Abstract
Objective:
Antinociceptive effect of morphine in offspring born of mothers that received saline or morphine during the gestation period was investigated.
Materials and Methods:
Wistar rats (200-250 g) received saline, morphine 0.5 mg/kg or 5 mg/kg during gestation days 14-16. All pups after weaning were isolated treatment/sex dependently and were allowed to fully mature. The antinociceptive effect of morphine was assessed in formalin test. Morphine (0.5-7.5 mg/kg) or saline (1 ml/kg) was injected intraperitoneally 10 min before formalin (50 μl of 2.5% solution in right hind-paw).
Results:
Male offspring born of saline-treated mothers were less morphine-sensitive than females. On the contrary, male offspring exposed prenatally to morphine (5 mg/kg) were more sensitive to morphine-induced antinociceptive response in formalin test. However, no difference in antinociceptive effect was observed amongst offspring of either sex born of mothers treated with morphine 0.5 mg/kg, identifying a lower dose effect of the opioid.
Conclusion:
The exposure to morphine during the developmental period may result in altered development of tolerance to morphine and thus involved in drug abuse.
KEY WORDS: Formalin test, gestation, morphine, offspring, pain
Introduction
Evidence suggests an important gender difference in susceptibility to pain. According to the literature review, the males and females exhibit differences in their pain experiences. The females experience more pain and report more negative responses to pain than the males.[1]
Difference between males and females in analgesic effect following morphine administration is though generally accepted,[2] the mechanism of this sexual dimorphism is unclear. The persistence of analgesic sex differences after morphine injection in experimental animals, however, clearly implicates contributions from sites within the central nervous system[3] or strains. Particularly morphine analgesia subjects to sex differences in rats and mice.[3] Both opioid and non-opioid drugs that modulate morphine analgesia also do so sex-dependently.[4]
Exposure to morphine during gestation differentially alters the development of brain systems that mediate stress responses, such as norepinephrine (NE), and opioids, in the two sexes.[5] The exposure to morphine on gestation days 11-18 has long-term, sex-dependent effects on mu-opioid receptor binding in the brains of adult rats. This gestational morphine exposure reduces mu-opioid receptor binding by 25% in the hypothalamus and preoptic area (POA) of adult, ovariectomized female rats.[5]
Till date no study assessing the pain behavior in rat formalin test in the offspring born of mothers exposed to morphine during the gestational period have been performed. Present data provide surprising sex and dose differences in the analgesic effect induced by morphine in the offspring born of morphine exposed mothers.
Materials and Methods
Animals
Females Wistar rats approximately 120-140 days of age, 200-250 g (provided by Pasteur Institute of Iran) were used in the study. Animals were maintained under standard conditions; a 12 h light/dark cycle (light on at 7:00 a.m.), 24°C ± 2°C temperature, 50-55% humidity and were allowed free access to water and food ad lib. The local Committee for Ethics confirmed the protocol.
Drugs
Morphine sulphate (provided by TEMAD, active pharmaceutical ingredients, co., Iran) was prepared freshly by dissolving in saline at the desired concentration. In control group, saline was administered in a volume of 1 ml/kg. The acetate buffered formalin 2.5% mixture was prepared with extra-pure formalin (Merck Co., Germany).
Experimental Procedure
The female rats were housed with adult male rats. The observation of vaginal plugs was graded as embryonic day zero (E0). Each pregnant animal then was housed individually and received saline or morphine (0.5 mg/kg or 5 mg/kg, s.c.) through the 14th-16th days of pregnancy. All pups were weaned after 20 days passed of delivery. Post-weaning pups were grouped based on sex and prenatal treatment with saline/morphine dose treatment and were allowed to fully mature. To avoid of effect of changes in estrous cycling on nociceptive responses in female animals they were housed separately. Without exposing with male rats, the female rats are always in the diestrous phase; their phases will progress only after mating with a male rat.[6] The offspring were then grouped (n = 6 each sex) and examined for morphine antinociceptive effect using the formalin test.
Formalin Test
Each rat was placed in the transparent acrylic cage for 15-20 min to habituate. A mirror placed under the cage allowed an unobstructed view of the animal's paws to the observer. The antinociceptive effect of morphine was tested after 10 min using formalin injection. This time is sufficient for morphine to achieve its acute, but not complete antinociceptive effect. As evidence suggests, antinociceptive effect of morphine (early phase) appears very rapidly : The potency of morphine develops long-lasting through activation of both the endogenous antinociceptive systems and change in receptors’ sensitivities.[7] After (10 min) injection of morphine (0.5-7.5), each rat was restrained and received a 50-ìl (s.c.) injection of buffered formalin (2.5%) into the right hind-paw and placed in the observation box for 60 min. The pain response in the formalin test consists of an initial display of nociceptive behaviors that subsides after approximately 5 min and reappears after an additional 10-15 min; it then slowly diminishes over the subsequent 40-60 min and these behaviors have been described in detail.[8] Subjects in this experiment were observed for 60 min following formalin injection and the rats were rated every 15 s continuously by a 4-point scale : A score of 0 denotes normal use of the injected paw (i.e., the plantar surface of the paw comes into full contact with the floor of the observation box and the animal's weight is evenly distributed between hind-paws). A score of 1 indicates careful use of the injured paw, with some part of the paw in contact with the floor; the animal limps when walking. A score of 2 indicates elevation of the paw. A score of 3 denotes vigorous shaking or licking of the injured paw (distinct from normal grooming behavior). Scores were calculated as previously described[9] in which, pain behaviors are expressed during the initial early phase (0-5 min) or the second, late phase (15-60 min).
Statistical Analysis
Three-way ANOVA was used for prenatally treatment*sex*challenge dose. One-way analysis of variance (one-way ANOVA) was performed for each condition for each sex. Tukey-Kramer post hoc analysis was carried out if permitted. Differences with P < 0.05 were considered statistically significant.
Results
Morphine Antinociception in Male and Female Offspring Born of Saline or Low/High Dose Morphine Treated Mothers
Data were analyzed using three-way ANOVA, which significantly indicated an interaction. Three-way ANOVA provided that sex*treatment interaction is significant; FSex*Treatment (1, 150) = 21.005; P < 0.0001. The analysis also showed significantly interaction of treatment*dose and sex*dose in a respective manner: FTreatment*Dose (14, 150) = 13.367; P < 0.001 and FSex*Dose (14, 150) = 2.638; P < 0.01.
Treatment*dose interaction for males but not females was indicated as significant (FTreatment*Dose (8, 75) = 6.031; P < 0.0001 in early and FTreatment*Dose (8, 75) = 3.310; P < 0.001 in late phases).
Administration of morphine (0.5-7.5 mg/kg) in offspring born of saline-treated mothers caused antinociceptive effect significantly (both in early phase: F (4, 24) = 6.205; P < 0.01 and late phase: F (4, 24) = 17.890; P < 0.0001 in female offspring, and males: F (4, 24) = 144.90; P < 0.0001 and F (4, 24) = 140.100; P < 0.0001 respectively [Figure 1]. Post hoc analysis further indicated differences in the anti-pain effect induced by morphine in this category as males are less morphine-sensitive than females (late phase only).
Figure 1.
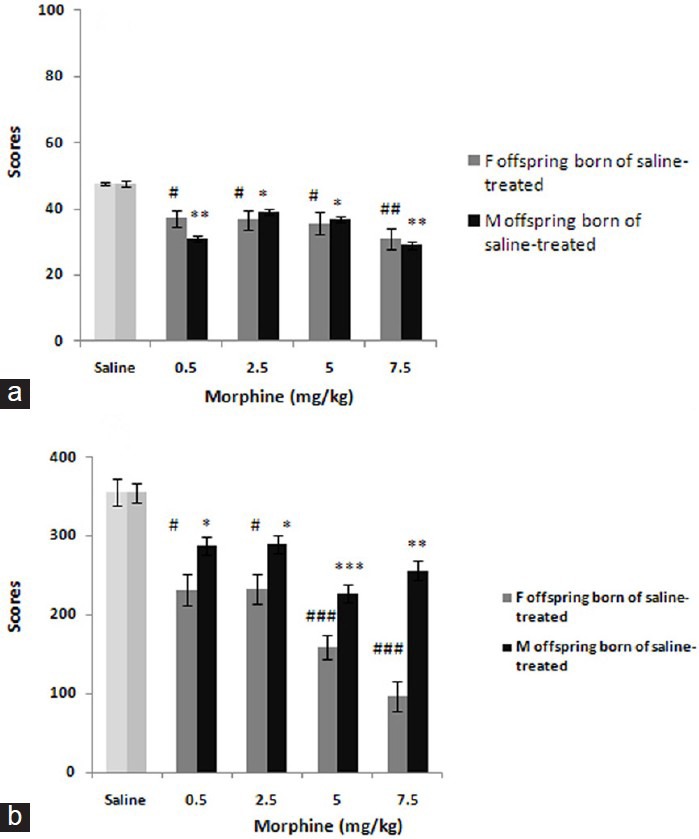
This figure shows the dose-response curves to morphine (0.5-7.5 mg/kg) in offspring born of saline-treated mothers. The scores were calculated according to the protocol detailed in material and method section and shown as mean ± SEM.*P< 0.05, **P< 0.01, ***P<0.001, #P< 0.05, ##P< 0.01, ###P< 0.001 are differences to controls according to post hoc analysis
In addition, pre-testing administration of morphine in offspring born of mothers received low-dose of morphine [Figure 2] showed also significant effects both in early phase: F (4, 24) = 3.117; P < 0.05 and late phase: F (4, 24) = 7.756; P < 0.001 in females [Figure 1] and respectively in males: F (4, 24) = 3.763; P < 0.05 and F (4, 24) = 14.043; P < 0.0001. Post hoc analysis identified no differences in this category.
Figure 2.
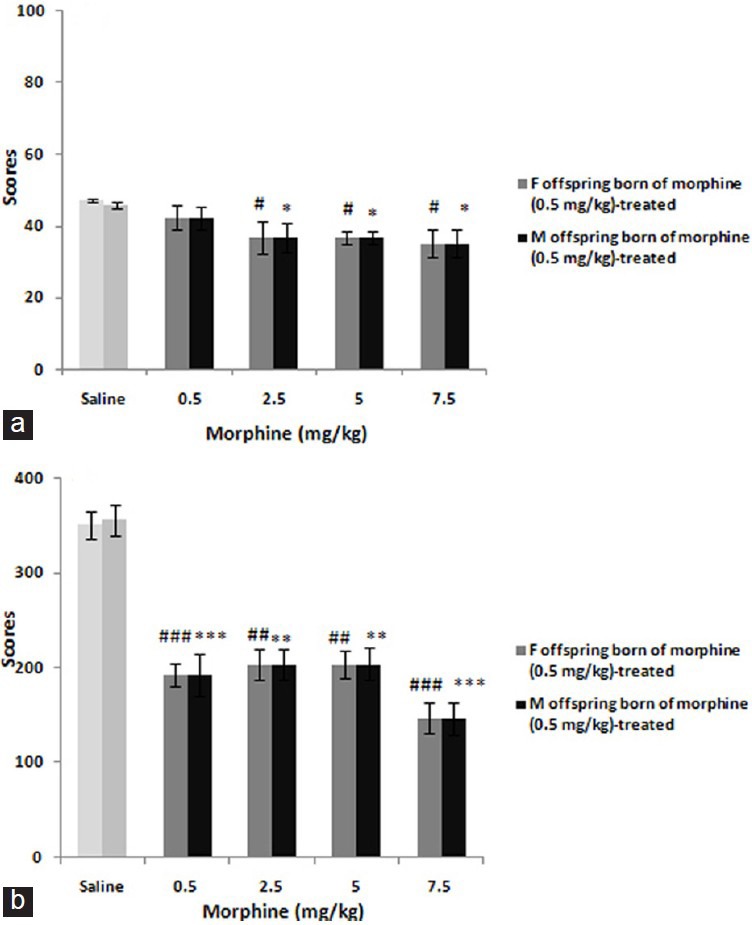
The figure shows the dose-response curves for morphine (0.5-7.5 mg/kg) in offspring born of morphine 0.5 mg/kg-treated mothers. The scores were calculated according to the protocol described in material and method section and shown as mean ± SEM. Based on post hoc analyses *P<0.05, **P<0.01, ***P<0.001, #P<0.05, ##P<0.01, ###P<0.001 are differences to controls
Furthermore, pre-testing injection of morphine in offspring born of mothers received high-dose of morphine revealed the antinociceptive effect of drug significantly both in early F (4, 24) = 21.496; P < 0.0001 and late F (4, 24) = 6.220; P < 0.01 phases in female offspring [Figure 3] and males respectively: F (4, 24) = 8.582; P < 0.001 and F (4, 24) = 11.458; P < 0.0001. Post hoc analysis showed differences in the category: Males are more sensitive than females in both phases.
Figure 3.
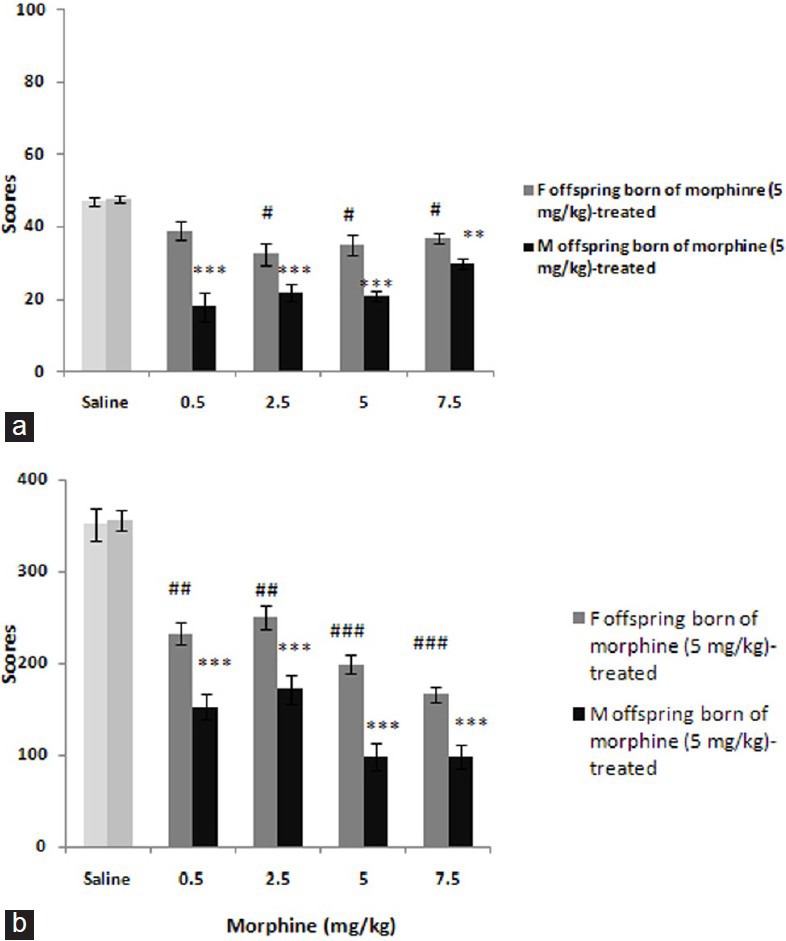
The figure shows the dose-response curves to morphine (0.5-7.5 mg/kg) in offspring born of morphine 5 mg/kg-treated mothers. The scores were calculated according to the protocol detailed in main text and shown as mean ± SEM. Post hoc analyses show**P< 0.01, ***P< 0.001, #P< 0.05, ##P< 0.01, ###P< 0.001 differences to controls
Discussion
This study discuses, the difference in morphine antinociception in male and female offspring born of saline or morphine treated mothers. Based on this result, the administration of morphine during the pregnancy induced change in behavior to pain. As present data provide a short-time exposure of pregnant female rats to morphine altered the pain behavior of their adult offspring exposed to the drug during the developmental period in a dependent manner to the sex.
There are few findings in support of the present data. It has been postulated that the analgesic differences show persistency after central morphine microinjection in mice.[10] Opioids accumulate selectively in the brains and nervous tissues of fetus and pre-weaning rats presumably due to increase in permeability of the blood-brain barrier in developing organisms.[11] By considering the gender differences in rate and timing of brain development,[12] the prenatal drug exposure can differently produce responses in male and female offspring. As been evidenced the catecholamine neurons differ in developing male and female rats during gestation days 14-16.[13] The brain sexual differentiation of dopamine and NE neurons has been deleted in the absence of gonadal steroids[14] showing a dependence of sexual differentiation to the steroids. Exposures to morphine during gestation, thus, induce differentially an alteration on developing brain systems that mediate pain responses in the two sexes.[5]
Prenatal morphine exposure evidently concerns the endogenous opioid systems and alters the estrogen regulation of μ, δ, and κ opioid receptor binding in specific brain regions in adult female rats.[5,15] Thus, our findings that prenatal morphine exposure significantly (P < 0.0001) enhances the sex-dependent anti-nociceptive effects of an acute morphine injection may identify the altered receptive responses to environmental stimuli through modifying both the pain stress-sensitive brain circuitry and the opioid-receptor mediated signaling.
Considering that in female rats, morphine exposure during pregnancy significantly changes the regional development of [3H] Met-enkephalin binding sites in the brains of the offspring,[16] a possible explanation may be that the prenatal exposure to morphine altered the ì-opioid receptors in brain regions or changed endogenous opioid binding sites in the offspring brain areas involved in pain behavior.
Exposure of rats to morphine during early development may also alter the brain opioid system, suggesting the possible consequences for morphine-mediated functions. As previous data suggest the prenatal morphine exposure alters estrogen regulation of kappa receptors in the frontal cortex and POA of females, but, it has no effects on 8-receptors in any of the examined brain regions in male rats.[17] This may explain the present observation effects showing that males are normally less morphine sensitive (late phase only), although, a morphine pre-treatment makes them more sensitive (both phases). In support, it has been demonstrated that the hormone responses to stimuli sex-dependently alters by prenatal exposure to morphine in adult rats.[18]
Steingart et al.,[19] furthermore, concluded that normal adult female rats react to stressors more intensely than adult male rats due to alteration in the glucocorticoid negative feedback inhibition.
Though, our results show a gender difference in anti-nociceptive behavior of morphine in offspring receiving high-dose of morphine prenatally, the antinociceptive response to the opioid did not reveal sex difference in prenatally morphine low-dose exposed offspring. The results may indicate that the morphine-induced antinociceptive response is most likely a net outcome of opioid acting on different classes of receptors having different regulating functions on opioid-induced antinociception. However, no previous studies show discordant results that prenatal exposure to low dose of morphine changed antinociceptive response to the drug in adulthood dependently to the sex. In a previous study on morphine induced analgesia in mice using tail-flick a low-dose of the opioid (1 mg/kg) has been accordingly reported as non-effective dose of morphine.[20] Previous data additionally show that even prolonged exposure of pregnant Sprague-Dawley rats to low-doses of morphine (up to 4 mg/kg) during days 3-20 of gestation did not result in sex-differences in formalin tests of the offspring,[21] the finding, which has been discussed by considering of tolerance development to morphine in prenatally morphine exposed rats. Other data however, show that prenatal morphine-induced analgesia altered pain sensitivities in a gender dependent manner in adulthood.[22] The present results, so, merely propose that the status of endogenous opiate systems may alter the expression of many opioid-regulated behaviors in adulthood.
The significance of the present data may be that a short-time opioid high-dose therapy during days of pregnancy may possibly cause sex-related change in pain response in adulthood.
It is known that in some phases of pregnancy, embryos are more sensitive to the effects of exogenous substances like abused drugs.[23] Our data reveals that an exposure to low-dose morphine prenatally is more effective in causing tolerance to morphine antinociceptive effect. One explanation for is that this observation morphine acts on several types of opioid receptors that have different affinity for the drug. Moreover, the populations of these receptors are gender dependently different in the developing embryo.[10,24] Therefore, the opioid receptor subtypes, those that are considered as responsible for the action of morphine are more sensitive to the lower dose of the drug. Alternatively, the action of the drug may be masked by activities of other opioid receptor subtypes when dose of morphine is increased. Another reason is that the opioid receptors located on the placenta are more sensitive to the lower doses of morphine.[25]
Our results show that short-time fetal morphine administration may produce long-term consequences in the brain. Morphine exposure prenatally may cause a change in somatic parameters such as analgesia, and responsiveness to inflammatory pain. This may be discussed in a way that morphine exposure prenatally may result in altered development of tolerance to morphine and thus may be involved in drug abuse.
Acknowledgments
The authors thank the Deputy of Research at Shahed University and Neurophysiology Research Center of Shahed University for supporting of educational programs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Fillingim RB, Gear RW. Sex differences in opioid analgesia: Clinical and experimental findings. Eur J Pain. 2004;8:413–25. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins E, Rossi G, Kest B. Sex differences in systemic morphine analgesic tolerance following intrathecal morphine injections. Brain Res. 2004;1014:244–6. doi: 10.1016/j.brainres.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 3.Kest B, Wilson SG, Mogil JS. Sex differences in supraspinal morphine analgesia are dependent on genotype. J Pharmacol Exp Ther. 1999;289:1370–5. [PubMed] [Google Scholar]
- 4.Hamann SR, Malik H, Sloan JW, Wala EP. Interactions of ultra-low doses of naltrexone and morphine in mature and young male and female rats. Receptors Channels. 2004;10:73–81. doi: 10.1080/10606820490464334. [DOI] [PubMed] [Google Scholar]
- 5.Rimanóczy A, Vathy I. Prenatal exposure to morphine alters brain mu opioid receptor characteristics in rats. Brain Res. 1995;690:245–8. doi: 10.1016/0006-8993(95)00638-7. [DOI] [PubMed] [Google Scholar]
- 6.Maeda KI, Kura SO, Tsukamura H. Physiology of reproduction. In: Hrinke CJ, editor. The Laboratory Rat: The Handbook of Experimental Animal. London: Academic Press; 2000. pp. 145–76. [Google Scholar]
- 7.Horvath G, Kekesi G, Dobos I, Klimscha W, Benedek G. Long-term changes in the antinociceptive potency of morphine or dexmedetomidine after a single treatment. Anesth Analg. 2005;101:812–8. doi: 10.1213/01.ane.0000166982.19796.ae. [DOI] [PubMed] [Google Scholar]
- 8.Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 1990;535:155–8. doi: 10.1016/0006-8993(90)91835-5. [DOI] [PubMed] [Google Scholar]
- 9.Zarrindast MR, Asgari-Afshar A, Sahebgharani M. Morphine-induced antinociception in the formalin test: Sensitization and interactions with D1 and D2 dopamine receptors and nitric oxide agents. Behav Pharmacol. 2007;18:177–84. doi: 10.1097/FBP.0b013e32813c5462. [DOI] [PubMed] [Google Scholar]
- 10.Zhu H, Barr GA. Opiate withdrawal during development: Are NMDA receptors indispensable? Trends Pharmacol Sci. 2001;22:404–8. doi: 10.1016/s0165-6147(00)01792-2. [DOI] [PubMed] [Google Scholar]
- 11.Vathy IU, Etgen AM, Barfield RJ. Effects of prenatal exposure to morphine on the development of sexual behavior in rats. Pharmacol Biochem Behav. 1985;22:227–32. doi: 10.1016/0091-3057(85)90382-x. [DOI] [PubMed] [Google Scholar]
- 12.Beyer C, Feder HH. Sex steroids and afferent input: Their roles in brain sexual differentiation. Annu Rev Physiol. 1987;49:349–64. doi: 10.1146/annurev.ph.49.030187.002025. [DOI] [PubMed] [Google Scholar]
- 13.Ossipov MH, Kovelowski CJ, Porreca F. The increase in morphine antinociceptive potency produced by carrageenan-induced hindpaw inflammation is blocked by naltrindole, a selective delta-opioid antagonist. Neurosci Lett. 1995;184:173–6. doi: 10.1016/0304-3940(94)11199-s. [DOI] [PubMed] [Google Scholar]
- 14.Engele J, Pilgrim C, Reisert I. Sexual differentiation of mesencephalic neurons in vitro: Effects of sex and gonadal hormones. Int J Dev Neurosci. 1989;7:603–11. doi: 10.1016/0736-5748(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 15.Rimanóczy A, Slamberová R, Vathy I. Prenatal morphine exposure alters estrogen regulation of kappa receptors in the cortex and POA of adult female rats but has no effects on these receptors in adult male rats. Brain Res. 2001;894:154–6. doi: 10.1016/s0006-8993(00)03326-6. [DOI] [PubMed] [Google Scholar]
- 16.Tsang D, Ng SC. Effect of antenatal exposure to opiates on the development of opiate receptors in rat brain. Brain Res. 1980;188:199–206. doi: 10.1016/0006-8993(80)90568-5. [DOI] [PubMed] [Google Scholar]
- 17.Slamberová R, Rimanóczy A, Schindler CJ, Vathy I. Cortical and striatal mu-opioid receptors are altered by gonadal hormone treatment but not by prenatal morphine exposure in adult male and female rats. Brain Res Bull. 2003;62:47–53. doi: 10.1016/j.brainresbull.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Rimanóczy A, Slamberová R, Bar N, Vathy I. Morphine exposure prevents up-regulation of MR and GR binding sites in the brain of adult male and female rats due to prenatal stress. Int J Dev Neurosci. 2006;24:241–8. doi: 10.1016/j.ijdevneu.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Steingart RA, Barg J, Maslaton J, Nesher M, Yanai J. Pre-and postsynaptic alterations in the septohippocampal cholinergic innervations after prenatal exposure to drugs. Brain Res Bull. 1998;46:203–9. doi: 10.1016/s0361-9230(97)00454-1. [DOI] [PubMed] [Google Scholar]
- 20.Karimfar MH, Gholizadeh S, Hajrasouliha AP, Miri AH, Tabrizian K, Azami K, et al. Low dose morphine enhances morphine antinociception effects in the animals pretreated with selective and non-selective phosphodiestrase inhibitors. Iran J Pharm Sci. 2009;5:73–81. [Google Scholar]
- 21.Chiang YC, Hung TW, Lee CW, Yan JY, Ho IK. Enhancement of tolerance development to morphine in rats prenatally exposed to morphine, methadone, and buprenorphine. J Biomed Sci. 2010;17:46. doi: 10.1186/1423-0127-17-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinsley CH, Mann PE, Bridges RS. Prenatal stress alters morphine-and stress-induced analgesia in male and female rats. Pharmacol Biochem Behav. 1988;30:123–8. doi: 10.1016/0091-3057(88)90434-0. [DOI] [PubMed] [Google Scholar]
- 23.Levitt P. Prenatal effects of drugs of abuse on brain development. Drug Alcohol Depend. 1998;51:109–25. doi: 10.1016/s0376-8716(98)00070-2. [DOI] [PubMed] [Google Scholar]
- 24.Leslie FM, Chen Y, Winzer-Serhan UH. Opioid receptor and peptide mRNA expression in proliferative zones of fetal rat central nervous system. Can J Physiol Pharmacol. 1998;76:284–93. [PubMed] [Google Scholar]
- 25.Nasiraei-Moghadam S, Sahraei H, Bahadoran H, Sadooghi M, Salimi SH, Kaka GR, et al. Effects of maternal oral morphine consumption on neural tube development in Wistar rats. Dev Brain Res. 2005;159:12–7. doi: 10.1016/j.devbrainres.2005.06.012. [DOI] [PubMed] [Google Scholar]


