Abstract
Background:
Breast cancer (BC) represents the highest incidence of malignancy in women throughout the world. Medicinal fungi can stimulate the body, reduce side-effects associated with chemotherapy and improve the quality of life in patients with cancer.
Aim:
To evaluate the effects of dietary supplementation of Agaricus sylvaticus on clinical and nutritional parameters in BC patients undergoing chemotherapy.
Materials and Methods:
A randomized, placebo-controlled, double-blind, clinical trial was carried out at the Oncology Clinic, Hospital of the Federal District-Brazil from September 2007 to July 2009. Forty six patients with BC, Stage II and III, were randomly assigned to receive either nutritional supplement with A. sylvaticus (2.1 g/day) or placebo. Patients were evaluated during treatment period.
Results:
Patient supplemented with A. sylvaticus improved in clinical parameters and gastrointestinal functions. Poor appetite decreased by 20% with no changes in bowel functions (92.8%), nausea and vomiting (80%).
Conclusion:
Dietary supplementation with A. sylvaticus improved nutritional status and reduced abnormal bowel functions, nausea, vomiting, and anorexia in patients with BC receiving chemotherapy.
KEY WORDS: Agaricus sylvaticus, chemotherapy side effects, nutritional status
Introduction
Breast cancer (BC) represents the highest incidence of malignancy in women throughout the world.[1] The Brazilian Ministry of Health estimates an increase in the incidence with more than 49,000 new cases in next 5 years.[2] Different studies have been carried out to identify the risk factors associated with BC. Some of them are well-established risk-factors such as early menarche, parity, and first pregnancy over age 30, the use of oral contraceptives, late menopause, and hormone replacement therapy. While it has been reported that breastfeeding, physical activity and healthy eating habits with maintenance of body weight may reduce BC risk.[3,4] A relationships between diet and cancer as well as other chronic non-transmissible diseases has also been suggested.[1]
Anticancer drugs induced side-effects may reduce caloric intake and decrease the absorption of nutrients. Adjuvant therapies, like dietary supplementation with Agaricus sylvaticus, could be useful to overcome these side-effects[1,5,6] and the aim of this study was to evaluate the effects of dietary supplementation with A. sylvaticus on clinical and nutritional status.
Materials and Methods
A randomized, double-blind, placebo-controlled trial was carried out at the Oncology Clinic, Hospital of the Federal District-Brazil from September 2007 to July 2009. The study protocol was approved by the Ethics Committee of the Ministry of Health of the Federal District, under protocolNo 041/2007. Written informed consent was obtained from patients included in the study.
Experimental Design
Extract with A. sylvaticus
The A. sylvaticus whose popular name is Cogumelo do Sol (Sun Mushroom) was obtained from a producer accredited by Empresa Brasileira de Agropecuária-Embrapa, from the region of Tapiraí, São Paulo state. The fungus extract was obtained by soaking the dried material in hot water for 30 min, liquified, sieved, and dried in a dissector. The composition analysis of A. sylvaticus was conducted by Japan Food Research Laboratories Center and revealed the presence of carbohydrates (18.51 g/100 g), lipid (0.04 g/100 g), ergosterol (624 mg/100 g), protein (4.99 g/100 g), amino acids (arginine - 1, 14%, lysine - 1, 23%, histidine - 0, 51%, phenylalanine - 0, 92%, tyrosine - 0, 67%, leucine - 1, 43% - methionine 0.32%, valine - 1, 03%, alanine - 1, 28% glycine - 0, 94% proline - 0, 95%, glutamic acid - 3, 93%, serine - 0, 96%, threonine 0.96%, aspartic acid - 1, 81%, tryptophan - 0, 32%, cysteine - 0, 25%), and micronutrients in quantities not quantified.
The fungus was administered in pills at the dosage of 2.1 g, divided into two daily administrations. Patients who received placebo tablets received the same quantities, with the same ingredients and calories, but without the extract of A. sylvaticus. All patients ingested six tablets a day (three in the morning and three in the afternoon between meals) for a period of 6 months.
Enrolment and subject selection
Forty six women with BC receiving chemotherapy were selected according to the inclusion criteria: (1) patients with BC, (2) aged between 40 and 65, and (3) in Stage II or III receiving chemotherapy. At the beginning patients were divided according to the chemotherapy cycles prescribed by the medical staff, 26 women with three cycles and 20 women with six cycles. Among women with three chemotherapy cycles, 14 were diagnosed in Stage II and 12 in Stage III of the disease. Among patients with 6 chemotherapy cycles, 10 were in Stage II and 10 in Stage III of the disease.
Patients were randomly separated in placebo group ([group of 3 cycles, n = 13], [group of 6 cycles, n = 10]), and supplemented with A. sylvaticus (group [3 cycles, n = 13], [group of six cycles, n = 10]). All patients were treated with their basic medication for BC (cyclofosfamide, methotrexate, fluorouracil) or (fluorouracil, adriblastina, cyclofosfamide) in cycles of 21 days as recommended the Brazilian guidelines and followed by the oncologists in charge in each case.
Data was stored and processed by the EpiInfo 6.04 computer program. Neither investigators nor patients had information on the drug used throughout the study. Adults who (a) were eligible, (b) met all inclusion criteria, and (c) had given written informed consent for the trial, were included in the study. A random table was developed to (blindly) allocate each of these 46 patients to receive either A. sylvaticus or placebo. The medication was provided at no charge. Sample size for each treatment group (n) needed to ensure sufficient statistical power (80%), confidence level of 90%, and odds ratio = 7, was calculated.
A standard form from each patient was filled out to collect all information related with the trial. The form was applied on the 1st day of consultation and in subsequent appointments. Data was collected was performed by trained researchers. The characteristics of the study population, socioeconomic (family income, place of residence, race, marital status, education), life-style (smoking, alcohol consumption, and physical inactivity) and information regarding the number of pregnancies and first-born breastfeeding duration was obtained. Patients were followed-up for 3-6 months, and assessed every 30 days or every chemotherapy cycle. Patients were asked to maintain a normal diet. Safety assessment was carried out by a standardized questionnaire.
Adverse event was defined as the development of any sign or symptoms that did not exist before, or became more serious following the commencement of the treatment. Serious adverse events were defined as death, any life-threatening, disabling or incapacitating events, or those requiring hospitalization.
Assessment of compliance
One of the following requirements was considered to indicate treatment non-compliance: (1) failure to attend the follow-up visits; (2) not taking one or more dose at the instructed level and duration; (3) discontinued the drug without asking the treating physician.
Data management and statistical analysis
The data regarding treatment response and adverse events were noted on pre-designed record forms and subsequently analyzed to determine the frequency of each response/effect using EpiInfo 6.04 software (Public Health Domain Software, CDC, Atlanta, GA, USA). The statistical significance of differences between mean values was determined using the Student t-test. The Fisher exact test was used to establish the significance of differences in proportions. P d” 0.05 was considered to be statistically significant.
Results
After follow-up, 46 women with BC completed the study, 26 (56.5%) in the group of three chemotherapy cycles and 20 (43, 4%) in six chemotherapy cycles. Of these patients, 23 (50%) received placebo and the other received A. sylvaticus. Patients in placebo group (n = 23) had a mean age of 49.5 years while in the group supplemented with A. sylvaticus (n = 23) the mean age was 52.7 years. The placebo group of patients belonged to Stage II while supplemented group was in Stage III of the disease [Table 1].
Table 1.
General data of patients with breast cancer
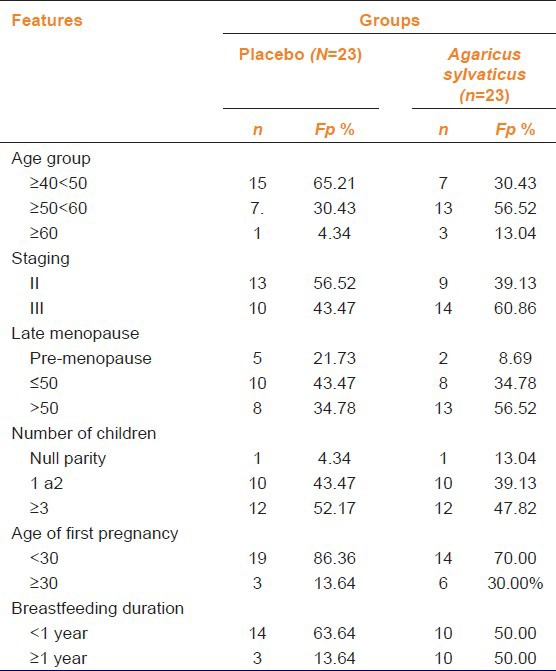
The menopause, in supplemented patients was at 50 years while placebo group had late menopause. In the supplemented group, three patients were nulliparous whereas in the placebo group it was only one patient [Table 1]. Most women had their first pregnancy before age 30, 86.36% (n = 19) in placebo group and 70.0% in supplemented group [Table 2].
Table 2.
Socio-demographic characteristics and habits of life of patients with breast cancer
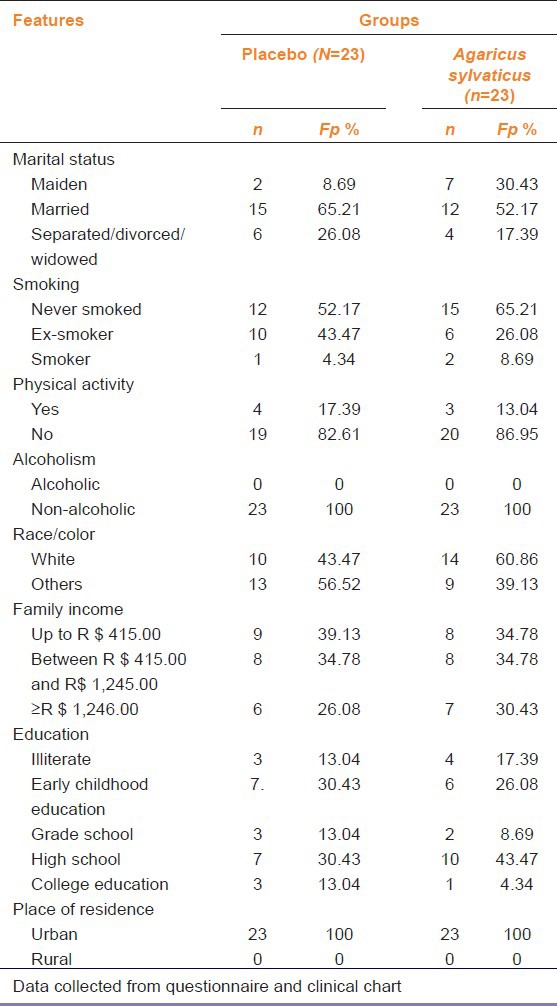
Twelve patients never smoked in supplemented and placebo group. All patients reported not drinking alcohol [Table 2]. Data analysis of physical activity revealed that majority (82.61% [n = 19]) of patients in placebo and supplemented group were sedentary [Table 2].
Both groups were analysed separately according to chemotherapy cycles and type of supplementation seeking for gastrointestinal symptoms. After 3 months of chemotherapy, 4 patients (30.77%) in placebo group showed loss of appetite, whereas patients treated supplemented with A. sylvaticus did not complain of anorexia [Table 3]. A good number of patients in the placebo group had complaints of diarrhea or constipation, while 92.31% (n = 12) of patients supplemented with mushroom had no such changes.
Table 3.
Clinical and gastrointestinal symptoms of patients with breast cancer who received three chemotherapy cycles
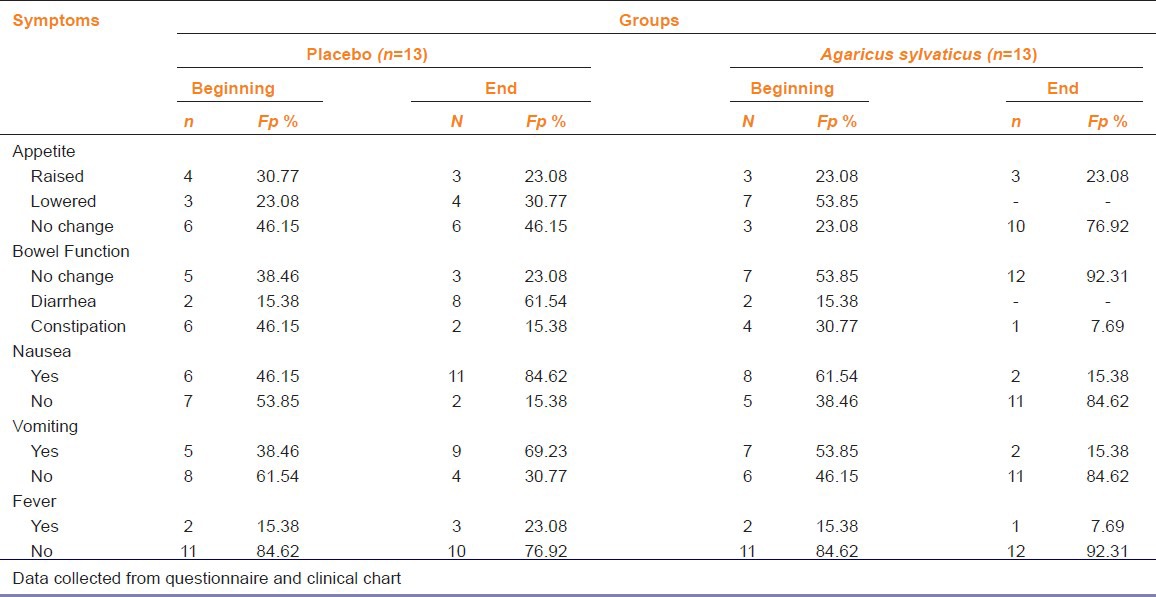
Only 2 (15.3%) patients treated with A. sylvaticus reported nausea and vomiting while majority of patients 11 (84.62%) in placebo group notified nausea and vomiting. Only 7.69% (n = 1) of the supplemented patients had fever during the first 10 days after chemotherapy, however 23.08% (n = 3) of placebo patients reported such complaints.
Patients with six chemotherapy cycles showed that 60% (6) of patients in the placebo group complained about loss of appetite. Upon completion of treatment 80% (n = 8) of patients reported reduction in appetite.
While 6 months with drug treatment bowel functions were not affected. However, 80% (n = 8) of patients in the placebo group had bowel function disturbances. Regarding nausea and vomiting, upon completion of after 6 months treatment, among supplemented patients only 20% (2) reported nausea and 10% (1) vomiting, whereas 100% (10) and 70% (7) among placebo group reported nausea or vomiting respectively [Table 4].
Table 4.
Clinical and gastrointestinal symptoms of patients with breast cancer who received six chemotherapy cycles
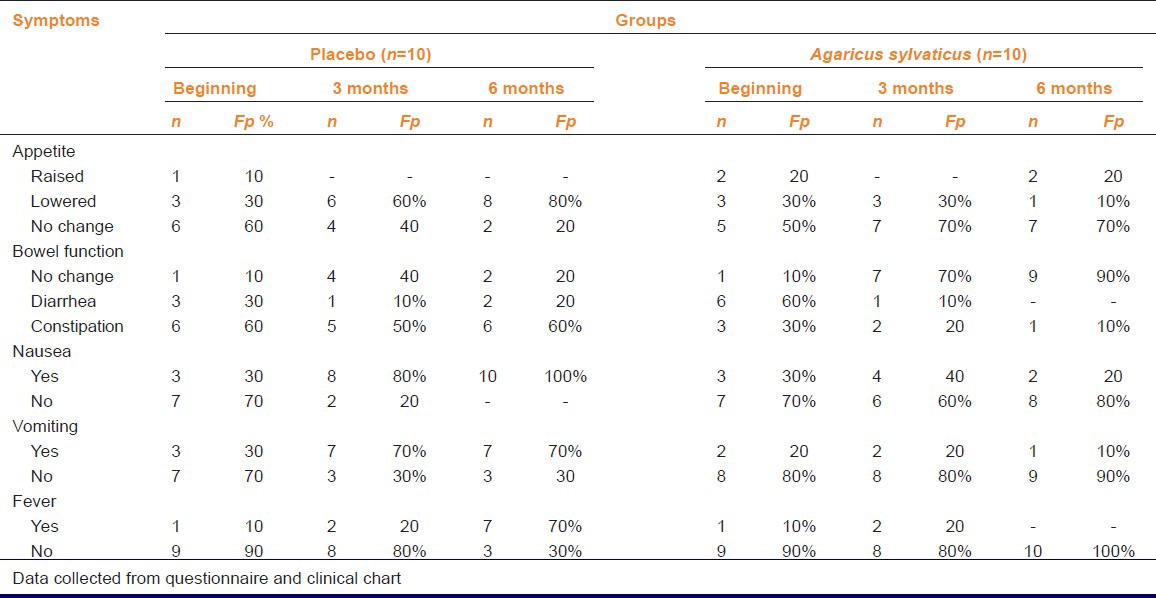
After 6 months 70% (n = 7) of placebo patients reported fever while no fever was notified from patients supplemented with A. sylvaticus [Table 4].
Nutritional assessment was performed utilizing : weight parameters, body mass index (BMI), waist-hip ratio (W/HR), triceps percentage adequacy and skin fold sum (triceps skinfold [TSF]). For the statistical analysis the t test was performed for paired samples [Table 5].
Table 5.
Nutritional parameters of patients with breast cancer undergoing three or six chemotherapy cycles with Agaricus sylvaticus dietary supplementation
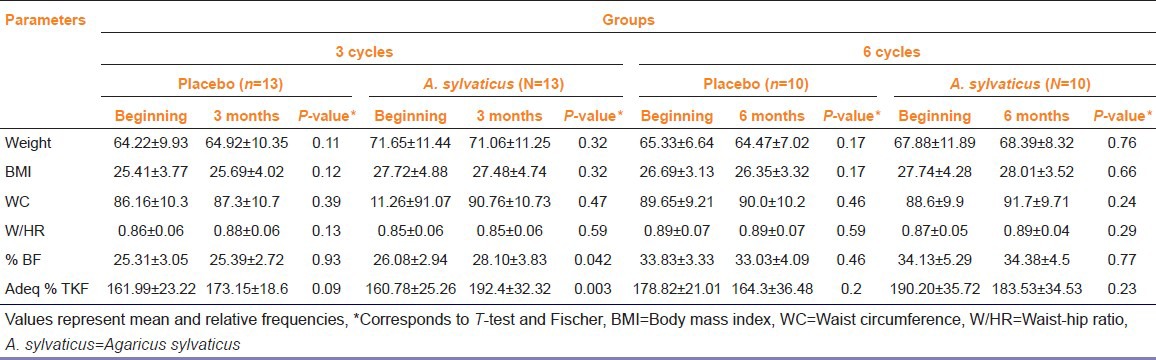
There was significant difference in parameters when comparing the first and 3rd months of treatment of appropriate triceps percentage and skin fold sum (TSF). As for BMI and W/HR, regardless of the type of treatment, patients were classified to be overweight and have cardiovascular risk due to accumulation of visceral fat.
Discussion
In this study 60.86% of patients in A. sylvaticus groups belonged to Stage III and 56.52% of placebo group belonged to Stage II by the TNM system. Similar results were found in the study published by Machetti et al. , Moraes et al. and Brito et al., demonstrating that late diagnosis have negative effect on patient prognosis.[7,8,9] For many years studies has been carried out in order to define risk-factors associated with BC.[10] Null parity, first pregnancy at 30 years or older and late menopause are well-established as risk-factors for BC. The present study showed that 13.04% of patients supplemented with A. sylvaticus and 4.34% of patients in the placebo group were nulliparous, 30% of supplemented women were aged 30 or older at first pregnancy and 56.52% of these reached menopause at 50 years or later. Only 17.39% and 13.04% patients in the placebo and A. sylvaticus group practiced some physical activity. One study carried out in India demonstrated that women who practiced regular physical activity were 38% more protected against BC than those who were sedentary.[11] Scientific evidence shows that exposure to cigarette smokes may increase the risk of BC, especially, when consumption occurs for longer periods. Similarly, in individuals who excessively consume alcohol, there is an increased risk of cancer.[1]
Several studies reported that patients in stable marital union have higher chances of recovery and improved quality of life during treatment.[10,12] This study found a higher prevalence of married women in both groups, 65.21%, and 52.17% respectively. In both groups, women had income up to the minimum monthly salary. Some studies conducted in the unified health system hospitals in Brazil, has related low family income as a risk factor for BC mainly in cases of late diagnoses.[1,12,13]
A healthy diet is essential in all stages.[1,3] Patients with three chemotherapy cycles at baseline reported poor appetite, whereby 23.08% of these patients were in the placebo group, and 53.85% of these patients were in the A. sylvaticus group. After 3 months, 30.77% of patients in placebo group reported reduction in appetite, while in the supplemented group there were no such complaints. Compared to patients with six chemotherapy cycles and longer use of mushroom A. sylvaticus, data show that towards the end of treatment, complaints of appetite loss reduced with time, unlike that of the placebo group, which after 6 months treatment showed 80% of these patients with complaints of poor appetite. These data reveal potential bioactive effects of supplementation with A. sylvaticus.
Different studies have shown that the use of medicinal fungi as adjuvant therapy in the treatment of malignancies is able to promote significant improvement in appetite, reduce fatigue, and stabilize hematological, and immunological parameters thus, enhancing the well-being of these patients.[1,14,15] The main substance found in fungi that has medicinal functional action is the â-Glucan and they have a large amount of other dietary fibre; the possible benefits caused by these fungi in bowel function may be due to fibres contained in their composition. â-Glucan acts in the human body by increasing immune functions, stimulating natural killer cells, T lymphocytes, B lymphocytes, and complementary cells; by increasing the number and function of macrophages and monocytes, promoting proliferation and/or production of antibodies and various cytokines such as IL-2 and IL-6, so as to avoid cancer metastasis.[14,16]
Studies reinforce the essential role of dietary fiber on intestinal metabolism due to increased concentrations of SCFA, which also act on the integrity of colonic cells intensifying the re-absorption of sodium and water, taking crucial importance for diarrheal cases.[1,3]
The data concerning the nutritional status of patients diagnosed with BC found in literature legitimize the data found in this study.[17,18] The literature shows that the majority of patients with BC have nutritional profile of overweight and obesity. Patients with three chemotherapy cycles began treatment with an average BMI of 26.53 ± 4.65 kg/m2 and average weight of 67.93 ± 11.61 kg. Patients with six chemotherapy cycles had a mean weight of 66.60 ± 9.44 kg and BMI 27.21 ± 3.68 kg/m2. BMI is the nutritional parameter most used for classification of nutritional profile. Nevertheless, this is a fact that isolated does not report the actual nutritional status of patients. Friedenreich connected obesity, uncontrolled weight gain, high BMI and W/HR to the risk of BC and mainly to a poor prognosis of the disease, with greater chances of recurrence for such patients.[19] Kim in a study with 833 women found a strong association between high BMI and diagnosis of BC in stadium equal or greater than II of disease.[20]
Epidemiological studies utilize the relation W/HR and waist circumference (WC) for assessing body fat distribution. Among the established cut-off point to discriminate appropriate from inappropriate values regarding W/HR, the most widely used has been 0.85 for females. However, Pereira et al.,[21] in a population-based study conducted in Rio de Janeiro, showed that using the cut-off point of 0.80 for women had a better association with prediction of diseases linked to obesity.
Ronco found women diagnosed with BC having high skin folds values and WC (93.12 ± 14.12). He also noted that TSF values were strongly related to risk of BC, with a P value of 0.0004.[4] Data analysis on WC, W/HR, % body fat and adequacy of the percentage of triceps skinfold in this study were well above normal limits, revealing that nearly the entire population in the study held the risk of diseases associated with obesity, such as BC.
Clinical studies have shown that supplements such as A. sylvaticus (Cogumelo do Sol) are capable of reducing the side-effects of anticancer drugs, improving bowel function, sustaining nutritional status, stimulating the immune, and hematological systems, and improving the quality of life of cancer patients.[1,5,6]
Conclusion
Dietary supplementation with A. sylvaticus improved nutritional status and reduced adverse drug reactions in bowel functions, nausea, vomiting, anorexia, and fever in patients with BC treated with chemotherapy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Novaes MR, Valadares F, Reis MC, Gonçalves DR, Menezes Mda C. The effects of dietary supplementation with Agaricales mushrooms and other medicinal fungi on breast cancer: Evidence-based medicine. Clinics (Sao Paulo) 2011;66:2133–9. doi: 10.1590/S1807-59322011001200021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brazil Ministry of Health. Estimate 2010: Cancer incidence in Brazil. Rio de Janeiro: INCA; 2009. National Health Care Cancer Institute [INCA] [Google Scholar]
- 3.Guerra MR, Mendonça GA, Bustamante-Teixeira MT, Cintra JR, Carvalho LM, Magalhães LM. Five-year survival and prognostic factors in a cohort of breast cancer patients treated in Juiz de Fora, Minas Gerais State, Brazil. Cad Saude Publica. 2009;25:2455–66. doi: 10.1590/s0102-311x2009001100015. [DOI] [PubMed] [Google Scholar]
- 4.Ronco AL, Mendoza B, Varas X, Jaumandreu S, DeStefano E, Febles, et al. Somatotype walk risk of breast cancer : A case-control study in Uruguay. Rev Bras Epidemiol. :2008, 11215–27. [Google Scholar]
- 5.Costa Fortes R, Carvalho Garbi Novaes MR. The effects of Agaricus sylvaticus fungi dietary supplementation on the metabolism and blood pressure of patients with colorectal cancer during post surgical phase. Nutr Hosp. 2011;26:176–86. [PubMed] [Google Scholar]
- 6.Costa Fortes R, Lacorte Recôva V, Lima Melo A, Carvalho Garbi Novaes MR. Life quality of postsurgical patients with colorectal cancer after supplemented diet with agaricus sylvaticus fungus. Nutr Hosp. 2010;25:586–96. [PubMed] [Google Scholar]
- 7.Machetti A. Staging of breast cancer diagnosed in the public health system of São Carlos. Medicina. :2007, 394–402. [Google Scholar]
- 8.de Moraes AB, Zanini RR, Turchiello MS, Riboldi J, de Medeiros LR. Survival study of breast cancer patients treated at the hospital of the Federal University in Santa Maria, Rio Grande do Sul, Brazil. Cad Saude Publica. 2006;22:2219–28. doi: 10.1590/s0102-311x2006001000028. [DOI] [PubMed] [Google Scholar]
- 9.Brito C, Portela MC, Vasconcellos MT. Survival of breast cancer women in the state of Rio de Janeiro, Southeastern Brazil. Rev Saude Publica. 2009;43:481–9. doi: 10.1590/s0034-89102009000300012. [DOI] [PubMed] [Google Scholar]
- 10.Pinho VF, Coutinho ES. Variables associated with breast cancer in clients of primary healthcare units. Cad Saude Publica. 2007;23:1061–9. doi: 10.1590/s0102-311x2007000500008. [DOI] [PubMed] [Google Scholar]
- 11.Mathew A, Gajalakshmi V, Rajan B, Kanimozhi VC, Brennan P, Binukumar BP, et al. Physical activity levels among urban and rural women in south India and the risk of breast cancer: A case-control study. Eur J Cancer Prev. 2009;18:368–76. doi: 10.1097/CEJ.0b013e32832e1c46. [DOI] [PubMed] [Google Scholar]
- 12.da Silva EP, Pelloso SM, Carvalho MD, Toledo MJ. Exploring breast cancer risk factors in Kaingáng women in the Faxinal Indigenous Territory, Paraná State, Brazil, 2008. Cad Saude Publica. 2009;25:1493–500. doi: 10.1590/s0102-311x2009000700007. [DOI] [PubMed] [Google Scholar]
- 13.Conde DM, Pinto-Neto AM, Cabello C, Santos-Sá D, Costa-Paiva L, Martinez EZ. Quality of life in Brazilian breast cancer survivors age 45-65 years: Associated factors. Breast J. 2005;11:425–32. doi: 10.1111/j.1075-122X.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- 14.Tartari RF, Busnello FM, Nunes CHA. Nutritional profile of patients undergoing chemotherapy treatment at a clinic specializing in chemotherapy. Rev Bras Oncol. 2010;56:43–50. [Google Scholar]
- 15.Terry PD, Rohan TE. Cigarette smoking and the risk of breast cancer in women: A review of the literature. Cancer Epidemiol Biomarkers Prev. 2002;11:953–71. [PubMed] [Google Scholar]
- 16.Strong RC, Taveira VC, Novaes MR. The role of immunomodulator â-D-Glucan co-adjuvant for cancer therapy. Rev Bras Nutr Clin. 2006;21:163–8. [Google Scholar]
- 17.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292–3. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 18.Amorim VM, Barros MB, César CL, Carandina L, Goldbaun M. Factors associated with not having a mammogram and breast examination : A population-based study and in Campinas, São Paulo, Brazil. Cad Saúde Pública. 2008;24:2623–32. doi: 10.1590/s0102-311x2008001100017. [DOI] [PubMed] [Google Scholar]
- 19.Friedenreich CM. Review of anthropome TRIc factors and breast cancer risk. Eur J Cancer Prev. 2001;10:15–32. doi: 10.1097/00008469-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Kim YA, Lee CW. Effects of obesisity on breast cancer stage at diagnosis in Korean women. Eur J Cancer Prev. 2004;13:13–7. doi: 10.1097/00008469-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Pereira RA, Sichieri R, Marins VM. Waist:hips girth ratio as a predictor of arterial hypertension. Cad Saude Publica. 1999;15:333–44. doi: 10.1590/s0102-311x1999000200018. [DOI] [PubMed] [Google Scholar]


