Abstract
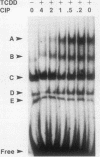
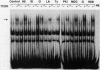
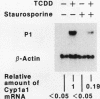
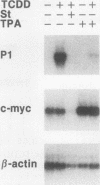
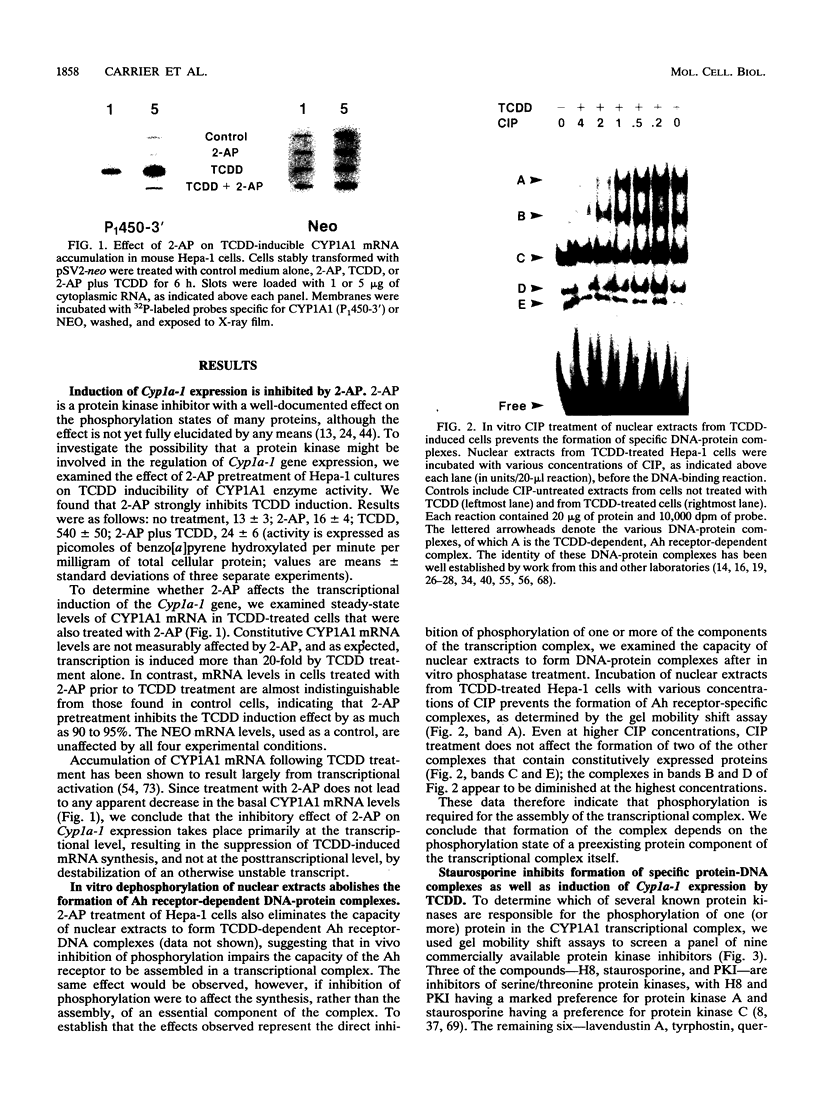
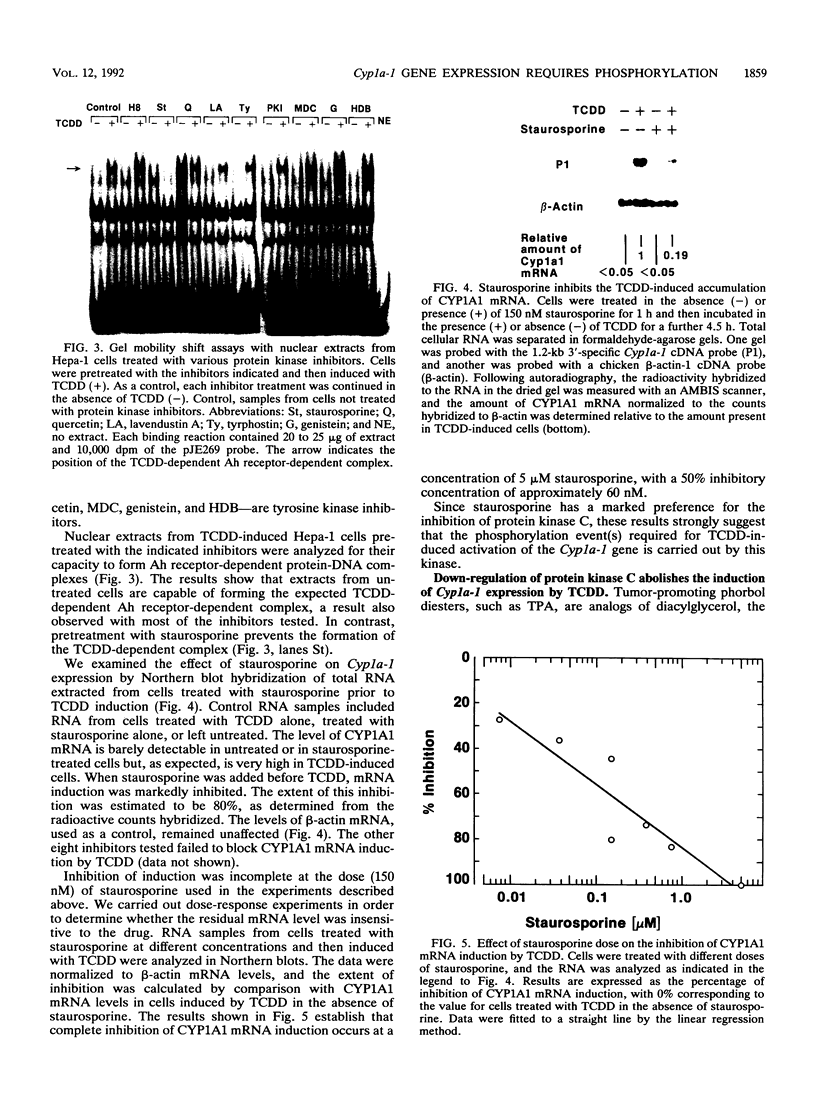
Transcriptional activation of the murine Cyp1a-1 (cytochrome P(1)450) gene by inducers such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (dioxin) requires the aromatic hydrocarbon (Ah) receptor and the interaction of an inducer-receptor complex with one or more of the Ah-responsive elements (AhREs) located about 1 kb upstream from the transcriptional initiation site. We find that treatment of mouse hepatoma Hepa-1 cells with 2-aminopurine, an inhibitor of protein kinase activity, inhibits CYP1A1 mRNA induction by TCDD as well as the concomitant increase in CYP1A1 enzyme activity. Formation of DNA-protein complexes between the Ah receptor and its AhRE target is also inhibited by 2-aminopurine, as determined by gel mobility shift assays. Phosphorylation is required for the formation of Ah receptor-specific complexes, since in vitro dephosphorylation of nuclear extracts from TCDD-treated Hepa-1 cells abolishes the capacity of the Ah receptor to form specific complexes with its cognate AhRE sequences. To determine whether any one of several known protein kinases was involved in the transcriptional regulation of the Cyp1a-1 gene, we treated Hepa-1 cells with nine other protein kinase inhibitors prior to induction with TCDD; nuclear extracts from these cells were analyzed for their capacity to form specific DNA-protein complexes. Only extracts from cells treated with staurosporine, a protein kinase C inhibitor, were unable to form these complexes. In addition, staurosporine completely inhibited CYP1A1 mRNA induction by TCDD. Depletion of protein kinase C by prolonged treatment with phorbol ester led to the complete suppression of CYP1A1 mRNA induction by TCDD. We conclude that (i) phosphorylation is necessary for the formation of a transcriptional complex and for transcriptional activation of the Cyp1a-1 gene; (ii) the phosphorylation site(s) exists on at least one of the proteins constituting the transcriptional complex, possibly the Ah receptor itself; and (iii) the enzyme responsible for the phosphorylation is likely to be protein kinase C.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Ballester R., Rosen O. M. Fate of immunoprecipitable protein kinase C in GH3 cells treated with phorbol 12-myristate 13-acetate. J Biol Chem. 1985 Dec 5;260(28):15194–15199. [PubMed] [Google Scholar]
- Benedict W. F., Gielen J. E., Owens I. S., Niwa A., Bebert D. W. Aryl hydrocarbon hydroxylase induction in mammalian liver cell culture. IV. Stimulation of the enzyme activity in established cell lines derived from rat or mouse hepatoma and from normal rat liver. Biochem Pharmacol. 1973 Nov 1;22(21):2766–2769. doi: 10.1016/0006-2952(73)90138-x. [DOI] [PubMed] [Google Scholar]
- Bernhard H. P., Darlington G. J., Ruddle F. H. Expression of liver phenotypes in cultured mouse hepatoma cells: synthesis and secretion of serum albumin. Dev Biol. 1973 Nov;35(1):83–96. doi: 10.1016/0012-1606(73)90008-0. [DOI] [PubMed] [Google Scholar]
- Bombick D. W., Jankun J., Tullis K., Matsumura F. 2,3,7,8-Tetrachlorodibenzo-p-dioxin causes increases in expression of c-erb-A and levels of protein-tyrosine kinases in selected tissues of responsive mouse strains. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4128–4132. doi: 10.1073/pnas.85.12.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombick D. W., Madhukar B. V., Brewster D. W., Matsumura F. TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) causes increases in protein kinases particularly protein kinase C in the hepatic plasma membrane of the rat and the guinea pig. Biochem Biophys Res Commun. 1985 Feb 28;127(1):296–302. doi: 10.1016/s0006-291x(85)80158-3. [DOI] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Cheng H. C., Kemp B. E., Pearson R. B., Smith A. J., Misconi L., Van Patten S. M., Walsh D. A. A potent synthetic peptide inhibitor of the cAMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):989–992. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cohen P., Cohen P. T. Protein phosphatases come of age. J Biol Chem. 1989 Dec 25;264(36):21435–21438. [PubMed] [Google Scholar]
- De Benedetti A., Baglioni C. Phosphorylation of initiation factor eIF-2 alpha, binding of mRNA to 48 S complexes, and its reutilization in initiation of protein synthesis. J Biol Chem. 1983 Dec 10;258(23):14556–14562. [PubMed] [Google Scholar]
- Dean N. M., Kanemitsu M., Boynton A. L. Effects of the tyrosine-kinase inhibitor genistein on DNA synthesis and phospholipid-derived second messenger generation in mouse 10T1/2 fibroblasts and rat liver T51B cells. Biochem Biophys Res Commun. 1989 Dec 15;165(2):795–801. doi: 10.1016/s0006-291x(89)80036-1. [DOI] [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr Protein-DNA interactions at recognition sites for the dioxin-Ah receptor complex. J Biol Chem. 1989 Oct 5;264(28):16478–16482. [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr The DNA recognition site for the dioxin-Ah receptor complex. Nucleotide sequence and functional analysis. J Biol Chem. 1988 Nov 25;263(33):17221–17224. [PubMed] [Google Scholar]
- Denison M. S., Vella L. M., Okey A. B. Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin in rat liver: lack of sensitivity to alkaline phosphatase when compared with that of glucocorticoid receptor. Arch Biochem Biophys. 1989 Sep;273(2):458–465. doi: 10.1016/0003-9861(89)90505-5. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio V., Huber B. E., Jacobs S. Partial down-regulation of protein kinase C reverses the growth inhibitory effect of phorbol esters on HepG2 cells. J Cell Physiol. 1990 Nov;145(2):381–389. doi: 10.1002/jcp.1041450225. [DOI] [PubMed] [Google Scholar]
- Durrin L. K., Whitlock J. P., Jr 2,3,7,8-Tetrachlorodibenzo-p-dioxin-inducible aryl hydrocarbon receptor-mediated change in CYP1A1 chromatin structure occurs independently of transcription. Mol Cell Biol. 1989 Dec;9(12):5733–5737. doi: 10.1128/mcb.9.12.5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrin L. K., Whitlock J. P., Jr In situ protein-DNA interactions at a dioxin-responsive enhancer associated with the cytochrome P1-450 gene. Mol Cell Biol. 1987 Aug;7(8):3008–3011. doi: 10.1128/mcb.7.8.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Elferink C. J., Whitlock J. P., Jr 2,3,7,8-Tetrachlorodibenzo-p-dioxin-inducible, Ah receptor-mediated bending of enhancer DNA. J Biol Chem. 1990 Apr 5;265(10):5718–5721. [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Nishi C., Fujii-Kuriyama Y. Regulatory DNA elements localized remotely upstream from the drug-metabolizing cytochrome P-450c gene. Nucleic Acids Res. 1986 Feb 11;14(3):1465–1477. doi: 10.1093/nar/14.3.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y. Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 1987 May 26;15(10):4179–4191. doi: 10.1093/nar/15.10.4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Yamane M., Fujii-Kuriyama Y. A DNA-binding factor specific for xenobiotic responsive elements of P-450c gene exists as a cryptic form in cytoplasm: its possible translocation to nucleus. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5859–5863. doi: 10.1073/pnas.85.16.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit A., Yaish P., Gilon C., Levitzki A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J Med Chem. 1989 Oct;32(10):2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- Graziani Y., Erikson E., Erikson R. L. The effect of quercetin on the phosphorylation activity of the Rous sarcoma virus transforming gene product in vitro and in vivo. Eur J Biochem. 1983 Oct 3;135(3):583–589. doi: 10.1111/j.1432-1033.1983.tb07692.x. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hapgood J., Cuthill S., Denis M., Poellinger L., Gustafsson J. A. Specific protein-DNA interactions at a xenobiotic-responsive element: copurification of dioxin receptor and DNA-binding activity. Proc Natl Acad Sci U S A. 1989 Jan;86(1):60–64. doi: 10.1073/pnas.86.1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989 May 25;264(15):8646–8652. [PubMed] [Google Scholar]
- Hershey J. W. Protein phosphorylation controls translation rates. J Biol Chem. 1989 Dec 15;264(35):20823–20826. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Huber B. E., Thorgeirsson S. S. Analysis of c-myc expression in a human hepatoma cell line. Cancer Res. 1987 Jul 1;47(13):3414–3420. [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Jones P. B., Durrin L. K., Galeazzi D. R., Whitlock J. P., Jr Control of cytochrome P1-450 gene expression: analysis of a dioxin-responsive enhancer system. Proc Natl Acad Sci U S A. 1986 May;83(9):2802–2806. doi: 10.1073/pnas.83.9.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. The murine Ah locus. Comparison of the complete cytochrome P1-450 and P3-450 cDNA nucleotide and amino acid sequences. J Biol Chem. 1984 Sep 10;259(17):10705–10713. [PubMed] [Google Scholar]
- Knutson J. C., Poland A. Response of murine epidermis to 2,3,7,8-tetrachlorodibenzo-p-dioxin: interaction of the ah and hr loci. Cell. 1982 Aug;30(1):225–234. doi: 10.1016/0092-8674(82)90028-9. [DOI] [PubMed] [Google Scholar]
- Landers J. P., Bunce N. J. The Ah receptor and the mechanism of dioxin toxicity. Biochem J. 1991 Jun 1;276(Pt 2):273–287. doi: 10.1042/bj2760273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legon S., Brayley A., Hunt T., Jackson R. J. The effect of cyclic AMP and related compounds on the control of protein synthesis in reticulocyte lysates. Biochem Biophys Res Commun. 1974 Feb 4;56(3):745–752. doi: 10.1016/0006-291x(74)90668-8. [DOI] [PubMed] [Google Scholar]
- Levy J., Teuerstein I., Marbach M., Radian S., Sharoni Y. Tyrosine protein kinase activity in the DMBA-induced rat mammary tumor: inhibition by quercetin. Biochem Biophys Res Commun. 1984 Sep 28;123(3):1227–1233. doi: 10.1016/s0006-291x(84)80264-8. [DOI] [PubMed] [Google Scholar]
- Mahadevan L. C., Wills A. J., Hirst E. A., Rathjen P. D., Heath J. K. 2-Aminopurine abolishes EGF- and TPA-stimulated pp33 phosphorylation and c-fos induction without affecting the activation of protein kinase C. Oncogene. 1990 Mar;5(3):327–335. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthies H. J., Palfrey H. C., Hirning L. D., Miller R. J. Down regulation of protein kinase C in neuronal cells: effects on neurotransmitter release. J Neurosci. 1987 Apr;7(4):1198–1206. doi: 10.1523/JNEUROSCI.07-04-01198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio A., Di Domenico M., Green S., de Falco A., Kajtaniak E. L., Blasi F., Chambon P., Auricchio F. Phosphorylation on tyrosine of in vitro synthesized human estrogen receptor activates its hormone binding. Mol Endocrinol. 1989 Jul;3(7):1061–1069. doi: 10.1210/mend-3-7-1061. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem. 1968 Dec 10;243(23):6242–6249. [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Jones J. E. Regulation of the mammalian cytochrome P1-450 (CYP1A1) gene. Int J Biochem. 1989;21(3):243–252. doi: 10.1016/0020-711x(89)90182-1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. Proposed role of drug-metabolizing enzymes: regulation of steady state levels of the ligands that effect growth, homeostasis, differentiation, and neuroendocrine functions. Mol Endocrinol. 1991 Sep;5(9):1203–1214. doi: 10.1210/mend-5-9-1203. [DOI] [PubMed] [Google Scholar]
- Nebert D. W. The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol. 1989;20(3):153–174. doi: 10.3109/10408448909017908. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Gonzalez F. J., Jaiswal A. K., Nebert D. W. Dioxin-inducible enhancer region upstream from the mouse P(1)450 gene and interaction with a heterologous SV40 promoter. DNA. 1986 Oct;5(5):403–411. doi: 10.1089/dna.1986.5.403. [DOI] [PubMed] [Google Scholar]
- Neuhold L. A., Shirayoshi Y., Ozato K., Jones J. E., Nebert D. W. Regulation of mouse CYP1A1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989 Jun;9(6):2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah E., Kote Z., Natsumeda Y., Yamaji Y., Jarai G., Lapis E., Financsek I., Weber G. Down-regulation of c-myc and c-Ha-ras gene expression by tiazofurin in rat hepatoma cells. Cancer Biochem Biophys. 1990 Apr;11(2):107–117. [PubMed] [Google Scholar]
- Onoda T., Iinuma H., Sasaki Y., Hamada M., Isshiki K., Naganawa H., Takeuchi T., Tatsuta K., Umezawa K. Isolation of a novel tyrosine kinase inhibitor, lavendustin A, from Streptomyces griseolavendus. J Nat Prod. 1989 Nov-Dec;52(6):1252–1257. doi: 10.1021/np50066a009. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Nebert D. W. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev. 1982 Jun;34(2):189–222. [PubMed] [Google Scholar]
- Pitot H. C., Goldsworthy T., Campbell H. A., Poland A. Quantitative evaluation of the promotion by 2,3,7,8-tetrachlorodibenzo-p-dioxin of hepatocarcinogenesis from diethylnitrosamine. Cancer Res. 1980 Oct;40(10):3616–3620. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Poland A., Palen D., Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982 Nov 18;300(5889):271–273. doi: 10.1038/300271a0. [DOI] [PubMed] [Google Scholar]
- Pongratz I., Strömstedt P. E., Mason G. G., Poellinger L. Inhibition of the specific DNA binding activity of the dioxin receptor by phosphatase treatment. J Biol Chem. 1991 Sep 5;266(25):16813–16817. [PubMed] [Google Scholar]
- Prochownik E. V., Kukowska J. Deregulated expression of c-myc by murine erythroleukaemia cells prevents differentiation. 1986 Aug 28-Sep 3Nature. 322(6082):848–850. doi: 10.1038/322848a0. [DOI] [PubMed] [Google Scholar]
- Puga A., Nebert D. W. Evolution of the P450 gene superfamily and regulation of the murine Cyp1a1 gene. Biochem Soc Trans. 1990 Feb;18(1):7–10. doi: 10.1042/bst0180007. [DOI] [PubMed] [Google Scholar]
- Richards C. A., Short S. A., Thorgeirsson S. S., Huber B. E. Characterization of a transforming N-ras gene in the human hepatoma cell line Hep G2: additional evidence for the importance of c-myc and ras cooperation in hepatocarcinogenesis. Cancer Res. 1990 Mar 1;50(5):1521–1527. [PubMed] [Google Scholar]
- Saatcioglu F., Perry D. J., Pasco D. S., Fagan J. B. Multiple DNA-binding factors interact with overlapping specificities at the aryl hydrocarbon response element of the cytochrome P450IA1 gene. Mol Cell Biol. 1990 Dec;10(12):6408–6416. doi: 10.1128/mcb.10.12.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. D., Glickman J. F., Chang K. J. The antiproliferative effects of staurosporine are not exclusively mediated by inhibition of protein kinase C. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1250–1256. doi: 10.1016/s0006-291x(88)80767-8. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tiwari R. K., Kusari J., Kumar R., Sen G. C. Gene induction by interferons and double-stranded RNA: selective inhibition by 2-aminopurine. Mol Cell Biol. 1988 Oct;8(10):4289–4294. doi: 10.1128/mcb.8.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa K., Hori T., Tajima H., Imoto M., Isshiki K., Takeuchi T. Inhibition of epidermal growth factor-induced DNA synthesis by tyrosine kinase inhibitors. FEBS Lett. 1990 Jan 29;260(2):198–200. doi: 10.1016/0014-5793(90)80102-o. [DOI] [PubMed] [Google Scholar]
- Whitlock J. P., Jr Genetic and molecular aspects of 2,3,7,8-tetrachlorodibenzo-p-dioxin action. Annu Rev Pharmacol Toxicol. 1990;30:251–277. doi: 10.1146/annurev.pa.30.040190.001343. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Huang D. P., Chiu D. K., Chiu J. F. The expression of oncogenes in human developing liver and hepatomas. Biochem Biophys Res Commun. 1987 Feb 13;142(3):932–938. doi: 10.1016/0006-291x(87)91503-8. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Wang Z., Lee A., Huang D. P., Chiu J. F. Differential expression of cellular oncogenes during rat liver development. Cancer Lett. 1988 Aug 15;41(2):147–155. doi: 10.1016/0304-3835(88)90111-5. [DOI] [PubMed] [Google Scholar]
- Zinn K., Keller A., Whittemore L. A., Maniatis T. 2-Aminopurine selectively inhibits the induction of beta-interferon, c-fos, and c-myc gene expression. Science. 1988 Apr 8;240(4849):210–213. doi: 10.1126/science.3281258. [DOI] [PubMed] [Google Scholar]