Abstract
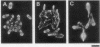
The Schizosaccharomyces pombe sxa1 and sxa2 mutants showed an exaggerated response to mating pheromones, producing excessively long conjugation tubes and exhibiting mating deficiency. This phenotype was similar to phenotypes of cells bearing an activated allele of ras1, such as ras1Val-17 or ras1Leu-66, and phenotypes of cells defective in gap1. However, genetic evidence suggested that the sxa1 and sxa2 gene products are not directly involved in the Ras1 pathway. The gene products of sxa1 and sxa2, as deduced from their nucleotide sequences, were homologous to aspartyl proteases and serine carboxypeptidases, respectively. The sxa1 gene function was required for efficient mating only in h+ cells, although even disruption of sxa1 did not completely abolish the mating ability. Conversely, the sxa2 gene function was required only in h- cells. Wild-type cells produced a diffusible substance, which may be the sxa2 gene product itself, that could confer fertility to sxa2 mutant cells placed at a distance. These observations are consistent with the possibility that the sxa gene products are involved in degradation or processing of the mating pheromones and that their loss cause a persistent response to the pheromones.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammerer G., Hunter C. P., Rothman J. H., Saari G. C., Valls L. A., Stevens T. H. PEP4 gene of Saccharomyces cerevisiae encodes proteinase A, a vacuolar enzyme required for processing of vacuolar precursors. Mol Cell Biol. 1986 Jul;6(7):2490–2499. doi: 10.1128/mcb.6.7.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma T., Pals G., Mohandas T. K., Couvreur J. M., Taggart R. T. Human gastric cathepsin E. Predicted sequence, localization to chromosome 1, and sequence homology with other aspartic proteinases. J Biol Chem. 1989 Oct 5;264(28):16748–16753. [PubMed] [Google Scholar]
- Beach D., Piper M., Nurse P. Construction of a Schizosaccharomyces pombe gene bank in a yeast bacterial shuttle vector and its use to isolate genes by complementation. Mol Gen Genet. 1982;187(2):326–329. doi: 10.1007/BF00331138. [DOI] [PubMed] [Google Scholar]
- Cooper A., Bussey H. Characterization of the yeast KEX1 gene product: a carboxypeptidase involved in processing secreted precursor proteins. Mol Cell Biol. 1989 Jun;9(6):2706–2714. doi: 10.1128/mcb.9.6.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Azzo A., Hoogeveen A., Reuser A. J., Robinson D., Galjaard H. Molecular defect in combined beta-galactosidase and neuraminidase deficiency in man. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4535–4539. doi: 10.1073/pnas.79.15.4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmochowska A., Dignard D., Henning D., Thomas D. Y., Bussey H. Yeast KEX1 gene encodes a putative protease with a carboxypeptidase B-like function involved in killer toxin and alpha-factor precursor processing. Cell. 1987 Aug 14;50(4):573–584. doi: 10.1016/0092-8674(87)90030-4. [DOI] [PubMed] [Google Scholar]
- Egel R., Egel-Mitani M. Premeiotic DNA synthesis in fission yeast. Exp Cell Res. 1974 Sep;88(1):127–134. doi: 10.1016/0014-4827(74)90626-0. [DOI] [PubMed] [Google Scholar]
- Elder R. T., Loh E. Y., Davis R. W. RNA from the yeast transposable element Ty1 has both ends in the direct repeats, a structure similar to retrovirus RNA. Proc Natl Acad Sci U S A. 1983 May;80(9):2432–2436. doi: 10.1073/pnas.80.9.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Kaziro Y. Molecular cloning and sequence analysis of a ras gene from Schizosaccharomyces pombe. EMBO J. 1985 Mar;4(3):687–691. doi: 10.1002/j.1460-2075.1985.tb03684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Kaziro Y., Yamamoto M. Mating pheromone-like diffusible factor released by Schizosaccharomyces pombe. EMBO J. 1986 Aug;5(8):1991–1993. doi: 10.1002/j.1460-2075.1986.tb04454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Kozasa T., Kaziro Y., Takeda T., Yamamoto M. Role of a ras homolog in the life cycle of Schizosaccharomyces pombe. Cell. 1986 Jan 31;44(2):329–336. doi: 10.1016/0092-8674(86)90767-1. [DOI] [PubMed] [Google Scholar]
- Fukui Y., Miyake S., Satoh M., Yamamoto M. Characterization of the Schizosaccharomyces pombe ral2 gene implicated in activation of the ras1 gene product. Mol Cell Biol. 1989 Dec;9(12):5617–5622. doi: 10.1128/mcb.9.12.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galjart N. J., Gillemans N., Harris A., van der Horst G. T., Verheijen F. W., Galjaard H., d'Azzo A. Expression of cDNA encoding the human "protective protein" associated with lysosomal beta-galactosidase and neuraminidase: homology to yeast proteases. Cell. 1988 Sep 9;54(6):755–764. doi: 10.1016/s0092-8674(88)90999-3. [DOI] [PubMed] [Google Scholar]
- Grimm C., Kohli J., Murray J., Maundrell K. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol Gen Genet. 1988 Dec;215(1):81–86. doi: 10.1007/BF00331307. [DOI] [PubMed] [Google Scholar]
- Harris T. J., Lowe P. A., Lyons A., Thomas P. G., Eaton M. A., Millican T. A., Patel T. P., Bose C. C., Carey N. H., Doel M. T. Molecular cloning and nucleotide sequence of cDNA coding for calf preprochymosin. Nucleic Acids Res. 1982 Apr 10;10(7):2177–2187. doi: 10.1093/nar/10.7.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R., Moore S., Stein W. H. Serine at the active center of yeast carboxypeptidase. J Biol Chem. 1973 Dec 25;248(24):8366–8369. [PubMed] [Google Scholar]
- Hemmings B. A., Zubenko G. S., Hasilik A., Jones E. W. Mutant defective in processing of an enzyme located in the lysosome-like vacuole of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 Jan;78(1):435–439. doi: 10.1073/pnas.78.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hobart P. M., Fogliano M., O'Connor B. A., Schaefer I. M., Chirgwin J. M. Human renin gene: structure and sequence analysis. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5026–5030. doi: 10.1073/pnas.81.16.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogeveen A. T., Verheijen F. W., Galjaard H. The relation between human lysosomal beta-galactosidase and its protective protein. J Biol Chem. 1983 Oct 25;258(20):12143–12146. [PubMed] [Google Scholar]
- Horiuchi H., Yanai K., Okazaki T., Takagi M., Yano K. Isolation and sequencing of a genomic clone encoding aspartic proteinase of Rhizopus niveus. J Bacteriol. 1988 Jan;170(1):272–278. doi: 10.1128/jb.170.1.272-278.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D. A., Fukui Y., Yamamoto M. Homologous activators of ras in fission and budding yeast. Nature. 1990 Mar 22;344(6264):355–357. doi: 10.1038/344355a0. [DOI] [PubMed] [Google Scholar]
- Imai Y., Miyake S., Hughes D. A., Yamamoto M. Identification of a GTPase-activating protein homolog in Schizosaccharomyces pombe. Mol Cell Biol. 1991 Jun;11(6):3088–3094. doi: 10.1128/mcb.11.6.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. W. The synthesis and function of proteases in Saccharomyces: genetic approaches. Annu Rev Genet. 1984;18:233–270. doi: 10.1146/annurev.ge.18.120184.001313. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Shimoda C. The Schizosaccharomyces pombe mam2 gene encodes a putative pheromone receptor which has a significant homology with the Saccharomyces cerevisiae Ste2 protein. EMBO J. 1991 Dec;10(12):3743–3751. doi: 10.1002/j.1460-2075.1991.tb04943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronstad J. W., Holly J. A., MacKay V. L. A yeast operator overlaps an upstream activation site. Cell. 1987 Jul 31;50(3):369–377. doi: 10.1016/0092-8674(87)90491-0. [DOI] [PubMed] [Google Scholar]
- MacKay V. L., Welch S. K., Insley M. Y., Manney T. R., Holly J., Saari G. C., Parker M. L. The Saccharomyces cerevisiae BAR1 gene encodes an exported protein with homology to pepsin. Proc Natl Acad Sci U S A. 1988 Jan;85(1):55–59. doi: 10.1073/pnas.85.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus S., Xue C. B., Naider F., Becker J. M. Degradation of a-factor by a Saccharomyces cerevisiae alpha-mating-type-specific endopeptidase: evidence for a role in recovery of cells from G1 arrest. Mol Cell Biol. 1991 Feb;11(2):1030–1039. doi: 10.1128/mcb.11.2.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin-Davis S. A., Nasim A., Beach D. Involvement of ras in sexual differentiation but not in growth control in fission yeast. EMBO J. 1986 Nov;5(11):2963–2971. doi: 10.1002/j.1460-2075.1986.tb04593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadin-Davis S. A., Yang R. C., Narang S. A., Nasim A. The cloning and characterization of a RAS gene from Schizosaccharomyces pombe. J Mol Evol. 1986;23(1):41–51. doi: 10.1007/BF02100997. [DOI] [PubMed] [Google Scholar]
- Obara T., Nakafuku M., Yamamoto M., Kaziro Y. Isolation and characterization of a gene encoding a G-protein alpha subunit from Schizosaccharomyces pombe: involvement in mating and sporulation pathways. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5877–5881. doi: 10.1073/pnas.88.13.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Okazaki N., Kume K., Jinno S., Tanaka K., Okayama H. High-frequency transformation method and library transducing vectors for cloning mammalian cDNAs by trans-complementation of Schizosaccharomyces pombe. Nucleic Acids Res. 1990 Nov 25;18(22):6485–6489. doi: 10.1093/nar/18.22.6485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Riezman H. Detection of an intermediate compartment involved in transport of alpha-factor from the plasma membrane to the vacuole in yeast. J Cell Biol. 1990 Jun;110(6):1911–1922. doi: 10.1083/jcb.110.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipiczki M., Ferenczy L. Protoplast fusion of Schizosaccharomyces pombe Auxotrophic mutants of identical mating-type. Mol Gen Genet. 1977 Feb 28;151(1):77–81. doi: 10.1007/BF00446915. [DOI] [PubMed] [Google Scholar]
- Sogawa K., Fujii-Kuriyama Y., Mizukami Y., Ichihara Y., Takahashi K. Primary structure of human pepsinogen gene. J Biol Chem. 1983 Apr 25;258(8):5306–5311. [PubMed] [Google Scholar]
- Valls L. A., Hunter C. P., Rothman J. H., Stevens T. H. Protein sorting in yeast: the localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell. 1987 Mar 13;48(5):887–897. doi: 10.1016/0092-8674(87)90085-7. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Lino Y., Furuhata K., Shimoda C., Yamamoto M. The S.pombe mei2 gene encoding a crucial molecule for commitment to meiosis is under the regulation of cAMP. EMBO J. 1988 Mar;7(3):761–767. doi: 10.1002/j.1460-2075.1988.tb02873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford C. A., Daniels L. B., Park F. J., Jones E. W., Van Arsdell J. N., Innis M. A. The PEP4 gene encodes an aspartyl protease implicated in the posttranslational regulation of Saccharomyces cerevisiae vacuolar hydrolases. Mol Cell Biol. 1986 Jul;6(7):2500–2510. doi: 10.1128/mcb.6.7.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubenko G. S., Park F. J., Jones E. W. Mutations in PEP4 locus of Saccharomyces cerevisiae block final step in maturation of two vacuolar hydrolases. Proc Natl Acad Sci U S A. 1983 Jan;80(2):510–514. doi: 10.1073/pnas.80.2.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst G. T., Galjart N. J., d'Azzo A., Galjaard H., Verheijen F. W. Identification and in vitro reconstitution of lysosomal neuraminidase from human placenta. J Biol Chem. 1989 Jan 15;264(2):1317–1322. [PubMed] [Google Scholar]