Abstract
Multifactorial metabolic diseases, for instance diabetes develop several complications like hyperlipidemia, hepatic toxicity, immunodeficiency etc., Hence, instead of mono-drug therapy the management of the disease requires the combination of herbs. Marketed herbal drugs comprise of irrational combinations, which makes their quality control more difficult. Phytoconstituents, despite having excellent bioactivity in vitro demonstrate less or no in vivo actions due to their poor lipid solubility, resulting in high therapeutic dose regimen; phospholipids encapsulation can overcome this problem. Hence, present study was designed to develop a phospholipids encapsulated polyherbal anti-diabetic formulation. In the present study, polyherbal formulation comprises of lyophilized hydro-alcoholic (50% v/v) extracts of Momordica charantia, Trigonella foenum-graecum and Withania somnifera 2:2:1, respectively, named HA, optimized based on oral glucose tolerance test model in normal Wistar rats. The optimized formulation (HA) entrapped in the phosphatidylcholine and cholesterol (8:2) vesicle system is named HA lipids (HAL). The vesicles were characterized for shape, morphology, entrapment efficiency, polar-dispersity index and release profile in the gastric pH. The antidiabetic potential of HA, marketed polyherbal formulation (D-fit) and HAL was compared in streptozotocin-induced diabetic rat model of 21 days study. The parameters evaluated were behavioral changes, body weight, serum glucose level, lipid profile and oxidative stress. The antidiabetic potential of HA (1000 mg/kg) was at par with the D-fit (1000 mg/kg). However, the potential was enhanced by phospholipids encapsulation; as HAL (500 mg/kg) has shown more significant (P < 0.05) potential in comparison to HA (1000 mg/kg) and at par with metformin (500 mg/kg).
Keywords: Antihyperglycemic, antioxidant, metformin, streptozotocin
INTRODUCTION
According to WHO, diabetes mellitus will be the single largest non-communicable disease worldwide by the year 2025 with the largest diabetic population in India.[1] Current pharmaco-therapeutics/medicines are not able to reverse hyperglycemia completely, have limited tolerability, and induce side effects.[2] Therefore, a need for alternative therapy especially the natural plant sources having minimal side effects has arisen. Even the WHO expert committee on diabetes has recommended the need of investigation of their effectiveness in the management of the disease.[3] In majority of traditional systems, diabetes is better managed by the herbs combination (Polyherbal) instead of single herb because of synergism and less side effects.
About two hundreds of polyherbal formulations are available in the market (Data un-published) for the management of diabetes, majority of them contain eight or more drugs in the combination, quite complex to standardize; therefore, quality of the product cannot be assured. Since, FDA recommends the number of ingredients to be three or less than three in any combination;[4] hence, in the present study the polyherbal formulation comprising of lyophilized hydro-alcoholic extracts of only three drugs combination was optimized.
In the study, the lyophilization was done in order to improve the physical state, bioavailability, and more importantly stability of thermo-liable constituents present in the herbs.[5]
The fruits of Momordica charantia, seeds of Trigonella foenum-graecum and roots of Withania somnifera are the major component in numbers of marketed as well as traditional antidiabetic formulations.[6,7]
Momordica charantia (Karela), a climber belongs to family, Cucurbitaceae, commonly known as bitter gourd or bitter melon in English. Karela fruits have scientifically proven for antidiabetic, cholesterol-lowering and antioxidant properties.[8,9,10]
Trigonella foenum-graecum (Methi), a green leafy vegetable belongs to the Leguminosae family. Its seed possesses antidiabetic, anti-hyperlipidemic and antioxidant properties.[11,12,13]
Withania somnifera (Ashwagandha) is a green shrub of family Solanaceae. Its roots are potential source of antihyperglycemic and hypo-cholesterolemic agents.[14] The root is also reported for its free radical scavenging and immuno-modulatory properties. Therefore, these three drugs (karela, methi, and roots of ashwagandha) have been selected for the development of a polyherbal antidiabetic formulation.
The problem associated with the use of herbal preparation is of their poor bioavailability.[15] Phytoconstituents like flavonoids, tannins, terpenoids etc., are poorly absorbed due to their large molecular size (which cannot be absorbed by passive diffusion). Also due to their poor lipid solubility, it severely limits its ability to pass across the lipid-rich biological membranes.[15] The vesicle system composed of phospholipids can increase the therapeutic value, reduce toxicity and can increase the bioavailability of the phytomedicines. The phospholipid-based vesicle system, mainly phytosomes, has been applied recently to many popular herbal extracts including Ginkgo biloba, grape seed, hawthorn, milk thistle, green tea and ginseng.[16] The flavonoid and terpenoid components of these herbal extracts lend themselves quite well for the direct binding to phosphatidylcholine. The binding of components of herbal extracts to phosphatidylcholine results in a dosage form that is better absorbed and thus produces better results than the conventional herbal extracts.[16] The reports indicate that the absorption of Silybin from Silybin phytosome is approximately seven times higher compared to the absorption of Silybin from regular milk thistle extract.[17] Bombardelli et al. (1991) reported that the silymarin phospholipid complex showed much higher antioxidant, free radical scavenging properties and a long lasting action than the silymarin alone.[17] Hence, in the proposed study they developed polyherbal antidiabetic formulation incorporated in the phospholipids vesicle system.
MATERIALS AND METHODS
Drugs, Chemicals and Instruments
The selected plant materials viz karela (fruit), methi (seed) and ashwagandha (root) were procured from the authentic supplier and further authenticated by Dr. H.B. Singh Director, Department of Raw Material Herbarium and Museum, National Institute of Sciences Communication and Information Resources (NISCAIR), New Delhi. The voucher specimens are deposited in the department of Pharmacognosy at our institute (specimen numbers HERB/COG/2009-10/1096/89 for karela; HERB/COG/2009-10/1096/91 for ashwagandha and HERB/COG/2009-10/1096/94 for methi). The polyherbal antidiabetic formulation, D-fit capsules (Dhanwantri Pharmaceuticals, Amritsar), comprising of karela, methi, ashwagandha along with other 12 ingredients, was used for the comparison of antidiabetic potential of the developed formulation. Streptozotocin (STZ) was procured from Sigma Chemical Co. (St Louis, MO, USA). The kit for glucose estimation was purchased from Beacon Diagnostics Pvt. Ltd, Navsari, India. Total cholesterol, triglycerides, HDL-C kits were obtained from Carol Company, Goa, India. All other chemicals and reagents were of the highest commercial grade available. Cooling Centrifuge (REMI), Rotary evaporator (Roteva), UV/Visible Spectrophotometer (UV 1700, Pharmaspec, Shimadzu), Lyophilizer (Daihan, Korea) and Transmission Electron Microscope (TEM-200 Tokyo, Japan) were used during the study.
Extraction and Lyophilization
The selected plants materials were shade dried, grinded, using mixer grinder and then sieved by passing through 100 number sieves and stored in an airtight container. Five hundred grams of each drug coarsely powdered and were extracted with hydro-alcohol (50% v/v) by triple maceration (1 × 3 L). The prepared hydro-alcohol extracts were concentrated under vacuum using rotary evaporator at 40° C temperature (removal of alcohol). The concentrated extracts were freeze dried at -20° C for 12 h then lyophilized using lyophilizer. The lyophilized extract(s) powders were stored in an airtight container and kept in the desiccator till used.
Formulation Development
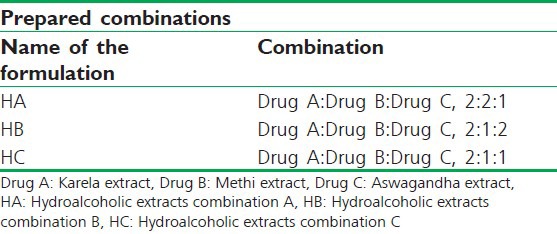
The lyophilized powders of hydro-alcoholic extracts of karela, methi and ashwagandha were evaluated for antihyperglycemic potentials in OGTT model in normal Wistar rats at a single dose of 1000 mg/kg, where karela extract had shown maximum antihyperglycemic potential (Data un-published). Therefore, different extract combinations were prepared for the formulation development by varying the ratios of methi and ashwagandha, whereas karela was kept constant [Table 1].
Table 1.
Lyophilized hydro-alcoholic extracts of karela (A), methi (B) and aswagandha (C) combinations
Animals
The animal study was carried out on 3-5 months old Wistar albino rats of either sex weighing (180-220 g), procured from the animal house, ISF College of Pharmacy, Moga. The rats were housed in polypropylene cages under standard conditions with a 12-h light–dark cycle. The experiment was conducted with the approval of Institutional Animal Ethical Committee (IAEC) and CPCSEA guidelines for the use and care of experimental animals (vide approval no ISF/CPCSEA/IAEC/2011/33).
Optimization of the Formulation Based on Oral Glucose Tolerance Test Model
The OGTT study was conducted on overnight fasted glucose (2 g/kg) induced hyperglycemic normal rats. The rats were divided into six groups (n = 6); Group I normal control (0.5% w/v carboxy methyl cellulose (CMC) solution pretreated rats), Group II (glucose load + 0.5% w/v CMC pretreated rats) and Group III-VI received HA, HB, HC and D-fit, respectively, at single dose level (1000 mg/kg per oral) prior to one hour of glucose load. The blood samples were withdrawn from the retro orbital plexus under mild anesthesia, before and 0, 60 and 120 min after glucose administration. The serum glucose level (SGL) was estimated within 30 min of withdrawal of the blood sample by glucose oxidase–peroxidase method.[18]
Acute Toxicity Study
Acute oral toxicity study was performed as per revised organization for economic co-operation and development (OECD) guidelines in the albino mice with the OGTT optimized formulation. The formulation was administered to the overnight fasted animals in different dose levels up to 5 g/kg body weight. The animals were observed for 14 days for any kind of abnormal symptoms such as behavioral changes, locomotion and mortality.[19]
Quantification of Total Phenolic and Flavonoid Contents
The phytochemical screening of HA showed the presence of polyphenols and flavonoids (data unpublished). The amount of total phenolic and flavonoid contents was determined in order to characterize the phospholipids vesicle system for their entrapment efficiency and release profile. The quantification was done using the colorimetric method i.e., Folin Ciocalteu's reagent[20] and aluminium chloride methods.[21]
Preparation and Characterization of Vesicle Entrapped Formulation (HAL)
The formulation (HA) had shown maximum check on rising of SGL in OGTT model. The HA was incorporated into the vesicle system, prepared by solvent evaporation method. In this method the weighed amount of cholesterol and phosphatidyl choline (PC) were dissolved in acetone. This prepared solution was stirred on the hot plate using magnetic stirrer; the temperature was maintained at 60° C in order to prevent the solidification of the PC and cholesterol. To this a solution of HA, prepared in phosphate buffer solution was added slowly. The process was continued till the acetone was not removed completely. The resultant product was centrifuged for 15 minutes at 10,000 rpm in the cooling centrifuge and the vesicles settled at the bottom were lyophilized and characterized.[22] This process was repeated using PC and cholesterol in different ratios to optimize the effective combination of these two. The vesicles prepared were evaluated for their shape and morphology using Transmission Electron Microscope. The particle size and polarity dispersive index were analyzed using Delsa Nano C particle analyzer. The entrapment efficiency and release profile of the prepared vesicles were evaluated based on total phenolic (Gallic acid equivalent) and total flavonoid contents (Rutin equivalent) using UV spectrophotometer.
Antidiabetic Study in Streptozotocin-Induced Diabetic Model
The effectiveness of HA and HAL in the management of diabetes was further evaluated in STZ-induced diabetic rat model.
Induction of Experimental Diabetes
Diabetes was induced by freshly prepared STZ (50 mg/kg injection i.p.) in 0.1 M citrate buffer (pH 4.5). STZ-treated rats were allowed free access of 20% w/v glucose solution for 24 h to prevent STZ-induced hypoglycemic mortality. The SGL, determined at 72 h, animals with fasting serum glucose 250 mg/dl and above were considered as diabetic in the present study.
Experimental Design
The diabetic rats were randomly divided into eight groups (n = 6):
Group I: Normal control (received 0.5% w/v CMC solution)
Group II: Diabetic control (received STZ 50 mg/kg + 0.5% w/v CMC solution)
Group III: Diabetic rats treated with HA 1000 mg/kg
Group IV: Diabetic rats treated with HAL 250 mg/kg
Group V: Diabetic rats treated with HAL 500 mg/kg
Group VI: Diabetic rats treated with HAL 1000 mg/kg
Group VII: Diabetic rats treated with D-fit 1000 mg/kg
Group VIII: Diabetic rats treated with metformin 500 mg/kg
The test as well as standard drugs were suspended in the 0.5% w/v carboxy methyl cellulose (CMC) solution (vehicle) in distilled water and administered orally using an intra-gastric canula once daily for 21 days. The parameters studied included behavioral, biochemical and oxidative stress.
Behavioral Parameters
The effect of developed formulation, on body weight, feed intake, water intake and urine output were evaluated and recorded.
Biochemical Parameters
The blood samples were collected on day 0, 7, 14 and 21 of the study by puncturing the retro orbital plexus under mild anesthesia. On 21st day, 18 h fasted animals were sacrificed by cervical dislocation. Blood was collected in a dry test tube and allowed to coagulate at ambient temperature for 30 min. Serum was separated by centrifugation at 3000 rpm for 10 min for the estimation of glucose, total cholesterol,[23] total triglycerides, low density lipoprotein (LDL), very low density lipoprotein (VLDL) and high density lipoprotein (HDL) levels.[24] The liver of the sacrificed animals was dissected out, weighed, washed with saline, blotted dry and homogenate using tris and phosphate buffers for the purpose. The homogenate was analyzed for total glycogen content.[25]
Oxidative Stress
The obtained liver homogenate was used to evaluate the anti-oxidant potential of test drugs on the oxidative stress obtained during diabetes. For this two oxidative parameters viz. thio barbituric acid reactive substances (TBARS) level[26] and reduced glutathione (GSH) level[27] have been selected.
Antioxidant Activity
The antioxidant potential of HAL was evaluated by established in-vitro methods; 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical[27] superoxide dismutase (SOD) and nitric oxide (NO) radical scavenging activity models.[28,29]
Statistical Analysis
All the results are expressed as mean ± SD, analyzed by two-way ANOVA followed by Tukey's multiple comparison analysis as post-hoc test. The P < 0.05 was considered to be statistically significant.
RESULTS
Optimization of the Formulation Using Ogtt Model
The normal vehicle treated rats have shown non-significant change in the SGL. The group of positive control animals has showed 48.93% rise in SGL after 60 min of glucose administration [Figure 1]. In comparison HA (1000 mg/kg) and D-fit (1000 mg/kg) pretreated groups showed maximum check on SGL levels and allowed 25.84% and 23.57% of rise, respectively [Figure 1]. However, SGL was normalized in all the animals of tested groups within 2 h [Figure 1].
Figure 1.
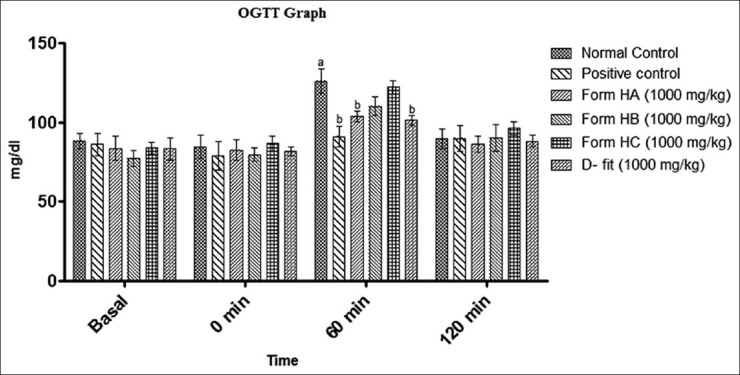
The effect of HA, HB, HC and D-fit at the dose of 1000 mg/kg body wt on serum glucose level of glucose loaded normal rats (OGTT model). Where a = P < 0.05 vs normal group; b = P < 0.05 vs positive control group
The results of the study showed that HA have most potent antihyperglycemic combination compared to other combinations evaluated. Hence, HA was further evaluated for its anti-diabetic potential in experimental-induced diabetic model in rats.
Acute Toxicity Study
The HA-treated animals have not shown any sign of behavioral changes up to the dose of 5 g/kg body wt.
Total Phenolic and Flavonoid Contents
The OGTT-based optimized formulation (HA) showed the presence of 13.22 mg gallic acid/g equivalents of total phenolic and 8.16 mg rutin/g equivalents of total flavonoid contents.
Antidiabetic Study in STZ-Induced Diabetic Rats
In comparison to normal rats, the diabetic control rats showed consistent and gradual rise in the fasting SGL during the study. The HA (1000 mg/kg) treated animals showed fall in elevated SGL by 12.66%, 31.29% and 45.62% on 7th , 14th and 21st days of the experiment, respectively, in comparison to onset of study. These results were close to the D-fit (1000 mg/kg) treated diabetic rats [Figure 2].
Figure 2.
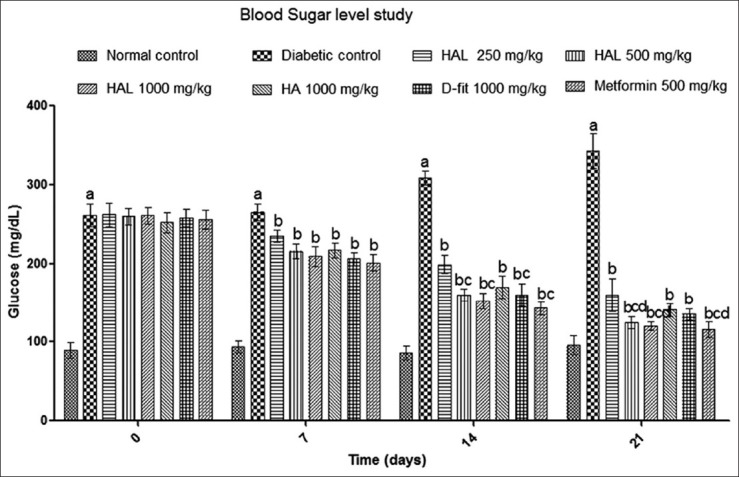
The effect of HA (1000 mg/kg), HAL (250 mg/kg), HAL (500 mg/kg), HAL (1000 mg/kg), D-fit (1000 mg/kg) and metformin (500 mg/kg) on serum glucose level in STZ-induced diabetic rats. All values represent Mean ± SD (n = 6). Where a = P < 0.05 vs normal group; b = P < 0.05 vs diabetic control group; c = P < 0.05 vs HA, d = P < 0.05 vs D-fit
Preparation and Characterization of HAL
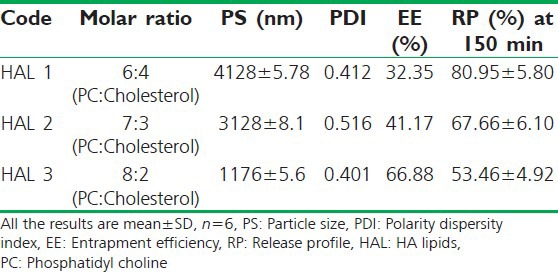
The HAL with molar ratio of PC and cholesterol (8: 2) was optimized for the preparation of phospholipids vesicle. The vesicle (HAL 3) had shown the highest entrapment efficiency, smallest size and resistance (up to 150 min) in the gastric pH and released only 53% of the formulation contents [Table 2].
Table 2.
Optimization of phospholipids vesicle based on entrapment efficiency, particle size, polarity dispersity index and release profile at gastric pH
Antihyperglycemic Study of HAL in OGTT Model
The HAL 500 mg/kg and 1000 mg/kg pretreated groups have shown 20.25% and 17.24% rise in SGL, respectively, after 60 min of glucose load. However, vehicle pretreated group of rats have shown the SGL rise by 52.94% [Figure 1].
Antidiabetic Study of HAL
Serum glucose level estimation
Diabetic control rats showed significant (P < 0.05) and gradual rise in the SGL up to 21st day of the study as compared to the onset day. The diabetic rats treated with the HAL (500 mg/kg) have shown the consistent fall in raised SGL on 7th, 14th and 21st days by 17.11%↓, 38.39%↓ and 52.11%↓, respectively, in comparison to onset of study. The results were at par with metformin (500 mg/kg) treated diabetic rats, which had shown consistent fall in serum glucose by 21.56%↓, 43.92%↓ and 54.76%↓ in comparison to onset day. The HAL (500 mg/kg) treated groups were more prominent in lowering the SGL compared to HA (1000 mg/kg) and D-fit (1000 mg/kg) treated animals [Figure 2].
Behavioral Parameters
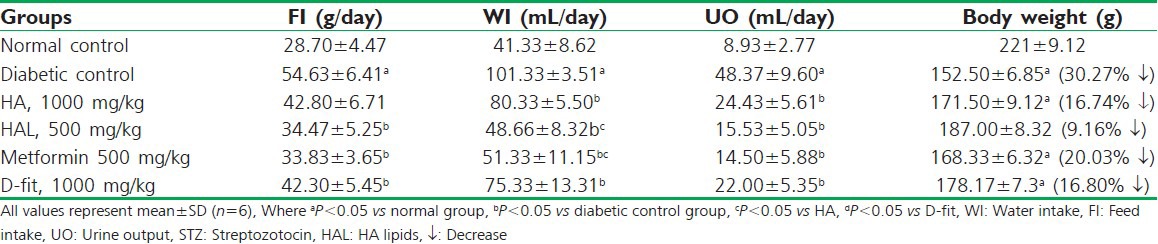
Diabetic control group (vehicle treated) animals have shown an abnormal rise in feed intake (54.63 g/day), water intake (101.33 ml/day) and urine output (48.37 ml/day). These effects were attenuated with HAL (500 mg/kg), which has shown 34.47 g/day, 48.66 ml/day and 15.53 ml/day, feed intake, water intake and urine output, respectively. Metformin (500 mg/kg) and D-fit (1000 mg/kg) also altered these modified levels [Table 3]. The reduction in body weight of diabetic control group was 30.27%↓ observed on 21st day of study, compared to 0 day. Whereas, HAL (500 mg/kg) treated group registered check on the loss in body weight showed the reduction by 9.16%↓. However, animals treated with HA (1000 mg/kg) have shown check on the loss of body weight by 16.74%↓ [Table 3].
Table 3.
The effect of 21 days test drugs treatment on feed intake, water intake, urine output and body weight of the STZ induced diabetic rats
Lipid Profile
On 21st day of study, diabetic animals have shown the rise in triglyceride (75.00%↑) total cholesterol (92.92%↑), LDL (150.33%↑) and VLDL (135.85%↑) levels, whereas HDL level was decreased (39.85%↓), compared to normal control animals [Table 4]. The animals treated with HAL (500 mg/kg), metformin (500 mg/kg) and D-fit (1000 mg/kg) have shown significant (P < 0.05) improvement in these altered levels [Figure 3].
Table 4.
The effect of 21 days test drugs treatment on TBARS, GSH and total hepatic glycogen levels in STZ-induced diabetic Wistar rats
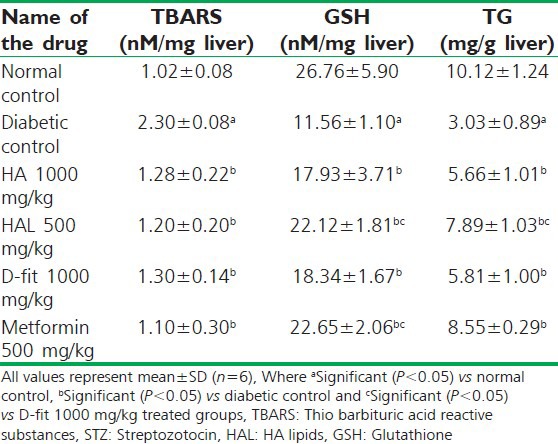
Figure 3.
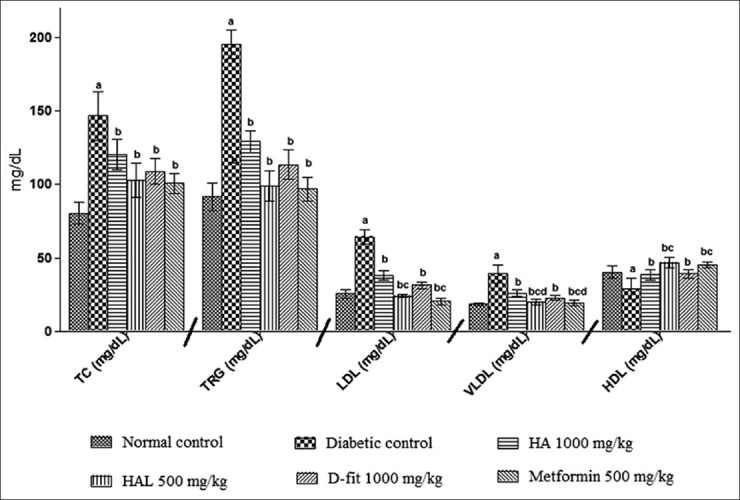
The effect of HA (1000 mg/kg), HAL (500 mg/kg), D-fit (1000 mg/kg) and metformin (500 mg/kg) on lipid profile in STZ-induced diabetic rats. All values represent Mean ± SD (n = 6). Where a = P < 0.05 vs normal group; b = P < 0.05 vs diabetic control group; c = P < 0.05 vs HA
Oxidative Stress and Total Glycogen Content
The liver homogenate of diabetic animals had shown the increased level of TBARS (2.3 nM/mg liver) when compared with normal control rats (1.02 nM/mg liver). Twenty days treatment with both HAL (500 mg/kg) and metformin (500 mg/kg) has significantly reversed the raised TBARS level (1.2 and 1.1 nM/mg liver, respectively) in STZ-induced diabetic animals. Moreover, in diabetic animals the GSH level was reduced (11.56 nM/mg liver) with respect to the normal control group (26.76 nM/mg liver). The treatment with both HAL (500 mg/kg) and metformin (500 mg/kg) has effectively improved the GSH level in diabetic rats [Table 4]. The glycogen content in diabetic control rats was found to be 3 mg/g on 21st day. This level is improved in HAL (500 mg/kg) treated diabetic animals (7.89 mg/g). In comparison, HA (1000 mg/kg) treated animals have shown 5.66 mg/g of total glycogen [Table 4].
Antioxidant Activity of HAL
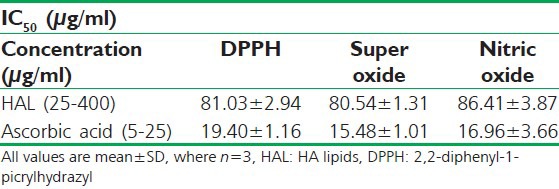
The HAL evaluated for its antioxidant potential had exhibited powerful free radical scavenging affect against all three selected models DPPH, SOD and NO free radicals with low IC50 values [Table 5].
Table 5.
In-vitro antioxidant activity of developed formulation (HAL) using DPPH free radical scavengers, super oxide radicals and nitric oxide radical as a marker for the activity
DISCUSSION
The previous studies suggest that karela (fruits) increases peripheral utilization of glucose,[9] enhanced numbers of beta cells increased numbers of GLUT4 transporter protein of muscles[10] and inhibit the glucose-6-phosphatase and fructose-1,6-bisphosphatase in liver and stimulate hepatic glucose-6-phosphate dehydrogenase activities.[30] Whereas, methi (seeds) extracts reported to exhibit antidiabetic potentials by delayed gastric emptying time, glucose absorption rate,[31] reduced uptake of glucose in the small intestine;[13] stimulation of insulin release from the remnant β-cells and improve insulin action at the cellular level.[31] Oxidative stress has been suggested to be a common pathway linking diverse mechanisms for the pathogenesis of complications in diabetes.[32] Oxidative stress may play an important role in the onset and progression of diabetic vascular complications. This suggests the possibility that such complications can be prevented and treated with antioxidants. Ashwagandha is well known for its antioxidant, anti-stress and immuno-modulatory activities. These available reports on the selected plants encouraged us to their incorporation in the development of a polyherbal formulation in order to achieve the synergism.
In present study, the formulation was prepared by mixing different ratios of lyophilized hydro-alcohol (50%) extracts of the selected plant materials, prepared by triple maceration method, which protects thermo labile compounds and resulted good yield.[33,34] In the study the combinations of extracts were prepared by keeping karela extract at higher ratio, based on the results of comparative antidiabetic study carried on the hydro-alcohol extracts of karela, methi and ashwagandha in our department (un-published data). The formulation (HA) comprised of karela, methi and ashwagandha extracts in the ratio of 2:2:1, respectively. Table 1 shows significant (P < 0.05) antihyperglycemic potential in OGTT model [Figure 1]. This effect of HA may be due to the insulin release and increased peripheral absorption of glucose,[12] as methi and karela extracts were present in the higher ratios. Therefore, HA (1000 mg/kg) was selected for further study in STZ-induced diabetic rat model. STZ-induced diabetic rats showed elevated levels of serum glucose (>250 mg/dl), due to the destruction of islets of Langerhans, which results into decrease in serum insulin level. The HA (1000 mg/kg) treated diabetic animals showed significant (P < 0.05) gradual fall in SGL in comparison to diabetic control group of animals. The results were at par with D fit (1000 mg/kg) and were comparable to metformin (500 mg/kg) treated animals [Figure 2]. The antihyperglycemic activity of HA may be attributed to the synergistic effect of the karela and methi present in the formulation.
Serum lipid levels are usually elevated in diabetes, which represents a high risk factor for coronary heart disease. Under normal condition, insulin activates the enzyme lipoprotein lipase, which hydrolyses triglycerides. However, in a diabetic state, lipoprotein lipase does not function properly due to insulin deficiency resulting in hyper-triglyceridemia.[35] On 21st day of the study, diabetic animals showed significant (P < 0.05) rise in total cholesterol, triglycerides, LDL and VLDL levels, and lower HDL level in comparison to normal rats. The HA (1000 mg/kg), D-fit (1000 mg/kg) and metformin (500 mg/kg) treated animals led to a significant (P < 0.05) normalization of lipid profile [Figure 3]. It implies that HA can reduce the diabetic complications of lipid metabolism and associated cardiovascular risk factors. This action of HA (1000 mg/kg) is probably due to the Karela which restores the action of lipoprotein lipase;[36] methi which up-regulate the peroxisome proliferators activator receptors (PPAR) activity and also increased insulin secretion and also due to the aswagandha that scavenge the free radicals generates during the diabetes.[14]
In diabetic rats, lack of insulin results in decreased hepatic glycogen contents. The HA (1000 mg/kg) treated group of animals showed significant (P < 0.05) improvement in the glycogen content [Table 4]. This may be because of synergistic effect of karela and methi on the reactivation of the glycogen synthase system, increased serum insulin level or it may also be due to the up-regulation of GLUT-4 and PPAR-γ activity of HA, which facilitates uptake of glucose by the peripheral tissues and excess of glucose stored as glycogen.[37]
The hyperglycemia leads to auto-oxidation and generates the reactive oxygen species (ROS) during the process of glycation.[38] The oxidative stress can impair the internalization of insulin by endothelial, thus limiting hormone delivery to target tissues and interfere with GLUT-4-mediated glucose transport.[39] The oxidative stress acts on signal transduction and, via nF-kB, affects gene expression. Thereby, the expression of antioxidant enzyme can be reduced. Thus, usefulness of antioxidants and the PPAR-α activator has been suggested in the control of NF-kB activity.[40]
The diabetic animals have shown elevated level of lipid peroxidation and decreased GSH level; those are clear manifestation of excessive formation of free radicals and resulting in tissue damage. The present study demonstrates that the 21 days treatment of diabetic rats with HA (1000 mg/kg) have significantly (P < 0.05) returned the altered levels of lipid peroxides and GSH towards their normal values [Table 4]. It indicates that HA (1000 mg/kg) has antioxidant potential, which may be due to the presence of triterpenoids, phenolic and flavonoids and steroidal lactones in the HA.
The diabetes results in loss of body weight, attributed to the abnormality in carbohydrate metabolism such as lipolysis, glycogenolysis, and acidosis that result in protein deficiency.[41] Diabetic rats treated with HA (1000 mg/kg) and D-fit (1000 mg/kg) have shown significant (P < 0.05) check on body weight loss. The antidiabetic action of HA is probably due to the synergistic effect of drugs of different mechanism of action such as, insulinomimetic, insulin sensitive, insulin secretagogue,[10,36] GLUT-4 and PPAR–γ up-regulations,[31] free radical scavenger and β cells rejuvenation.
The phospholipids encapsulation of HA resulted in significant improvement of its antidiabetic action and dose was reduced (up to 50%). Moreover, it has been observed that the effect of HAL (500 mg/kg) were more significant (P < 0.05) than the HA (1000 mg/kg) in normalizing hyperglycemia [Figure 2] as well as lipid profile [Figure 3] of the diabetic animals. The probable reason for this improvement is that, drugs those contain the macromolecules as well as protein/peptides may degrade pre-systemically in the gastrointestinal tract leading to a reduced fraction reaching the intestinal wall. The formulation/drugs with more hydrophilic contents do not absorb properly through the GIT. This usually results in poor bioavailability of the drug sometimes only one percent of the total drug contents reaches into the blood.[42] The HAL have shown the high entrapment efficiency, minimum drug release and stability in gastric pH up to 150 minutes [Table 2]. The in-vitro characterization details of HAL suggest that the vesicle-entrapped formulation might have reached into small intestine without its degradation. The phytochemical screening of HA had shown the presence of polyphenols, flavonoids, triterpenoids, and steroids and amino acids (data unpublished). The flavonoid and terpenoidal components are reported to lend themselves quite well for the direct binding to phosphatidylcholine resulting in the protection of the phytoconstituents from the gastric pH and better absorption of them through the small intestine.[42] Phospholipids vesicle, protects the valuable components of the herbal drugs from destruction in the gastric environment and gut bacteria, thus producing better results in comparison to the conventional polyherbal formulation. These results further support the concept of pinocytosis of liposomes by intestinal epithelial cells, which transfer it to the serosal side of the gut. The stability of the phospholipids vesicle in gastrointestinal environment can increase the potential of the incorporated drugs. The results of both in vitro and in vivo studies have shown that there may be the possibility of enhancing the uptake process to deliver a range of drugs by the oral route.
CONCLUSION
In this study, a polyherbal antidiabetic formulation with three drugs (components of marketed polyherbal formulation with fifteen drugs) was developed by optimization on the basis of OGTT study in normal Wistar rats. The developed formulation, HA (1000 mg/kg) was further evaluated for its antidiabetic activity in STZ-induced diabetic rat models and found therapeutically at par with a marketed polyherbal formulation, D-fit (1000 mg/kg) and with standard drug metformin (500 mg/kg). Furthermore, HA was encapsulated in the phospholipids-based vesicle system. On antidiabetic evaluation in STZ-induced diabetic rat model, it was observed that its therapeutic efficacy was improved two times; hence, dose was reduced by 50%. Hence, concept of our hypotheses of both the synergism of the selected drugs and encapsulation of the herbal extracts are scientifically validated that, incorporation of more numbers of herbs in the formulation can be minimized by selecting the drugs, produce their action by targeting the different sites/receptors. Furthermore, the encapsulation of conventional extracts/dosage form in vesicular system can potentiate the efficacy of the herbal formulation/products.
ACKNOWLEDGMENTS
The authors are grateful to AICTE, New Delhi for financial support through Research Promotion Scheme (Vide No. 8023/BOR/RID/RPS-136/2009-10). The author(s) also acknowledge Shri Praveen Garg for providing the institutional and instrumental facilities to carry out this research project.
Footnotes
Source of Support: AICTE, New Delhi for financial supporta through Research Promotion Scheme (Vide No. 8023/BOR/RID/RPS-136/2009-10).
Conflict of Interest: Nil.
REFERENCES
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes care. 2010;33:S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eurich DT, McAlister FA, Blackburn DF, Majumdar SR, Tsuyuki RT, Varney J, et al. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: Systematic review. BMJ. 2007;335:497. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilburt JC, Kaptchuk TJ. Herbal medicine research and global health: An ethical analysis. Bull World Health Org. 2008;86:594–9. doi: 10.2471/BLT.07.042820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao V. Modern approaches to herbal medicine. East Pharm. 2000;12:35–8. [Google Scholar]
- 5.Kennedy J, Cabral J. Chichester: Wiley; 1992. Recovery processes for biological materials. [Google Scholar]
- 6.Kumar BD, Mitra A, Manjunatha M. In vitro and in vivo studies of antidiabetic Indian medicinal plants: A review. J Herbal Med Toxicol. 2009;3:9–14. [Google Scholar]
- 7.Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam TP. Indian herbs and herbal drugs used for the treatment of diabetes. J Clin Biochem Nutr. 2007;40:163–73. doi: 10.3164/jcbn.40.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller AC, Ma J, Kavalier A, He K, Brillantes AM, Kennelly EJ. Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine. 2011;19:32–7. doi: 10.1016/j.phymed.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan MJ, Ye JM, Turner N, Hohnen-Behrens C, Ke CQ, Tang CP, et al. Antidiabetic activities of tri-terpenoids isolated from bitter melon associated with activation of the AMPK pathway. Chem Biol. 2008;15:263–73. doi: 10.1016/j.chembiol.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Shih CC, Lin CH, Lin WL, Wu JB. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol. 2009;123:82–90. doi: 10.1016/j.jep.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 11.Hamza N, Berke B, Cheze C, Le Garrec R, Umar A, Agli AN, et al. Preventive and curative effect of Trigonella foenum-graecum L. seeds in C57BL/6J models of type 2 diabetes induced by high-fat diet. J Ethnopharmacol. 2012;142:516–22. doi: 10.1016/j.jep.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Belguith-Hadriche O, Bouaziz M, Jamoussi K, El Feki A, Sayadi S, Makni-Ayedi F. Lipid-lowering and antioxidant effects of an ethyl acetate extract of fenugreek seeds in high-cholesterol-fed rats. J Agric Food Chem. 2010;58:2116–22. doi: 10.1021/jf903186w. [DOI] [PubMed] [Google Scholar]
- 13.Sharma R, Sarkar A, Hazara D, Mishra B, Singh J, Sharma S, et al. Use of Fenuqreek seed powder in the management of non-insulin dependent diabetes mellitus. Nutr Res. 1996;16:1331–9. [Google Scholar]
- 14.Andallu B, Radhika B. Hypoglycemic, diuretic and hypocholesterolemic effect of winter cherry (Withania somnifera, Dunal) root. Indian J Exp Biol. 2000;38:607–9. [PubMed] [Google Scholar]
- 15.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: Food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 16.Barzaghi N, Crema F, Gatti G, Pifferi G, Perucca E. Pharmacokinetic studies on IdB 1016, a silybin-phosphatidylcholine complex, in healthy human subjects. Eur J Drug Metab Pharmacokinet. 1990;15:333–8. doi: 10.1007/BF03190223. [DOI] [PubMed] [Google Scholar]
- 17.Bombardelli E, Spelta M, Della Loggia R, Sosa S, Tubaro A. Aging Skin: Protective effect of silymarin-PHYTOSOME. Fitoterapia. 1991;62:115–22. [Google Scholar]
- 18.Baron AD. Postprandial hyperglycaemia and α-glucosidase inhibitors. Diab Res Clin Pract. 1998;40:S51–5. doi: 10.1016/s0168-8227(98)00043-6. [DOI] [PubMed] [Google Scholar]
- 19.Botham P. Acute systemic toxicity-prospects for tiered testing strategies. Toxicol In Vitro. 2004;18:227–30. doi: 10.1016/s0887-2333(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 20.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth Enzymol. 1999;299:152–78. [Google Scholar]
- 21.Woisky RG, Salatino A. Analysis of propolis: Some parameters and procedures for chemical quality control. J Apic Res. 1998;37:99–105. [Google Scholar]
- 22.Niu M, Lu Y, Hovgaard L, Wu W. Liposomes containing glycocholate as potential oral insulin delivery systems: Preparation, in vitro characterization, and improved protection against enzymatic degradation. Int J Nanomed. 2011;6:1155–66. doi: 10.2147/IJN.S19917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roeschlau P, Bernt E, Gruber W. Enzymatic determination of total cholesterol in serum. J Clin Chem Biochem. 1974;12:403–7. [PubMed] [Google Scholar]
- 24.Rajasekaran S, Ravi K, Sivagnanam K, Subramanian S. Beneficial effects of Aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Phys. 2006;33:232–7. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 25.Kits Van Heijningen AJ, Kemp A. Free and fixed glycogen in rat muscle. Biochem J. 1955;59:487–91. doi: 10.1042/bj0590487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okaha H. Assay for lipid peroxide in animal tissue by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Kunchandy E, Rao M. Oxygen radical scavenging activity of curcumin. Int J Pharm. 1990;58:237–40. [Google Scholar]
- 28.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 29.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 30.Shibib BA, Khan LA, Rahman R. Hypoglycaemic activity of Coccinia indica and Momordica charantia in diabetic rats: Depression of the hepatic gluconeogenic enzymes glucose-6-phosphatase and fructose-1, 6-bisphosphatase and elevation of both liver and red-cell shunt enzyme glucose-6-phosphate dehydrogenase. Biochem J. 1993;292:267–70. doi: 10.1042/bj2920267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madar Z. Fenugreek (Trigonella foenum-graecum) as a means of reducing postprandial glucose level in diabetic rats [Trigonella foenum-graecum] Nutr Rep Int. 1984;29:1267–73. [Google Scholar]
- 32.Naziroğlu M, Butterworth PJ. Protective effects of moderate exercise with dietary vitamin C and E on blood antioxidative defense mechanism in rats with streptozotocin-induced diabetes. Can J App Phys. 2005;30:172–85. doi: 10.1139/h05-113. [DOI] [PubMed] [Google Scholar]
- 33.Poussier M, Guilloux-Benatier M, Torres M, Heras E, Adrian M. Influence of different maceration techniques and microbial enzymatic activities on wine stilbene content. Am J Enol Vitic. 2003;54:261–6. [Google Scholar]
- 34.Wang H, Ng T. Natural products with hypoglycemic, hypotensive, hypocholesterolemic, antiatherosclerotic and antithrombotic activities. Life Sci. 1999;65:2663–77. doi: 10.1016/s0024-3205(99)00253-2. [DOI] [PubMed] [Google Scholar]
- 35.Pinkney JH, Stehouwer C, Coppack SW, Yudkin JS. Endothelial dysfunction: Cause of the insulin resistance syndrome. Diabetes. 1997;46:S9. doi: 10.2337/diab.46.2.s9. [DOI] [PubMed] [Google Scholar]
- 36.Yadav UC, Moorthy K, Baquer NZ. Combined treatment of sodium orthovanadate and Momordica charantia fruit extract prevents alterations in lipid profile and lipogenic enzymes in alloxan diabetic rats. Mol Cell Biochem. 2005;268:111–20. doi: 10.1007/s11010-005-3703-y. [DOI] [PubMed] [Google Scholar]
- 37.Mandard S, Stienstra R, Escher P, Tan NS, Kim I, Gonzalez FJ, et al. Glycogen synthase 2 is a novel target gene of peroxisome proliferator-activated receptors. Cell Mol life Sci. 2007;64:1145–57. doi: 10.1007/s00018-007-7006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Penckofer S, Schwertz D, Florczak K. Oxidative stress and cardiovascular disease in type 2 diabetes: The role of antioxidants and pro-oxidants. J Cardiovas Nurs. 2002;16:68–85. doi: 10.1097/00005082-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Rudich A, Tirosh A, Potashnik R, Hemi R, Kanety H, Bashan N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes. 1998;47:1562–9. doi: 10.2337/diabetes.47.10.1562. [DOI] [PubMed] [Google Scholar]
- 40.Delerive P, Fruchart J, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453–9. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- 41.Rang H, Dale M, Ritter J, Moore P. UK: Churchill Livingstone; 1999. Textbook of pharmacology; pp. 199–242. [Google Scholar]
- 42.Ziv E, Bendayan M. Intestinal absorption of peptides through the enterocytes. Micro Res Tech. 2000;49:346–52. doi: 10.1002/(SICI)1097-0029(20000515)49:4<346::AID-JEMT3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]


