Abstract
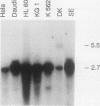
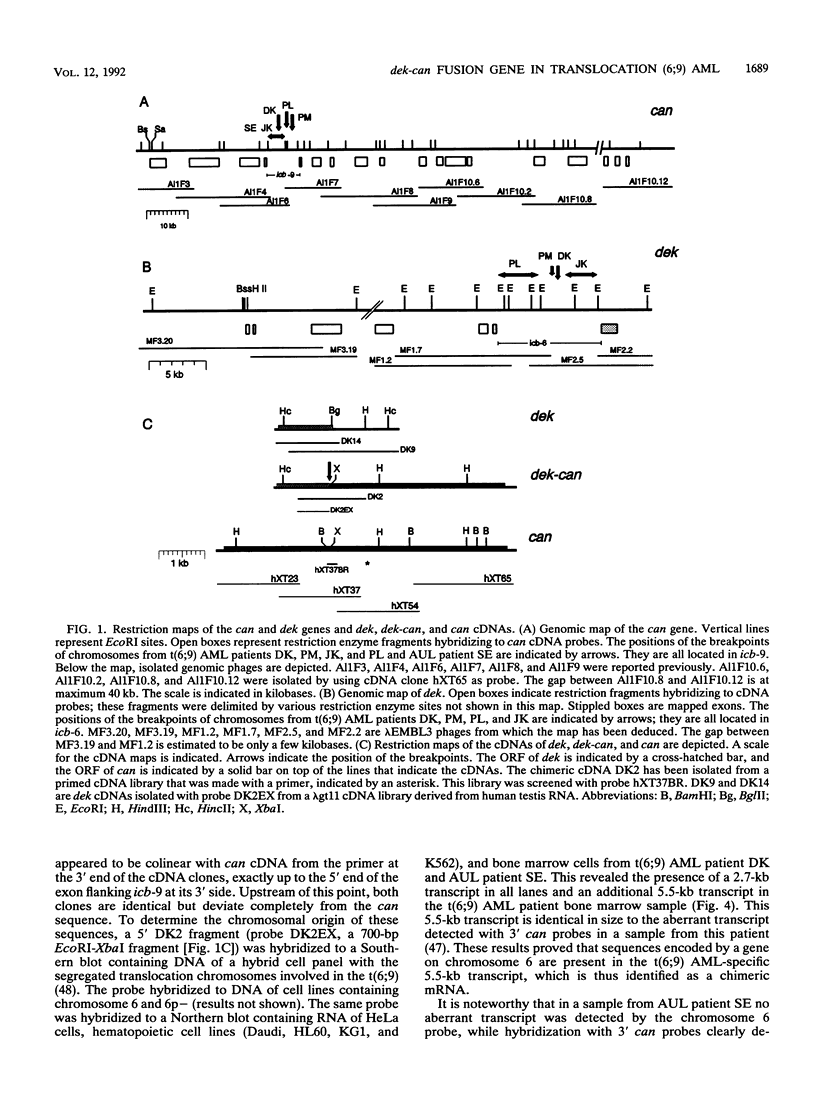
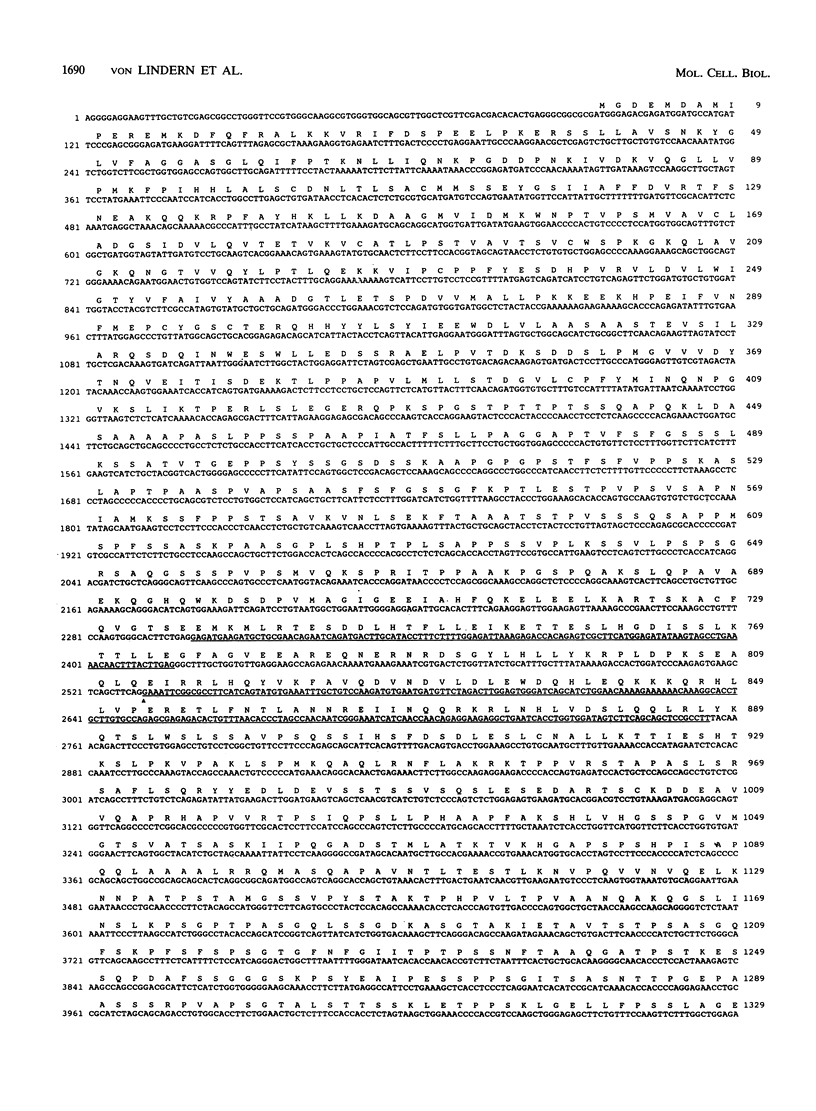
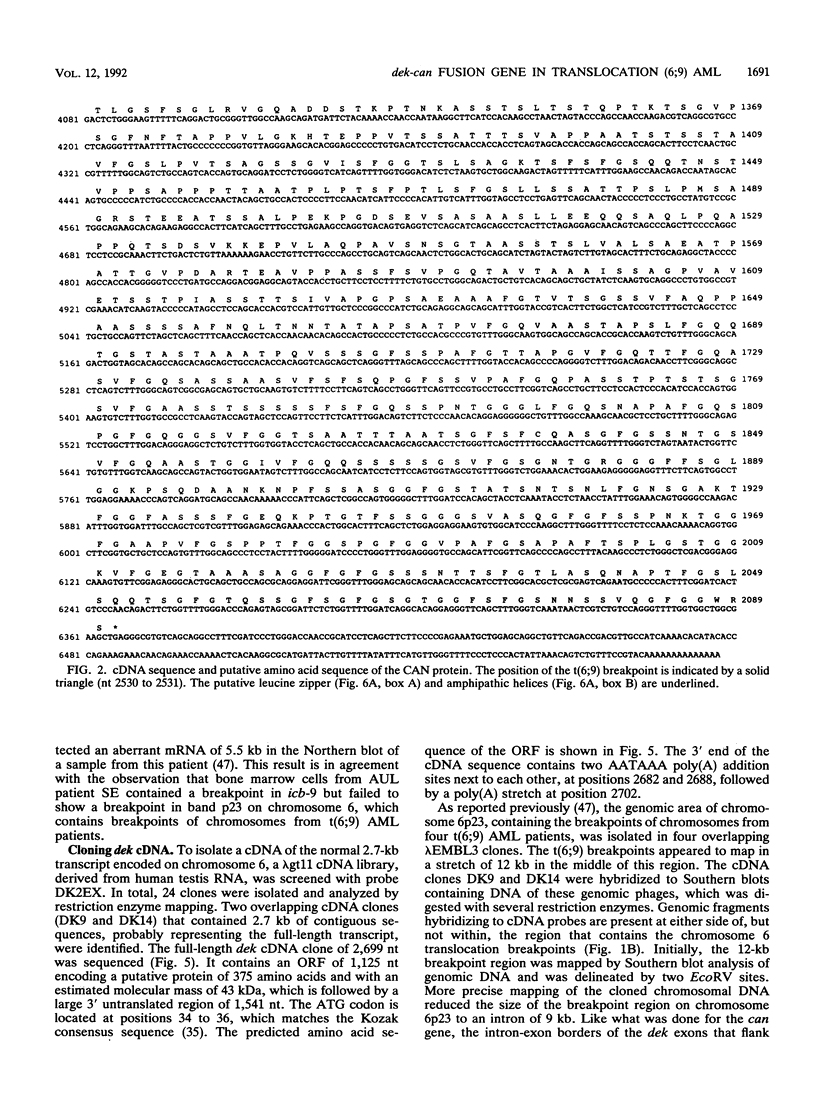
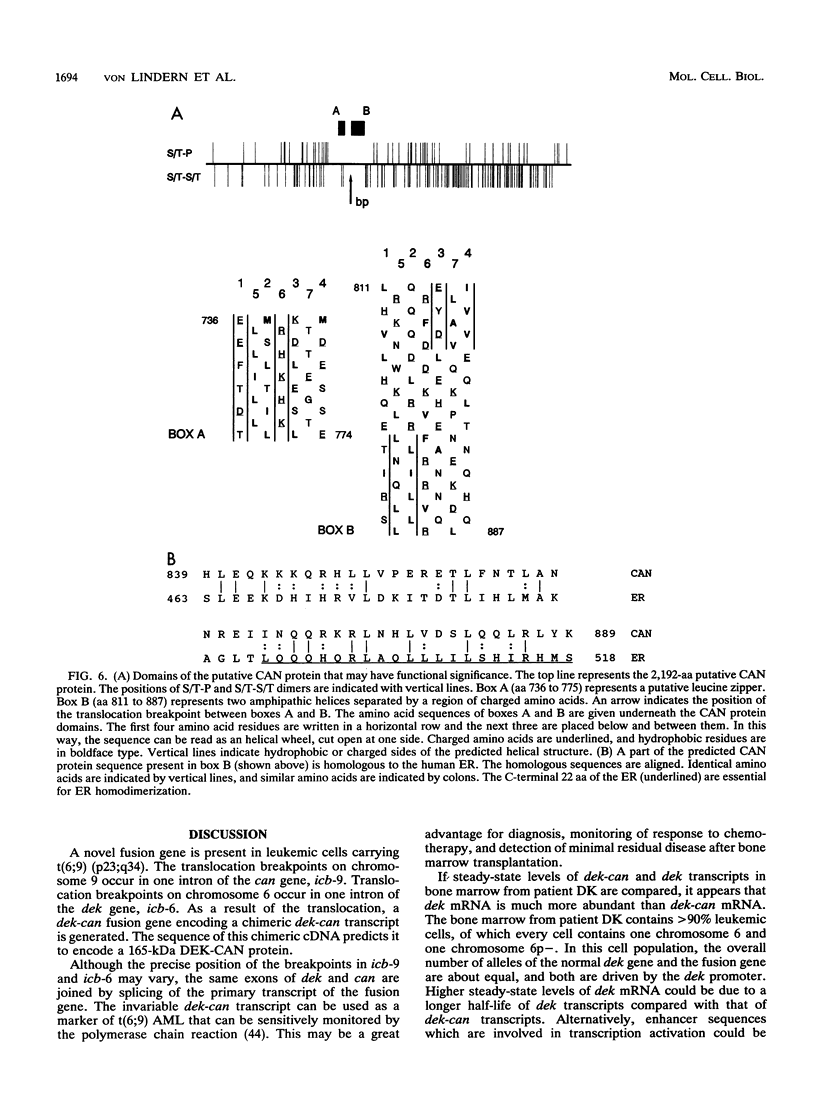
The translocation (6;9) is associated with a specific subtype of acute myeloid leukemia (AML). Previously, it was found that breakpoints on chromosome 9 are clustered in one of the introns of a large gene named Cain (can). cDNA probes derived from the 3' part of can detect an aberrant, leukemia-specific 5.5-kb transcript in bone marrow cells from t(6;9) AML patients. cDNA cloning of this mRNA revealed that it is a fusion of sequences encoded on chromosome 6 and 3' can. A novel gene on chromosome 6 which was named dek was isolated. In dek the t(6;9) breakpoints also occur in one intron. As a result the dek-can fusion gene, present in t(6;9) AML, encodes an invariable dek-can transcript. Sequence analysis of the dek-can cDNA showed that dek and can are merged without disruption of the original open reading frames and therefore the fusion mRNA encodes a chimeric DEK-CAN protein of 165 kDa. The predicted DEK and CAN proteins have molecular masses of 43 and 220 kDa, respectively. Sequence comparison with the EMBL data base failed to show consistent homology with any known protein sequences.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adriaansen H. J., van Dongen J. J., Hooijkaas H., Hählen K., van 't Veer M. B., Löwenberg B., Hagemeijer A. Translocation (6;9) may be associated with a specific TdT-positive immunological phenotype in ANLL. Leukemia. 1988 Mar;2(3):136–140. [PubMed] [Google Scholar]
- Alcalay M., Zangrilli D., Pandolfi P. P., Longo L., Mencarelli A., Giacomucci A., Rocchi M., Biondi A., Rambaldi A., Lo Coco F. Translocation breakpoint of acute promyelocytic leukemia lies within the retinoic acid receptor alpha locus. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1977–1981. doi: 10.1073/pnas.88.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison L. A., Moyle M., Shales M., Ingles C. J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- Allison L. A., Wong J. K., Fitzpatrick V. D., Moyle M., Ingles C. J. The C-terminal domain of the largest subunit of RNA polymerase II of Saccharomyces cerevisiae, Drosophila melanogaster, and mammals: a conserved structure with an essential function. Mol Cell Biol. 1988 Jan;8(1):321–329. doi: 10.1128/mcb.8.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Biggs J., Searles L. L., Greenleaf A. L. Structure of the eukaryotic transcription apparatus: features of the gene for the largest subunit of Drosophila RNA polymerase II. Cell. 1985 Sep;42(2):611–621. doi: 10.1016/0092-8674(85)90118-7. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Borrow J., Goddard A. D., Sheer D., Solomon E. Molecular analysis of acute promyelocytic leukemia breakpoint cluster region on chromosome 17. Science. 1990 Sep 28;249(4976):1577–1580. doi: 10.1126/science.2218500. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell. 1985 Dec;43(3 Pt 2):729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Busch S. J., Sassone-Corsi P. Dimers, leucine zippers and DNA-binding domains. Trends Genet. 1990 Feb;6(2):36–40. doi: 10.1016/0168-9525(90)90071-d. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomienne C., Ballerini P., Balitrand N., Huang M. E., Krawice I., Castaigne S., Fenaux P., Tiollais P., Dejean A., Degos L. The retinoic acid receptor alpha gene is rearranged in retinoic acid-sensitive promyelocytic leukemias. Leukemia. 1990 Dec;4(12):802–807. [PubMed] [Google Scholar]
- Corden J. L., Cadena D. L., Ahearn J. M., Jr, Dahmus M. E. A unique structure at the carboxyl terminus of the largest subunit of eukaryotic RNA polymerase II. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7934–7938. doi: 10.1073/pnas.82.23.7934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley G. Q., Van Etten R. A., Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990 Feb 16;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C. Anionic regions in nuclear proteins. J Cell Biol. 1987 Oct;105(4):1479–1482. doi: 10.1083/jcb.105.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass C. K., Devary O. V., Rosenfeld M. G. Multiple cell type-specific proteins differentially regulate target sequence recognition by the alpha retinoic acid receptor. Cell. 1990 Nov 16;63(4):729–738. doi: 10.1016/0092-8674(90)90139-6. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Jenster G., ten Hoeve J., Zovich D., Pattengale P. K., Groffen J. Acute leukaemia in bcr/abl transgenic mice. Nature. 1990 Mar 15;344(6263):251–253. doi: 10.1038/344251a0. [DOI] [PubMed] [Google Scholar]
- Hermans A., Gow J., Selleri L., von Lindern M., Hagemeijer A., Wiedemann L. M., Grosveld G. bcr-abl oncogene activation in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 1988 Oct;2(10):628–633. [PubMed] [Google Scholar]
- Hermans A., Heisterkamp N., von Linden M., van Baal S., Meijer D., van der Plas D., Wiedemann L. M., Groffen J., Bootsma D., Grosveld G. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell. 1987 Oct 9;51(1):33–40. doi: 10.1016/0092-8674(87)90007-9. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Hu Y. F., Lüscher B., Admon A., Mermod N., Tjian R. Transcription factor AP-4 contains multiple dimerization domains that regulate dimer specificity. Genes Dev. 1990 Oct;4(10):1741–1752. doi: 10.1101/gad.4.10.1741. [DOI] [PubMed] [Google Scholar]
- Kadowaki T., Kadowaki H., Taylor S. I. A nonsense mutation causing decreased levels of insulin receptor mRNA: detection by a simplified technique for direct sequencing of genomic DNA amplified by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Jan;87(2):658–662. doi: 10.1073/pnas.87.2.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizuka A., Miller W. H., Jr, Umesono K., Warrell R. P., Jr, Frankel S. R., Murty V. V., Dmitrovsky E., Evans R. M. Chromosomal translocation t(15;17) in human acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991 Aug 23;66(4):663–674. doi: 10.1016/0092-8674(91)90112-c. [DOI] [PubMed] [Google Scholar]
- Konopka J. B., Watanabe S. M., Witte O. N. An alteration of the human c-abl protein in K562 leukemia cells unmasks associated tyrosine kinase activity. Cell. 1984 Jul;37(3):1035–1042. doi: 10.1016/0092-8674(84)90438-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. A profusion of controls. J Cell Biol. 1988 Jul;107(1):1–7. doi: 10.1083/jcb.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne G., Franke W. W. A major soluble acidic protein located in nuclei of diverse vertebrate species. Exp Cell Res. 1980 Sep;129(1):167–189. doi: 10.1016/0014-4827(80)90341-9. [DOI] [PubMed] [Google Scholar]
- Lapeyre B., Bourbon H., Amalric F. Nucleolin, the major nucleolar protein of growing eukaryotic cells: an unusual protein structure revealed by the nucleotide sequence. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1472–1476. doi: 10.1073/pnas.84.6.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees J. A., Fawell S. E., White R., Parker M. G. A 22-amino-acid peptide restores DNA-binding activity to dimerization-defective mutants of the estrogen receptor. Mol Cell Biol. 1990 Oct;10(10):5529–5531. doi: 10.1128/mcb.10.10.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson M. G., Vardiman J. W., Le Beau M. M., Rowley J. D., Schwartz S., Kerman S. L., Cohen M. M., Fleischman E. W., Prigogina E. L. Increased numbers of marrow basophils may be associated with a t(6;9) in ANLL. Am J Hematol. 1985 Apr;18(4):393–403. doi: 10.1002/ajh.2830180409. [DOI] [PubMed] [Google Scholar]
- Sandberg A. A., Morgan R., McCallister J. A., Kaiser-McCaw B., Hecht F. Acute myeloblastic leukemia (AML) with t(6;9) (p23;q34): a specific subgroup of AML? Cancer Genet Cytogenet. 1983 Oct;10(2):139–142. doi: 10.1016/0165-4608(83)90117-6. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985 Jun 13;315(6020):550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- Suzuki M. SPXX, a frequent sequence motif in gene regulatory proteins. J Mol Biol. 1989 May 5;207(1):61–84. doi: 10.1016/0022-2836(89)90441-5. [DOI] [PubMed] [Google Scholar]
- Suzuki M. The heptad repeat in the largest subunit of RNA polymerase II binds by intercalating into DNA. Nature. 1990 Apr 5;344(6266):562–565. doi: 10.1038/344562a0. [DOI] [PubMed] [Google Scholar]
- Walker L. C., Ganesan T. S., Dhut S., Gibbons B., Lister T. A., Rothbard J., Young B. D. Novel chimaeric protein expressed in Philadelphia positive acute lymphoblastic leukaemia. 1987 Oct 29-Nov 4Nature. 329(6142):851–853. doi: 10.1038/329851a0. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Transcription factors: a new family gathers at the cAMP response site. Trends Genet. 1990 Mar;6(3):69–72. doi: 10.1016/0168-9525(90)90081-g. [DOI] [PubMed] [Google Scholar]
- de Thé H., Chomienne C., Lanotte M., Degos L., Dejean A. The t(15;17) translocation of acute promyelocytic leukaemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990 Oct 11;347(6293):558–561. doi: 10.1038/347558a0. [DOI] [PubMed] [Google Scholar]
- de Thé H., Lavau C., Marchio A., Chomienne C., Degos L., Dejean A. The PML-RAR alpha fusion mRNA generated by the t(15;17) translocation in acute promyelocytic leukemia encodes a functionally altered RAR. Cell. 1991 Aug 23;66(4):675–684. doi: 10.1016/0092-8674(91)90113-d. [DOI] [PubMed] [Google Scholar]
- von Lindern M., Poustka A., Lerach H., Grosveld G. The (6;9) chromosome translocation, associated with a specific subtype of acute nonlymphocytic leukemia, leads to aberrant transcription of a target gene on 9q34. Mol Cell Biol. 1990 Aug;10(8):4016–4026. doi: 10.1128/mcb.10.8.4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lindern M., van Agthoven T., Hagemeijer A., Adriaansen H., Grosveld G. The human pim-1 gene is not directly activated by the translocation (6;9) in acute nonlymphocytic leukemia. Oncogene. 1989 Jan;4(1):75–79. [PubMed] [Google Scholar]