Abstract
Objective
To provide guidance for clinical disease prevention and treatment, this study examined the epidemiology, antibiotic susceptibility, and serotype distribution of Streptococcus pneumoniae (S. pneumoniae) associated with invasive pneumococcal diseases (IPDs) among children less than 14 years of age in Shenzhen, China.
Materials and Methods
All the clinical strains were isolated from children less than 14 years old from January 2009 to August 2012. The serotypes and antibiotic resistance of strains of S. pneumoniae were determined using the capsular swelling method and the E-test.
Results
A total of 89 strains were isolated and 87 isolates were included. The five prevailing serotypes were 19F (28.7%), 14 (16.1%), 23F (11.5%), 19A (9.2%) and 6B (6.9%). The most common sequence types (ST) were ST271 (21.8%), ST876 (18.4%), ST320 (8.0%) and ST81 (6.9%) which were mainly related to 19F, 14, 19A and 23F, respectively. The potential coverage by 7-, 10-, and 13-valent pneumococcal conjugate vaccine were 77.0%, 77.0%, and 89.7%, respectively. Among the 87 isolates investigated, 11.5% were resistant to penicillin, and for meningitis isolates, the resistance rate was 100%. Multi-drug resistance (MDR) was exhibited by 49 (56.3%) isolates. Eighty-four isolates were resistance to erythromycin, among which, 56 (66.7%) carried the ermB gene alone and 28 (33.3%) expressed both the ermB and mefA/E genes.
Conclusions
The potential coverage of PCV13 is higher than PCV7 and PCV10 because high rates of serotypes 19A and 6A in Shenzhen. The clinical treatment of IPD needs a higher drug concentration of antibiotics. Continued surveillance of the antimicrobial susceptibility and serotypes distribution of IPD isolates may be necessary.
Introduction
Streptococcus pneumoniae (S. pneumoniae) is one of the most common causative pathogens of severe invasive infections (e.g., meningitis and sepsis) among infants and young children. The morbidity and mortality rates of IPD worldwide are high, particularly in developing countries. The World Health Organization (WHO) estimated that the annual incidence of IPD ranges from 10 to 100 cases per 100 000 population in Europe and the United States. In developing countries, the incidence of IPD in children aged <5 years is several times higher than it is in industrialized countries [1]. A surveillance study in Asia estimated the mean incidence of pneumococcal meningitis in children aged 1 to 23 months at 5.1 cases per 100 000 childbirths from 1999 to 2003 [2]. More recently, Chinese data showed that there were 12 815 cases/100 000/year of all-cause pneumonia among children between 1 month and 59 months, with 526 deaths/100 000 annually and there were 14 meningitis cases/100 000/year between 1980 and 2008 [3]. However, data from IPD cases are rare in many parts of Asia because of the low culture rate caused by the inappropriate use of antibiotics [4], [5], [6] and difficulties in culturing S. pneumoniae in vitro [7].
This situation has been worsened by the rapid spread of antimicrobial-resistant S. pneumoniae. Antibiotic-resistant pneumococci have been increasing since the 1990s and are becoming a major problem worldwide. The emergence of multidrug-resistant S. pneumoniae (MDRSP) has been observed in various countries over the past decades. The growing resistance of S. pneumoniae to commonly used antibiotics underlines the urgent need for vaccines to be used to control pneumococcal disease.
There are two types of S. pneumoniae vaccine available that can be classified as polysaccharide vaccine and glycoconjugated polysaccharide vaccine. At present, the 7-valent glycoconjugated vaccine (PCV7, which included pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) has been regularly used for all infants and children aged two to 59 months who are at increased risk for pneumococcal disease in Europe and the United States [8]. Since the introduction of PCV7, it had showed high effectiveness in controlling IPD [9]. However, the coverage of PCV7 varies with the different population being vaccinated and may range from 60% in Asia to 70% to 85% in the United States and Europe [1]. At present, two new vaccines based on PCV7, PCV10 (including additional serotypes 1, 5 and 7F to PCV7) and PCV13 (including additional serotypes 1, 3, 5, 6A, 7F and 19A to PCV7) had been used in some developed countries [1]. To be effective in controlling pneumococcal disease, the composition of vaccine must match as closely as possible the prevalence of serotypes in each region. Therefore, it is necessary to monitor pneumococcal serotypes at local regions for the design of effective vaccine formulations.
The current study is conducted to examine the serotype distribution and antimicrobial resistance pattern of S. pneumoniae in Shenzhen, a city located in the south of China, for guiding clinical usage of antimicrobial agents and application of pneumococcal vaccines in public health control programs.
Materials and Methods
2.1 Clinical Isolates
The study protocol was in accordance with the ethical standards of the responsible regional committee on human experimentation and the Helsinki Declaration of 1975 (as revised in 1983). It was approved by the ethics committee of Shenzhen Children’s Hospital.
A total of 89 isolates were identified as S. pneumoniae during a clinical work in Shenzhen Children’s Hospital from January 2009 to August 2012. All presumptive S. pneumoniae isolates were delivered to the Laboratory of Microbiology and Immunology, Beijing Children’s Hospital, Beijing, China. The isolates were identified based on their alpha-hemolytic colony, alpha hemolytic, and Gram positive diplococci. The identification was confirmed by optochin sensitivity test (Oxoid, Basingstoke, Britain), bile solubility test, and Omni serum assay (Statens Serum Institute, Copenhagen, Denmark). Only 87 of these isolates were covered in this study because of the loss of two isolates from the blood sample collected in 2012.
Among the 87 isolates, 24 were collected from 2009, 32 from 2010, 23 from 2011, and eight from 2012. In addition, 71 (81.6%) strains were isolated from blood samples, nine (10.4%) from cerebral spinal fluid samples, three (3.4%) from abscess samples, and one (4.6%) each from pleural fluid, joint cavity fluid, abdominal fluid, and broncho-alveolar lavage samples. Most of these strains (78/87, 89.7%) were isolated from children aged <5 years and nine (10.3%) from children aged ≥5 years.
S. pneumoniae strains isolated from normal sterile specimens from children aged <14 years were collected. All isolates were stored at −80°C in a fat-free milk preservation medium until further analysis. When two isolates express the same serotype from the same subject, only one isolate was included. All isolates were typed by a capsule-quelling test using type-specific antisera (Statens Serum Institute, Copenhagen, Denmark) against the serotypes present in the 23-valent pneumococcal polysaccharide vaccine (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, and 33F) and serotype 6A. Typing was conducted by phase-contrast microscopy according to the published procedure [10]. Strains that could not be typed using the abovementioned method were denoted as un-typed strains.
2.2 Antimicrobial Susceptibility Testing
The minimum inhibitory concentrations (MICs) for penicillin, amoxicillin–clavulanic acid, cefaclor, ceftriaxone, cefuroxime, erythromycin, azithromycin, sulfamethoxazole–trimethoprim (SXT), vancomycin, tetracycline, chloramphenicol, levofloxacin, and imipenem were determined using E-test strips (AB Biodisk, Solna, Sweden) [11]. Breakpoints were based on the 2010 criteria of the Clinical and Laboratory Standards Institute (CLSI) [12]. S. pneumoniae ATCC 49619 was used as the quality control. Isolates not susceptible to three or more classes of antimicrobials were considered MDRSP.
2.3 Detection of Macrolide Resistance Genes
The ermB and mefA/E resistance genes were amplified by polymerase chain reaction (PCR) for all erythromycin-resistant strains using the primers and PCR conditions as previously described [13]. Each PCR reaction contained 500 ng of template DNA, 50 mM potassium chloride, 10 mM Tris–hydrochloride (pH 8.3), 200 µM of each deoxynucleotide triphosphate, 2.5 U Taq DNA polymerase (Takara Bio, Dalian, China), 1.5 mM magnesium chloride, and 1.5 µM of each primer. The PCR products were visualized by 1.5% agarose gel electrophoresis and gold-view staining.
2.4 Multilocus Sequence Typing (MLST)
Internal fragments ∼450 bp long from the aroE, gdh, gki, recP, spi, xpt, and ddl genes were amplified by PCR as previously described [14]. All the STs absent in the pneumococcal MLST database were submitted to the MLST S. pneumoniae database for designation. The eBURST algorithm (http://eburst.mlst.net) was used to estimate the relationships among isolates. STs that share six identical alleles of the seven MLST loci with another ST in the group were subdivided into one group as a clone complex (CC).
2.5 Statistical Methods
All data were analyzed using WHONET software version 5.6 (WHO). The χ2 test, calculated using SPSS version 10.0 (SPSS Inc, Chicago, USA), was used for statistical comparisons. A two-tailed cutoff of P<0.05 was considered statistically significant.
Results
3.1 Distribution of Serotypes and Coverage of PCVs
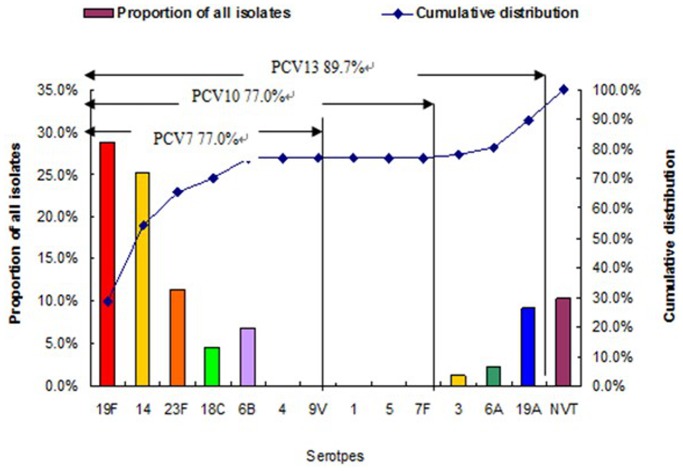
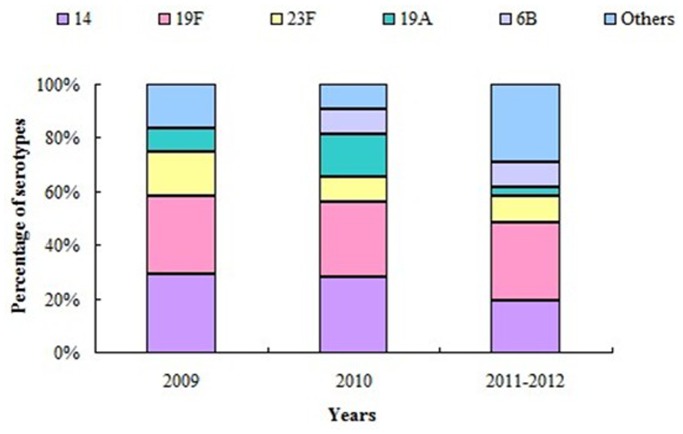
The serotype distribution of the 87 S. pneumoniae isolates is shown in Figure 1. Among the 87 isolates, 19F (n = 25, 28.7%), 14 (n = 22, 25.3%), 23F (n = 10, 11.5%), 19A (n = 8, 9.2%), and 6B (n = 6, 6.9%) were the most common serotypes. These five serotypes accounted for 81.6% of all the isolates. Two strains (2.3%) were un-typed. The overall coverage rates of PCV-7, PCV-10, and PCV-13 were 77.0%, 77.0%, and 89.7%, respectively (Figure 1). No significant differences in the distribution of the common serotypes were observed among the different years (Figure 2).
Figure 1. Proportionate and cumulative serotype distributions of 87 S. pneumoniae isolates causing invasive infections among children aged <14 years in Shenzhen Children’s Hospital from 2009 to 2012.
NVT, non-vaccine serotypes not included in PCV13.
Figure 2. Frequency of common serotypes among the different years in Shenzhen Children’s Hospital.
(For serotype 14: χ2 = 0.9, 19F: χ2 = 0.01, 19A: χ2 = 2.94, 23F: χ2 = 0.87, 6B: χ2 = 0.71; all P value >0.05.).
3.2 Antimicrobial Susceptibility Testing
The antibiotic activities of the 87 S. pneumoniae isolates against the 13 antimicrobials are presented in Table 1. According to the revised CLSI breakpoints for parenteral penicillin (resistant ≥8 µg/mL for non-meningitis isolates and ≥0.12 µg/mL for meningitis isolates), the prevalence rates of penicillin resistance were 1.3% and 100% in the non-meningitis and meningitis isolates, respectively. The proportion of isolates resistant to ceftriaxone was 3.8% for the non-meningitis and 33.3%meningitis isolates. The cefuroxime and cefaclor resistance rates were 79.3% and 81.6%, respectively. All the isolates were susceptible to vancomycin, amoxicillin–clavulanic acid, and levofloxacin, except for one (1.1%) isolate that was resistant to levofloxacin. Most of these isolates showed high resistance to macrolide antimicrobials, whereby 84 (96.6%) were resistant to both erythromycin and azithromycin with an MIC of >256 µg/mL. The non-susceptibility rates to imipenem, tetracycline, and SXT were 72.9%, 94.3%, and 55.1%, respectively.
Table 1. Susceptibility and minimum inhibitory concentrations of 13 antimicrobials for the 87 S. pneumoniae isolates.
| Antimicrobials | No of isolates | Susceptibility | MIC(µg/ml) | ||||
| Resistant | Intermediate | Susceptible | 50% | 90% | Range | ||
| Penicillin | |||||||
| Meningitis | 9 | 9(100%) | 0 | 0 | 1 | 2 | 0.125–2 |
| Non-meningitis | 78 | 1(1.3%) | 2(2.6%) | 75(96.1%) | 1 | 2 | 0.016–8 |
| Amoxicillin–clavulanic acid | 87 | 0 | 5(5.7%) | 82(94.3%) | 0.75 | 2 | 0.023–2 |
| Cefuroxime | 87 | 69(79.3%) | 3(3.4%) | 15(17.2%) | 3 | 6 | 0.016–24 |
| Ceftriaxone | |||||||
| Meningitis | 9 | 3(33.3%) | 5(55.6%) | 1(11.1%) | 1.5 | 2 | 0.032–2 |
| Non-meningitis | 78 | 3(3.8%) | 26(33.3%) | 49(62.9%) | 1 | 2 | 0.016–8 |
| Cefaclor | 87 | 71(81.6%) | 2(2.3%) | 14(16.1%) | 32 | 256 | 0.19–512 |
| Erythromycin | 87 | 84(96.6%) | 1(1.1%) | 2(2.3%) | >256 | >256 | 0.094–512 |
| Azithromycin | 87 | 85(97.7%) | 2(2.3%) | 0 | >256 | >256 | 1–512 |
| Vancomycin | 87 | 0 | 0 | 87(100%) | 0.5 | 0.75 | 0.38–0.75 |
| Levofloxacin | 87 | 0 | 1(1.1%) | 86(98.9%) | 0.75 | 1 | 0.38–4 |
| Imipenem | 87 | 0 | 63(72.4%) | 24(27.6%) | 0.19 | 0.38 | 0.19–0.5 |
| Tetracycline | 87 | 65(74.7%) | 17(19.6%) | 5(5.7%) | 12 | 24 | 0.094–48 |
| Chloramphenicol | 87 | 13(14.9%) | 0 | 74(85.1%) | 3 | 16 | 1.5–256 |
| Sulfamethoxazole–trimethoprim | 87 | 31(35.6%) | 17(19.5%) | 39(44.9%) | 4 | 32 | 0.064–32 |
The overall rate of MDRSP was 56.3% (n = 49) (55.1% and 66.7% in the non-meningitis and meningitis isolates, respectively). The most common pattern of MDR was resistance to β-lactam antibiotics, macrolides, and SXT (n = 38, 77.6%), followed by resistance to β-lactam antibiotics, macrolides, SXT and chloramphenicol (n = 5, 10.2%). All strains with MDR were resistant to at least one of the macrolides tested. Among the 49 MDR isolates, 19F (n = 22/25, 88%), 23F (n = 10/10, 100%), 19A (n = 5/8, 62.5%), 14 (n = 4/22, 18.2%), and 18C (n = 3/4, 75%) were the most common serotypes.
3.3 Detection of Macrolide Resistance Genes
A total of 84 isolates of erythromycin-resistant S. pneumoniae were examined for macrolide-resistance genes. There were 56 (66.7%) isolates were only positive for ermB, but negative for mefA. Twenty-eight (33.3%) pneumococcal isolates contained both ermB and mefA/E. Most of (n = 27, 96.4%) the 28 isolates that carried both the ermB and mefA/E genes were typed into serotypes 19F (n = 21) and 19A (n = 6).
3.4 MLST
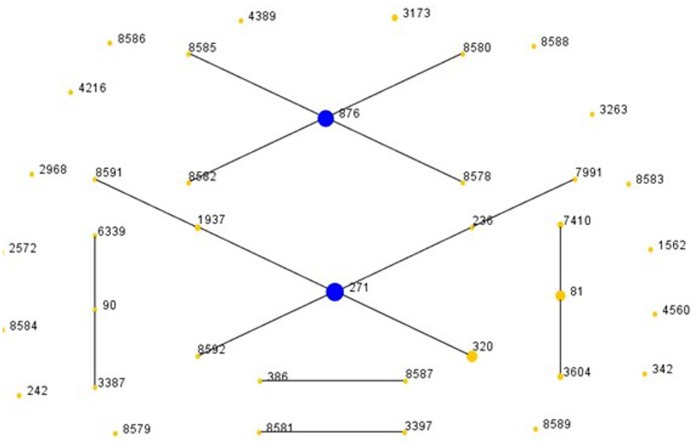
A total of 38 STs were detected in the 87 S. pneumoniae isolates, of which 17 were newly assigned (2572, 6339, 8577 to 8589, 8591 and 8592) via MLST analysis. All of the new STs were novel combinations of known alleles. The four predominant STs for all pneumococci were ST271 (22.9%, 20/87), ST876 (18.4%, 16/87), ST320 (8.0%, 7/87), and ST81 (6.9%, 6/87; Figure 3), which were mainly related to serotypes 19F, 14, 19A, and 23F, respectively. Moreover, all the isolates with ST320 were typed into 19A, and the strains with ST876 were typed to serotype 14. The eBURST analysis showed six CCs and 17 singletons (Figure 3). Among all the isolates, CC271 was the most frequent CC, with a proportion of 35.6% (31/87), followed by CC876, with a proportion of 22.9% (20/87).
Figure 3. Population snapshot of the 87 S. pneumoniae strains through eBURST analysis.
One spot indicates one ST. The size of one spot corresponds to the number of pneumococcal isolates with the same ST. The lines indicate the presence of single locus variant (SLV) links among particular STs.
Discussion
The serotypes distribution of S. pneumoniae reportedly varied with age, geography, diseases, and time. In this study, the most prevalent serotypes were different from previous report in either ranking order or the proportion of the predominant serotypes. Throughout Europe, the most common serotypes causing IPD were 14, 6B, 19F, and 23F. However, since the introduction of PCV7, serotypes 1, 3, 6A, 7F, and 19A have become the leading cause among the IPD isolates in several European countries [15]. Among the 115 invasive S. pneumoniae isolates from Thai children aged <5 years, 6B, 23F, 14, and 19F are the most common serotypes, accounting for 68% of all isolates [16]. Our colleges recently reported that the most common serotypes of IPD isolates in Chinese pediatric patients were 19F, 14, 19A, 6B, and 23F [17]. However, we found that the rank order of serotypes was 19F, 14, 23F, 19A, and 6B. Notably, eight (9.2%) of the isolates expressing serotype 19A, which were not found in the study between 2006 and 2008 [17], were found in the present study. Xue et al [17] indicated that serotype 19A is very common in northern cities but is rarely found in southern and eastern cities, including Shenzhen. This change has two potential causes. First, serotype 19A originated from Northern to Southern China. Second, 19A, as an MDR serotype, was selected under the pressure of antibiotics and easier to be transferred. As reported previously [17], PCV7 is not responsible for this increasing trend because its immunization rate is less than 1% in this city for the past four years. These data indicated that the prevailing serotypes vary in different regions and time worldwide. So, the continuous surveillance for IPD isolates, especially MDRSP such as 19A, 19F, and 23F was necessary.
PCV7, the first conjugate vaccine for preventing pneumococcal infections for children, was introduced into the routine infant immunization programs in the US in 2000. Within two years after its introduction, the incidence of IPD among children aged <5 years decreased by 75% [1]. Recent studies have introduced two conjugate vaccines: PCV10 and PCV13. Data suggested that after changing from PCV7 to PCV10, the proportion of serotypes covered would increase to varying degrees in USA, Europe, Africa, and Asia. Changing from PCV10 to PCV13 would further improve the coverage of serotypes by 4% to 7% globally [18]. A study in central Thailand from 2006 to 2009 indicated that PCV7 and PCV13 covered 70.3% and 81.2% of patients aged <5 years, respectively [19]. In Europe, the vaccine serotype coverage ranged from 37% to 100% for PCV7, with mean increases of 7% and 16% for PCV10 and PCV13, respectively [15]. However, data regarding vaccine coverage are rare in China. Among children with pneumonia in five Chinese hospitals, the change from PCV7 to PCV10 increased the vaccine coverage rates by approximately 0.6%, whereas the change from PCV10 to PCV13 increased the vaccine coverage rates by approximately 15.4% [20]. A multicenter study on IPD isolates of children in China [17] showed that PCV7, PCV10, and PCV13 could cover 60.3%, 66.7%, and, 87.8% of all isolates, respectively. In the present study, the coverage rates of PCV7 and PCV10 were same, whereas the vaccine coverage from PCV10 to PCV13 increased by 12.7%. These findings are similar to the results of a previous study conducted by Zhou [21]. Eight (9.2%) isolates with serotype 19A and two (2.3%) isolates with serotype 6A may be responsible for the significant increasing from PCV7 and PCV10 to PCV13. Therefore, PCV13 should be introduced in China because it can protect more pneumococcal diseases in Chinese children.
Pneumococcal infections are treated mainly with antibiotics, especially penicillin. However, the emergence of penicillin-resistant S. pneumoniae has been a major challenge. In Asian countries, the incidence rate of penicillin-resistant S. pneumoniae in children has increased from 9.1% in 1998 to 1999 [22] to 29.4% in 2000 to 2001 [23]. Before 2000, the rate of penicillin resistance was 10.1% in Japan, 5.2% in the UK, 0.7% in Germany, 24.7% in Spain, 33.9% in France, and 4.9% in Italy [24]. In the present study, only 3 (3.9%) non-meningitis isolates were non-susceptible to penicillin based on the 2010 CLSI criteria. This finding was much lower than that in previous studies because of the change in penicillin breakpoints. When the breakpoint of CLSI 2007 (susceptible when MIC was ≤0.06 µg/mL but resistant when MIC was ≥2 µg/mL) was adopted, the penicillin intermediate rate of the non-meningitis isolates was up to 74.3%, and the resistant rate was 11.5%. Moreover, the resistance rate of penicillin reportedly decreased to 70.6% [25] for children aged <5 years with the use of penicillin oral breakpoints (resistant when MIC was ≥2 µg/mL). Although the resistant rate of S. pneumoniae to penicillin decreased dramatically, the tendency of increasing pneumococcal resistance was certain. In the present study, the MIC50 of penicillin in the isolates was 1.0 µg/mL. This value was much higher than that in the non-invasive pneumococci (from 0.032 µg/mL to 0.25 µg/mL) [21], [26] but similar to the results of IPD isolates [17]. Thus, the IPD isolates may have a higher MIC level for penicillin. In other words, to get good clinical outcomes, a higher concentration of antibiotics was required in the treatment of IPD.
In recent years, the resistance rates of S. pneumoniae to other β-lactam antibiotics have also increased in China [25]. In the present study, the resistant rates of S. pneumoniae to cefaclor and cefuroxime were 81.6% and 79.3%, respectively, which are higher than those in our previous study [17] and in a recent study by Zhao et al. [25]. Ceftriaxone resistance rates were 3.8% and 33.3% in the non-meningitis and meningitis isolates, respectively. In a previous study on the antimicrobial susceptibility of S. pneumoniae in eight European countries, the resistance rates to cefotaxime, cefuroxime, and cefpodoxime were only 5.1%, 17.7%, and 17.5%, respectively [27]. Moreover, 56.3% of IPD isolates collected were MDRSP, which is similar to the results of a previous study in Asian countries [28]. This data indicated that the resistance of antibiotics has been a serious problem in China. The inappropriate using of antibiotics may have caused the high resistance rate, and MDR serotypes such as 19F, 19A, 23F, and 14 may have an important effect on this phenomenon. Fortunately, PCV7 and PCV13 covered 75.5% and 85.7% of the MDR pneumococcal isolates, respectively. Thus, they all have potential in controlling MDRSP. Given that serotype 19A plays an important role in China, PCV13 is more useful than PCV7 and the conjugate vaccines could prevent the spread of MDRSP to some extent.
In our study, most of the isolates showed high resistance rate and MIC level to erythromycin (96.6% of isolates with MIC >256 µg/mL), which is similar to the results (96.4%) reported by the Asian Network for Surveillance of Resistant Pathogens (ANSORP) [28]. The two primary mechanisms of macrolide resistance in S. pneumoniae are ribosomal methylation and macrolide efflux encoded by the ermB and mefA genes, respectively. Target-site modification through substitutions in the 23S rRNA and in the L4 and L22 riboproteins is also rare [29], [30]. Macrolide resistance in Pneumococci from Asia, Europe, and South Africa is predominantly mediated by the ermB gene, which is usually associated with high-level resistance. In North America, the mefA gene-encoded efflux shows low-level resistance and accounts for 61% to 85% of macrolide resistance [30]–[32]. In the present study, the mechanism of the ermB gene-encoded ribosomal methylation was found to be the primary resistance mechanism in the IPD isolates from Southern China. Furthermore, no significant differences were associated with the serotype and erythromycin resistance levels. Thus, all serotypes showed similar resistance levels to erythromycin. However, erythromycin resistance in Europe is often associated with pneumococcal serotype 14 [15]. In this study, most of the isolates harboring both the ermB and mefA genes belong to serotypes 19A and 19F, which is consistent with the results of previous studies [33], [34]. However, ANSORP reported that most pneumococcal isolates that contain both the ermB and mefA genes belong to serotypes 19F and 14 [35]. The results of the present study showed that the prevalence and distribution of serotype 19F or 19A isolates may have contributed to the high rate of macrolide-resistant Pneumococci in China.
In summary, the current study provided updated information and changing trends in the antimicrobial resistance and serotype distribution of IPD isolates in Southern China. The data showed an extremely high prevalence of macrolide resistance and an increasing prevalence of MDRSP. Given the high prevalence of resistance and its clinical impact, continuous surveillance of pneumococcal epidemiology is strongly warranted. This study also showed the serotype distribution of S. pneumoniae and the coverage of 7-, 10-, and 13-valent conjugate vaccines. Based on our results, the introduction of PCVs, especially PCV13, can reduce pneumococcal disease and the prevalence of MDRSP in China, as what had been achieved in other countries.
The present study also has several limitations. For instance, all the IPD isolates were from one hospital and the situation of antibiotics using was unclear, leading to bias and affecting the results of this study. In addition, only a small sample size of isolates was used in this study. Therefore, isolates from more pediatric patients should be included.
Acknowledgments
The authors sincerely thank all members who took part in this study, especially co-workers in Shenzhen Children’s Hospital, for their valuable assistance in collecting the isolates.
Funding Statement
This work was supported by the Ministry of Science and Technology [2008BA166B01], the National Natural Science Funds of the People’s Republic of China [30801259], and the Beijing Guidance Teacher Technology Item of Excellent Doctorship Thesis [YB20091002502]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. WHO (2007) Pneumococcal conjugated vaccine for childhood immunization. WHO position paper-Weekly Epidemiological Record 82: 93–104. [PubMed] [Google Scholar]
- 2.Kilgore PE, Kennedy WA, Anh DD, Nyambat B, Kim JS, et al. (2005) Epidemiology of childhood pneumococcal meningitis in China, South Korea and Vietnam, 1999 through 2003: implications for estimating the burden of disease and prevention through immunization. In: Presented at: 43rd Annual Meeting of the Infectious Diseases Society of America October 6–9. p. 142 [Abstract].
- 3. Chen Y, Deng W, Wang SM, Mo QM, Jia H, et al. (2011) Burden of pneumonia and meningitis caused by Streptococcus pneumoniae in China among children under 5 years of age: A systematic literature review. PLoS One 6(11): e27333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho HJ, Hong SJ, Park S (2004) Knowledge and beliefs of primary care physicians, pharmacists, and parents on antibiotic use for the pediatric common cold. Soc Sci Med 58: 623–629. [DOI] [PubMed] [Google Scholar]
- 5. Bharathiraja R, Sridharan S, Chelliah LR, Suresh S, Senguttuvan M (2005) Factors affecting antibiotic prescribing pattern in pediatric practice. Indian J Pediatr 72: 877–879. [DOI] [PubMed] [Google Scholar]
- 6. Osatakul S, Puetpaiboon A (2007) Appropriate use of empirical antibiotics in acute diarrhoea: a cross-sectional survey in southern Thailand. Ann Trop Paediatr 27: 115–122. [DOI] [PubMed] [Google Scholar]
- 7. P.L Ho, T.L Que, T.K Ng, S.S Chiu, R.W Yung, et al. (2006) Clinical outcomes of bacteremic pneumococcal infections in an area with high resistance. Eur J Clin Microbiol Infect Dis 25: 323–327. [DOI] [PubMed] [Google Scholar]
- 8. Advisory Committee on Immunization Practices (2000) Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 49: 1–35. [PubMed] [Google Scholar]
- 9. Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, et al. (2006) Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae . N Engl J Med 354: 1455–1463. [DOI] [PubMed] [Google Scholar]
- 10. Sorensen U (1993) Typing of pneumococci by using 12 pooled antisera. J Clin Microbiol 31(8): 2097–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kelly LM, Jacobs MR, Appelbaum PC (1999) Comparison of agar dilution, microdilution, E-test, and disk diffusion methods for testing activity of cefditoren against Streptococcus pneumoniae . Journal of Clinical Microbiology 37: 3296–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute (CLSI). (2010) Performance standards for antimicrobial susceptibility testing: 20th informational supplement. Wayne, PA: Clinical and Laboratory Standards Institute M100–S20.
- 13. Sutcliffe JT, Tait-Kamradt A, Wondrack L (1996) Detection of erythromycin-resistant determinants by PCR. Antimicrobial Agents and Chemotherapy 40: 2562–2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Enright MC, Spratt BG (1998) A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology 144: 3049–3060. [DOI] [PubMed] [Google Scholar]
- 15. Isaacman DJ, McIntosh ED, Reinert RR (2010) Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis 14(3): e197–209. [DOI] [PubMed] [Google Scholar]
- 16. Phongsamart W, Srifeungfung S, Dejsirilert S, Chatsuwan T, Nunthapisud P, et al. (2007) Serotype distribution and antimicrobial susceptibility of S. pneumoniae causing invasive disease in Thai children younger than 5 years old, 2000–2005. Vaccine 25(7): 1275–1280. [DOI] [PubMed] [Google Scholar]
- 17. Xue L, Yao KH, Xie GL, Zheng YJ, Wang CQ, et al. (2010) Serotype distribution and antimicrobial resistance of Streptococcus pneumoniae isolates that cause invasive disease among Chinese children. Clinical Infectious Diseases 50: 741–744. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention (CDC) (2005) Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease–United States, 1998–2003. MMWR Morb Mortal Wkly Rep 54(36): 893–897. [PubMed] [Google Scholar]
- 19. Srifeungfung S, Tribuddharat C, Comerungsee S, Chatsuwan T, Treerauthanaweeraphong V, et al. (2010) Serotype coverage of pneumococcal conjugate vaccine and drug susceptibility of Streptococcus pneumoniae isolated from invasive or non-invasive diseases in central Thailand,2006–2009. Vaccine 28(19): 3440–3444. [DOI] [PubMed] [Google Scholar]
- 20. Yao KH, Wang LB, Zhao GM, Zheng YJ, Deng L, et al. (2011) Pneumococcal serotype distribution and antimicrobial resistance in Chinese children hospitalized for pneumonia. Vaccine 29(12): 2296–2301. [DOI] [PubMed] [Google Scholar]
- 21. Zhou L, Yu SJ, Gao W, Yao KH, Shen AD, et al. (2011) Serotype distribution and antibiotic resistance of 140 pneumococcal isolates from pediatric patients with upper respiratory infections in Beijing, 2010. Vaccine 29: 7704–7710. [DOI] [PubMed] [Google Scholar]
- 22. Lee NY, Song JH, Kim S, Peck KR, Ahn KM, et al. (2001) Carriage of antibioticresistant pneumococci among Asian children: a ultinational surveillance by the Asian Network for Surveillance of Resistant Pathogens (ANSORP). Clin Infect Dis 32(10): 1463–1469. [DOI] [PubMed] [Google Scholar]
- 23. Song JH, Jung SI, Ko KS, Kim NY, Son JS, et al. (2004) High prevalence of antimicrobial resistance among clinical Streptococcus pneumoniae isolates in Asia (an ANSORP study). Antimicrob Agents Chemother 48(6): 2101–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sahm DF, Jones ME, Hickey ML, Diakun DR, Mani SV, et al. (2000) Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in Asia and Europe, 1997–1998. J Antimicrob Chemother 45(4): 457–466. [DOI] [PubMed] [Google Scholar]
- 25. Chunjiang Zhao, Hongli Sun, Hui Wang, Yudong Liu, Bijie Hu, et al. (2012) Antimicrobial resistance trends among 5608 clinical Gram-positive isolates in China: results from the Gram-Positive Cocci Resistance Surveillance program (2005–2010).Diagnostic microbiology and infectious disease. 73(2): 174–181. [DOI] [PubMed] [Google Scholar]
- 26. Yu SJ, Yao KH, Shen XZ, Zhang W, Liu X, Yang YH (2008) Serogroup distribution and antimicrobial resistance of nasopharyngeal isolates of Streptococcus pneumoniae among Beijing children with upper respiratory infections (2000–2005). Eur J Clin Microbiol Infect Dis 27(8): 649–655. [DOI] [PubMed] [Google Scholar]
- 27. Reinert RR, Reinert S, van der Linden M, Cil MY, Al-Lahham A, et al. (2005) Antimicrobial susceptibility of Streptococcus pneumoniae in eight European countries from 2001 to 2003. Antimicrob Agents Chemother 49(7): 2903–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, et al. (2012) Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study. Antimicrob Agents Chemother. 56(3): 1418–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tait-Kamradt A, Davies T, Appelbaum PC, Depardieu F, Courvalin P, et al. (2000) Two new mechanisms of macrolide resistance in clinical strains of Streptococcus pneumoniae from Eastern Europe and North America. Antimicrob Agents Chemother 44: 3395–3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frederic Fitoussi, Catherine Doit, Pierre Geslin, Naima Brahimi, Edouard Bingen (2001) Mechanism of macrolide resistance in the clinical pneumoncoccal isolates in France. Antimicrob Agents Chemother 45: 636–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johnston NJ, De Azavedo JC, Kellner JD, Low DE (1998) Prevalence and characterization of the mechanisms of macrolide, lincosamide, and streptogramin resistance in isolates of Streptococcus pneumoniae . Antimicrob Agents Chemother 42: 2425–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schmitz FJ, Perdikouli M, Beeck A, Verhoef J, Fluit AC (2001) Molecular surveillance of macrolide, tetracycline and quinolone resistance mechanisms in 1191 clinical European Streptococcus pneumoniae isolates. Int J Antimicrob Agents 18: 433–436. [DOI] [PubMed] [Google Scholar]
- 33. Toltzis P, Dul M, O’Riordan MA, Jacobs MR, Blumer J (2006) Serogroup 19 pneumococci containing both mef and erm macrolide resistance determinants in an American city. Pediatr Infect Dis J 25: 19–24. [DOI] [PubMed] [Google Scholar]
- 34. Bae S, Lee K (2009) Distribution of capsular serotypes and macrolide resistance mechanisms among macrolide-resistant Streptococcus pneumoniae isolates in Korea. Diagn Microbiol Infect Dis 63: 213–216. [DOI] [PubMed] [Google Scholar]
- 35. Song JH, Chang HH, Suh JY, Ko KS, Jung SI, et al. (2004) Macrolide resistance and genotypic characterization of Streptococcus pneumoniae in Asian countries: a study of the Asian Network for Surveillance of Resistant Pathogens (ANSORP). J Antimicrob Chemother 53: 457–463. [DOI] [PubMed] [Google Scholar]