in much the same way as a stress fiber or cytokinetic furrow. NMII-mediated contraction of the circumferential actin would pull junctions inward, thus providing one mechanism to disrupt junctions and increase permeability. Alternatively, if the linkages between cells are strong enough to bear the load, NMII-mediated contraction of the actin belt could shrink the circumference of the apical domain, thus generating an apical contraction that could facilitate morphogenetic tissue movements such as gastrulation. NMII clearly has several central roles in junctions, and global inhibition of NMII with blebbistatin disrupts recruitment of E-cadherin (epithelial cadherin) to initial cell-cell contacts,33 junction assembly and disassembly,12,15 and paracellular permeability of the tight junction.34 Since these and other experiments on the global functions of NMII and actin in junctions have been previously reviewed,35 here we will focus on recent developments and the discovery of distinct roles for the different NMII isoforms.
Isoform-specific knockout studies indicate roles for both NMII-A and NMII-B in cell adhesion and polarized cell junctions. NMII-A knockout mice are lethal at embryonic day 7.5 (E7.5)36; the visceral endoderm in knockout embryos shows loss of polarized columnar morphology, whereas knockout embryoid bodies exhibit cell shedding, which suggests a cell adhesion defect. Interestingly, E-cadherin and β-catenin are reduced at cell-cell contacts in the absence of NMII-A.36 NMII-B is enriched in brain, particularly in the neuroepithelial layers. NMII-B mutants are also lethal (~E14.5) and show structural defects and discontinuities in the apical domain of the neuroepithelial layers that appear to lead to spinal canal obstruction, hydrocephalus and embryonic lethality.37
In polarized epithelial cells, both NMII-A and NMII-B are present in the apical junctional complex12,33 (Fig. 3A). NMII-A clearly functions in the dynamics of junction assembly and disassembly. NMII-A knockdown cells show a delay in junction formation, measured by transepithelial resistance (TER) or by localization of tight junction and adherens junction markers. Despite the delay of several hours, apical junctions do eventually form.38 NMII-A knockdown also affects junction disassembly, as F-actin reorganization into actin rings was disrupted and the translocation of the tight junction protein occludin from cell-cell junctions was attenuated.38 Thus, NMII-A is required for the proper kinetics of junction assembly and disassembly.
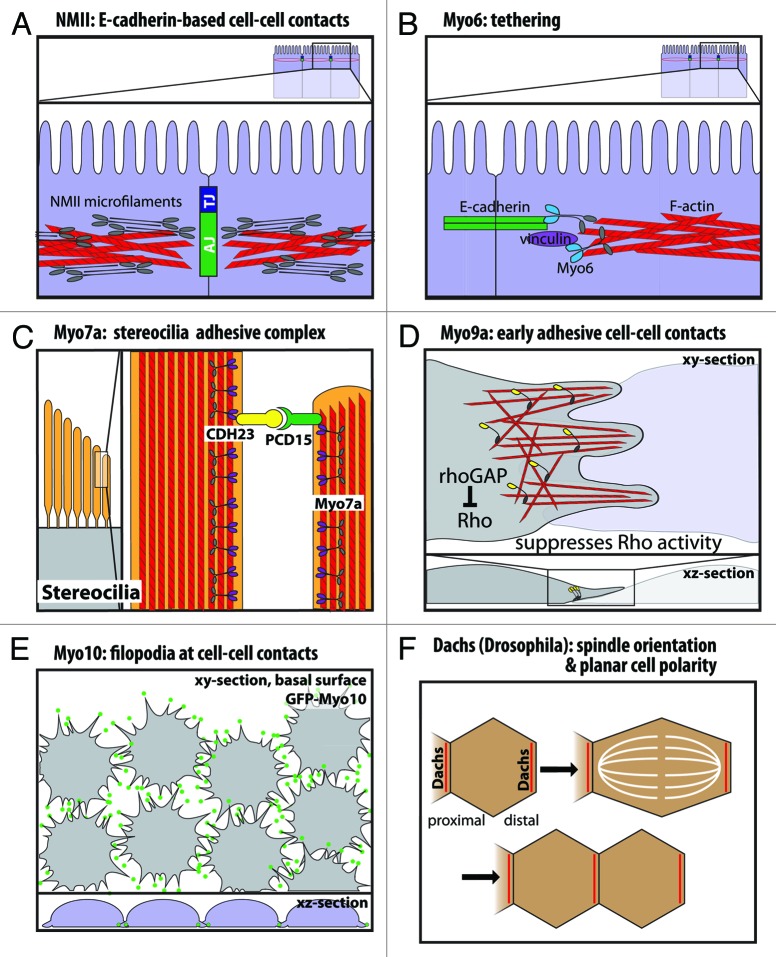
Figure 3. Functional roles of myosins in cell junctions and cell-cell contacts. (A) Nonmuscle myosin-II localizes to the adherens junction-associated circumferential actin belt, and knockdown on nonmuscle myosin-II disrupts E-cadherin-based cell-cell contacts. (B) Myo6 and vinculin have been suggested to tether E-cadherin to the perijunctional actin cytoskeleton. (C) In stereocilia, Myo7a binds cadherin-23 and protocadherin-15 in a stereocilia adhesive tip complex. (D) Myo9a is a RhoGAP that localizes to cell-cell contacts and suppresses Rho activity. Myo9a knockdown shows defects in formation and stabilization of early cell-cell contacts, resulting in a cell scattering phenotype. (E) Myo10 localizes to the tips of filopodia at nascent cell-cell contacts during junction assembly, and Myo10 knockdown delays junction assembly. (F) Dachs has a planar polarized distribution in Drosophila wing disc epithelia. Loss of Dachs disrupts orientation of the mitotic spindle and cell division along the proximal-distal axis.
Isoform-specific knockdown also reveals differential roles for NMII-A and NMII-B in E-cadherin-based cell-cell contacts. NMII-A is required for proper E-cadherin organization at the adherens junction, as NMII-A siRNA reduces the amount of E-cadherin at cell-cell contacts and decreases cadherin-based homophilic adhesion.39 Interestingly, an E-cadherin blocking antibody decreased NMII-A staining at cell-cell contacts.33 Thus, NMII-A activity is needed for E-cadherin localization to the junctions and vice versa. Furthermore, NMII-A knockdown results in increased and irregular oscillatory movement of E-cadherin at cell-cell contacts.40 In contrast, NMII-B maintains the integrity of the actin cytoskeleton at the adherens junction, as NMII-B knockdown decreases actin and its dynamics at cell junctions and fragments the localization of E-cadherin.39 The authors of this study suggest that the high duty ratio of NMII-B may allow this motor to generate tension to support dynamic actin at the cell junctions. Consistent with this, live cell imaging of NMII-B knockdown cells shows significantly less translational movement of actin and E-cadherin.40 Interestingly, double knockdown of NMII-A and B leads to disruption of the tight junction, visualized by zonula occludens-1 (ZO-1) localization.39 One potential link between the actin cytoskeleton and the tight junction complex is cingulin, a submembranous tight junction-associated phosphoprotein that binds to NMII and to ZO-1, ZO-2 and ZO-3.41 Recent work shows that simultaneous knockdown of ZO-1 and ZO-2 leads to a dramatic increase in F-actin, NMII-B, Rho kinase and phosphorylated regulatory light chain at the circumferential actin belt, indicating increased contractility at the adherens junction.42 This intriguing result indicates that ZO-1 and ZO-2, scaffolding proteins found at the tight junction, normally act to negatively regulate NMII-B contraction in the circumferential actin belt.
Together, these studies suggest specific roles for the different isoforms of NMII: NMII-A is required for normal kinetics of junction assembly and organizes E-cadherin at the adherens junction, whereas NMII-B supports the integrity and dynamics of the circumferential actin belt. In addition, recent studies with inhibitors show that NMII is largely responsible for generating the forces sensed by mechanosensory molecules at cell junctions.24-26,43 It should also be noted that in addition to NMII-mediated contraction of the circumferential actin bundle, a more diffuse network of NMII and actin filaments in the apical region can also contract to power apical constriction, and this force is transmitted from cell to cell via junctions to drive gastrulation in Drosophila.44 Interestingly, during C. elegans gastrulation, apical constriction appears to be triggered by activation of a clutch-like linkage between the apical junction and an apically-directed actin flow generated by NMII contraction.45 As a major force generator and component of adherens junctions, NMII may also have yet to be discovered roles at the junction.
Myo1e at Specialized Glomerular Junctional Complexes
Class I myosins are single-headed motors with short tails that bind to lipid membranes.46 They are phylogenetically ancient and are found in amoebae, fungi and animals. Many organisms express several class I myosins; the slime mold Dictyostelium discoideum expresses seven46 and humans express eight class I myosins.3 Myo1a, one of the best known class I myosins, forms a link between the plasma membrane and the actin filaments of intestinal microvilli.47 Myo1e (initially called human myosin-1c or myr3) has a longer tail that contains both a membrane-binding domain and an SH3 domain48 (Fig. 1). Myo1e is ubiquitously expressed, with the highest levels in kidney, prostate, colon, liver and ovary.49 Notably, Myo1e in kidney is predominantly found in the glomerulus and its podocytes, which are epithelial cells that extend “foot processes” to wrap around glomerular capillaries.50
Myo1e localizes to cell junctions in several cell types. Myo1e localizes with β-catenin at the adherens junction in intestine and kidney.51,52 In Caco-2 cells, a human colon carcinoma cell line used as a model for small intestine, Myo1e is enriched at the apical cell junctions in spreading cells and mature monolayers.51 In cultured monolayers of mouse podocyte cells, Myo1e frequently localizes to cell-cell contacts and may be needed for proper actin organization.52 Renal glomeruli from Myo1e knockout mice show disrupted podocyte foot processes as well as thickened and disorganized glomerular basement membranes, leading to impaired renal function.50,52 Disruption in the cytoskeleton of the glomerular intercellular junctional complexes has also been shown to impair renal function.53 As a class I myosin, Myo1e could function to stabilize the actin cytoskeleton by binding the surrounding glomerular membrane.
In Myo1e knockout studies, Myo1e-deficient mice exhibit podocyte injury and impaired renal function.52 Mutations in human Myo1e are associated with familial focal segmental glomerulosclerosis, an autosomal recessive disease of podocytes.50 Thus far, no extrarenal defects have been identified in knockout mice or patients with Myo1e mutations. Apart from Myo1e, little is known regarding class I myosins and epithelial cell junctions. Do any of the other seven class I myosins localize to or function in cell junctions? With the Myo1a knockout mouse available, are there observable junctional defects? As investigations into class I myosins move forward, it will be important to consider functional redundancy54,55 given the many class I myosins and the central importance of junctions in metazoan physiology and survival.
Myosin-VI at Cadherin-Based Cell-Cell Contacts
Myosin-VI (Myo6) is unique in that it is the only known motor that moves toward the minus end of actin filaments56 (Fig. 1). In general, the plus ends of actin filaments are oriented toward the plasma membrane,57 so Myo6 might be expected to transport vesicles inward or push actin filaments outward. Myo6 arose early during the evolution of metazoans3 and is ubiquitously expressed in mammalian cells.58 Myo6 is a processive motor that can dimerize via cargo binding to the tail,59,60 and as a high duty ratio motor, Myo6 spends most of its ATPase cycle bound to actin.61 Thus, as a processive myosin, a single Myo6 dimer is theoretically sufficient to transport a vesicle along an actin filament. Myo6 is well-known for its roles in clathrin-mediated endocytosis62 as well as endocytic trafficking and sorting.63 In epithelial cells, Myo6 is also required for the polarized transport of certain proteins to the basolateral membrane.64
Importantly, loss of Myo6 causes deafness in both humans65 and the Snell’s waltzer mouse.66 In the inner ear hair cells, Myo6 is enriched both in the vesicle-rich pericuticular necklace and in stereocilia, which are mechanosensing actin-based protrusions on hair cells. In the Snell’s waltzer mouse, the inner ear hair cells develop disorganized and fused stereocilia,67 and a similar phenotype is observed in intestinal microvilli.68 Furthermore, loss of function studies in Drosophila also indicate Myo6 is critical for epithelial morphogenesis. Drosophila Myo6 (Jaguar) deficiency disrupts dorsal closure, a process of epithelial sheet fusion at the dorsal midline in late embryogenesis.69,70 Drosophila Myo6 deficiency also disrupts border cell migration during oogenesis,71,72 where tethering of Myo6 to fixed integral membrane proteins could potentially provide the pushing force required to protrude cell extensions.
Myo6 has functional roles in cadherin-based cell-cell contacts and the perijunctional actin cytoskeleton. In vitro studies in MCF-7 cells show that Myo6 localizes to mature cell-cell contacts and co-localizes with E-cadherin.73 Immunoprecipitation studies indicate Myo6 interacts with E-cadherin73 and β-catenin.72 Myo6 also binds Echinoid,69 a nectin-like cell adhesion molecule that cooperates with Drosophila epithelial cadherin (DE-cadherin).74 Interestingly, Myo6 and E-cadherin/β-catenin each appear to stabilize the other protein. In Myo6-null or mutant Drosophila border cells, E-cadherin and β-catenin levels are reduced.70,72 Conversely, Drosophila Myo6 protein levels are reduced in cells lacking either E-cadherin or β-catenin.72 Similar results were obtained in vitro by Myo6 knockdown in monolayers of vertebrate epithelial cells.73 Thus, Myo6 stabilizes E-cadherin at epithelial cell-cell contacts.
Given its high duty ratio, Myo6 may also stabilize the perijunctional actin cytoskeleton and/or tether membrane proteins, such as E-cadherin or β-catenin, to the perijunctional actin cytoskeleton. Myo6 has been previously reported to stabilize actin filament networks in unpolarized cells,75 and Myo6 serves a similar membrane anchoring role in stereocilia.67,76 In epithelial cells, loss of Myo6 leads to disrupted perijunctional actin70,73 as well as loss of tight junction markers at cell-cell contacts.73 Vinculin, a cytoskeletal protein found in focal adhesions and adherens junctions, is a downstream effector of Myo6, with Myo6 and vinculin acting together to stabilize perijunctional actin and cadherin-based cell-cell contacts (Fig. 3B).73 An important role for Myo6 at the adherens junction is also shown by recent work where Caco-2 monolayers were treated with HGF (hepatocyte growth factor/scatter factor), a growth factor that is secreted by many tumors and that induces epithelial to mesenchymal transition.77 HGF led to a rapid loss of F-actin from the adherens junction, and this loss was blocked by overexpression of Myo6. Given these in vitro roles for Myo6, it will be important to determine if the humans and mice that lack Myo6 exhibit in defects in their cell junctions, especially given that mammals only express a single Myo6 gene, and Myo6 is the only myosin known to move toward the pointed end.
Myosin-VIIa Interacts With Junction-Associated Proteins at Epithelial Cell-Cell Contacts
Myosin-VIIa (Myo7a) is a member of the “MyTH-FERM” family of myosins (Fig. 1), an evolutionarily ancient family that is characterized by the presence of a tail containing a myosin tail homology 4 (MyTH4) domain located N-terminal to a protein 4.1, ezrin, radixin, moesin (FERM) domain. Functionally, these MyTH-FERM myosins often localize to actin-based extensions such as filopodia and stereocilia, and several MyTH-FERM myosins are implicated in cell adhesion.78-80 The structure of the MyTH4-FERM domain from Myo7a was recently solved, revealing that the MyTH4-FERM domain forms a structural supermodule.81 Drosophila Myo7a can form an inactive monomer regulated by head-to-tail folding82 but appears to function as a processive dimer.83,84 Biochemical experiments indicate Myo7a has a high duty ratio.85,86 In mammals, Myo7a is predominantly expressed in epithelial cells87 with highest levels in the testis, cochlea and retina and lower expression in kidney.88
Mutations in human Myo7a are responsible for Usher Syndrome 1B (USH1B), a disease of congenital deafness, vestibular dysfunction and pre-pubertal onset of retinitis pigmentosa leading to blindness.89 Mouse Myo7a is encoded by the shaker-1 locus, and homozygous shaker-1 mutants are characterized by deafness and vestibular dysfunction.90 In cochlear hair cells, loss of Myo7a function results in disruption in stereocilia structure and organization.91,92 Myo7a binds cadherin-23 (CDH23),80 an atypical cadherin93 present at the extracellullar links between stereocilia that have been proposed to apply the tension needed for proper stereocilia organization80,94,95 (Fig. 3C). Myo7a has also been reported to interact with protocadherin-15 (PCD15),96 and protocadherin-15 in turn binds to cadherin-23 to form the stereocilia tip links required for hearing and balance.97 Finally, mutations in Drosophila Myo7a are responsible for crinkled, a fly mutant that is deaf and has defects in actin-based bristles and hairs.98,99 The shaker-1 and crinkled phenotypes both indicate a role for Myo7a in the formation of complex actin-based protrusions.
In polarized epithelial cells, endogenous Myo7a100 or the GFP-Myo7a tail100,101 localize to cell-cell contacts. In addition, in the testis, Myo7a is found in a dynamic adhesive structure called the ectoplasmic specialization.102 Interestingly, Myo7a interacts with several junction-associated proteins at epithelial cell-cell contacts. First, the FERM domain of Myo7a interacts with vezatin, a transmembrane protein at the adherens junction.101 Vezatin, which also binds E-cadherin and α- and β-catenin, has been proposed to bridge Myo7a to the cadherin-catenin complex at the adherens junction. Functionally, Myo7a and vezatin are needed for the bacterial entry of Listeria monocytogenes into epithelial cells.100 The MyTH4-FERM domain of Myo7a also mediates the interaction with Shroom2, a tight junction-associated protein.103 Thus, Shroom2 may link Myo7a and the actin cytoskeleton to the tight junction.103 Finally, Myo7a binds the actin-associated protein Keap1 at the specialized adhesion junctions in testis.102 Thus, Myo7a may link the apical junctional complex to perijunctional actin via its interactions with junction-associated proteins in epithelial cells. It should be noted, however, that the localizations of vezatin, Shroom2 and Keap1 are Myo7a-independent, as the localization of each Myo7a-binding protein in shaker-1 mutants is unchanged.101-103 Given that humans who lack Myo7a suffer from deafness and blindness, it will be important to determine if they also have defects in cell-cell junctions.
Class IX Myosins: Motorized Rho GAPs that Regulate Cell-Cell Junctions and Migration
Class IX myosins are metazoan-specific motor proteins which appear to act as single-headed myosins that can move processively on actin.104,105 Humans express two myosin IX genes, myosin-IXa (Myo9a, formerly known as myr 7) and myosin-IXb (Myo9b, formerly known as myr 5), and the two proteins share approximately 57% amino acid sequence identity in their motor domains. Unique among myosins, the tails of class IX myosins are Rho GTPase activating proteins (GAPs) that negatively regulate Rho GTPases106 (Fig. 1). The RhoGAP domains of the class IX myosins are specific for Rho and have little effect on Rac1 or Cdc42.107,108 Rho regulates many processes, including actin organization and cell migration,109,110 and a major Rho effector is Rho-kinase, which activates nonmuscle myosin-II. Importantly, Rho and its effectors are involved in epithelial junction assembly.111,112 Until recently, however, little was known about the functions of the class IX myosins and their Rho-GAP activity in epithelia and junctions.
Myo9a localizes at cell-cell junctions both in vivo and in vitro,113 and it is ubiquitously expressed during development.114 In adult tissues, Myo9a is particularly abundant in the brain and testis, with lower levels of expression in the adrenal gland, kidney, lung and spleen.107 In the brain, Myo9a is highly expressed in the ciliated ependymal cells that form the epithelial layer lining the ventricles.113 In knockout studies, loss of Myo9a results in a distorted ependymal layer, blockage of the canals in the ventricular system and severe hydrocephalus. Remarkably, the hydrocephalus could be suppressed by pharmacologically inhibiting Rho-kinase, a major Rho effector. At the cellular level, Myo9a knockouts have defects in the localization of E-cadherin and β-catenin as well as loss of occludin localization to the tight junction.113 Knockdown of Myo9a in Caco-2 cells also reveals defects in junction assembly, although junctions do eventually form and the cells have normal apico-basal polarity.113
A recent study by Omelchenko and Hall (2012) highlights the role of Myo9a in collective cell migration and the use of its RhoGAP activity at cell-cell junctions (Fig. 3D). In cultured human bronchial epithelial cells (16HBE), Myo9a is enriched at the leading edge and at nascent cell-cell contacts, and FRET experiments indicate that it negatively regulates Rho GTPase activity (RhoA) as cells contact one another.115 Similar to the knockout mouse, Myo9a siRNA knockdown leads to loss of ZO-1 and irregular E-cadherin staining at cell-cell contacts. In addition, actin at cell-cell contacts fails to reorganize into the radial actin bundles thought to stabilize contacts. Importantly, Myo9a knockdown leads to impaired wound healing and a dramatic cell scattering phenotype, as cells fail to stabilize initial cell-cell contacts.115
Myo9b is of particular interest because Myo9b polymorphisms have been associated with Crohn's disease, ulcerative colitis and celiac disease.116-118 These diseases are also associated with increased paracellular permeability,119 although it is not clear if the changes in paracellular permeability are a cause or an effect of the inflammation. Like Myo9a, Myo9b appears to use its motor activity to target itself to the leading edge of lamellipodia, thus inhibiting Rho at sites of protrusion.120 In monolayers of Caco-2 cells, Myo9b localizes both to the lateral membrane and to the cytoplasm, but after cell wounding, it shows a dramatic relocalization to the leading edge.121 In monolayers, Myo9b knockdown cells showed dramatically decreased staining of tight junction components including ZO-1, claudin and occludin. Knockdown cells also have defects in wound healing; rather than extending lamellipodia like control cells, the knockdown cells instead formed stress fiber-like arrays of actin and nonmuscle myosin-II along the leading edge.121 Myo9b thus appears to act as a motorized RhoGAP that transports itself to the leading edge, where it inhibits Rho activity and thus inhibits processes such as nonmuscle myosin-II-mediated contraction. Since Myo9b null mice have recently been generated and macrophages from these mice exhibit defects in spreading and chemotaxis in vitro and defective recruitment in vivo,122 it will be important to determine whether Myo9b null mice exhibit any defects in junction formation and if they provide a model for inflammatory bowel disease.
Myosin-X: A MyTH-FERM Myosin at the Basolateral Domain of Polarized Epithelial Cells
Myosin-X (Myo10) is a MyTH-FERM myosin best known for localizing to filopodial tips. It appears to have arisen just prior to the evolution of the metazoans, but was apparently lost in the lineages leading to flies and worms.3 The tail of Myo10 has several unique domains. These include three pleckstrin homology (PH) domains, one of which binds inositol phospholipids with high affinity for phosphatidylinositol (3,4,5)-triphosphate (PIP3).123-125 In addition, a C-terminal MyTH-FERM domain can bind microtubules126 and β-integrins79 (Fig. 1). Myo10 is ubiquitously expressed, with relatively high levels of expression in epithelial tissues such as kidney, but it appears to be a low abundance myosin that is orders of magnitude less abundant than nonmuscle myosin-II.127 Notably, Myo10's IQ motifs can bind either calmodulin or calmodulin-like protein (CLP), which is an epithelia-specific light chain.128
In unpolarized cells such as fibroblasts, Myo10 localizes to tips of filopodia and is required for the formation of filopodia.129 In polarized MDCK cells, Myo10 localizes to the lateral membrane during junction assembly130 while in kidney it localizes basolaterally130 and fractionates with basolateral membranes.78 The basolateral targeting of Myo10 is likely to be mediated by interaction of its PH domains with PIP3, since inositol phospholipids in epithelial cells are segregated with PIP3 enriched basolaterally and PIP2 enriched apically.131 Consistent with this, the PH domains of Myo10 localize to the basolateral membrane in MDCK cells.125
Recent work reveals that Myo10 has important roles in junction assembly and epithelial morphogenesis.130 As observed with knockdown of key junctional components, ZO-1 and E-cadherin,132,133 Myo10 knockdown results in a delay in junction assembly, as measured by a delay in the localization of tight junction and adherens junction markers to cell-cell contacts and a delay in peak transepithelial resistance (TER).130 Importantly, the delay in junction assembly following Myo10 knockdown does not appear to be due to a defect in epithelial polarity since apico-basal polarity of several markers was normal, as was the TER of mature monolayers. Although Myo10 has been reported to interact with VE-cadherin and to undergo co-transport with it in filopodia during early stages of junction formation in endothelial cells,134 no interaction between Myo10 and E-cadherin has been detected thus far.130 Filopodia linked to radial actin cables are theorized to form and stabilize initial cell-cell contacts at the early stages of junction formation.16 Since GFP-Myo10 shows localization to the tips of dynamic filopodia-like structures at the basal surface of MDCK cells during junction assembly,130 Myo10 is likely to function at the initial stages of cell-cell contact (Fig. 3E).
In three-dimensional culture, Myo10 knockdown cells form cysts with multiple lumens rather than the normal, single lumen cysts in MDCK cells. Since defects in spindle orientation can result in multi-lumen cysts,135-137 and Myo10 is required for normal spindle positioning and orientation,126,130,138-140 Myo10 knockdown may lead to multiple lumens because of defects in spindle orientation. Consistent with this, knockdown of Myo10 disrupts the normally horizontal orientation of spindles in MDCK monolayers.130 Although the mechanisms by which Myo10 orients spindles remain unclear, in epithelial cells of Xenopus embryos, Myo10 is reported to be present at both the cell cortex and the mitotic spindle.140 Intriguing new data indicates that the vertical position of the spindle in this system is determined by the balance between a basally directed force that depends on Myo10 and microtubules and an apically directed actin flow that depends on nonmuscle myosin-II and actin.141
Myosin-XVa in Stereocilia and Drosophila Sisyphus in Dorsal Closure
Myosin-XVa (Myo15a) is another member of the MyTH-FERM family of myosins, and in mammals it exists as two alternatively spliced isoforms with or without a large N-terminal extension142,143 (Fig. 1). Expression of Myo15a in mammals appears to be limited to only a few cell types, including the neurosensory cells of the inner ear.144
Human mutations in Myo15a are responsible for human non-syndromic autosomal recessive deafness (DFNB3).145 In mice, Myo15a mutations (shaker-2) result in deafness and vestibular dysfunction.145,146 Myo15a localizes to the tips of stereocilia in the inner ear,143 and shaker-2 mice have short and disorganized stereocilia that fail to form their characteristic staircase structure.146 Therefore, Myo15a is necessary for the graded elongation of stereocilia. Moreover, Myo15a interacts with whirlin,147 a scaffolding protein associated with Usher syndrome type 2,148 and with Eps8, an actin capping protein, to form a stereocilia tip complex.149
Initial investigations of class XV myosins in epithelial cells have been performed by studying Sisyphus, a Drosophila MyTH-FERM myosin that shares similarity to Myo15a, but that is expressed in many tissues.150 Liu et al. found that Sisyphus is required for epithelial morphogenesis and functions in cell adhesion. As epithelial sheets close during dorsal closure, Sisyphus accumulates in contact-making filopodia and newly formed junctions. Sisyphus-deficient mutants show defects in epithelial alignment, and delay and/or failure in fusion of epithelial sheets. Sisyphus was reported to co-localize with and bind Drosophila E-cadherin, and Sisyphus-deficient embryos show reduced E-cadherin at the dorsal side of leading edge cells during dorsal closure.150
It thus appears that Myo15a and Sisyphus have distinct roles. In mammals, Myo15a functions in the inner ear to regulate stereocilia length and organization, while in Drosophila, Sisyphus interacts with E-cadherin and is critical for epithelial sheet alignment and adhesion during dorsal closure. This raises the question of whether other MyTH-FERM myosins expressed in mammals such as Myo10 might fill the roles played by Sisyphus in Drosophila.
Dachs: A Drosophila Unconventional Myosin is Planar Polarized at Apical Junctions
Dachs is an unconventional myosin in Drosophila151-153 that lacks a clear mammalian homolog153 (Fig. 1). The Dachs sequence includes an N-terminal extension preceding the head and neck, and a relatively short tail that shows no sequence similarity to other proteins. Dachs mRNA is broadly expressed throughout embryonic and wing disc development.153 Recent investigations reveal that Dachs is required for proper orientation of cell division and tissue growth.154 In the wing disc epithelium, Dachs protein is planar polarized along the proximal-distal axis, localizing to the distal side of each cell near the adherens junction.153,155
Loss of Dachs results in reduced tissue growth, shortened legs and wings and often fused tarsal segments.153,156 In dachs mutants, wing discs are rounded and shortened in the proximal-distal axis due to tissue undergrowth and disrupted wing disc elongation.154 Importantly, dachs mutants show mitotic spindle misorientation154 (Fig. 3F), a defect that can arise in several ways, including inhibition of junctional proteins such as E-cadherin.157 Interestingly, dachs mutants have dilated apical cell surfaces, which suggests that the Dachs myosin is required for exertion of a polarized contractile force that constricts apical junctions and orients the mitotic spindle along the proximal-distal axis to produce net elongation and tissue growth along this axis.154
Dachs is a component of the Fat signaling pathway,153 a pathway that involves two large protocadherins, Dachsous and Fat.158 Dachsous on a given cell appears to act as a ligand for Fat on the adjacent cell, and the planar polarized distribution of Dachs is disrupted in the absence of Fat or other Fat signaling regulators.155 Recent work indicates that Dachsous recruits Dachs to one side of a junction and that Fat signaling on the other side of the junction inhibits Dachs localization there.159 This leads to anisotropic tension across the junction and oriented cell rearrangements that generate extension in the proximal-distal axis. Almost nothing is known about the structural and motor properties of Dachs, and it would be of interest to learn whether Dachs generates tension on junctions directly by linking the protocadherin Dachsous to actin or if it regulates tension indirectly by regulating nonmuscle myosin-II. Since virtually all of the components of the Fat signaling pathway are conserved in vertebrates and several are tumor suppressors, it would also be of great interest to determine if a different myosin takes the place of Dachs in humans.
Conclusions
It is now clear that many different myosins function at cell-cell junctions. This includes the different forms of nonmuscle myosin-II and an increasing number of unconventional myosins. As knowledge of these actin-based motors continues to grow, several themes begin to emerge. The different forms of nonmuscle myosin-II are major components of the circumferential actin belt and clearly have central roles in junction assembly and disassembly. One of the most exciting developments in the field has been the growing recognition that cell-cell junctions are mechanosensitive, and nonmuscle myosin-II exerts much of the force that is sensed by junctions. Several myosins act at the early stages of junction formation by facilitating the formation or stabilization of actin-based protrusions and initial cell-cell contacts. Myosins can also function by facilitating cell adhesion, organizing actin, tethering junctional proteins and orienting the mitotic spindle. Another important function for myosin motors is cargo transport, which raises the question of whether there are specific unconventional myosins that deliver molecules to cell junctions. A major challenge in the field is that we currently lack drugs analogous to blebbistatin that specifically target the different unconventional myosins. In addition, cell junctions are so fundamental to metazoan physiology that the molecular mechanisms underlying their function may be highly redundant. Despite these challenges, recent studies have revealed important roles at cell-cell junctions for several unconventional myosins, and there are several others whose functions remain unknown. In the next few years, it is likely that the growing numbers of myosin knockout models, along with large scale sequencing of human patients, will reveal even more roles for myosins in cell junctions and human health.
Acknowledgments
We regret that space considerations prevent us from discussing other exciting contributions in this area of research. We thank Drs. Alan Fanning and Mark Peifer for helpful discussions. This work was supported by a grant from the National Institutes of Health to REC (National Institute on Deafness and Other Communication Disorders R01DC03299) and a National Research Service Award fellowship to KCL (National Institute of Digestive and Kidney Disease 1F30DK089695).
Footnotes
Previously published online: www.landesbioscience.com/journals/BioArchitecture/article/21791
References
- 1.Hartman MA, Finan D, Sivaramakrishnan S, Spudich JA. Principles of unconventional myosin function and targeting. Annu Rev Cell Dev Biol. 2011;27:133–55. doi: 10.1146/annurev-cellbio-100809-151502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woolner S, Bement WM. Unconventional myosins acting unconventionally. Trends Cell Biol. 2009;19:245–52. doi: 10.1016/j.tcb.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berg JS, Powell BC, Cheney RE. A millennial myosin census. Mol Biol Cell. 2001;12:780–94. doi: 10.1091/mbc.12.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10:778–90. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–29. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 6.Fanning AS, Anderson JM. Zonula occludens-1 and -2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Ann N Y Acad Sci. 2009;1165:113–20. doi: 10.1111/j.1749-6632.2009.04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–55. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–14. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- 9.Hirokawa N, Keller TC, 3rd, Chasan R, Mooseker MS. Mechanism of brush border contractility studied by the quick-freeze, deep-etch method. J Cell Biol. 1983;96:1325–36. doi: 10.1083/jcb.96.5.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madara JL. Intestinal absorptive cell tight junctions are linked to cytoskeleton. Am J Physiol. 1987;253:C171–5. doi: 10.1152/ajpcell.1987.253.1.C171. [DOI] [PubMed] [Google Scholar]
- 11.Drenckhahn D, Dermietzel R. Organization of the actin filament cytoskeleton in the intestinal brush border: a quantitative and qualitative immunoelectron microscope study. J Cell Biol. 1988;107:1037–48. doi: 10.1083/jcb.107.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16:2636–50. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kametani Y, Takeichi M. Basal-to-apical cadherin flow at cell junctions. Nat Cell Biol. 2007;9:92–8. doi: 10.1038/ncb1520. [DOI] [PubMed] [Google Scholar]
- 14.Cereijido M, Robbins ES, Dolan WJ, Rotunno CA, Sabatini DD. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978;77:853–80. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15:2639–51. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–19. doi: 10.1016/S0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 17.Ando-Akatsuka Y, Yonemura S, Itoh M, Furuse M, Tsukita S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J Cell Physiol. 1999;179:115–25. doi: 10.1002/(SICI)1097-4652(199905)179:2<115::AID-JCP1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 18.Madara JL, Barenberg D, Carlson S. Effects of cytochalasin D on occluding junctions of intestinal absorptive cells: further evidence that the cytoskeleton may influence paracellular permeability and junctional charge selectivity. J Cell Biol. 1986;102:2125–36. doi: 10.1083/jcb.102.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 20.Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778:562–71. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yonemura S. Cadherin-actin interactions at adherens junctions. Curr Opin Cell Biol. 2011;23:515–22. doi: 10.1016/j.ceb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Gomez GA, McLachlan RW, Yap AS. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 2011;21:499–505. doi: 10.1016/j.tcb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, et al. Mechanical tugging force regulates the size of cell-cell junctions. Proc Natl Acad Sci U S A. 2010;107:9944–9. doi: 10.1073/pnas.0914547107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M. alpha-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol. 2010;12:533–42. doi: 10.1038/ncb2055. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi K, Ishiuchi T, Takeichi M. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J Cell Biol. 2011;194:643–56. doi: 10.1083/jcb.201104124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons M, Wang M, McBride OW, Kawamoto S, Yamakawa K, Gdula D, et al. Human nonmuscle myosin heavy chains are encoded by two genes located on different chromosomes. Circ Res. 1991;69:530–9. doi: 10.1161/01.RES.69.2.530. [DOI] [PubMed] [Google Scholar]
- 28.Golomb E, Ma X, Jana SS, Preston YA, Kawamoto S, Shoham NG, et al. Identification and characterization of nonmuscle myosin II-C, a new member of the myosin II family. J Biol Chem. 2004;279:2800–8. doi: 10.1074/jbc.M309981200. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–61. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 30.Wang F, Kovacs M, Hu A, Limouze J, Harvey EV, Sellers JR. Kinetic mechanism of non-muscle myosin IIB: functional adaptations for tension generation and maintenance. J Biol Chem. 2003;278:27439–48. doi: 10.1074/jbc.M302510200. [DOI] [PubMed] [Google Scholar]
- 31.Kovács M, Wang F, Hu A, Zhang Y, Sellers JR. Functional divergence of human cytoplasmic myosin II: kinetic characterization of the non-muscle IIA isoform. J Biol Chem. 2003;278:38132–40. doi: 10.1074/jbc.M305453200. [DOI] [PubMed] [Google Scholar]
- 32.Kovács M, Tóth J, Hetényi C, Málnási-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279:35557–63. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 33.Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, et al. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol Biol Cell. 2005;16:4531–42. doi: 10.1091/mbc.E05-04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, et al. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci. 2006;119:2095–106. doi: 10.1242/jcs.02915. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci. 2008;13:6662–81. doi: 10.2741/3180. [DOI] [PubMed] [Google Scholar]
- 36.Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279:41263–6. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- 37.Ma X, Bao J, Adelstein RS. Loss of cell adhesion causes hydrocephalus in nonmuscle myosin II-B-ablated and mutated mice. Mol Biol Cell. 2007;18:2305–12. doi: 10.1091/mbc.E07-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One. 2007;2:e658. doi: 10.1371/journal.pone.0000658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, et al. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smutny M, Wu SK, Gomez GA, Mangold S, Yap AS, Hamilton NA. Multicomponent analysis of junctional movements regulated by myosin II isoforms at the epithelial zonula adherens. PLoS One. 2011;6:e22458. doi: 10.1371/journal.pone.0022458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordenonsi M, D’Atri F, Hammar E, Parry DA, Kendrick-Jones J, Shore D, et al. Cingulin contains globular and coiled-coil domains and interacts with ZO-1, ZO-2, ZO-3, and myosin. J Cell Biol. 1999;147:1569–82. doi: 10.1083/jcb.147.7.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fanning AS, Van Itallie CM, Anderson JM. Zonula occludens-1 and -2 regulate apical cell structure and the zonula adherens cytoskeleton in polarized epithelia. Mol Biol Cell. 2012;23:577–90. doi: 10.1091/mbc.E11-09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, et al. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol. 2010;189:1107–15. doi: 10.1083/jcb.201001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–9. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roh-Johnson M, Shemer G, Higgins CD, McClellan JH, Werts AD, Tulu US, et al. Triggering a cell shape change by exploiting preexisting actomyosin contractions. Science. 2012;335:1232–5. doi: 10.1126/science.1217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soldati T. Unconventional myosins, actin dynamics and endocytosis: a ménage à trois? Traffic. 2003;4:358–66. doi: 10.1034/j.1600-0854.2003.t01-1-00095.x. [DOI] [PubMed] [Google Scholar]
- 47.Tyska MJ, Mackey AT, Huang JD, Copeland NG, Jenkins NA, Mooseker MS. Myosin-1a is critical for normal brush border structure and composition. Mol Biol Cell. 2005;16:2443–57. doi: 10.1091/mbc.E04-12-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bement WM, Wirth JA, Mooseker MS. Cloning and mRNA expression of human unconventional myosin-IC. A homologue of amoeboid myosins-I with a single IQ motif and an SH3 domain. J Mol Biol. 1994;243:356–63. doi: 10.1006/jmbi.1994.1662. [DOI] [PubMed] [Google Scholar]
- 49.Bement WM, Hasson T, Wirth JA, Cheney RE, Mooseker MS. Identification and overlapping expression of multiple unconventional myosin genes in vertebrate cell types. Proc Natl Acad Sci U S A. 1994;91:6549–53. doi: 10.1073/pnas.91.14.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mele C, Iatropoulos P, Donadelli R, Calabria A, Maranta R, Cassis P, et al. PodoNet Consortium MYO1E mutations and childhood familial focal segmental glomerulosclerosis. N Engl J Med. 2011;365:295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skowron JF, Bement WM, Mooseker MS. Human brush border myosin-I and myosin-Ic expression in human intestine and Caco-2BBe cells. Cell Motil Cytoskeleton. 1998;41:308–24. doi: 10.1002/(SICI)1097-0169(1998)41:4<308::AID-CM4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 52.Krendel M, Kim SV, Willinger T, Wang T, Kashgarian M, Flavell RA, et al. Disruption of Myosin 1e promotes podocyte injury. J Am Soc Nephrol. 2009;20:86–94. doi: 10.1681/ASN.2007111172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 2007;17:428–37. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Jung G, Wu X, Hammer JA., 3rd Dictyostelium mutants lacking multiple classic myosin I isoforms reveal combinations of shared and distinct functions. J Cell Biol. 1996;133:305–23. doi: 10.1083/jcb.133.2.305. [see comments] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novak KD, Peterson MD, Reedy MC, Titus MA. Dictyostelium myosin I double mutants exhibit conditional defects in pinocytosis. J Cell Biol. 1995;131:1205–21. doi: 10.1083/jcb.131.5.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wells AL, Lin AW, Chen LQ, Safer D, Cain SM, Hasson T, et al. Myosin VI is an actin-based motor that moves backwards. Nature. 1999;401:505–8. doi: 10.1038/46835. [DOI] [PubMed] [Google Scholar]
- 57.Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: mechanism of cell body translocation. J Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buss F, Kendrick-Jones J, Lionne C, Knight AE, Côté GP, Paul Luzio J. The localization of myosin VI at the golgi complex and leading edge of fibroblasts and its phosphorylation and recruitment into membrane ruffles of A431 cells after growth factor stimulation. J Cell Biol. 1998;143:1535–45. doi: 10.1083/jcb.143.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rock RS, Rice SE, Wells AL, Purcell TJ, Spudich JA, Sweeney HL. Myosin VI is a processive motor with a large step size. Proc Natl Acad Sci U S A. 2001;98:13655–9. doi: 10.1073/pnas.191512398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phichith D, Travaglia M, Yang Z, Liu X, Zong AB, Safer D, et al. Cargo binding induces dimerization of myosin VI. Proc Natl Acad Sci U S A. 2009;106:17320–4. doi: 10.1073/pnas.0909748106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robblee JP, Olivares AO, de la Cruz EM. Mechanism of nucleotide binding to actomyosin VI: evidence for allosteric head-head communication. J Biol Chem. 2004;279:38608–17. doi: 10.1074/jbc.M403504200. [DOI] [PubMed] [Google Scholar]
- 62.Buss F, Luzio JP, Kendrick-Jones J. Myosin VI, a new force in clathrin mediated endocytosis. FEBS Lett. 2001;508:295–9. doi: 10.1016/S0014-5793(01)03065-4. [DOI] [PubMed] [Google Scholar]
- 63.Hasson T. Myosin VI: two distinct roles in endocytosis. J Cell Sci. 2003;116:3453–61. doi: 10.1242/jcs.00669. [DOI] [PubMed] [Google Scholar]
- 64.Au JS, Puri C, Ihrke G, Kendrick-Jones J, Buss F. Myosin VI is required for sorting of AP-1B-dependent cargo to the basolateral domain in polarized MDCK cells. J Cell Biol. 2007;177:103–14. doi: 10.1083/jcb.200608126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmed ZM, Morell RJ, Riazuddin S, Gropman A, Shaukat S, Ahmad MM, et al. Mutations of MYO6 are associated with recessive deafness, DFNB37. Am J Hum Genet. 2003;72:1315–22. doi: 10.1086/375122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, et al. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11:369–75. doi: 10.1038/ng1295-369. [DOI] [PubMed] [Google Scholar]
- 67.Self T, Sobe T, Copeland NG, Jenkins NA, Avraham KB, Steel KP. Role of myosin VI in the differentiation of cochlear hair cells. Dev Biol. 1999;214:331–41. doi: 10.1006/dbio.1999.9424. [DOI] [PubMed] [Google Scholar]
- 68.Hegan PS, Giral H, Levi M, Mooseker MS. Myosin VI is required for maintenance of brush border structure, composition, and membrane trafficking functions in the intestinal epithelial cell. Cytoskeleton (Hoboken) 2012;69:235–51. doi: 10.1002/cm.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin HP, Chen HM, Wei SY, Chen LY, Chang LH, Sun YJ, et al. Cell adhesion molecule Echinoid associates with unconventional myosin VI/Jaguar motor to regulate cell morphology during dorsal closure in Drosophila. Dev Biol. 2007;311:423–33. doi: 10.1016/j.ydbio.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 70.Millo H, Leaper K, Lazou V, Bownes M. Myosin VI plays a role in cell-cell adhesion during epithelial morphogenesis. Mech Dev. 2004;121:1335–51. doi: 10.1016/j.mod.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Deng W, Leaper K, Bownes M. A targeted gene silencing technique shows that Drosophila myosin VI is required for egg chamber and imaginal disc morphogenesis. J Cell Sci. 1999;112:3677–90. doi: 10.1242/jcs.112.21.3677. [DOI] [PubMed] [Google Scholar]
- 72.Geisbrecht ER, Montell DJ. Myosin VI is required for E-cadherin-mediated border cell migration. Nat Cell Biol. 2002;4:616–20. doi: 10.1038/ncb830. [DOI] [PubMed] [Google Scholar]
- 73.Maddugoda MP, Crampton MS, Shewan AM, Yap AS. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol. 2007;178:529–40. doi: 10.1083/jcb.200612042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei SY, Escudero LM, Yu F, Chang LH, Chen LY, Ho YH, et al. Echinoid is a component of adherens junctions that cooperates with DE-Cadherin to mediate cell adhesion. Dev Cell. 2005;8:493–504. doi: 10.1016/j.devcel.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 75.Noguchi T, Lenartowska M, Miller KG. Myosin VI stabilizes an actin network during Drosophila spermatid individualization. Mol Biol Cell. 2006;17:2559–71. doi: 10.1091/mbc.E06-01-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seiler C, Ben-David O, Sidi S, Hendrich O, Rusch A, Burnside B, et al. Myosin VI is required for structural integrity of the apical surface of sensory hair cells in zebrafish. Dev Biol. 2004;272:328–38. doi: 10.1016/j.ydbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 77.Mangold S, Wu SK, Norwood SJ, Collins BM, Hamilton NA, Thorn P, et al. Hepatocyte growth factor acutely perturbs actin filament anchorage at the epithelial zonula adherens. Curr Biol. 2011;21:503–7. doi: 10.1016/j.cub.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 78.Yonezawa S, Yoshizaki N, Sano M, Hanai A, Masaki S, Takizawa T, et al. Possible involvement of myosin-X in intercellular adhesion: importance of serial pleckstrin homology regions for intracellular localization. Dev Growth Differ. 2003;45:175–85. doi: 10.1034/j.1600-0854.2004.00688.x. [DOI] [PubMed] [Google Scholar]
- 79.Zhang H, Berg JS, Li Z, Wang Y, Lång P, Sousa AD, et al. Myosin-X provides a motor-based link between integrins and the cytoskeleton. Nat Cell Biol. 2004;6:523–31. doi: 10.1038/ncb1136. [DOI] [PubMed] [Google Scholar]
- 80.Bahloul A, Michel V, Hardelin JP, Nouaille S, Hoos S, Houdusse A, et al. Cadherin-23, myosin VIIa and harmonin, encoded by Usher syndrome type I genes, form a ternary complex and interact with membrane phospholipids. Hum Mol Genet. 2010;19:3557–65. doi: 10.1093/hmg/ddq271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu L, Pan L, Wei Z, Zhang M. Structure of MyTH4-FERM domains in myosin VIIa tail bound to cargo. Science. 2011;331:757–60. doi: 10.1126/science.1198848. [DOI] [PubMed] [Google Scholar]
- 82.Umeki N, Jung HS, Watanabe S, Sakai T, Li XD, Ikebe R, et al. The tail binds to the head-neck domain, inhibiting ATPase activity of myosin VIIA. Proc Natl Acad Sci U S A. 2009;106:8483–8. doi: 10.1073/pnas.0812930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y, Kovács M, Sakamoto T, Zhang F, Kiehart DP, Sellers JR. Dimerized Drosophila myosin VIIa: a processive motor. Proc Natl Acad Sci U S A. 2006;103:5746–51. doi: 10.1073/pnas.0509935103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sakai T, Umeki N, Ikebe R, Ikebe M. Cargo binding activates myosin VIIA motor function in cells. Proc Natl Acad Sci U S A. 2011;108:7028–33. doi: 10.1073/pnas.1009188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe S, Ikebe R, Ikebe M. Drosophila myosin VIIA is a high duty ratio motor with a unique kinetic mechanism. J Biol Chem. 2006;281:7151–60. doi: 10.1074/jbc.M511592200. [DOI] [PubMed] [Google Scholar]
- 86.Haithcock J, Billington N, Choi K, Fordham J, Sellers JR, Stafford WF, et al. The kinetic mechanism of mouse myosin VIIA. J Biol Chem. 2011;286:8819–28. doi: 10.1074/jbc.M110.163592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sahly I, El-Amraoui A, Abitbol M, Petit C, Dufier JL. Expression of myosin VIIA during mouse embryogenesis. Anat Embryol (Berl) 1997;196:159–70. doi: 10.1007/s004290050088. [DOI] [PubMed] [Google Scholar]
- 88.Hasson T, Heintzelman MB, Santos-Sacchi J, Corey DP, Mooseker MS. Expression in cochlea and retina of myosin VIIa, the gene product defective in Usher syndrome type 1B. Proc Natl Acad Sci U S A. 1995;92:9815–9. doi: 10.1073/pnas.92.21.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weil D, Blanchard S, Kaplan J, Guilford P, Gibson F, Walsh J, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–1. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 90.Gibson F, Walsh J, Mburu P, Varela A, Brown KA, Antonio M, et al. A type VII myosin encoded by the mouse deafness gene shaker-1. Nature. 1995;374:62–4. doi: 10.1038/374062a0. [DOI] [PubMed] [Google Scholar]
- 91.Chen ZY, Hasson T, Kelley PM, Schwender BJ, Schwartz MF, Ramakrishnan M, et al. Molecular cloning and domain structure of human myosin-VIIa, the gene product defective in Usher syndrome 1B. Genomics. 1996;36:440–8. doi: 10.1006/geno.1996.0489. [DOI] [PubMed] [Google Scholar]
- 92.Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–66. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- 93.Bolz H, von Brederlow B, Ramírez A, Bryda EC, Kutsche K, Nothwang HG, et al. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet. 2001;27:108–12. doi: 10.1038/83667. [DOI] [PubMed] [Google Scholar]
- 94.Di Palma F, Holme RH, Bryda EC, Belyantseva IA, Pellegrino R, Kachar B, et al. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet. 2001;27:103–7. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- 95.Boëda B, El-Amraoui A, Bahloul A, Goodyear R, Daviet L, Blanchard S, et al. Myosin VIIa, harmonin and cadherin 23, three Usher I gene products that cooperate to shape the sensory hair cell bundle. EMBO J. 2002;21:6689–99. doi: 10.1093/emboj/cdf689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Senften M, Schwander M, Kazmierczak P, Lillo C, Shin JB, Hasson T, et al. Physical and functional interaction between protocadherin 15 and myosin VIIa in mechanosensory hair cells. J Neurosci. 2006;26:2060–71. doi: 10.1523/JNEUROSCI.4251-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, et al. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449:87–91. doi: 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- 98.Kiehart DP, Franke JD, Chee MK, Montague RA, Chen TL, Roote J, et al. Drosophila crinkled, mutations of which disrupt morphogenesis and cause lethality, encodes fly myosin VIIA. Genetics. 2004;168:1337–52. doi: 10.1534/genetics.104.026369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Todi SV, Franke JD, Kiehart DP, Eberl DF. Myosin VIIA defects, which underlie the Usher 1B syndrome in humans, lead to deafness in Drosophila. Curr Biol. 2005;15:862–8. doi: 10.1016/j.cub.2005.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sousa S, Cabanes D, El-Amraoui A, Petit C, Lecuit M, Cossart P. Unconventional myosin VIIa and vezatin, two proteins crucial for Listeria entry into epithelial cells. J Cell Sci. 2004;117:2121–30. doi: 10.1242/jcs.01066. [DOI] [PubMed] [Google Scholar]
- 101.Küssel-Andermann P, El-Amraoui A, Safieddine S, Nouaille S, Perfettini I, Lecuit M, et al. Vezatin, a novel transmembrane protein, bridges myosin VIIA to the cadherin-catenins complex. EMBO J. 2000;19:6020–9. doi: 10.1093/emboj/19.22.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Velichkova M, Guttman J, Warren C, Eng L, Kline K, Vogl AW, et al. A human homologue of Drosophila kelch associates with myosin-VIIa in specialized adhesion junctions. Cell Motil Cytoskeleton. 2002;51:147–64. doi: 10.1002/cm.10025. [DOI] [PubMed] [Google Scholar]
- 103.Etournay R, Zwaenepoel I, Perfettini I, Legrain P, Petit C, El-Amraoui A. Shroom2, a myosin-VIIa- and actin-binding protein, directly interacts with ZO-1 at tight junctions. J Cell Sci. 2007;120:2838–50. doi: 10.1242/jcs.002568. [DOI] [PubMed] [Google Scholar]
- 104.Liao W, Elfrink K, Bähler M. Head of myosin IX binds calmodulin and moves processively toward the plus-end of actin filaments. J Biol Chem. 2010;285:24933–42. doi: 10.1074/jbc.M110.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bähler M, Elfrink K, Hanley PJ, Thelen S, Xu Y. Cellular functions of class IX myosins in epithelia and immune cells. Biochem Soc Trans. 2011;39:1166–8. doi: 10.1042/BST0391166. [DOI] [PubMed] [Google Scholar]
- 106.Reinhard J, Scheel AA, Diekmann D, Hall A, Ruppert C, Bähler M. A novel type of myosin implicated in signalling by rho family GTPases. EMBO J. 1995;14:697–704. doi: 10.1002/j.1460-2075.1995.tb07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chieregatti E, Gärtner A, Stöffler HE, Bähler M. Myr 7 is a novel myosin IX-RhoGAP expressed in rat brain. J Cell Sci. 1998;111:3597–608. doi: 10.1242/jcs.111.24.3597. [DOI] [PubMed] [Google Scholar]
- 108.Müller RT, Honnert U, Reinhard J, Bähler M. The rat myosin myr 5 is a GTPase-activating protein for Rho in vivo: essential role of arginine 1695. Mol Biol Cell. 1997;8:2039–53. doi: 10.1091/mbc.8.10.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 110.Ridley AJ. Rho: theme and variations. Curr Biol. 1996;6:1256–64. doi: 10.1016/S0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- 111.Braga VM, Yap AS. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr Opin Cell Biol. 2005;17:466–74. doi: 10.1016/j.ceb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 112.Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, et al. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–66. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abouhamed M, Grobe K, San IV, Thelen S, Honnert U, Balda MS, et al. Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus. Mol Biol Cell. 2009;20:5074–85. doi: 10.1091/mbc.E09-04-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gorman SW, Haider NB, Grieshammer U, Swiderski RE, Kim E, Welch JW, et al. The cloning and developmental expression of unconventional myosin IXA (MYO9A) a gene in the Bardet-Biedl syndrome (BBS4) region at chromosome 15q22-q23. Genomics. 1999;59:150–60. doi: 10.1006/geno.1999.5867. [DOI] [PubMed] [Google Scholar]
- 115.Omelchenko T, Hall A. Myosin-IXA regulates collective epithelial cell migration by targeting RhoGAP activity to cell-cell junctions. Curr Biol. 2012;22:278–88. doi: 10.1016/j.cub.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Monsuur AJ, de Bakker PI, Alizadeh BZ, Zhernakova A, Bevova MR, Strengman E, et al. Myosin IXB variant increases the risk of celiac disease and points toward a primary intestinal barrier defect. Nat Genet. 2005;37:1341–4. doi: 10.1038/ng1680. [DOI] [PubMed] [Google Scholar]
- 117.van Bodegraven AA, Curley CR, Hunt KA, Monsuur AJ, Linskens RK, Onnie CM, et al. Genetic variation in myosin IXB is associated with ulcerative colitis. Gastroenterology. 2006;131:1768–74. doi: 10.1053/j.gastro.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 118.Cooney R, Cummings JR, Pathan S, Beckly J, Geremia A, Hancock L, et al. Association between genetic variants in myosin IXB and Crohn’s disease. Inflamm Bowel Dis. 2009;15:1014–21. doi: 10.1002/ibd.20885. [DOI] [PubMed] [Google Scholar]
- 119.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–91. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- 120.van den Boom F, Düssmann H, Uhlenbrock K, Abouhamed M, Bähler M. The Myosin IXb motor activity targets the myosin IXb RhoGAP domain as cargo to sites of actin polymerization. Mol Biol Cell. 2007;18:1507–18. doi: 10.1091/mbc.E06-08-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chandhoke SK, Mooseker MS. A role for myosin IXb, a motor-RhoGAP chimera, in epithelial wound healing and tight junction regulation. Mol Biol Cell. 2012;23:2468–80. doi: 10.1091/mbc.E11-09-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hanley PJ, Xu Y, Kronlage M, Grobe K, Schön P, Song J, et al. Motorized RhoGAP myosin IXb (Myo9b) controls cell shape and motility. Proc Natl Acad Sci U S A. 2010;107:12145–50. doi: 10.1073/pnas.0911986107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Plantard L, Arjonen A, Lock JG, Nurani G, Ivaska J, Strömblad S. PtdIns(3,4,5)P₃ is a regulator of myosin-X localization and filopodia formation. J Cell Sci. 2010;123:3525–34. doi: 10.1242/jcs.069609. [DOI] [PubMed] [Google Scholar]
- 124.Umeki N, Jung HS, Sakai T, Sato O, Ikebe R, Ikebe M. Phospholipid-dependent regulation of the motor activity of myosin X. Nat Struct Mol Biol. 2011;18:783–8. doi: 10.1038/nsmb.2065. [DOI] [PubMed] [Google Scholar]
- 125.Lu Q, Yu J, Yan J, Wei Z, Zhang M. Structural basis of the myosin X PH1(N)-PH2-PH1(C) tandem as a specific and acute cellular PI(3,4,5)P(3) sensor. Mol Biol Cell. 2011;22:4268–78. doi: 10.1091/mbc.E11-04-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Weber KL, Sokac AM, Berg JS, Cheney RE, Bement WM. A microtubule-binding myosin required for nuclear anchoring and spindle assembly. Nature. 2004;431:325–9. doi: 10.1038/nature02834. [DOI] [PubMed] [Google Scholar]
- 127.Berg JS, Derfler BH, Pennisi CM, Corey DP, Cheney RE. Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci. 2000;113:3439–51. doi: 10.1242/jcs.113.19.3439. [DOI] [PubMed] [Google Scholar]
- 128.Rogers MS, Strehler EE. The tumor-sensitive calmodulin-like protein is a specific light chain of human unconventional myosin X. J Biol Chem. 2001;276:12182–9. doi: 10.1074/jbc.M010056200. [DOI] [PubMed] [Google Scholar]
- 129.Bohil AB, Robertson BW, Cheney RE. Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci U S A. 2006;103:12411–6. doi: 10.1073/pnas.0602443103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu KC, Jacobs DT, Dunn BD, Fanning AS, Cheney RE. Myosin-X functions in polarized epithelial cells. Mol Biol Cell. 2012;23:1675–87. doi: 10.1091/mbc.E11-04-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–97. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McNeil E, Capaldo CT, Macara IG. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2006;17:1922–32. doi: 10.1091/mbc.E05-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Capaldo CT, Macara IG. Depletion of E-cadherin disrupts establishment but not maintenance of cell junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell. 2007;18:189–200. doi: 10.1091/mbc.E06-05-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Almagro S, Durmort C, Chervin-Pétinot A, Heyraud S, Dubois M, Lambert O, et al. The motor protein myosin-X transports VE-cadherin along filopodia to allow the formation of early endothelial cell-cell contacts. Mol Cell Biol. 2010;30:1703–17. doi: 10.1128/MCB.01226-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–33. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, Mostov K, et al. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol. 2010;189:725–38. doi: 10.1083/jcb.201002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zheng Z, Zhu H, Wan Q, Liu J, Xiao Z, Siderovski DP, et al. LGN regulates mitotic spindle orientation during epithelial morphogenesis. J Cell Biol. 2010;189:275–88. doi: 10.1083/jcb.200910021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kwon M, Godinho SA, Chandhok NS, Ganem NJ, Azioune A, Thery M, et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 2008;22:2189–203. doi: 10.1101/gad.1700908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Toyoshima F, Nishida E. Integrin-mediated adhesion orients the spindle parallel to the substratum in an EB1- and myosin X-dependent manner. EMBO J. 2007;26:1487–98. doi: 10.1038/sj.emboj.7601599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Woolner S, O’Brien LL, Wiese C, Bement WM. Myosin-10 and actin filaments are essential for mitotic spindle function. J Cell Biol. 2008;182:77–88. doi: 10.1083/jcb.200804062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Woolner S, Papalopulu N. Spindle position in symmetric cell divisions during epiboly is controlled by opposing and dynamic apicobasal forces. Dev Cell. 2012;22:775–87. doi: 10.1016/j.devcel.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liang Y, Wang A, Belyantseva IA, Anderson DW, Probst FJ, Barber TD, et al. Characterization of the human and mouse unconventional myosin XV genes responsible for hereditary deafness DFNB3 and shaker 2. Genomics. 1999;61:243–58. doi: 10.1006/geno.1999.5976. [DOI] [PubMed] [Google Scholar]
- 143.Belyantseva IA, Boger ET, Friedman TB. Myosin XVa localizes to the tips of inner ear sensory cell stereocilia and is essential for staircase formation of the hair bundle. Proc Natl Acad Sci U S A. 2003;100:13958–63. doi: 10.1073/pnas.2334417100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lloyd RV, Vidal S, Jin L, Zhang S, Kovacs K, Horvath E, et al. Myosin XVA expression in the pituitary and in other neuroendocrine tissues and tumors. Am J Pathol. 2001;159:1375–82. doi: 10.1016/S0002-9440(10)62524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, et al. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science. 1998;280:1447–51. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]
- 146.Probst FJ, Fridell RA, Raphael Y, Saunders TL, Wang A, Liang Y, et al. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science. 1998;280:1444–7. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- 147.Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, et al. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7:148–56. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 148.Ebermann I, Scholl HP, Charbel Issa P, Becirovic E, Lamprecht J, Jurklies B, et al. A novel gene for Usher syndrome type 2: mutations in the long isoform of whirlin are associated with retinitis pigmentosa and sensorineural hearing loss. Hum Genet. 2007;121:203–11. doi: 10.1007/s00439-006-0304-0. [DOI] [PubMed] [Google Scholar]
- 149.Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, et al. Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol. 2011;21:167–72. doi: 10.1016/j.cub.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liu R, Woolner S, Johndrow JE, Metzger D, Flores A, Parkhurst SM. Sisyphus, the Drosophila myosin XV homolog, traffics within filopodia transporting key sensory and adhesion cargos. Development. 2008;135:53–63. doi: 10.1242/dev.011437. [DOI] [PubMed] [Google Scholar]
- 151.Bridges C, Morgan T. Contributions to the genetics of Drosophila melanogaster. II. The second-chromosome group of mutant characters. Carnegie Inst Washington Publ. 1919;278:123–304. [Google Scholar]
- 152.Tzolovsky G, Millo H, Pathirana S, Wood T, Bownes M. Identification and phylogenetic analysis of Drosophila melanogaster myosins. Mol Biol Evol. 2002;19:1041–52. doi: 10.1093/oxfordjournals.molbev.a004163. [DOI] [PubMed] [Google Scholar]
- 153.Mao Y, Rauskolb C, Cho E, Hu WL, Hayter H, Minihan G, et al. Dachs: an unconventional myosin that functions downstream of Fat to regulate growth, affinity and gene expression in Drosophila. Development. 2006;133:2539–51. doi: 10.1242/dev.02427. [DOI] [PubMed] [Google Scholar]
- 154.Mao Y, Tournier AL, Bates PA, Gale JE, Tapon N, Thompson BJ. Planar polarization of the atypical myosin Dachs orients cell divisions in Drosophila. Genes Dev. 2011;25:131–6. doi: 10.1101/gad.610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–21. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Waddington C. The genetic control of wing development in Drosophila. J Genet. 1940;41:75–113. doi: 10.1007/BF02982977. [DOI] [Google Scholar]
- 157.den Elzen N, Buttery CV, Maddugoda MP, Ren G, Yap AS. Cadherin adhesion receptors orient the mitotic spindle during symmetric cell division in mammalian epithelia. Mol Biol Cell. 2009;20:3740–50. doi: 10.1091/mbc.E09-01-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mahoney PA, Weber U, Onofrechuk P, Biessmann H, Bryant PJ, Goodman CS. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell. 1991;67:853–68. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- 159.Bosveld F, Bonnet I, Guirao B, Tlili S, Wang Z, Petitalot A, et al. Mechanical control of morphogenesis by Fat/Dachsous/Four-jointed planar cell polarity pathway. Science. 2012;336:724–7. doi: 10.1126/science.1221071. [DOI] [PubMed] [Google Scholar]