Abstract
Drug information centre refer to facility specially set aside for, and specializing in the provision of drug information and related issues. The purpose of drug information center is to provide authentic individualized, accurate, relevant and unbiased drug information to the consumers and healthcare professionals regarding medication related inquiries to the nation for health care and drug safety aspects by answering their call regarding the all critical problems on drug information, their uses and their side effects. Apart from that the center also provides in-depth, impartial source of crucial drug information to meet the needs of the practicing physicians, pharmacists and other health care professionals to safeguard the health, financial and legal interests of the patient and to broaden the pharmacist role visible in the society and community. The service should include collecting, reviewing, evaluating, indexing and distributing information on drugs to health workers. Drug and poisons information centers are best established within major teaching hospitals. This allows access to clinical experience, libraries, research facilities and educational activities. Information present in the current paper will not only enlighten the role of drug information center but also focused on the rational use of drug.
Keywords: Drug information center, drug information services, health care, illegal drug information center, Role of WHO
INTRODUCTION
Drug information is the provision of written and/or verbal information about drugs and drug therapy in response to a request from other healthcare providing organizations, committees, patients, and public community. Drug information services refer to the activities undertaken by pharmacists in providing information to drug use. Drug information center provides in-depth, unbiased source of crucial drug information to meet needs of the practicing physicians, pharmacists and other health care professionals. In the country like India the national policies are industry focused rather than health focused so, it became crucial to enlighten the role of drug information center to spread the awareness about drug information services and rational use of drugs.[1]
Poor drug regulations and lack of independent, unbiased drug information's are the main contributing reasons for irrational drug use in India. About 40% of the health care service's budget is consumed by medicines and with a limited resource available, it becomes essential to promote rational drug use.[2]
Access to authoritative and independent information is fundamental for the rational and effective use of drugs. Information must be available in a format suitable for health practitioners and relevant to current clinical practice.[3] WHO recognizes independent drug information centers as a core component of national programs to promote the rational use of drugs.[2,4]
Hospital based DIC: The hospital-based DIC perform various activities which include answering the in-house call, assist in formulary decision, participate in drug use evaluation, coordinate adverse drug reaction reporting, publishing newsletter, provide in service education, assist in Pharmacy and Therapeutic committee (P and T) committee, oversee investigational drug activity.[5]
Industry based DIC
Community based DIC: Drug statistics: Drug sales (1 million Swedish Kroner =0.1 million Euros) by ACT class for 1st. quarter 1995–2000 in Stockholm. This function is updated monthly at the website: http://www.janusinfo.org
Main Objectives
The objectives of DIC are:
To provide an organized database of specialized information on medicines and therapeutics to meet the drug information needs of practitioners.
To educate pharmacy students to serve as effective providers of medicines information.
To provide accurate and unbiased medicines information service to the pharmacists, physicians and other health care professionals in the hospital and community.
To promote patient care through rational use of medicines.[6]
Requirements for Establishing Drug Information Center
Organization and Space[7]: The various parameters are considered while determining the requirements of space and organization. These factors include- Type of activities offered, Space available, Budget, Staff, Resources[8]
Resource[9]: The can be classified based on the era in which they emerged[10]
Dic – Resources: Pre-Computer Era
Resources can be categorized into[11]
Primary sources[14] : These are the foundation on which all other drug information is based. These include journal publications on drug-related subjects, such as reports of clinical drug trials, case reports, and pharmacological research. Evaluating primary literature is difficult [Table 1].[15] The most reliable evidence comes from reports on randomized controlled trials. Proper evaluation of these trials requires considerable experience, and systematic reviews of combined trials (meta-analyses) may be necessary. This work is being undertaken by the Cochrane Collaboration.[16]
-
Secondary sources: Function as a guide to or review of the primary literature. Secondary sources include review articles, meta-analyses, indexes (Index Medicus), abstracts (International Pharmaceutical Abstracts), and combinations of abstracts and full-text reprints.
Examples of such services include Medline, Current Contents, International Pharmaceutical Abstracts, Index Medicus, Excerpta Medica, and the Iowa Drug Information Service (which also includes full-text reprints of articles).
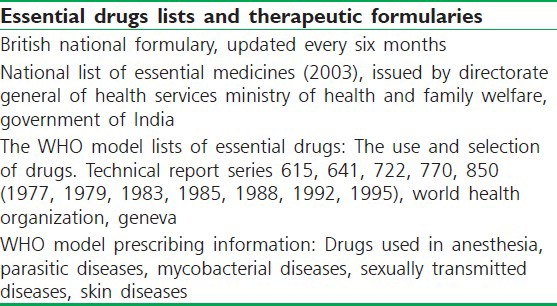
Tertiary or general sources: Present documented information in a condensed format. Examples include formulary manuals, standard treatment manuals, textbooks, general reference books, drug bulletins, and drug compendia [Table 2].[17]
Table 1.
Primary information
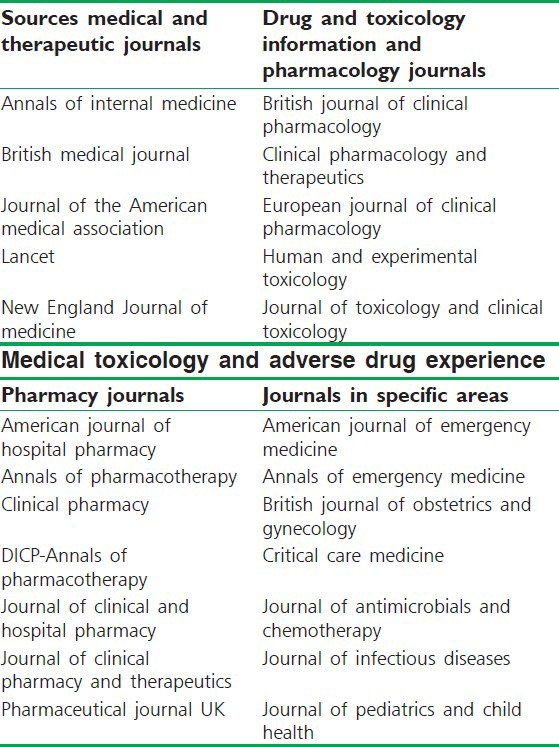
Table 2.
List of most respected tertiary resources
-
3.
Personnel: The number of personnel required depend on the range of activities offered and the hours of service. A center aim is to provide a direct service during periods of major demand by its clients. For patient-related enquiries this is likely to be when clinic consultations occur and during peak periods for hospital functions.[18]
-
4.
Policy and Procedures (P and P): Policy means general outlines (framework) and Procedures means detailed outlines (how to). Both help in smoothing the operation of the DIC. P and P development depends on type of the DIC and scope of service. It is subdivided into Administrative and professional guides.[19]
GLOBAL SCENARIO
In 1962, the first drug information center was opened at the University of Kentucky Medical Center and was intended to be utilized as a source of selected, comprehensive drug information for staff physicians, which allowed them to evaluate and compare drugs besides catering to the information needed for nursing staff. The staffs of the drug information center were expected to take an active role in the education of health professionals within the institution. In 1973, the first formal survey identified 54 drug information centers in the USA. According to a report published in 1995, there are about 120 full-fledged pharmacist-operated drugs information centers in the United States, which accept a broad scope of request from health care professionals.[1]
Role of WHO in Drug Information Center
WHO was first alerted to the circulation of counterfeit pharmaceuticals at the Conference of Experts on the Rational Use of Drugs held in Nairobi, in 1985. The most important resolution was adopted in May 1988 (1) and requested WHO “to initiate program for the prevention and detection of the export, import and smuggling of falsely labeled, spurious, counterfeited or substandard pharmaceutical preparations and to cooperate with the Secretary-General of the United Nations in such cases when the provisions of international drug treaties are violated.[20,21,22,23,24,25]
WHO has developed a network of technically competent officials within national drug regulatory authorities with a view to ensuring timely exchange of information, both on cases of counterfeiting and on any countermeasures taken at national level by means of drug information centers worldwide.
Regional Drug and Therapeutics Center
The Regional Drug and Therapeutics Center (RDTC) is responsible for a range of issues relating to medicines use and drug safety. These include monitoring and advising on prescribing and medicines use in primary and secondary care, and the provision of quality assurance for medicines to stakeholder organizations in the North of England.
The RDTC aims to promote the safe, effective and economical use of medicines to the National Health Service in the North of England; promoting the highest quality of care for people exposed to the toxic effects of drugs or chemicals; and disseminating and developing knowledge in these areas through teaching and research. The center was established in 1991 as collaboration between the University and the former Northern Regional Health Authority. With changing regional NHS boundaries, it increased its activities to cover the then Northern and Yorkshire Region in 1994. Following the abolition of Regional Health Authorities in 1996 the Center has been managed as a partnership with stakeholder Primary Care Organizations and other stakeholders throughout the North of England. The Center is housed on the University of Newcastle campus and is hosted by the Newcastle upon Tyne Hospitals NHS Trust, which is responsible for employing most of the staff. It is staffed by a multi-disciplinary team comprising pharmacists, physicians, medical information scientists and support staff.
INDIAN SCENARIO
Recognizing the need to provide organized drug information to health care professionals as well as consumers, the WHO India Country Office in collaboration with the Karnataka State Pharmacy Council (KSPC) is supporting the establishment of 5 drug information centers. These centers have been established in Haryana (Sirsa), Chhattisgarh (Raipur), Rajasthan (Jaipur), Assam (Dibrugarh), and Goa (Panaji) [Figure 1].
Figure 1.
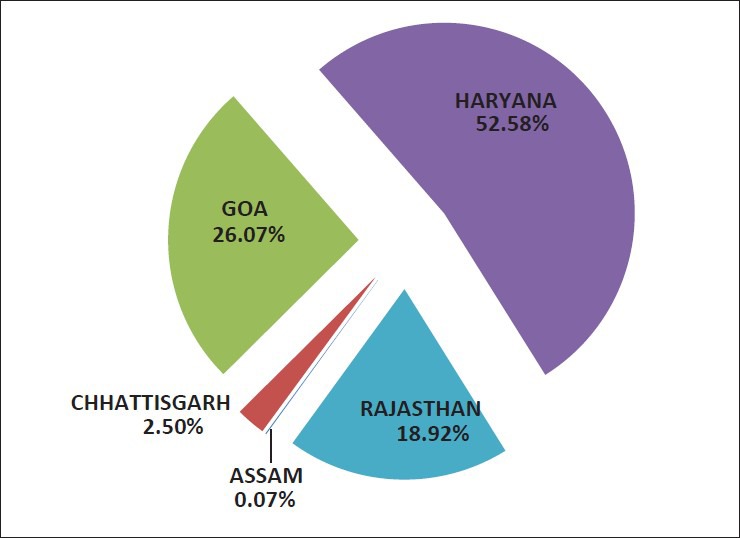
Queries received by centers established by WHO in India
The Karnataka State Pharmacy Council established its Drug Information Center (DIC) in August 1997 to disseminate unbiased drug information to healthcare professionals. In India, this was the first independent DICstarted. The center is registered with IRDIS, an International Register of Drug Information Services.[26]
List of Drug Information Centers Run at State Pharmacy Councils in India (Regional)
Drug Information Center, Maharashtra State Pharmacy Council, Maharashtra
Andhra Pradesh state pharmacy council, Andhra Pradesh
Drug information center, Jaipur, Rajasthan
Drug information center, Raipur, Chhattisgarh
Karnataka state pharmacy council (Kspc), Bangalore, Karnataka
DIC's Set Up By Karnataka State Pharmacy Council, CDSCO, and Who India Country Office
Drug information center, Jaipur, Rajasthan
Drug information center, Raipur, Chhattisgarh
Drug information center, Panaji, Goa
Drug information center, Dibrugarh, Assam
Other Drug, Poison or Alcohol Information Centers in India
Alcohol and drug information center (ADIC), Trivandrum, Kerala
Bowring and Lady Curzon hospital, Bangalore
Bulletin on drug and health information (BIDI), Kolkata
CDMU documentation center, Calcutta
Christian medical college hospital Vellore, Tamilnadu
Department of pharmacy practice, Chidambaram, Tamilnadu
Department of pharmacy practice, national institute of pharmaceutical education and research (NIPER), Chandigarh
Drug information center, division of pharmacy practice department of pharmacy, Annamalai University
Drug information center, (KSPC), Bowring and Lady Curzon hospital, Bangalore, Karnataka
Drug information center, (KSPC), Victoria hospital, Bangalore, Karnataka
Jawaharlal Nehru medical college hospital (JNMC), Belgaum, Karnataka
JIPMER drug information center, department of pharmacology JIPMER hospital Gorimedu, Pondicherry - 605 006
JSS, Mysore, Karnataka
JSS, Ooty
Kasturba medical college (KMC), Manipal, Karnataka
Kempagowda institute of medical sciences (KIMS), Bangalore, Karnataka
N.R.S. medical college and hospital, Calcutta
National poisons information center, all India institute of medical sciences, Ansari nagar, New Delhi
Pharma information center, Chennai, Tamilnadu
Poison control center and department of analytical toxicology, amrita institute of medical sciences and research, Cochin
Poison control, training and research center, government general hospital Chennai
Poison information center, national institute of occupational health, Ahmadabad
RIPER poison and drug information center, RDT Hospital, Bathalapalli, A. P.
Sri Ramachandra Hospital, Porur, Chennai
Sri Ramakrishna mission hospital, Coimbatore, Tamilnadu
Toxicology and IMCU unit, government general hospital, Chennai
Trivandrum medical college, Trivandrum, Kerala
Central Drug Research Institute
R and D activities in CDRI are supported by a modern knowledge Resource Center which comprises a fully computerized library with a rich collection of relevant books and periodicals, on-line subscription of a large number of databases and periodicals and an Information Centerwhich provides many services as well as on-line response to queries. The Center publishes value added Current Awareness Periodicals, besides providing services to the In House Scientific and Technical staff as well as to academia and industry from within the country and even to overseas users [Table 3].
Table 3.
CDRI Library has received following recognitions from national and international organizations

The inception of this modern Center dates back to 1951 when a small library was set up with a small collection of about two thousand Publications to start with the Library was set up to primarily to meet the information needs of the Scientific and Technical Staff of the Institute. It systematically and gradually grew as a collection of specialized reading materials such as books, periodicals, reference works, serials and various macro and micro-documents in the areas of Biomedical Research and Drugs and Pharmaceuticals.
National Information System for Science and Technology (NISSAT), decided to house National Information Center for Drugs and Pharmaceuticals (NICDAP), a Sectoral Information Center on Drugs and Pharmaceuticals in the CDRI library. NICDAP came into being in 1977 with metamorphosis of this library into a National Information Center in the early eighties and wide spectrums of Information Technologies were introduced in the library.
Recognitions received by CDRI are as follows:
Indian Drug Center and Clinical Pharmacy Department
There is several drug information centers exist in the country,[27] many of them are attached to the pharmacy colleges or hospitals, where clinical pharmacy programs exist or they offer. There are few independent drug information centers not attached to health facilities but often associated with state pharmacy councils.
Some of the drug information centers are:
Drug Information Center, Karnataka State Pharmacy Council, Bangalore
Drug Information Center, Maharashtra State Pharmacy Council, Mumbai
Pharma Information Center at Chennai; Drug Information Center at National Institutes of Pharmaceutical Education and Research, Mohali, JSS College of Pharmacy, Mysore; JSS College of Pharmacy, Ooty
Ramakrishna College of Pharmacy, Coimbatore
RMMC Hospital, Annamalai University, Annamalai Nagar; Al-Ameen College of Pharmacy, Bangalore
College of Pharmaceutical Sciences, Manipal
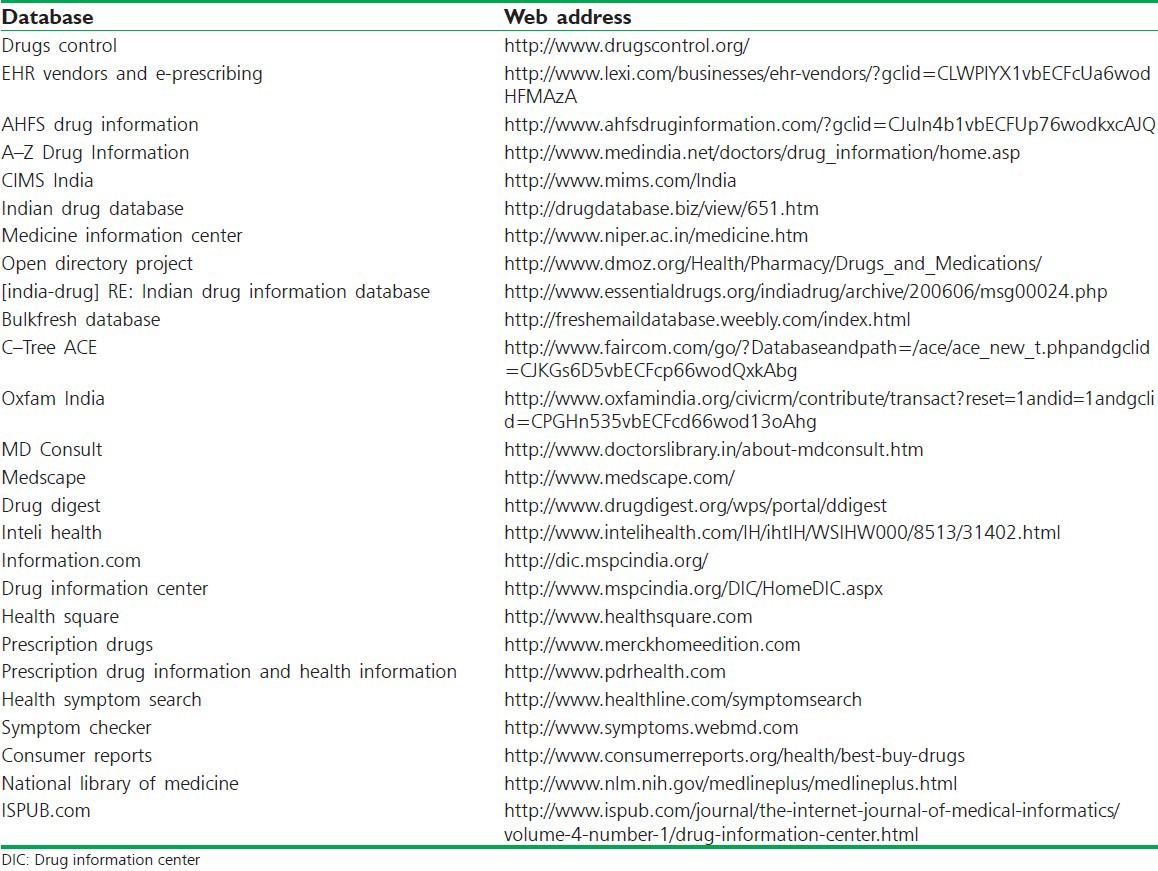
Delhi Institute of Pharmaceutical Education and Research, New Delhi. Most of these centers are located in southern art of the country [Table 4].
Table 4.
List of some websites working as databases in India
There is a need to have some centers in eastern and northern part of the country.
METHODOLOGY
The following methodology was used to implement the project:
Selection of five states for the establishing drug information center.
Preliminary discussion with the identified hospitals/pharmacy councils in selected states.
Training of staffs that are intended to manage the DICs on provision of Drug information.
Establishing and functioning of centers.
Documenting the process of establishing the centers with difficulties faced and lesson learnt.
CDSCO India Central Drugs Standard Control Organization
The specific aims of the Program run under CDSCO are to:
Contribute to the regulatory assessment of benefit, harm, effectiveness and risk of medicines, encouraging their safe, rational and more effective (including cost effective) use
Improve patient care and safety in relation to use of medicines and all medical and paramedical interventions
Improve public health and safety in relation to use of medicines promote effective communication to the public.
World Wide Web Sites
For even more pharmacology and toxicology (http://hsc.virginia.edu/hslibrary info_serv/erd/genref/pharmacology.html) Web sites - check out the Library's Electronic Reference Desk! (http://www.med.virginia.edu/hs-library/info_serv/erd/)[28]
AIDS Clinical Trials Information Service
Federally and privately funded HIV/AIDS clinical trials information from the US Department of Health and Human Services.
Center Watch Drug Directories
(http://www.centerwatch.com/patient/drugs/drugdirectories.html)
This source calls itself “The information source for the clinical trials industry.” The following three drug directories from this site are extremely helpful.
Drugs Approved by the FDA
(http://www.centerwatch.com/patient/drugs/druglist.html)
This section can be searched by year and a medical specialty, such as Cardiology.
Drugs Currently in Clinical Research
(http://www.centerwatch.com/patient/cwpipeline/default.asp)
According to the company, this site contains: “A listing of 2,000 active drugs in Phase I - Phase III trials are profiled sometimes including NDA submissions and withdrawals. Each summary contains information about the new treatment: the branded and generic name of the new therapy, the company sponsoring the research, and the phase of the clinical trials.” It can be searched by medical condition.
Clinical Trials Results Database
(http://www.centerwatch.com/patient/results/default.asp)
The information in this section dates back to May, 2000 and is updated weekly. It is pulled from published materials from medical conferences, journals, and company reports. The clinical trial result summaries typically include the name and phase of the new therapy, the company sponsoring the research, a description of the trial design, and information on how well the drug has performed.
Clinical Trials.Gov
ClinicalTrials.gov provides patients, family members, health care professionals, and members of the public easy access to information on clinical trials for a wide range of diseases and conditions brought to you through a collaborative effort of NIH, NLM, and the FDA.
Drugtopics.Com
(http://www.drugtopics.com/be_core/d/index.jsp)
The online news magazine for pharmacists.
FDA - Food And Drug Administration - Main Site With News and More
This site includes news, product reports, and safety alerts for all FDA-regulated products.
FDA/Center for Drug Evaluation and Research
According to their Web site, Center for Drug Evaluation and Research (CDER) serves as a consumer watchdog by evaluating new drugs before they can be sold. Their goal is to ensure that prescription and over-the-counter drugs (brand name and generic) work correctly and that their health benefits outweigh the known risks. Three sections of special interest on the site are:
New and Generic Drug Approvals
(http://www.fda.gov/cder/approval/index.htm)
This is an alphabetical listing of all prescription drugs approved during 1998 through 2001. It is updated on a daily basis and contains links to labels, approval letters, and reviews.
FDA Drug Approvals List
(http://www.fda.gov/cder/da/da.htm)
This is a reverse chronological listing of all drugs approved since September 1996. It is updated on a weekly basis.
Electronic Orange Book - Approved Drug Products With Therapeutic Equivalence Evaluations
(http://www.fda.gov/cder/ob/default.htm)
Allows searching by Active Ingredient, Applicant Holder, Proprietary Name, and Application Number.
FDA Enforcement Report Index
(http://www.fda.gov/opacom/Enforce.html)
A weekly report that contains information on actions taken in connection with agency regulatory activities.
FDA – Med Watch
(http://www.fda.gov/medwatch/safety)
The FDA safety information and adverse-event reporting program.
The Institute for Safe Medication Practices
A nonprofit organization that works closely with health care practitioners and institutions, regulatory agencies, professional organizations, and the pharmaceutical industry to provide education about adverse-drug events and their prevention. The Institute provides an independent review of medication errors that have been voluntarily submitted by practitioners to a national Medication Errors Reporting Program (MERP) operated by the United States Pharmacopeia (USP) in the USA.
Mosby's Genrx Top 200 Most Prescribed Drugs (Ranked by Sales Volume)
(http://www.genrx.com/genrxfree/Top_200_2000/Top_200_2000.html)
Includes information about each drug listed.
NIH Clinical Alerts and Advisories
(http://www.nlm.nih.gov/databases/alerts/clinical_alerts.html)
Clinical alerts are provided to expedite the release of findings from the NIH funded clinical trials where such release could significantly affect morbidity and mortality.
Electronic Journals in Clinical Pharmacology and Toxicology:
*Complete List of Electronic Journals at the Uva Health Sciences Library
(http://hslnet.med.virginia.edu/ejrnl.html).
Indian electronic databases The databases used in India are thoroughly revised and very much reliable. These sites are strictly under control of government regulations. Some examples are listed below:
Drug Information Centers Established Under WHO in India
WHO has played an important role in establishing DICs in India. It initiated in following five states:[29]
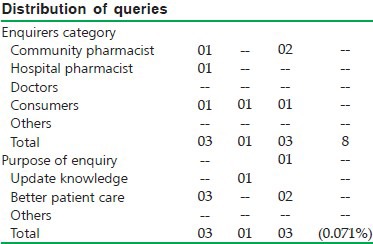
ASSAM: The Assam drug information center started functioning from January 2007 and was formally inaugurated on 23rd March 2007. It has conducted promotional programs to create awareness on drug information service at Assam Medical College where the community pharmacists and doctors participated. The center has also organized two days continuing education programs for community pharmacists where more than 100 pharmacists participated [Figure 2].
Figure 2.
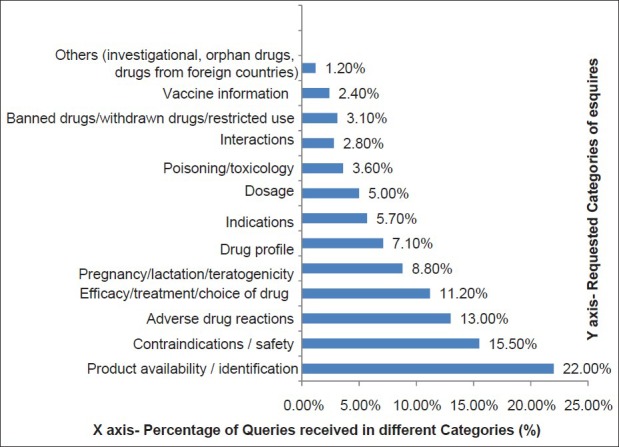
Statistical data of queries received in different categories
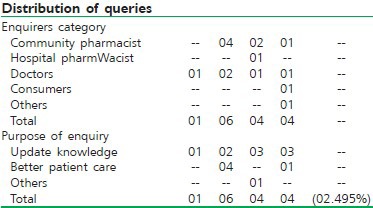
Chhattisgarh: Chhattisgarh drug information center started functioning from January 2007 and was formally inaugurated on 9th April 2007. The center has initiated several steps to inform all concerns of the establishment of the center. The center has given presentation on the objective and functioning of the DIC in Chief District Medical Officers meet and IMA meet. It has also published apromotional article in IMA's Newsletter.
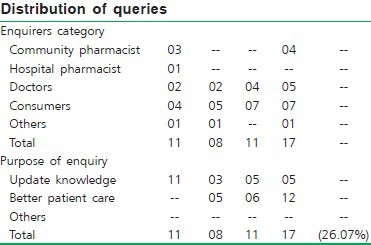
Goa: The drug information center, Goa, started functioning since January 2007 and was formally inaugurated on 25th March 2007. Promotional advertisement was published in bulletin of Chemist and Druggist Association, and distributed to pharmacists of Goa Dental College, Goa9 Medical and Homeopathic Colleges. Letters informing about the existence and the functioning of the DIC had been sent to Indian Pharmaceutical Association President, Chemist and Druggist Association, President - Goa Pharmacy council. E-mails were sent to doctors about the utilization of drug information services.
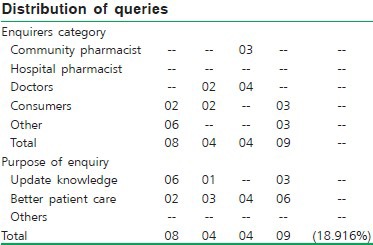
Rajasthan: The Rajasthan Drug Information Center (sponsored by Rajasthan Pharmacy Council) at LBS College of Pharmacy, Jaipur, has started functioning since January 2007 and was formally inaugurated on 25th March 2007. It has initiated several programs to inform the stake holders that the DIC is established. The DIC team has communicated to the ethics committee of the SMS Medical College, and made presentations in other hospitals. The continuing education programs were organized at divisional level in association with state pharmacy council.
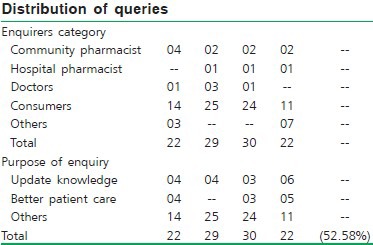
Haryana: The Haryana Drug Information Center started functioning from January 2007 and was formally inaugurated on 6th April 2007. A write up was published in local Hindi daily. The pamphlets and other promotional materials were distributed informing the establishment of drug information center. A presentation was made to gathering of doctors and pharmacists at government hospital, Sirsa.
Drug Information Centers of Other States
Gujarat: The INFLIBNET Library plays a vital role in the collection development and dissemination of scientific and technical information to meet the present and future needs of the Center. The Library maintains databases of
participants who attended various training programs at INFLIBNET Center;
SOUL installations; and
universities and its contact details including e-mail and website addresses. These databases are accessible through the library website at http://www.inflibnet.ac.in/universitydirectory.
The CD-ROM databases available at the INFLIBNET Library has EMBASE Drugs and Pharmacology CD (1986-2003).
Tamil Nadu: The first Drug Information and Pharmaco - Vigilance Center, which was set up last year on the campus of the Directorate of Medical Services campus is a joint venture between the Directorate of Drugs Control and the Tamil Nadu Pharmacy Council. The center had received many calls from patients who had questions about drugs prescribed for them. Self-medication and evasive use of drugs were major issues in drug abuse. There are over 600 drug manufacturing units in Tamil Nadu producing drugs worth Rs. 500 crore. There are a total of 40,000 drug stores in the State.
Maharashtra: Recognizing the need to provide organized drug information to health care professionals as well as consumer Maharashtra State Pharmacy Council started Drug Information Center in September 2003. This center provides in-depth, unbiased crucial drug information to practicing physicians, pharmacists and patients in India. The center is committed to provide information on following areas:
Drug or its Product Information/Identification
Contraindications/Safety
Adverse Drug Reactions/Drug Interactions
Efficacy/Treatment/Choice of drug
Pregnancy/Lactation/Pediatrics
Drug Profile/Indications/Dosage
Toxicology
Counseling information to pharmacist
Illegal Drug Information Center
Illegal drug information centers report the abused drugs for victimizing some innocent. The Illinois State Police has started an information center to report such cases of drug abuse and spread awareness in both public and US officials to ban such drugs over the counter selling. It is governed by DEA Drug Enforcement Administration.[30]
Date Rape Drugs
The use of “date rape drugs” to facilitate sexual assault is increasingly being reported in some parts of the United States—Illinois included. In most cases, the method of operation is the same—the illicit substance is added to the drink of an intended victim without the person's knowledge. In some cases, eye droppers containing the liquid have been used for ease in administration.[31]
GHB (Gamma Hydroxybutyrate) Available by prescription in Europe and North Africa, GHB was originally conceived as a preoperative anesthetic and a sleep aid. The Food and Drug Administration in 1990 ruled the drug had no legal use. It has since been banned by the FDA and is illegal in the United States. This had been misused to involuntarily sedate individuals for sexual assault. Currently, GHB is showing up on Illinois college campuses, in dance clubs, and the “rave” scene.
Ketamine
Ketamine, along with GHB, is another of the “club” or “rave” drugs being used recreationally among many young people. Nicknamed “Special K” or “cat,” Ketamine is reportedly accessible at about any college in the country. Aside from recreational drug abuse, this Schedule III substance is being used for the purpose of rendering victims unable to resist sexual assault.
Rohypnol
Rohypnol is considered the original date rape drug and is currently termed the “date-rape drug-of-choice.” Offenders are using the drug on individuals and raping them while they sleep.
Importation has been banned as well. Despite these facts, it has been reported most often in Texas and Florida and recently in Connecticut, Rhode Island, and Illinois. Rohypnol is relatively inexpensive and can be purchased illegally for $2 to $3 per pill.
Post-Market Drug Safety Information for Patients and Providers
In 2005 the FDA proposed creating the Drug Watch Web Page, a new program that would communicate “the most up-to-date information possible on emerging [drug] safety issues to the public, even before FDA.[32] decides whether a regulatory action is appropriate.” Includes guidance from the FDA, drug specific information for health professionals and consumers, and related information. From the U.S. Food and Drug Administration (FDA), Center for DrugEvaluation and Research (CDER).
Conclusion
Drug Information Centers are regarded as a gateway of drug information. The future of drug information centers in India lies in the quality of service, credibility among users and the evaluation of its progress. The future of clinical pharmacy and drug information center is very bright so the government, private hospitals and regulatory bodies should come forward to establish more number of DIC in future time so that clinical pharmacist and drug information center can work to locate the quality in community.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Pradhan SC. The Performance of drug information center at the university of Kansas medical center Kansas city, USA-Experiences and Evaluation. Ind J Pharmacol. 2002;34:123–9. [Google Scholar]
- 2.Geneva: WHO Policy Perspectives on Medicines; 2002. Promoting rational use of medicines: Core components. [Google Scholar]
- 3.Godlee F, Pakenham Walsh N, Ncayiyana D, Cohen B, Packer A. Can we achieve health information for all by 2015.? Lancet. 2004;364:295–300. doi: 10.1016/S0140-6736(04)16681-6. [DOI] [PubMed] [Google Scholar]
- 4.Geneva: World Health Organization; 2004. “WHO medicines strategy: Countries at the core”, 2004-2007. [Google Scholar]
- 5.Medicine Information Center, Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research (NIPER), S. A. S. Nagar-160062, Punjab INDIA. [Last accessed date April, 2012]. Available from: http://www.niper.gov.in/medicine.htm .
- 6.Venkatraghavan S, Rama M, Leelavathi DA. Performance of a drug information center in a South Indian teaching hospital. Int J Pharm Tech Research. 2010;2:390–403. [Google Scholar]
- 7.Beena G, Padma GM. Assessment and evaluation of drug information services provided in a South Indian teaching hospital. Ind J Pharmacol. 2005;37:315–8. [Google Scholar]
- 8.Hansen KN, Nahata MC, Parthasarathi G. Drug Information. In: Rajendran SD, editor. A textbook of clinical pharmacy practice, essential concepts and skills. 1st ed. Andhra Pradesh, India: Orient Longman; 2004. pp. 267–86. [Google Scholar]
- 9.Joy ME, Arana CJ, Gallo GR. Use of information sources at a university hospital drug information service. Am J Hosp Pharm. 1986;43:1226–9. [PubMed] [Google Scholar]
- 10.Llerena A, Ohman B, Alvan G. References used in a drug information center. Eur J Clin Pharmacol. 1995;49:87–9. doi: 10.1007/BF00192364. [DOI] [PubMed] [Google Scholar]
- 11.Guide to good prescribing. WHO/DAP/94.11. Geneva: WHO/DAP; 1994. WHO/DAP (World Health Organization/Action Programme on Essential Drugs). 1994b. [Google Scholar]
- 12.Shields KM, Lust E. Drug information resources. In: Malone PM, editor. Drug Information: A Guide for Pharmacists. 3rd ed. New York, NY: McGraw Hill Inc; 2006. p. 61. [Google Scholar]
- 13.University of Minnesota Libraries. Primary, secondary and tertiary sources in the health sciences. [Last accessed on 2010 Oct 1]. Available from: http://www.biomed.lib.umn.edu/inst/sourcesinhs.pdf .
- 14.Nibu P, Ramesh M, Parthasarathi G. Review of a drug information service in an Indian teaching hospital. Aus J Hos Pharm. 2001;2:144–5. [Google Scholar]
- 15.Cuddy PG, Elenbaas RM, Elenbaas JK. Evaluating the medical literature. Part I: Abstract, introduction, methods. Ann Emerg Med. 1983;12:549–55. doi: 10.1016/s0196-0644(83)80296-0. [DOI] [PubMed] [Google Scholar]
- 16.Elenbaas RM, Elenbaas JK, Cuddy PG. Evaluating the medical literature. Part II: Statistical analysis. Ann Emerg Med. 1983;12:610–20. doi: 10.1016/s0196-0644(83)80205-4. [DOI] [PubMed] [Google Scholar]
- 17.Elenbaas JK, Cuddy PG, Elenbaas RM. Evaluating the medical literature. Part III: Results and Discussion. Ann Emerg Med. 1983;12:679–86. doi: 10.1016/s0196-0644(83)80416-8. [DOI] [PubMed] [Google Scholar]
- 18.Roma E, Planells C, Carrera A, Cercos AC, Sanchez S, Gallego C. Quality management in drug information. Eur Hosp Pharm. 1995;1:61–6. [Google Scholar]
- 19.Managing Drug Supply: The Selection, Procurement, Distribution, and Use of Pharmaceuticals. 2nd ed. United States: Kumarian Press; 1997. Drug and therapeutic information; pp. 3–16. (121-36). [Google Scholar]
- 20.World Health Organization. Counterfeit drugs: report of a joint WHO/IFPMA workshop. WHO/DMP/CFD/92. Geneva: WHO; 1992. World Health Assembly resolution WHA 41.16.2. [Google Scholar]
- 21.WHO Technical Report Series, Number 823. Geneva: WHO; 1992. WHO Expert Commitee on Specifications for Pharma-ceutical Preparations, Thirty-second Report. [PubMed] [Google Scholar]
- 22.Communication from the TGA addressed to WHO dated 3 and 25 Mar. Geneva: WHO; 1997. Note from the Therapeutics Goods Administration (TGA) entitled Substitution of Active PharmaceuticalIngredients, distributed during PIC-PIC/S meeting of officials in Geneva in February 1997. [Google Scholar]
- 23.Geneva: WHO; 1997. Letters from the US FDA Office of Criminal Investigations addressed to WHO dated 28 February and 7 April. [Google Scholar]
- 24.Geneva: WHO; 1997. Letter from Medicines Control Agency, United Kingdom to WHO dated 2 June. [Google Scholar]
- 25.Deaths in Haiti renal failure epidemic. Washington D.C: PAHO News Release; 1996. Pan American Health Organization. [Google Scholar]
- 26.Karnataka state pharmacy council. [Last accessed on 2012 Oct 7]. Available from: http://www.kspcdic.com .
- 27.Health Systems Development (HSD) Essential Drugs and Medicines, Drug Information Centes. [Last accessed on 2011 Apr 6]. Available from: http://whoindia.org/en/Section2/Section427_1396.htm .
- 28.Grandage K. United States: Charlottesville; 2002. Drug Information Resources, University of Virginia Health System; pp. 1–10. [Google Scholar]
- 29.Drug Information Center Karnataka State Pharmacy Council, Setting up of Drug Information Centers in selected States in India, June, Bangalore. (8-12).2007:5–6. [Google Scholar]
- 30. [Last accessed on 2012 Oct 7]. Available from: http://www.justice.gov/dea/pubs/abuse/index.htm .
- 31. [Last accessed on 2012 Oct 7]. Available from: http://www.ncjrs.gov/spotlight/club_drugs/summary.html .
- 32. [Last accessed on 2012 Oct 7]. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProvider s/default.htm .


