Abstract
Warfarin is a well established oral anticoagulant for the treatment of thromboembolic disorders. Warfarin therapy is complicated by a narrow therapeutic index and marked inter-individual dose variability with therapeutic doses ranging from 1 mg to 10 mg/day.1 Recently genetic variation and resultant drug metabolizing polymorphisms have been found to contribute to warfarin dose variability with resultant hemorrhagic or thromboembolic complications. Cytochrome P450 2C9 alters the rate of warfarin metabolism and clearance. A second enzyme, vitamin K epoxide reductase comple (VKOR) binds and reduces vitamin K which is necessary for activation of clotting Factors II, VII, IX and X. The VKORC1 gene encodes for vitamin K epoxide reductase complex subunit 1, a key component of VKOR. The combination of physiologic factors (30%), CYP2C9 variations (20%) and VKORC1 variants (25%) accounts for approximately 75% of warfarin dose variability. This illustrative case report demonstrates the clinical importance of this new information. Clinicians need to incorporate these new genomic findings into appropriate management of warfarin dose anticoagulation.
Introduction
Warfarin is the most commonly prescribed oral anticoagulant in the United States with over 19 million prescriptions written in 2006. Warfarin dosing remains a challenging task.2 Warfarin is a narrow therapeutic index drug with frequent hemorrhagic complications despite dose adjustment for clinical variables including age, gender, weight, nutritional factors, dietary vitamin K intake and interactive medications. The cost of warfarin adverse drug reactions (ADRs) is high and is estimated to exceed $180 billion dollars annually.2 However, coagulation, and consequently, warfarin dose, is influenced by many other factors both physiological and genetic. For instance, a patient’s International Normalized Ratio (INR) varies inversely with dietary intake of vitamin K.3 Also, older patients often exhibit an enhanced dose response to warfarin, and require lesser dosing.4 Physically smaller patients and women compared to men require a lesser dose of warfarin.5–7 Currently, dosing algorithms based on the patient’s age, gender and body mass provide a more rational approach to dosing, but use of these algorithms in routine clinical practice is not the rule. Given warfarin’s narrow therapeutic range, dose estimate empiricism is hazardous, especially during the initial dose titration phase. Physicians typically estimate an initial daily dose and schedule frequent follow-up visits to test and reassess the dosing regimen. Determining the correct maintenance dose for each patient can also be difficult, requiring repeated adjustments through protracted trial-and-error periods. The consequences of over or under anticoagulation can be life threatening ranging from gastrointestinal (GI) bleeding to intracranial hemorrhage. The current warfarin empirical dosing approach often results in adverse drug reactions, and treating them is both expensive and time-consuming. Major and fatal bleeding events occur at a rate of 7.2 and 1.3/100 patient years, respectively, according to a meta-analysis of 33 studies.8 If DNA typing were performed for every patient before they were prescribed warfarin, an estimated 85,000 serious bleeding events and 17,000 strokes could be avoided annually, saving over one billion dollars in healthcare spending.2 The benefits of DNA typing for patients receiving warfarin therapy are substantial, saving billions of healthcare dollars, hours of patient and physician time and most importantly preventing adverse drug reactions that are at best debilitating and at times fatal.
Common polymorphisms in two genes, cytochrome P450 (CYP 2C9) and vitamin K epoxide reductase complex subunit 1 (VKORC1) affect warfarin dose through different mechanisms. Polymorphisms of CYP2C9 include *2 and *3 which are associated with a decrease in 2C9 enzyme activity to about 70% and 5% of the wild type respectively.9–13 The result of these polymorphisms is excessive warfarin accumulation with increased risk of elevated INR (> 4.0) and potential hemorrhagic complications. The CYP2C9 status accounts for approximately 15% to 20% of the variance in warfarin dose.7,14,15
Warfarin exerts its anticoagulant effect through inhibition of the vitamin K epoxide reductase (VKOR). The VKORC1 gene encodes for vitamin K epoxide reductase complex subunit one. VKOR is responsible for converting the oxidized vitamin K to reduced vitamin K hydroquinone which is an essential cofactor for the gamma carboxylation of clotting actors II, VII, IX and X.16 A common polymorphism of the VKORC1 promotor sequence (−1639) G>A) results in decreased vitamin K epoxide reductase enzyme activity. Patients who are carriers of this polymorphism require a lower warfarin maintenance dosage. In addition, six comparatively rare coding sequence mutations result in syndromes of marked warfarin resistance. In patients requiring anticoagulant therapy, the −1639 VKORC1 genotype can independently determine 20% to 25% of warfarin dose variance.7,15,17 Together the CYP2C9 and VKORC1 combined genotype can explain up to 45% of warfarin response variability.6,7,14,17 This case presentation is designed to provide guidelines in order to integrate this newly available genomic information into conventional risk assessment in order to provide optimal physician DNA guided clinical care.
Case Report
A 40-year-old black female patient of French Guianian extraction presented to the emergency department with aphasia and left sided upper extremity weakness. The patient had a background history of labile hypertension controlled with lisinopril and hypercholesterolemia controlled with atorvastatin. Physical examination confirmed the left sided neurological findings and was additionally positive for right lower extremity calf tenderness. Cardiac examination was unremarkable except for a mild systolic ejection murmur.
An echocardiogram demonstrated non-vegetative aortic valve lesions on both leaflets measuring a maximum of 0.5–0.6 mm. An ultrasound of the right lower extremity demonstrated deep vein thrombosis. A transesophageal echocardiogram (TEE) confirmed the earlier ultrasound findings of aortic valve nodules as well as aortic valve thickening. A saline bubble study demonstrated right to left shunting at the atrial level consistent with a patent foramen ovale (PFO). Blood cultures were negative. A paradoxical cardioembolic event was thought to be a probable cause of the patient’s stroke. The absence of fever and new murmurs together with negative blood cultures, the stability of the aortic valve lesion and the lack of aortic valve damage made endocarditis or a primary valve tumor with embolization unlikely causes.
Therapy was begun with warfarin for thrombophlebitis together with ampicillin/sulbactam and gentamycin coverage for possible endocarditis. A CYP2C9 assay was positive for a CYP2C9 polymorphism (2C9*1/*3) and a VKORC1 G>A polymorphism. The calf tenderness cleared. A repeat ultrasound of the right lower extremity demonstrated no residual clot. The neurologic symptoms cleared and the patient returned to full employment. Life time anticoagulation has been recommended.
Laboratory analysis
Five to 10 ml ethylene diamine tetraacetic acid (EDTA) blood samples were obtained at the time of routine PT/INR collections. Deoxyribonucleic acid (DNA) samples were extracted from whole blood using QIAamp DNA Blood Midi Kit (Qiagen, Valencia, CA) following manufacturers protocol. Extracted DNA was stored at −80°C in tris(hydroxymethyl) aminomethane deoxyribonucleic acid (TRI S-EDTA) buffer. Quantification of DNA was performed by fluorescent staining of double stranded DNA (PicoGreen® dsDNA Quantitation Kit, Molecular Probes, Eugene, OR). Fluorescent intensity was measured using a fluorescent micro-titer plate reader (POL ARstar OPTIM A, BMG LABTECH GmbH, Offenburg, Germany). The concentration of extracted DNA was adjusted to 5 ng/µl in DNase-free distilled water. A total of 15 ng of extracted DNA is required for the PCR reaction using the Tag-It™ Mutation Detection Kits from Luminex Molecular Diagnostics (formerly Tm Bioscience; Toronto, Ontario, Canada).
Genotyping was performed on a Luminex® 100 analyzer using xMAP® technology (Luminex® Corp., Austin, TX) installed at the Laboratory of Personalized Health (LPH) at Genomas Laboratory. The genotyping kits are from Luminex Molecular Diagnostics (Toronto, Ontario, Canada). The assay requires 3µl of sample normalized at 5 ng/µl to a total concentration of 15 ng of genomic DNA.
DNA Typing is performed at the Laboratory of Personalized Health (LPH), a division of Genomas Inc. (Hartford, CT). LPH is a high-complexity clinical DNA testing center licensed by the Connecticut Department of Health (CL-00644) and certified by the Centers for Medicare and Medicaid Services (ID# 07D1036625) under CLI A (Clinical Laboratory Improvement Amendments). The HILO met WARF ARIN DNA Typing system is available through the Clinical Laboratory Partners (CL P) network throughout Connecticut. The System determines an individual’s warfarin metabolic capacity and sensitivity by DNA-typing simultaneously the CYP2C9 and VKORC1 genes, at twelve variable sites; five alleles in CYP2C9 (Table 1) and seven alleles in VKORC1 (Table 2). The Tag-It Mutation Detection assays (Luminex Molecular Diagnostics, Toronto, Canada) were utilized for DNA typing.15 The wild-type allele, *1, is inferred by absence of the alleles typed in the assays. These assays employed PCR to amplify selectively the desired genes without co-amplifying pseudogenes or other closely related sequences.
Table 1.
Warfarin Doses and INR Responses
| Day Baseline |
INR | Coumadin Dose (mg./day) |
|---|---|---|
| 1 | 1.19 | |
| 2† | 1.18 | 5 |
| 3 | 1.15 | 7.5 |
| 4§ | 1.73 | 5 |
| 7 | 2.20 | 5 |
| 9 | 3.30* | 5 |
| 16 | 4.90* | 5/2.5 alt (3.8) |
| 22 | 5.30* | 3 |
| 32 | 2.10 | 3 |
| 48 | 1.40 | 3/3/5 alt (3.7) |
| 61 | 2.40 | 3/3/5 alt |
| 90 | 2.20 | 3/3/5 alt |
Therapeutic Range 2.0–3.0
above therapeutic range
2C9/VKORC1 drawn
2C9/VKORC1 reported
Table 2.
Frequency of CYP2C9*1 Genotype and VKORC −1693 G Allele in Ethnic Populations22
| Caucasian | Afro-American | Asian | Hispanic-American | |
|---|---|---|---|---|
| CYP2C9*1 | 74% | 94% | 95% | 93% |
| VKORC1 −1693G | 59% | 88% | 12% | 52% |
The kits use multiplexed Allele Specific Primer Extension (ASPE) to identify small nucleotide variations including single base changes and deletions of one or three bases. In brief, a PCR -derived target DNA with two universally-tagged allele-specific primers whose three foot ends define the alleles was used for each variation tested. A thermophilic DNA polymerase was used for primer extension and biotin-dCT P label incorporation. Because the two tagged allele-specific primers overlap the SNP site in the target DNA, only the correctly hybridized primers were extended to generate labeled products. Single tagged ASPE primers were used to detect the presence of unique PCR fragments generated for the deletion and duplication gene rearrangements. Following ASPE, tagged, extended products labeled with biotin were captured by their tag complements (antitags), which had been chemically coupled to spectrally addressable polystyrene microspheres. A fluorescent reporter molecule (streptavidin-phycoerythrin) was used to detect incorporated biotin. The fluorescent reporter signals generated for each bead population was measured on the Luminex xMAP™ system. (Luminex Corp., Austin TX).
Discussion and Analysis of Genetic Results
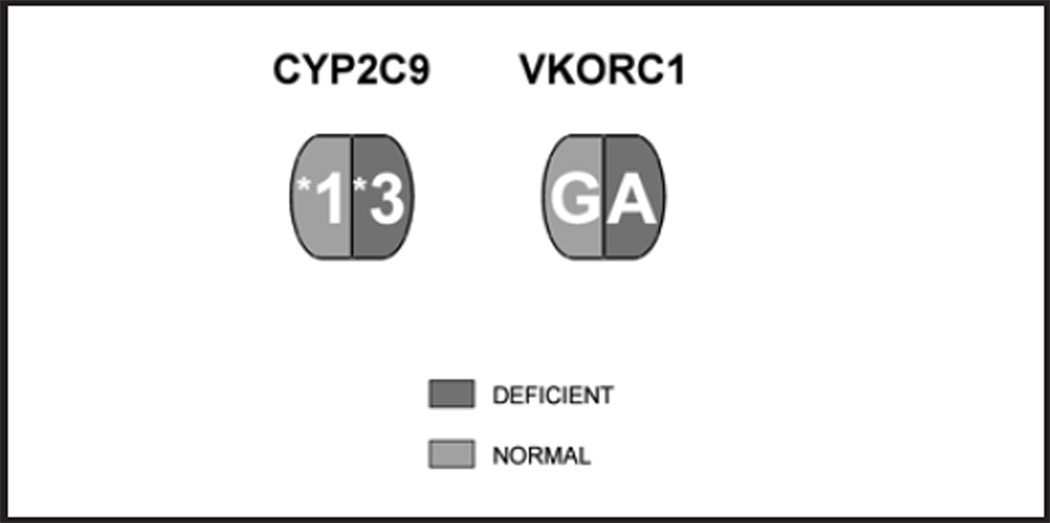
The patient has a CYP2C9 *1/*3 gene abnormality and a VKORC1 G>A polymorphism (Fig. 2). The CY P P450 subunit two family is expressed in the liver where it is responsible for ~ 90% of all drug metabolism. The CYP2C9 *3 variant codes for a A1075C mutation which reduces functional CY P 2C9 enzyme activity by 95%. The result is increased warfarin sensitivity, decreased warfarin clearance, higher peak concentration of warfarin and a delay in peak warfarin activity to day six to 12 or longer as opposed to day three to four in the functionally normal CYP2C9 *1/*1 wild type patient. These factors delay INR stability for several extra days or weeks often beyond hospital discharge and the ease of frequent INR monitoring.
Figure 2.
Functionally deficient CYP2C9 and VKORC G>A alleles in a patient recieving warfarin (Coumadin).
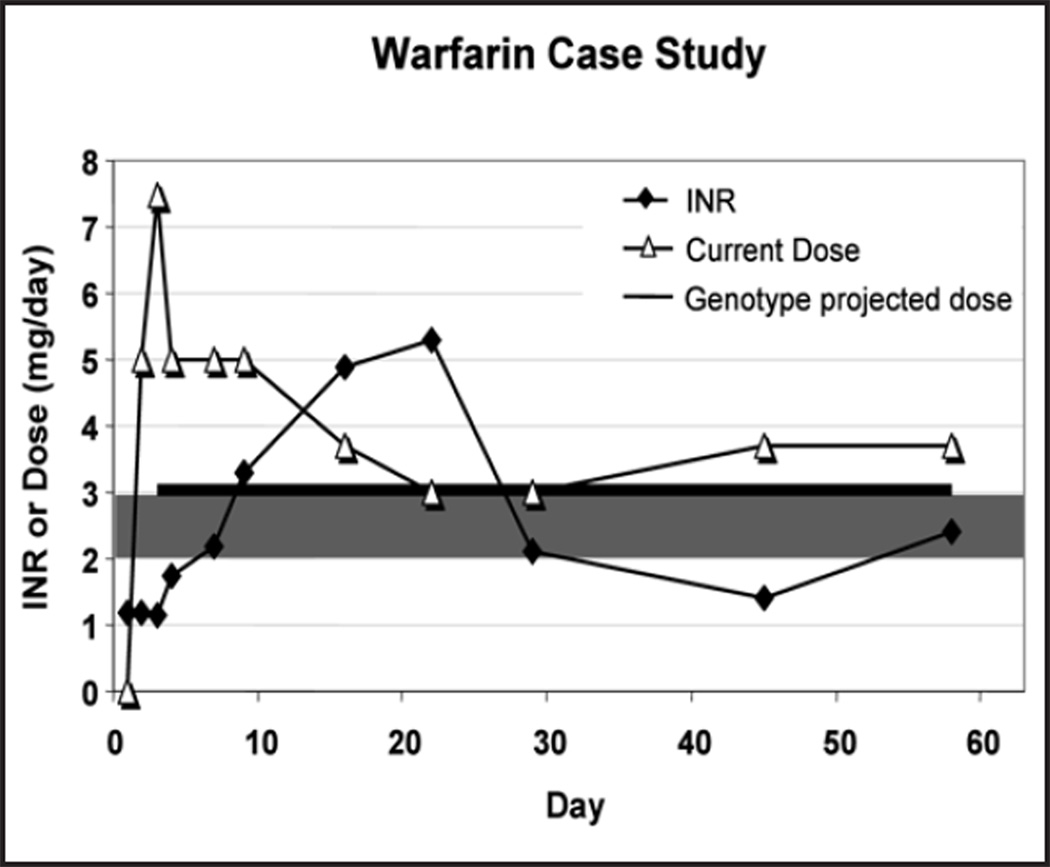
The patient’s INR levels are presented in Table 1. The patient demonstrated both excessive INR peak levels with conventional warfarin initiation doses and a delayed peak of INR at day 29. Optimal anticoagulation is critical in view of the clinical findings, the potential for a hemorrhagic infarct at the site of the initial cardio-embolic lesion and the patients ethnicity. Cerebrovascular disease has been reported to be increased in black patients with CYP2C9 polymorphisms both prior to initiation of warfarin therapy and during stable maintenance.18
A number of strategies have been proposed for incorporating genomic results into conventional physiologic based warfarin dose selection. These strategies serve to provide integrated DNA based target recommendations for clinicians. Therapeutic warfarin prescription can be divided into three phases; 1) initiation of warfarin therapy 2) transition to target maintenance dose, and finally 3) an INR based stable maintenance warfarin dose. EDTA blood samples for genotyping should be obtained at the earliest opportunity. For elective procedures including joint replacement and cardiac valvular surgery these can be incorporated into scheduled preadmission testing. For more emergent non-scheduled clinical situations such as cardiac arrythmias and thrombophlebitis genotyping should be ordered at the earliest opportunity. Recent developments have made it possible to shorten the turn around time for CYP2C9/VKORC1 test results to 24–48 hours. It is important to recognize that the initial warfarin dose is substantially independent of genotype status. Thus an initial warfarin does of 5 mg. daily on days one and two is generally recommended. By day three to four transitional physiologic and genotype based dose recommendations should be available. Finally an INR should be obtained on day four to six and the INR guided maintenance doses adjusted appropriately. The presence of the CYP2C9*3 polymorphism is associated with a decrease in enzyme activity to 5% of the wild type capacity. The result is excess warfarin accumulation, elevated INR>4.0) and increased frequency of hemorrhagic complications. The CYP2C9 status accounts for approximately 20% of variance in warfarin dose.19
In addition the patient had a −1639 VKORC1 G>A promoter polymorphism. VKOR (vitamin K epoxide reductase) is the target for vitamin K binding and action. Warfarin exerts its anticoagulant effect through its inhibition of VKOR. A key subunit of VKOR is coded for by the vitamin K reductase complex subunit 1 (VKORC1) gene and is the site of reduced vitamin K interaction with the vitamin K dependent clotting factors II, VII, IX and X.16 It should be appreciated that CYP2C9*2 and CYP2C9*3 polymorphisms which delay warfarin metabolism and half-life may have delayed peak anticoagulant effect and delayed initial INR response on days five to nine.
Published algorithms are available to estimate warfarin dose including physiologic factors such as age, gender, height, diet and weight as well as CYP2C9 and VKORC1 genetic status.7,10,20 In addition drugs such as amiodorone and fluvastatin which are metabolized by CYP2C9 may competitively inhibit CYP2C9 activity with a resultant decrease in functional activity.21 Warfarin doses should be decreased accordingly.
Black African Americans (AA) have an increase in major hemorrhagic complications during therapeutic anti-coagulation as opposed to their Caucasian counterparts. This makes optimal control of anticoagulation in AA essential.18 In addition cerebrovascular accidents secondary to embolic events have a propensity to convert to hemorrhagic strokes as a result of embolus fragmentation and down stream migration with resultant damage to the vascular wall and adventitia. It is important that every effort be made to provide not just adequate but optimal anti-coagulation for these patients.
Figure 1.
Illustrates the peak INR level at day 22 and the subsequent decline when the genomic CYP2C9 and VKORC1 DNA results became available. The horizontal line indicates the warfarin dose of 3.2 mg/d which was predicted by the therapeutic algorithm.7
Table 3.
Summary of Common Alleles of CYP2C9 and VKORC1
| Gene | Allele | DNA Sequence | Amino Acid Change | Enzymatic Phenotype |
|---|---|---|---|---|
| CYP2C9 | *1 | Most common | Reference | Normal |
| *2 | 430 C>T | Arg144Cys | Deficient | |
| *3 | 1075 A>C | Ile359Leu | ~ Null | |
| VKORC1 | G | Most common | Reference | Normal |
| A | −1639 G>A (Promoter) | Reduced Synthesis | Deficient |
Summary of CYP2C9 and VKORC1 alleles, molecular defects and resulting enzymatic phenotypes determined by DNA typing of the patient. The DNA sequence column indicates the base pair substitution or deletion, and the specific location in the gene. For CYP2C9, a transition from cytidine to thymidine (*2 allele) and a transversion from adenosine to cytidine (*3 allele) are noted. For VKORC1, a transition from guanosine to adenosine is noted at position −1639, located in the promoter region upstream from the start side of the coding DNA sequence.
The Amino Acid Change column indicates the amino acid substitution or protein synthesis alteration caused by the DNA change. Amino acid substitutions for CYP2C9 (*2 allele coding for an arginine to cysteine substitution and *3 allele coding for an isoleucine to leucine substitution) typically result in subfunctional proteins (deficient phenotype). Protein synthesis alteration from promoter DNA sequence variability for VKORC1 result in decreased expression of the gene and polypeptide production.
Acknowledgments
Dr. Ruano is President of Genomas, Inc. Dr. Windemuth and Mr. Kocherla are full time employees and shareholders of Genomas. Dr. Bower and Dr. Seip are consultants to the company. The Laboratory of Personalized Health (LPH), a division of Genomas, Inc., is a CLI A certified Clinical Laboratory (CL-0644) which performs DNA based diagnostic testing This work was supported by a research grant from Hartford Hospital and Genomas internal research funds.
Footnotes
Disclosures
Drs. White and Deconge have no disclosures to declare.
A glossary of genetic terminology is maintained by the National Human Genome Research Institute at www.genome.gov/glossary.cfm.
Contributor Information
Anthony LaSala, Hartford.
Bruce Bower, Senior Clinical Consultant, Hartford Hospital, Genomas, Inc., Hartford.
Andreas Windemuth, Genomas, Inc., Hartford.
C. Michael White, Associate Professor of Pharmacy Practice, University of Connecticut, Storrs.
Mohan Kocherla, Genomas, Inc., Hartford.
Richard Seip, Genomas, Inc., Hartford.
Jorge Duconge, Department Pharmaceutical Sciences, University of Puerto Rico, San Juan PR.
Gualberto Ruaño, Director of Genetics Research, Hartford Hospital, President, Genomas, Inc., Hartford.
REFERENCES
- 1.Wilkerson GR. Drug metabolism and variability among patients in drug response. N Engl J Med. 2005;352:2211–2224. doi: 10.1056/NEJMra032424. [DOI] [PubMed] [Google Scholar]
- 2.McWilliam A, Lutter R, Nardinelli C. Health care savings from personalized medicine using genetic testing: The case of warfarin. Rep. Working Paper 06–23,2006 AE1-Brookings Joint Center for Regulatory Studies [Google Scholar]
- 3.Couris R, Tataronis G, McCloskey W, et al. Dietary vitamin K variability affects international normalized ratio (INR) coagulation indices. Int J Vitam Nutr Res. 2006;76:65–74. doi: 10.1024/0300-9831.76.2.65. [DOI] [PubMed] [Google Scholar]
- 4.Ruano G, Thompson P, Villagra D, et al. High carrier prevalence of combitorial CYP2C9 and VKORC1 genotypes affecting warfarin dosing. Personalized Med. 2008;5:225–232. doi: 10.2217/17410541.5.3.225. [DOI] [PubMed] [Google Scholar]
- 5.Absher RK, Moore ME, Parker MH. Patient-specific factors predictive of warfarin dosage requirements. Ann Pharmacother. 2002;36:1512–1517. doi: 10.1345/aph.1C025. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Shennan M, Reynolds KK, et al. Estimation of warfarin maintenance dose based on VKORC1 (−1639 G>A) and CYP2C9 genotypes. Clin Chem. 2007;53:1199–1205. doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 7.Sconce EA, Khan TI, Wynne HA, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: Proposal for a new dosing regimen. Blood. 2005;106:2329–2333. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 8.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: A meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 9.Kamali F, Pirmohamed M. The future prospects of pharmacogenetics in oral anticoagulation therapy. Br J Clin Pharmacol. 2006;61:746–751. doi: 10.1111/j.1365-2125.2006.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linder MW, Looney S, Adams JE, et al. Warfarin dose adjustments based on CYP2C9 genetic polymorphisms. J Thromb Thrombolysis. 2002;14:227–232. doi: 10.1023/a:1025052827305. [DOI] [PubMed] [Google Scholar]
- 11.Rettie AE, Wienkers LC, Gonzalez FJ, et al. Impaired (S)-warfarin metabolism catalysed by the R144C allelic variant of CYP2C9. Pharmacogenetics. 1994;4:39–42. doi: 10.1097/00008571-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Scordo MG, Pengo V, Spina E, et al. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther. 2002;72:702–710. doi: 10.1067/mcp.2002.129321. [DOI] [PubMed] [Google Scholar]
- 13.Voora D, Eby C, Linder MW, et al. Prospective dosing of warfarin based on cytochrome P-450 2C9 genotype. Thromb Haemost. 2005;93:700–705. doi: 10.1160/TH04-08-0542. [DOI] [PubMed] [Google Scholar]
- 14.Hillman MA, Wilke RA, Caldwell MD, et al. Relative impact of covariates in prescribing warfarin according to CYP2C9 genotype. Pharmacogenetics. 14:539–547. doi: 10.1097/01.fpc.0000114760.08559.dc. YEAR? [DOI] [PubMed] [Google Scholar]
- 15.Wadelius M, Chen LY, Eriksson N, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121:23–34. doi: 10.1007/s00439-006-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suttie JW. The biochemical basis of warfarin therapy. Adv Exp Med Bio. 1987;214:3–16. doi: 10.1007/978-1-4757-5985-3_2. [DOI] [PubMed] [Google Scholar]
- 17.Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Eng. J Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 18.Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Mol Ther. 2008;88:312–321. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peyvandi F, Spreafico M, Siboni SM, et al. CYP2C9 genotypes and dose requirements during the induction of oral anticoagulant therapy. Clin Pharmacol Ther. 2004;75:198–203. doi: 10.1016/j.clpt.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Gage B. [Accessed March 2008]; http://WarfarinDosing.org. [Google Scholar]
- 21.Flockhart D. [Accessed March 2008]; http://medicine.iupui.edu/flockhart/ [Google Scholar]
- 22.Wu AHB, Wang P, Smith A, et al. Dosing Algorithm for using CY2C9 and VKORC1 genotyping from a multiethnic population: Comparison with other equations. Pharmacogenomics. 2008;9:169–178. doi: 10.2217/14622416.9.2.169. [DOI] [PubMed] [Google Scholar]