Abstract
Context:
Previous studies have suggested subtle anatomical brain differences between patients with schizophrenia and healthy control subjects. However, the results are inconsistent and there is no study investigating the various subtypes of this mental disorder separately.
Aim:
This study was conducted to compare the rate of absence of adhesio interthalamica (AI), a midline brain structure, between 3 subtypes of schizophrenia (paranoid, undifferentiated, and residual) and healthy control group, using magnetic resonance imaging (MRI).
Materials and Methods:
A total of 29 schizophrenia patients (21 men, 8 women) of three subtypes (paranoid, undifferentiated, and residual) were compared with 29 age- and gender-matched healthy controls. All subjects underwent 3-D brain MRI of full coronal series, 1.5-mm slices without interslice gaps. If the grey matter band connecting the thalami could not be identified on two or more coronal adjacent slices, the AI was considered as absent. The results were statistically analyzed.
Results:
The incidence rate of AI absence in patients with heterogenous subtypes of schizophrenia was was similar to control group, even when patients and controls of each gender were compared separately (P>0.05). In residual subtype, patients showed a significant priority in AI absence in comparison with the control group (P=0.041), which was not seen in paranoid and undifferentiated subtypes (P>0.05).
Conclusion:
Residual subtype of schizophrenia is associated with higher rate of AI absence in this study. Subsequent studies are required to determine if the absence of AI is a cause of residual schizophrenia or an effect.
Keywords: Adhesio interthalamica, magnetic resonance imaging, schizophrenia
INTRODUCTION
Schizophrenia is a common disabling mental disorder, affecting approximately 1% of the general population, presenting with delusions, hallucinations, disorganized behaviors, and negative symptoms (a deterioration in personal and social performance).[1] Some experts suggest that there is an association between this mental disorder and existence of subtle differences of the brain anatomy.[2] For instance, patients with schizophrenia have larger lateral and third ventricles and smaller thalamic, hippocampal, and superior temporal volumes when compared to control healthy subjects.[3] Among these structural differences, midline and medial abnormalities, such as those related to corpus callosum,[4,5] septum pellucidum,[6,7] and cerebellar vermis[8,9,10,11,12] have been suggested as a substrate of symptoms of schizophrenia, since midline circuits are responsible in mediating attention and information processing.[13] In particular, the thalamus region has been identified with a critical role in pathophysiology of the illness, because of its unique location and connectivity.[14,15,16]
The adhesio interthalamica (AI) is a diencephalic midline structure formed by fusion of medial borders of both thalami, composed of neurons and five commissural fiber systems connecting thalamic nuclei,[17,18,19] which develops in fetal period or after the birth.[20,21,22,23] However, post-mortem and radiological studies have shown that in 15-30% of the cases, this structure never develops, as a normal variety.[24,25] Absence of AI has been found to be more frequent in males than in females,[25,26,27] suggesting an important structural difference in connection of two hemispheres as an underlying factor for gender-related difference of cognitive function.[28] Some evidence also connected the presence and the size of AI structure with age, suggesting the atrophy and even disappearance of AI in elderly.[21] Nevertheless, studies have not found a relationship between development of AI and the size of the thalami[23] or brain weight.[28]
Recently, a few postmortem and MRI studies have evaluated the presence or absence of AI in patients with schizophrenia. Although Erbagci et al.,[29] and Takahashi et al.,[30] reported that absence of AI is more common in patients with schizophrenia, in comparison with healthy controls, Meisenzahl[31,32] and Agrawal[33] refused any difference between them. Others claimed that this difference is limited to certain groups of patients with schizophrenia.[34,35,36]
Unfortunately, these investigations have studied the patients with schizophrenia in a single group. Therefore, this discrepancy of results may be partly due to the heterogenicity of schizophrenia subtypes.[37] In order to address these issues, this study was designed to compare the rate of absent AI in patients suffering from three common clinical subtypes of schizophrenia (paranoid, undifferentiated, and residual) and a gender- and age-matched healthy group using MRI method.
MATERIALS AND METHODS
In this case-control study, 29 patients with schizophrenia (19 males and 10 females) referring to outpatient and inpatient departments of Avicenna educational psychiatry hospital of Mashhad, northeastern Iran during 2010 were selected using purposive sampling method. The diagnosis of schizophrenia was confirmed by two psychiatrists using a semi-structured interview based on DSM-IV criteria.[1] Paranoid, undifferentiated, and residual subtypes of schizophrenia included 10, 10, and 9 patients, respectively. All patients were receiving neuroleptic medications. Exclusion criteria were a history of head injury, neurological disorders, other mental co-morbidities, previous electroconvulsive therapy, and alcohol or drug abuse in the current 12 months.
As a control group, 29 gender-and age- (±5 years) matched healthy volunteers participated and their mental health was confirmed by a psychiatrist using GHQ-28 questionnaire. Subjects with history of head injury, neurological disorders, family history of mental disorders, and alcohol or drug abuse in current 12 months were excluded from the study.
All case and control participants signed an informed consent after being fully informed about the research process and approval by ethics committee of Mashhad University of Medical Sciences. Then, all participants underwent a 3-dimensional brain magnetic resonance imaging (MRI), using the brand named MAGNETOM Symphony Maestro Class with a 1.5-T scanner made by Siemens corporation, Germany. Images were taken in complete coronal planes with a 1.5-mm slices without inter-slice gaps, TR=1.920 ms, TE=3.93 ms, TI=1.100 ms, matrix size=256 × 179, FOV=240 × 240 mm, and scanning time=5 min and 40 s.
Presence or absence of the AI structure in MRI was assessed by a neuroanatomist of our team, who was unaware of the participants’ diagnosis, gender, and other variables. The images were displayed on a computer screen using Osiris® v. 409 software[38] interpolated to × 4 magnification, allowing easy side by side comparisons or a stack flow. When the grey matter band connecting the thalami could be identified, at least on two coronal adjacent slices, the AI was considered as present [Figure 1], otherwise it was considered as absent.
Figure 1.
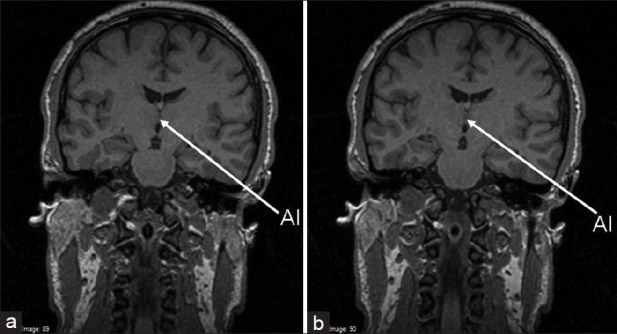
Two coronal adjacent slices showing adhesio interthalamica (AI) in a participant; the AI in such cases was considered as present
Data were analyzed by SPSS software version 15.0 and the rate of absence of AI was compared between two groups using Chi-square and Fisher's exact tests. The correlation between absence of AI with age and duration of schizophrenia was determined by Spearman's test. The level of significance was assumed to be <5%.
RESULTS
The mean age in case (n=29) and control (n=29) groups was 36.4±12.7 and 36.5±12.6 years, respectively. Mean body weight in two groups was 67.6±9.6 and 72.7±9.9 kg, respectively. Male/female proportion in both groups was 19/10. The rate right/left handedness was 27/2 and 23/6, respectively.
The AI structure was absent in 8 patients (27.58%, including 7 males and 1 female) and 4 healthy controls (13.79%, including 3 males and 1 female), but the difference was not statistically significant (Chi-square=0.84, df=1, P=0.11). There were no significant difference between patients and healthy controls regarding the absence of AI, when the comparisons were restricted to either male (Chi-square=2.17, df=1, P=0.35) or female subjects (P=0.53, Fisher's exact test, one-tailed). Also, there was not any difference in the absence of AI between females and males, neither in the case (Chi-square=2.36, df=1, P=0.12) or in the control group (P=0.40, Fisher's exact test, one-tailed). Spearman test showed that absence of AI was not significantly correlated with age, in either patients with schizophrenia (r=1.05, df=27, P=0.30) or healthy controls (r=1.36, df=27, P=0.18). Also, there were no significant correlation between the absence of AI and duration of schizophrenia (r=1.28, df=27, P=0.21).
Patients with paranoid schizophrenia (n=10, M: F=7/3) and their matched control group (n=10, M: F=7/3) were also similar regarding age, body weight, and handedness. One paranoid patient (10%, including one male) and 2 healthy controls (20%, including one male and one female) did not have AI, which was not significantly different (P=0.41, Fisher's exact test, one-tailed). There were no difference between males and females (P=0.7, Fisher's exact test, one-tailed) and also between male patients and male controls (P=0.54, Fisher's exact test, one-tailed) and between female patients and female controls (P=0.57, Fisher's exact test, one-tailed).
Patients with undifferentiated schizophrenia (n=10, M: F=7/3) and their matched control group (n=10, M: F=7/3) were similar considering age, body weight, and handedness. Three patients (30%, including 2 males and 1 female) and 2 healthy controls (20%, including 2 males) had absence of AI, which was not significantly different (P=0.37, Fisher's exact test, one-tailed). There were no difference between males and females (P=0.52, Fisher's exact test, one-tailed) and also between male patients and male controls (P=0.44, Fisher's exact test, one-tailed) and between female patients and female controls (P=0.6, Fisher's exact test, one-tailed).
Patients with residual schizophrenia (n=9, M: F=5/4) and their matched control group (n=9, M: F=5/4) were also similar considering age, body weight, and handedness. Four patients (80%, including 4 males) and none of healthy controls had absence of AI, which was significantly different (P=0.041, Fisher's exact test, one-tailed). The absence of AI was significantly more common in males than in females with residual schizophrenia (P=0.039, Fisher's exact test, one-tailed). Also, there were a significant difference between patients with residual schizophrenia and controls when the comparisons were restricted to males only (P=0.023, Fisher's exact test, one-tailed), but it was insignificant when the comparison was restricted to female subjects (P=0.50, Fisher's exact test, one-tailed).
DISCUSSION
As it was observed, patients with schizophrenia as well as paranoid and undifferentiated subtypes were not different from control group regarding the rate of AI absence. However, it was more common in residual subtype of schizophrenia as compared to healthy controls (P=0.041).
The first study in this field was performed by Snyder et al.[34] Their investigation included a MRI and a post-mortem study. The results of our study among all patients with schizophrenia are supported by post-mortem part of their study. They failed to detect any significant difference between post-mortem patient with schizophrenia and healthy controls. However, interestingly, their MRI data of live patients showed that AI absence is higher in patients with schizophrenia compared to controls. Since they used old MRI device and relative thick slices (3.1 mm), it is possible that they missed some small AIs in MRI, which may be detected by post-mortem technique. Therefore, post-mortem assessment seems to be more accurate than MRI, but, if required, MRI with thin slices is preferred.
The results of the present study is also consistent with the studies conducted by Meisenzahl,[31,32] Nopoulos,[35] and De Souza Crippa,[36] which could not find any significant differences in prevalence of AI absence between patients with schizophrenia and healthy controls. However, our results are not supported by Erbagci's[29] study in which higher prevalence of AI absence has been reported in patients in comparison with healthy controls.
Some researchers stated that this difference with healthy people is limited to certain groups of patients with schizophrenia. Snyder et al.,[34] confined this structural difference only to patients with first episode of schizophrenia and excluded the chronic patients. In contrast, Takahashi et al.,[39] reported that the absence of the AI was more common in patients with chronic schizophrenia, when compared to first-episode psychosis and Meisenzahl[31,32] and Takahashi[39] stated that the absence of AI may be associated with more severe negative symptoms (that are mostly observed in chronic patients). They claimed that patients with schizophrenia may manifest progressive brain changes, including atrophy of AI following the onset. However, this was inconsistent with our results, which showed that AI absence is not related to duration of illness.
Some others detected a gender exception in this pattern. Nopoulos et al.,[35] found that female patients with schizophrenia had a significantly higher prevalence of absent AI compared to female controls, but this difference was not present in males. In the present study, the difference between patients and controls was absent, even when the comparison was limited to either females or males. But in the residual subgroup, male patients had a higher rate of absent AI as compared to healthy males; this pattern was absent in females.
The present results was also consistent with the prior studies conducted by Erbagci[29] and Shimizu,[40] that did not show a difference between male and female patients. However, De Souza Crippa et al.,[36] found that absence of AI is more prevalent in male patients with schizophrenia than in female patients. This result was similar to our study on residual subtype of schizophrenia; males had a higher rate of absent AI than females.
In healthy controls, also, there was no differences between males and females in our study, as well as the studies performed by Erbagci[29] and De Souza Crippa.[36] However, Nopoulos[35] and Shimizu[40] found that AI absence was more common in healthy males than in healthy females. This inconsistency may be due to differences in sample size, which was much larger in Nopoulos and Shimizu's studies,[35,40] in comparison with Erbagci's,[29] De Souza Crippa's,[36] and ours. We believe that these controversies may be, also, partly due to differences in subtype distribution of the samples, which was not considered in prior studies. More precisely, if some subtypes of schizophrenia have a more constant relationship with AI absence than other subtypes, it can be concluded that studies including the more number of patients of these subtypes report an association between AI absence and schizophrenia, but studies with scant number of patients of these subtypes fail to show the relationship.
The present study was the first one that assessed the rate of absent AI in different subtypes of schizophrenia and showed a higher rate of absent AI in patients with residual schizophrenia. However, it seems consistent with the result of studies performed by Meisenzahl[31,32] and Takahashi,[39] who perceived that patients without AI structure are more prone to have negative symptoms (prominent symptoms of residual subtype of schizophrenia) than patients with AI.
On the other hand, AI has vast connections to the prefrontal cortex,[24,41] and prefrontal abnormalities have been accused for development of negative symptoms (most important symptoms of residual subtype) in patients with schizophrenia.[42,43,44,45] These series of relationships suggest that AI absence may be related to residual subtype of schizophrenia that again supports our results.
CONCLUSION
Although inconsistent with some prior studies, the rate of AI absence in patients with heterogenous subtypes of schizophrenia is not different with control group, even when patients and controls of each gender are compared separately. So, heterogenous subtypes of schizophrenia may have different neuroanatomical characteristics. In residual subtype of schizophrenia, patients showed a significant priority in AI absence in comparison with control group, which was not seen in paranoid and undifferentiated subtypes.
Limitations and suggestions
Small sample size and lack of a structured interview for a confident diagnosis of schizophrenia was the major limitations and should be corrected in subsequent studies. Although we tried to evaluate all patients of all different subtypes of schizophrenia, we could not find an adequate number of patients in disorganized and catatonic subtypes. Subsequent extensive investigations are required to assess this rare subtype and clarify the big deal of inconsistency in basic science about the anatomical differences in AI between patients with schizophrenia and control subjects. Longitudinal studies are also suggested to determine the probable role of this structural difference in development of schizophrenia: Is it a cause or an effect? We expect that the future enhancements in the basic knowledge about the schizophrenia result in the better treatment approaches and policies and even open the horizons toward cure or prevention.
ACKNOWLEDGMENTS
The authors wish to thank the managers, physicians, and stuff of the Iran and Razi Psychiatry hospitals, Tehran, Iran. Our special thanks also go to Dr. Mehrdad Eftekhar and Dr. Zia Ghaem Magham Farahani for the assistance in patient selection and interviews. We are also grateful to Dr. Jalal Jalal-Shokouhi, the CEO of Koorosh Imaging Center, Tehran, Iran.
This study was granted by the Deputy for Research, Mashhad University of Medical Sciences (under project number 82001).
Footnotes
Source of Support: Deputy for Research, Mashhad University of Medical Sciences (under project number 82001)
Conflict of Interest: None declared
REFERENCES
- 1.Sadock BJ, Sadock VA. Kaplan and Sadock's comprehensive textbook of psychiatry. 9th ed. Philadelphia: Lippincott Williams and Wilkins; 2009. Schizophrenia; pp. 1432–628. [Google Scholar]
- 2.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 3.Flaum M, Swayze VW, 2nd, O’Leary DS, Yuh WT, Ehrhardt JC, Arndt SV, et al. Effects of diagnosis, laterality, and gender on brain morphology in schizophrenia. Am J Psychiatry. 1995;152:704–14. doi: 10.1176/ajp.152.5.704. [DOI] [PubMed] [Google Scholar]
- 4.Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size schizophrenia. J Neurol Neurosurg Psychiatry. 1995;58:457–61. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tibbo P, Nopoulos P, Arndt S, Andreasen NC. Corpus callosum shape and size in male patients with schizophrenia. Biol Psychiatry. 1998;44:405–12. doi: 10.1016/s0006-3223(98)00096-1. [DOI] [PubMed] [Google Scholar]
- 6.Nopoulos P, Swayze V, Flaum M, Ehrhardt JC, Yuh WT, Andreasen NC. Cavum septi pellucidi in normals and patients with schizophrenia as detected by magnetic resonance imaging. Biol Psychiatry. 1997;41:1102–8. doi: 10.1016/S0006-3223(96)00209-0. [DOI] [PubMed] [Google Scholar]
- 7.Kwon JS, Shenton ME, Hirayasu Y, Salisbury DF, Fischer IA, Dickey CC, et al. MRI study of cavum septi pellucidi in schizophrenia, affective disorder and schizotypal personality disorder. Am J Psychiatry. 1998;155:509–15. doi: 10.1176/ajp.155.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weinberger DR, Torrey EF, Wyatt RJ. Cerebellar atrophy in chronic schizophrenia. Lancet. 1979;1:718–9. doi: 10.1016/s0140-6736(79)91164-4. [DOI] [PubMed] [Google Scholar]
- 9.Lippmann S, Manshadi M, Baldwin H, Drasin G, Rice J, Alrajeh S. Cerebellar vermis dimensions on computerized tomographic scans of schizophrenic and bipolar patients. Am J Psychiatry. 1982;139:667–8. doi: 10.1176/ajp.139.5.667. [DOI] [PubMed] [Google Scholar]
- 10.Sandyk R, Kay SR, Merriam AE. Atrophy of the cerebellar vermis: Relevance to the symptoms of schizophrenia. Int J Neurosci. 1991;57:205–12. doi: 10.3109/00207459109150694. [DOI] [PubMed] [Google Scholar]
- 11.Rossi A, Stratta P, Mancini F, de Cataldo S, Casacchia M. Cerebellar vermal size in schizophrenia: A male effect. Biol Psychiatry. 1993;33:354–7. doi: 10.1016/0006-3223(93)90324-7. [DOI] [PubMed] [Google Scholar]
- 12.Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC. An MRI study of cerebellar vermis morphology in patients with schizophrenia: Evidence in support of the cognitive dysmetria concept. Biol Psychiatry. 1999;46:703–11. doi: 10.1016/s0006-3223(99)00093-1. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen NC, Arndt S, Swayze V, 2nd, Cizadlo T, Flaum M, O’Leary D, et al. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266:294–8. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 14.Jones EG. Cortical development and thalamic pathology in schizophrenia. Schizophr Bull. 1997;23:483–501. doi: 10.1093/schbul/23.3.483. [DOI] [PubMed] [Google Scholar]
- 15.Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M. Defining the phenotype of schizophrenia: Cognitive dysmetria and its neural mechanisms. Biol Psychiatry. 1999;46:908–20. doi: 10.1016/s0006-3223(99)00152-3. [DOI] [PubMed] [Google Scholar]
- 16.Heckers S. Neuropathology of schizophrenia: Cortex, thalamus, basal ganglia, and neurotransmitter-specific projection systems. Schizophr Bull. 1997;23:403–21. doi: 10.1093/schbul/23.3.403. [DOI] [PubMed] [Google Scholar]
- 17.Crouch RL, Thomson JK. The efferent fibers of the thalamus of macacus rhesus. J Comp Neurol. 1938;69:255–71. [Google Scholar]
- 18.Zawitsch C. Commissural and other fiber systems in human massa intermedia thalami. Wien Z Nervenheilkd Grenzgeb. 1952;4:74–93. [Google Scholar]
- 19.Lumley JS. The role of the massa intermedia in motor performance in the rhesus monkey. Brain. 1972;95:347–56. doi: 10.1093/brain/95.2.347. [DOI] [PubMed] [Google Scholar]
- 20.Kollmann J. Jena: Eischer; 1889. Textbook of human evolution story; p. 149. [Google Scholar]
- 21.Rosales RK, Lemay MJ, Yakovlev PI. The development and involution of massa intermedia with regard to age and sex. J Neuropathol Exp Neurol. 1968;27:166. [PubMed] [Google Scholar]
- 22.Firbas W, Volavsek C. Ontogenesis of adhaesio interthalamica (massa intermedia) in man. Anat Anz. 1970;126:205–10. [PubMed] [Google Scholar]
- 23.Rabl R. Studies on the structure of the massa intermedia of the thalamus opticus. J Hirnforsch. 1958;4:78–112. [PubMed] [Google Scholar]
- 24.Kiernan JA. Philadelphia: Lippincott Williams and Wilkins; 2009. Barr's the human nervous system: An anatomical viewpoint; p. 194. [Google Scholar]
- 25.Samra KA, Cooper IS. Radiology of the massa intermedia. Radiology. 1968;91:1124–8. doi: 10.1148/91.6.1124. [DOI] [PubMed] [Google Scholar]
- 26.Davie JC, Baldwin M. Radiographic-anatomical study of the massa intermedia. J Neurosurg. 1967;26:483–7. doi: 10.3171/jns.1967.26.5.0483. [DOI] [PubMed] [Google Scholar]
- 27.Malobabic S, Puskas L, Blagotic M. Size and position of the human adhaesio interthalamica. Gegenbaurs Morphol Jahrb. 1987;133:175–80. [PubMed] [Google Scholar]
- 28.Allen LS, Gorski RA. Sexual dimorphism of the anterior commisure and massa intermedia of the human brain. J Comp Neurol. 1991;312:97–104. doi: 10.1002/cne.903120108. [DOI] [PubMed] [Google Scholar]
- 29.Erbagci H, Yildirim H, Herken H, Gumusburun E. A magnetic resonance imaging study of the adhesio interthalamica in schizophrenia. Schizophr Res. 2002;55:89–92. doi: 10.1016/s0920-9964(01)00199-2. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi T, Suzuki M, Zhou SY, Nakamura K, Tanino R, Kawasaki Y, et al. Prevalence and length of the adhesio interthalamica in schizophrenia spectrum disorders. Psychiatry Res. 2008;164:90–4. doi: 10.1016/j.pscychresns.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Meisenzahl EM, Frodl T, Zetzsche T, Leinsinger G, Heiss D, Maag K, et al. Adhesio interthalamica in male patients with schizophrenia. Am J Psychiatry. 2000;157:823–5. doi: 10.1176/appi.ajp.157.5.823. [DOI] [PubMed] [Google Scholar]
- 32.Meisenzahl EM, Frodl T, Zetzsche T, Leinsinger G, Maag K, Hegerl U, et al. Investigation of a possible diencephalic pathology in schizophrenia. Psychiatry Res. 2002;115:127–35. doi: 10.1016/s0925-4927(02)00044-6. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal N, Rambaldelli G, Perlini C, Dusi N, Kitis O, Bellani M, et al. Microstructural thalamic changes in schizophrenia: A combined anatomic and diffusion weighted magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:440–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder PJ, Bogerts B, Wu H, Bilder RM, Deoras KS, Lieberman JA. Absence of the adhesio interthalamica as a marker of early developmental neuropathology in schizophrenia: An MRI and postmortem histologic study. J Neuroimaging. 1998;8:159–63. doi: 10.1111/jon199883159. [DOI] [PubMed] [Google Scholar]
- 35.Nopoulos PC, Rideout D, Crespo-Facorro B, Andreasen NC. Sex differences in the absence of massa intermedia in patients with schizophrenia versus healthy controls. Schizophr Res. 2001;48:177–85. doi: 10.1016/s0920-9964(00)00067-0. [DOI] [PubMed] [Google Scholar]
- 36.de Souza Crippa JA, Zuardi AW, Busatto GF, Sanches RF, Santos AC, Araújo D, et al. Cavum septum pellucidum and adhesio interthalamica in schizophrenia: An MRI study. Eur Psychiatry. 2006;21:291–9. doi: 10.1016/j.eurpsy.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi T, Hotta N, Andoh T, Mori M, Fukatsu N, Suga H. Magnetic resonance imaging finding in schizophrenia and atypical psychoses. J Neural Transm. 2001;108:695–706. doi: 10.1007/s007020170046. [DOI] [PubMed] [Google Scholar]
- 38.Ligier Y, Ratib O, Logean M, Girard C. Osiris: A medical image-manipulation system. MD Comput. 1994;11:212–8. [PubMed] [Google Scholar]
- 39.Takahashi T, Yücel M, Yung AR, Wood SJ, Phillips LJ, Berger GE, et al. Adhesio interthalamica in individuals at high-risk for developing psychosis and patients with psychotic disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1708–14. doi: 10.1016/j.pnpbp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Shimizu M, Fujiwara H, Hirao K, Namiki C, Fukuyama H, Hayashi T, et al. Structural abnormalities of the adhesio interthalamica and mediodorsal nuclei of the thalamus in schizophrenia. Schizophr Res. 2008;101:331–8. doi: 10.1016/j.schres.2007.12.486. [DOI] [PubMed] [Google Scholar]
- 41.Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, et al. Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry. 2001;58:133–40. doi: 10.1001/archpsyc.58.2.133. [DOI] [PubMed] [Google Scholar]
- 42.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–24. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 43.Andreasen NC, Rezai K, Alliger R, Swayze VW, 2nd, Flaum M, Kirchner P, et al. Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry. 1992;49:943–58. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- 44.Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, et al. Image processing for the study of brain structure and function: Problems and programs. J Neuropsychiatry Clin Neurosci. 1992;4:125–33. doi: 10.1176/jnp.4.2.125. [DOI] [PubMed] [Google Scholar]
- 45.Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocorticalal terations with deficit syndrome. Arch Gen Psychiatry. 1992;49:522–30. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]


