Abstract
Objective
Placental growth factor (PlGF) is an angiogenetic factor and inducts the development of preeclampsia in a hypoxic environment. In this study, we examined maternal blood PlGF levels in a pregnant population between 16 and 19 weeks of gestation for determining the prospective value for early diagnosis of preeclampsia as a screening test.
Materials and Methods
In this prospective cross-sectional study, 114 nulliparous normotensive pregnant women were selected for the control group and 34 patients who have chronic hypertension or had a medical history of hypertensive disorders in previous pregnancies were selected for the study group.
Results
In the study group, the risk of preeclampsia increased 3.2 times when compared with the control with a confidence interval of 95 %. The cut-off value for PlGF for discriminating preeclamptic and non-preeclamptic patients was found to be 62.5 pg/ml.
Conclusion
Patients with a medical history of hypertensive disorders and low PIGF levels in early second trimester have an increased risk for preeclampsia.
Keywords: Preeclampsia, Angiogenesis, Placental growth factor
Introduction
Preeclampsia, a serious disorder and a multifactorial disease of pregnancy characterized by hypertension and proteinuria, complicates approximately 2–5 % of all pregnancies. Although several risk factors for this condition including first pregnancy, low and high maternal age, multiple pregnancy, obesity, and preexisting diabetes or hypertension are well recognized, the cause of preeclampsia remains unclear. As a consequence, no causal treatment is available and resolution of this syndrome occurs only after removal of the placenta from the maternal body. Preeclampsia is still defined as a "disease of theories," and previous hypotheses included pathogenic factors such as placental ischemia, inflammation, and oxidative stress [1].
PIGF is an angiogenetic protein which is a member of the sistein knot amino acids family. PIGF is especially synthesized by the placenta in the advancing weeks of gestation. Apart from gestation, PIGF expression increases with diseases such as ischemic heart disease or myocardial infarction. PIGF coordinates the activation of vascular endothelial growth factor (VEGF) which is the most potent of all angiogenetic inductive molecules [2]. Angiogenesis is the development of the vascular network by remodeling of the primer capillary plexus. VEGF and VEGF-like proteins are efficacious in this molecular complex phenomenon. VEGF is the most potent angiogenetic factor and induces the main two steps of vascular network development: the proliferation of the endothelial cells and their migration [2]. The VEGF family is formed of six different molecular-shaped proteins: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, and PIGF. PIGF contains 40 % similar aminoacid repetition to VEGF. The heterodimers of PIGF and VEGF are formed naturally and they are mitogenic to endothelial cells. The different pathophysiologic roles of different forms of PIGF are regulated by fms-like tyrosine kinase receptor-1 [3]. PIGF is expressed primarily in placental tissue and specifically by trophoblastic cells. Invitro studies determined that hypoxia has an inducing effect on VEGF and reducing effect on PIGF expression.
PIGF is an important component of placentation and angiogenesis during pregnancy. During placentation, the expression of this protein has a role for throphoblast invasion to the maternal decidua [4]. In pregnancy, the expression of PIGF protein and its mRNA increases during the second trimester. The second trimester is the characteristic time period when nonbranching villous angiogenesis and terminal villous formation occur. All this formation mentioned here is occurring by binding of PIGF to its receptor Flt-1 [5]. Recently, in 2012, Rajakumar et al. [6] declared that the preeclamptic placenta’s outermost layer, the syncytiotrophoblast, forms abundant “knots” that are enriched with sFlt1 protein. These syncytial knots easily detach from the syncytiotrophoblast, resulting in free, multinucleated aggregates (50–150 μm diameter) that are loaded with sFlt1 protein and mRNA, and are metabolically active. And, these placental microparticles in the maternal circulation appear to be greatly accelerated in preeclampsia and may contribute to the maternal vascular injury that characterizes this disorder [6].
One of the triggers of preeclampsia is the deficiency in invasion of trophoblastic cells to the maternal decidua. Uterine perfusion is the manipulator for angiogenetic factors to release into the maternal circulation. So, predicting preeclampsia, both by the uterine artery (UtA) pulsatility index and a set of biochemical markers in the first trimester of pregnancy, is being investigated by researchers. Youssef et al. [7] reported that the combination of maternal biochemical variables (serum placental biomarkers including pregnancy-associated plasma protein-A, placental growth factor, soluble fms-like tyrosine kinase 1, P-selectin, and neutrophil gelatinase-associated lipocalin) with the highest UtA pulsatility index could detect 77 % of preeclampsia at a 10 % false positive rate.
Placental lesions, like villous changes, isolated vascular lesions in the placenta, are reported to be associated with an imbalance in the maternal concentration of angiogenic/anti-angiogenic factors. There is a link between maternal underperfusion and an anti-angiogenic state characterized by the changes in the concentrations of angiogenic and anti-angiogenic factors in women with late onset preeclampsia [8]. The limited production of PIGF in placental tissue allowed it to be used as a predictive marker for preeclampsia. The aim of our study was to determine maternal serum PIGF levels at 16–19 weeks of gestation, comparing the levels between patients with maternal risk factors and a normal low-risk multiparous population and use these levels for prediction of preeclampsia.
In normal pregnancy, the expected levels of PIGF increase during the first and second trimester, peak at 29–30 weeks of gestation, and decrease after 30 weeks of gestation until the birth [9].
In many different studies, the normotensive control pregnant women are compared with the preeclamptic pregnant women. It is determined that starting from the early second trimester (as early as 10–11 weeks of gestation), PIGF levels are lower in preeclamptic pregnancies [10].
Materials and Methods
The blood pressure was measured with an automated device (Omron MIT, Kyoto, Japan) validated for use in pregnancy [11]. Preeclampsia is defined as new onset of a blood pressure >140/90 mm Hg on 2 separate occasions at least 4 h apart, accompanied by proteinuria 300 mg/24 h, in the absence of a urinary tract infection [12]. Blood samples were taken at 15–19 weeks. Serum and plasma were prepared from venous whole blood, frozen and stored anonymized at −70 °C until analyzed. Written informed content was given to all patients who volunteered to attend our study.
Between October 2009 and September 2010, 175 pregnant women who came to our antenatal clinic for their routine antenatal follow-up consecutively were selected for this cross-sectional study. All patients were under the age of 40, without a chronic disease, and they never had an operation previously. Twenty-three patients maladjusted with their antenatal follow-up and 4 patients had spontaneous abortion before 20 weeks of gestation. So, they are excluded from the study.
The patients’ gestational weeks were estimated by early first trimester ultrasonography before 11 weeks of gestation. A form which contains questions about the patients’ identity information was filled by a doctor. The patients’ age, height, weight, and body mass index (BMI) measurements were noted. All patients were questioned about their current and previous pregnancies. We formed two groups of patients: 114 nulliparous uncomplicated normotensive pregnancies were selected as the control group and 34 patients with chronic hypertension and with a medical history of preeclampsia or gestational hypertension in previous pregnancies were selected for the study group. The patients’ phone numbers and information processing numbers were enrolled for their antenatal follow-up. From this first antenatal interview, patients were followed-up until they give birth. Two from the control group and 2 from the study group of patients had spontaneous abortion before 20 weeks of gestation and they were excluded from the study.
Data were analyzed by SPSS for Windows, version 11.5 (SPSS Inc., Chicago, IL, United States). Comparisons between groups were made by the Mann–Whitney U test for continuous variables and the χ2 test for nominal variables.
Receiver operating characteristic (ROC) curves were generated to investigate whether PIGF could be used to differentiate between the preeclampsia and non-preeclampsia groups. The optimal cut-off point was determined as giving the maximum sum of sensitivity and specificity for the significant test. Sensitivity, specificity, and positive and negative predicted values associated with this cut-off level were calculated.
Whether the clinical factors (i.e., being in the study group and PIGF) had a statistically significant effect on preeclampsia was evaluated by logistic regression analyses. Odds ratio, 95 % confidence intervals for each independent variable were also calculated.
A P value of <0.05 was considered statistically significant.
Results
When we compared demographic features of our patients, no statistical difference between their median ages and body mass indexes was determined (P > 0.05) (Table 1).
Table 1.
Demographic features of the groups
| Groups | Number of patients (=n) | Median age (min–max) | Median BMI (min–max) |
|---|---|---|---|
| Control | n = 114 | 25 (16–39) | 23 (16–35) |
| Study | n = 34 | 26 (20–37) | 24.5 (19–35) |
| P value | 0.238 (P > 0.05) | 0.65 (P > 0.05) | |
There was no statistical difference between the two groups of patients’ median gestational week of serum sample collection. The median PIGF levels of the study group and control group were 87 and 93 pg/ml, respectively. No statistical difference was determined between the two groups (P > 0.05) (Table 2; Fig. 1).
Table 2.
Median gestational weeks and PIGF levels of the groups
| Groups | Mean ± std. deviation | Number of patients (=n) | Median (min–max) | P value |
|---|---|---|---|---|
| Gestational age | ||||
| Control | 16.81 ± 0.90 | 114 | 17 (15–19) | 0.952 (P > 0.05) |
| Study | 16.83 ± 0.98 | 34 | 17 (15–19) | |
| PIGF level | ||||
| Control | 101.19 ± 51.05 | 114 | 93 (20–343) | 0.409 (P > 0.05) |
| Study | 92.06 ± 39.13 | 34 | 87 (45–232) | |
Fig. 1.
Median PIGF values of the control and study groups
Six patients of the study group (17.1 % of the study group) and 7 patients of the control group (6.5 % of the control group) developed preeclampsia. The median PIGF level of the preeclamptic study group was 58.5 pg/ml and the median PIGF level of the control group was 57 pg/ml. There was no statistical difference between the median PIGF levels of the preeclamptic control group and the study group of patients (P: 0.17) (Table 3; Fig. 2).
Table 3.
Median PIGF of preeclamptic patients of the control and study groups
| Groups of patients | Number of patients (=n) | Median PIGF level (pg/ml) | P value |
|---|---|---|---|
| Preeclamptic study group | n = 6 (17.1 %) | 58.5 | 0.170 (P > 0.05) |
| Preeclamptic control group | n = 7 (6.5 %) | 57 |
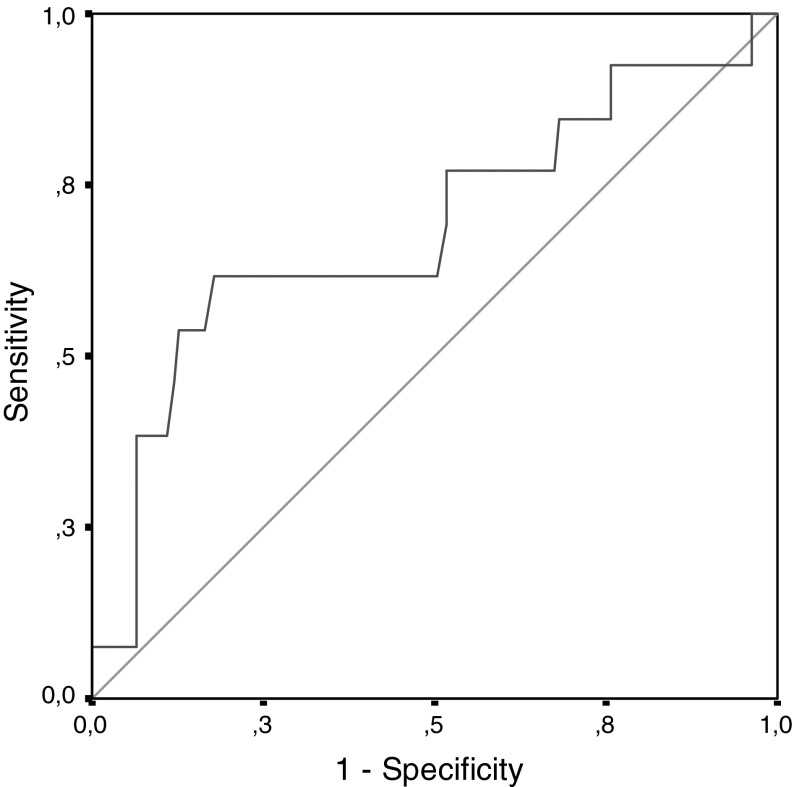
Fig. 2.
The ROC curve* of PIGF levels for all groups of patients. *If a ROC point is equal to “1,” we can speculate that all negatives are perfectly separated from the positives by a scanning test marker
We compared the percentage of developing preeclampsia for the study and control groups. When analyzed with χ2 test, no statistical difference was determined with a P value of 0.079, very close to 0.05 P value (Table 4). We reported this as the restriction of the study because of a limited number of pregnant women selected for both groups. In this study, our aim was to find out whether a recorded history of being hypertensive or preeclamptic has a statistically valuable effect on developing preeclampsia in current pregnancy or not. So, we reanalyzed our data by the single variable logistic regression analysis. According to the evaluation result of this analysis, being in the study group increases the risk of developing preeclampsia 3.2 times when compared with the control group with a confidence interval of 95 % (Table 5). For discriminating preeclamptic and non-preeclamptic patients by means of the PIGF values and to find out whether PIGF is a valuable marker for this discrimination or not. For this reason, receiver operating characteristic (ROC) analysis was done without separating the pregnant women into two groups like the "study" and "control." In signal detection theory, a ROC curve analysis is a graphical plot of the sensitivity, or true positive rate, versus false positive rate (1—specificity or 1—true negative rate), for a binary classifier system as its discrimination threshold is varied. The ROC can also be represented equivalently by plotting the fraction of true positives out of the positives (TPR = true positive rate) versus the fraction of false positives out of the negatives (FPR = false positive rate). It is also known as a relative operating characteristic curve because it is a comparison of two operating characteristics (TPR and FPR) as the criterion changes [13]. The result of ROC analysis showed us that the cut-off value for PIGF for discriminating preclamptic and non-preeclamptic patients is 62.5 pg/ml. Sensitivity, specificity, positive predictive value, and negative predictive value according to this cut-off point are 61.5, 82.2, 25, and 95.7 %, respectively (Table 5). Because of the higher rate of specifity and negative predictive value, we determined that PIGF is a statistically valuable scanning marker for predicting preeclampsia. The ROC curve which is drawn according to determined PIGF levels is shown in Fig. 1.
Table 4.
Percentage of developing preeclampsia for the study and control groups
| Groups | Preeclamptic | Non-preeclamptic | Total | P value |
|---|---|---|---|---|
| Control | 7 (6.1 %) | 107 (93.9 %) | 114 (100 %) | 0.079 (P > 0.05) |
| Study | 6 (17.1 %) | 28 (82.9 %) | 34 (100 %) | |
| Total | 13 (8.7 %) | 135 (91.3 %) | 148 (100 %) |
Table 5.
Percentage of developing preeclampsia for the study and control groups after single variable logistic regression analysis and cut-off value of PlGF for predicting preeclampsia
| Variables | Non-preeclamptic | Preeclamptic | P value | Odds ratio (95 % confidence interval) |
|---|---|---|---|---|
| Groups | 0.046 | 3.276 (1.020–10.523) | ||
| Control | 107 (79.3 %) | 7 (53.8 %) | ||
| Study | 28 (20.7 %) | 6 (46.2 %) | ||
| PlGF level | <0.001 | 7.400 (2.226–24.601) | ||
| ≥62.5 pg/ml | 111 (82.2 %) | 5 (38.5 %) | ||
| <62.5 pg/ml | 24 (17.8 %) | 8 (61.5 %) |
The determined PIGF cut-off level was applied to the control and study groups separately for investigating whether this cut-off level was still statistically valuable for predicting preeclampsia for each group of patients or not. We analyzed this data with multiple variables logistic regression analysis. Patients who were members of the study group with the PIGF levels below 62.5 pg/ml had an increased risk for developing preeclampsia. As mentioned before, patients in the study group had an independently increased preeclampsia risk. But, when PIGF levels were encountered for the risk assessment, the positive risk effect of being in the study group was eliminated. To explain in another way, the PIGF level affects the risk of developing preeclampsia independent from the maternal medical history of hypertensive disorders (Table 6).
Table 6.
Risk assessment of developing preeclampsia separately for being in the study group and a level of PIGF below the cut-off value
| Variables | Odds ratio | P value | 95 % confidence interval |
|---|---|---|---|
| Study group | 3.162 | 0.069 | 0.915–10.925 |
| PlGF < 62.5 pg/ml | 7.253 | 0.002 | 2.133–24.662 |
Discussion
In this study, we tried to determine a prior risk estimation by selecting multiparous women who had a history of preeclamptic or hypertensive pregnancies. Then, we combined this prior risk estimation with their early second trimester PIGF levels. All around the world now, preeclampsia risk estimation is evaluated like aneuploidy screening. The methodology which has been developed and refined over three decades is being adapted to screening for the adverse outcomes of pregnancy such as preeclampsia where many biochemical and physical markers have been discovered in recent years [14]. Tidwell et al. [15] determined PIGF levels in normal pregnancies in the first trimester and early second trimester (58.55 ± 7.5 pg/mL). The levels increase dramatically in the late second trimester (175.5 ± 21.64 pg/mL). And, this increase continues until the third trimester of pregnancy (753.0 ± 65.7 pg/mL) [15]. In our study, when we grouped all our patients as preeclamptic pregnancies and non-preeclamptic pregnancies without separating them as control or study, we determined a cut-off level of PIGF between 15 and 19 weeks of gestation to predict future preeclampsia development. If we use a ROC curve according to the preeclamptic groups, PIGF levels had a discriminating characteristic value (P < 0.05) (Table 5). With 82.2 % specificity and 95.7 % negative predictive value, PIGF levels lower than 62.5 pg/ml increase the risk of developing preeclampsia. These results confirm the use of early second trimester PIGF levels as a predictive marker for developing preeclampsia.
In the KCH (King’s College Hospital) cohort study, the latest published results relate to 8366 women of whom 165 (2.0 %) developed preeclampsia with relatively low frequency compared to the WHO statistics, indicating particularly strict diagnostic characteristics. In our study, among the control and study groups, 6.1 % (7/114) and 8.7 % (6/34) patients developed preeclampsia, respectively. We can say that our data is in accordance with the WHO statistics for the control group. In our study, the reason for the higher rate of developing preeclampsia in the study group is a maternal preeclamptic or hypertensive history.
Preeclampsia is a multisystem disease. Predicting preeclampsia at an early gestational week could decrease adverse maternal and neonatal outcomes. Bramham et al. investigated the recurrence rate of preeclampsia and neonatal outcomes in women with a history of preeclampsia that required preterm delivery. Five hundred women with previous preeclampsia were selected for the study. Preeclampsia recurred in 117 women (23 %). This study confirms that women with previous preeclampsia that required early delivery are at a high risk of the recurrence of preeclampsia development. The study also identifies risk factors for recurrence and illustrates that women with previous preeclampsia are at a greater risk of an adverse neonatal outcome. In our study, being a pregnant woman with a medical history of hypertensive disorders in previous pregnancies increases the risk of developing preeclampsia 3.2 times when compared with the control group with a confidence interval of 95 %. Maternal medical history is an independent risk factor for predicting preeclampsia.
Conclusion
A medical history of hypertensive disorders in previous pregnancies increases the risk of developing preeclampsia in current pregnancies. As a predictive marker, PIGF levels below cut-off values increase the risk of preeclampsia independent from the medical history. In our study, this cut-off value for early second trimester is 62.5 pg/ml. This is the first reported PIGF cut-off level for predicting preeclampsia in a Turkish pregnant population.
Acknowledgments
The authors offer special thanks to Dr. Leyla Mollamahmutoğlu, the head of the Zekai Tahir Burak Women’s Health, Education, and Research Hospital, the Biochemistry laboratory employees, and Dr. Üran Büyükkağnıcı, the laboratory chief.
Conflict of interest
None.
References
- 1.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 2.Iyer S, Acharya KR. Role of placenta growth factor in cardiovascular health. Trends Cardiovasc Med. 2002;12:128–134. doi: 10.1016/S1050-1738(01)00164-5. [DOI] [PubMed] [Google Scholar]
- 3.Kyle PM, Buckley D, Kissane J, et al. The angiotensin sensitivity test and low-dose aspirin are ineffective methods to predict and prevent hypertensive disorders in nulliparous pregnancy. Am J Obstet Gynecol. 1995;173(3 Pt 1):865–872. doi: 10.1016/0002-9378(95)90356-9. [DOI] [PubMed] [Google Scholar]
- 4.Vuorela P, Hatva E, Lymboussaki A. Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod. 1997;56:489–494. doi: 10.1095/biolreprod56.2.489. [DOI] [PubMed] [Google Scholar]
- 5.Kingdom J, Huppertz B, Seaward G. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/S0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 6.Rajakumar A, Cerdeira AS, Rana S, et al. Transcriptionally active syncytial aggregates in maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension. 2012;59(2):256–264. doi: 10.1161/HYPERTENSIONAHA.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youssef A, Righetti F, Morano D, et al. Uterine artery doppler and biochemical markers (PPAP-A, PlGF, sFlt-1, P-selectin, NGAL) at 11 + 0 and 13 + 6 weeks in the prediction of late (>34 weeks) pre-eclampsia. Prenat Diagn. 2011;31(12):1141–1146. doi: 10.1002/pd.2848. [DOI] [PubMed] [Google Scholar]
- 8.Soto E, Romero R, Kusanovic JP, et al. Late-onset preeclampsia is associated with an imbalance of angiogenic and anti-angiogenic factors in patients with and without placental lesions consistent with maternal underperfusion. J Matern Fetal Neonatal Med. 2011;25(5):498–507. doi: 10.3109/14767058.2011.591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torry DS, Wang HS, Wang TH, et al. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179(6 Pt 1):1539–1544. doi: 10.1016/S0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 10.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golara M, Benedict A, Jones C, et al. Inflationary oscillometry provides accurate measurement of blood pressure in pre-eclampsia. BJOG. 2002;109:1143–1147. doi: 10.1111/j.1471-0528.2002.01487.x. [DOI] [PubMed] [Google Scholar]
- 12.National High Blood Pressure Education Program Working Group Report of the National High Blood Pressure Education Program Working Group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. doi: 10.1067/mob.2000.107928. [DOI] [PubMed] [Google Scholar]
- 13.Swets JA. Signal detection theory and ROC analysis in psychology and diagnostics: collected papers. Mahwah: Lawrence Erlbaum Associates; 1996. [Google Scholar]
- 14.Cuckle HS. Screening for pre-eclampsia-lessons from aneuploidy screening. Placenta. 2011;32:42–48. doi: 10.1016/j.placenta.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 15.Tidwell SC, Ho HN, Chiu WH, et al. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184(6):1267–1272. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]