Abstract
Objective:
To evaluate the nephroprotective effect of methanolic extract of Hygrophila spinosa (HSME) (Acanthaceae) in (CP)-induced acute renal failure in rats.
Materials and Methods:
HSME (250 mg/kg and 500 mg/kg body weight), were administered orally to male wistar albino rats.CP was used to induce acute renal failure. The parameters studied included blood urea and serum creatinine and malondialdehyde (MDA), reduced glutathione (GSH), catalase (CAT), superoxide dismutase (SOD) and GSH peroxidase activities. Histopathological examination was also carried out.
Results:
The results revealed that HSME pretreatment signiûcantly reduced blood urea and serum creatinine levels elevated by CP administration. Furthermore, HSME signiûcantly attenuated CP-induced increase in MDA and decrease in reduced GSH, and CAT and SOD and GSH peroxidase activities in renal cortical homogenates. Additionally, histopathological examination showed that HSME markedly ameliorated CP-induced renal tubular necrosis.
Conclusion:
The results indicate that the aerial parts of H. spinosa are endowed with nephroprotective activity.
KEY WORDS: Cisplatin, Hygrophila spinosa, lipid peroxidation, nephrotoxicity
Introduction
Cisplatin (CP) is a highly effective antineoplastic DNA alkylating agent used against a wide variety of cancers. Although, higher doses of CP are more efficacious for the treatment of cancer many reversible and irreversible side-effects including, nephrotoxicity, neurotoxicity, bone marrow toxicity, gastrointestinal toxicity and ototoxicity often limit its utility and therapeutic profile.[1] Primary targets of CP in kidney are proximal straight and distal convoluted tubules where it accumulates and promotes cellular damage, by multiple mechanisms including oxidative stress, DNA damage, and apoptosis.[2,3,4] Several lines of evidence suggest the role of reactive oxygen species (ROS) in the pathogenesis of nephrotoxicity.[5] CP induces free radical production causing oxidative renal damage, possibly due to depletion of non-enzymatic and enzymatic antioxidant systems. Abnormal production of ROS may damage some macromolecules to induce cellular injury and necrosis via several mechanisms including, peroxidation of membrane lipids, protein denaturation and DNA damage.[6] Acute renal failure refers to the sudden and usually reversible loss of renal function which develops over a period of days or weeks. Among the causes of acute renal failure, acute tubular necrosis, which occurs due to ischemia or nephrotoxins like CP is most common, accounting for 85% of the incidence. There is a continuous search for agents which provide nephroprotection against the renal impairment caused by drugs like CP for which allopathy offers no remedial measures. Thus, it is imperative that mankind turns towards alternative systems of medicine. Hence, the present study is an attempt to screen the methanol extract of Hygrophila spinosa (HSME) for its nephroprotective activity. H. spinosa (K. Schum) Heine (syn.) Asteracantha longifolia Nees, Acanthaceae are described in ayurvedic literature as Ikshura, Ikshugandha and Kokilasha “having eyes like the Kokila or Indian Cuckoo”. The plant is widely distributed throughout India, Sri Lanka, Burma, Malaysia and Nepal. The leaves, roots, seeds and ashes of the plant are extensively used in traditional system of medicine for various ailments such as jaundice, hepatic obstruction, rheumatism, inflammation, pain, urinary infections, urinary calculi, edema and gout. It is classified in Ayurvedic system as seethaveeryam, mathuravipaka and used for the treatment of premeham (diabetes), athisaram (dysentery).[7,8] A literature survey revealed that H. spinosa is endowed with various chemical components such as flavonoids, steroids, polysaccharides, triterpenoids, lupeol, saponins, etc., which possibly contribute to its diverse uses in folklore medicine. The plant is known to possess antitumor,[9] hypoglycaemic, free radical scavenging and lipid peroxidation.[10] Hepatoprotective activity of seeds of Hygrophila auriculata in thioacetamide, paracetamol-induced liver damage in rats, ethylene glycol induced nephrolithiasis in rat.[11,12,13] A literature survey revealed that there are no scientific studies carried out regarding H. spinosa in CP induced nephrotoxicity. Hence the present study is focused to evaluate the nephroprotective and antioxidant potentials of the plant against CP induced nephrotoxicity in rats.
Materials and Methods
Drugs
CP (Biochem Pharmaceutical Industries, Mumbai, India) and silymarin (Ranbaxy Ltd.) were purchased from the local market. All other chemicals and reagents used were of analytical grade.
Animals
Male wistar rats, procured from commercial breeder, were kept for a week for acclimatization under environmentally controlled conditions with free access to standard food (Amrut Feed, Sangali, India) and water. Rats weighing 150-250 g were used for the experiments. All animal experiments were carried out according to the guidelines and approval of Institutional Animal Ethics Committee.
Plant material
The whole plant H. spinosa syn. A. longifolia, H. schulli (Acanthaceae) was collected in month of September from region of Indore, Madhya Pradesh, India, and authenticated at Botanical Survey of India (BSI), Government of India, Ministry of Environment and Forests, Pune, India by Dr. P. G. Diwakar. A voucher specimen of the plant was deposited in the BSI herbarium under the number BSI/WC/Tech/2010/851. The whole plant material was dried in shade and ground to obtain a coarse powder.
Preparation of extracts
Powder of dried H. spinosa was extracted with 70% methanol and concentrated. The concentrated mass was washed with petroleum ether several times to remove the resinous matter. Then the mass was filtered and concentrated under vacuum at temperature 60°C, dried to get the powdered form of the extract. The HSME was stored in tightly closed glass bottle in refrigerator at 2-8°C.
Preliminary phytochemical screening
Preliminary phytochemical screening[14] revealed the presence of phenolic compounds, steroids, alkaloids, flavonoids, and triterpenoids in the extract.
Acute toxicity studies
The acute oral toxicity study was carried out as per the guideline 423 set by Organization for Economic Cooperation and Development received from Committee for the Purpose of Control and Supervision of Experiments on Animals. One-tenth of the median lethal dose 50 was taken as an effective dose.[13]
Experimental design
Animals were randomly divided into five groups of six animals each. Group I treated with vehicle (distilled water, p.o.) was kept as normal. Group II was injected with a single dose of CP (7.5 mg/kg b.w., i.p.). Group III and Group IV were administered HSME 250 and 500 mg/kg body weight along with CP respectively. Group V was administered silymarin, 50 mg/kg b.w., i.p. along with CP. The extract and silymarin were administrated for 10 days and on 11th day CP injection was administered. At 72 h after the CP injection, animals were sacrificed using ether anesthesia; blood samples were collected by heart puncture for measuring serum urea and serum creatinine levels. The body weight of each animal was recorded at the beginning and at the end of the study.[15,16]
Kidneys were quickly removed and washed with ice-cold normal saline and homogenates (10%, w/v) were prepared in PBS. A part of the homogenate was used for the estimation of glutathione (GSH) and lipid peroxidation. The remaining homogenate was centrifuged at 5000 g for 10 min at 4°C; after removal of the cell debris, the supernatant was used for the assay of superoxide dismutase (SOD), catalase (CAT) andglutathione peroxidase Glutathione peroxidase (GPx).
Serum creatinine was determined by alkaline picric acid method using a diagnostic kit. Serum urea was determined by diacetylmonoxime reagent (modified Berthelot methodology) using a diagnostic kit.
The GSH level was measured colorimetrically using 5, 5'- Dithio.bis (2 – nitrobenzoic acid) (DTNB) as the substrate. The concentrations of malondialdehyde (MDA) as indices of lipid peroxidation were assessed. The SOD activity was determined by the Nitro blue tetrazolium (NBT) reduction method. The GPx activity was determined by the method. The CAT activity was determined from the rate of decomposition of H2 O2, method. Tissue protein was estimated using bovine serum albumin as standard. Histopathological sections of kidney from all the treated groups were evaluated using light microscopy. For this the tissues were fixed in 10% formalin, embedded in paraffin, sectioned at 5 mM and stained with hematoxylin-eosin.[1,5]
Statistical analysis
Results are given as mean ± SEM. Data was analyzed using one-way ANOVA followed by Dunnett's test. The statistical significance of difference was taken as P < 0.05.
Results
In acute oral toxicity study the extract was found to be safe up to a dose of 2000 mg/kg.
Effect of HSME on body weight
Final body weights in CP treated group decreased when compared to the control group. But pre-treatment with HSME significantly prevented decrease in body weight when compared with CP alone group as shown in Figure 1.
Figure 1.
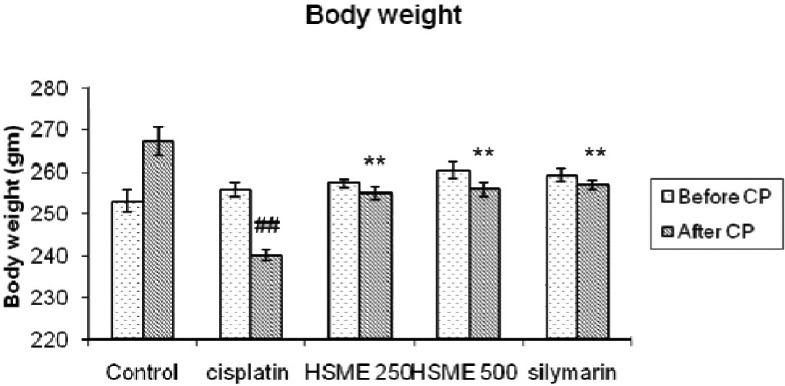
Effect of Hygrophila spinosa methanolic extracton body weight of rats with cisplatin induced nephrotoxicity. (Data are expressed as mean ± SEM., ##P<0.01 vs. control group, **P<0.01 vs. cisplatin group)
Effects of HSME blood urea and serum creatinine levels
In the present investigation, we have examined the CP treated animals showed a significant increase in blood urea and serum creatinine. As shown in Figures 2 and 3 serum creatinine and blood urea levels were significantly higher after 72 h respectively, after administration of single dose of CP when compared to the control group. The pre-treatment with HSME p.o. significantly (P < 0.01) lowered the elevated serum urea and creatinine when compared to the CP group. The pre-treatment with silymarin i.p. showed a marked decrease in concentrations of blood urea and serum creatinine compared to the control group (P < 0.01).
Figure 2.

Effect of Hygrophila spinosa methanolic extract on serum creatinine concentrations in rats treated with cisplatin. (Data are expressed as mean ± SEM., ##P< 0.01 vs. control group, **P< 0.01 vs. cisplatin group)
Figure 3.
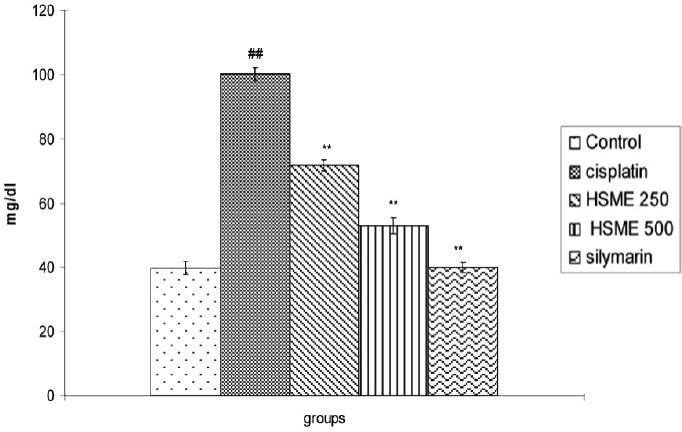
Effect of Hygrophila spinosa methanolic extract on blood urea concentrations in rats treated with cisplatin. (Data are expressed as mean ± SEM., ## P< 0.01 vs. control group, ** P< 0.01 vs. cisplatin group)
Effects of HSME on renal oxidant/antioxidant status
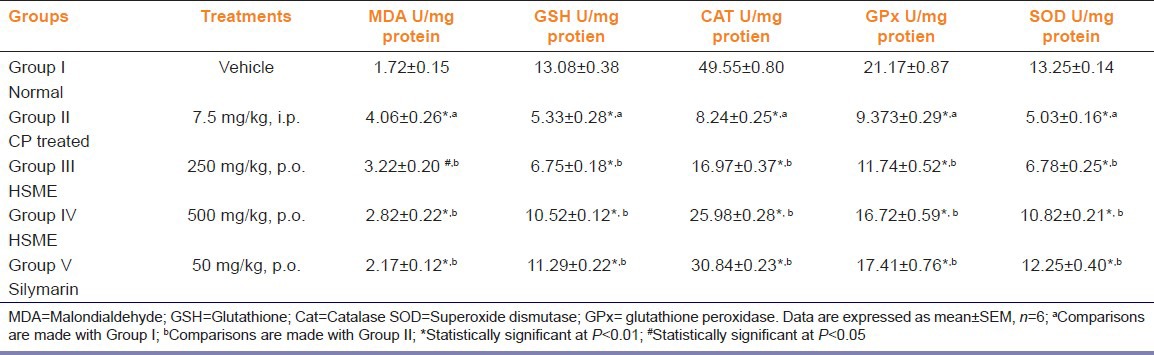
In CP treated group the activities of antioxidant enzymes like SOD, CAT and GPx and levels of GSH were found to be significantly decreased with marked increase in MDA as compared with control (P < 0.01). The pre-treatment of HSME or silymarin was found to significantly elevate the decreased activities of SOD, CAT and GPx (P < 0.01). The activities of renal SOD, CAT and GPx in the CP treated, CP plus HSME and CP plus silymarin administered groups were given in Table 1. Administration of HSME or silymarin also inhibited the CP-induced elevation in the MDA. The decrease in the GSH levels in renal tissues induced by CP was prevented by the administration of HSME or silymarin (P < 0.01) [Table 1].
Table 1.
Effect of Hygrophila spinosa extract on renal malondialdehyde, glutathione, catalase and superoxide dismutase, glutathione peroxidase GPx activities in rats treated with cisplatin
Effects of HSME on kidney histology
Treatment with CP caused a marked necrosis in proximal tubules and degeneration of the tubular epithelial cells [Figure 4b]. The pre-treatment with HSME and silymarin decreased the CP induced tubular necrosis when compared with CP treated group [Figure 4c-e].
Figure 4.

Light microscopy of renal tissue from rats injecting (a) control, (b) cisplatin (CP) alone, (c) pre-treatment with HSME 250 and CP, (d) pre-treatment with HSME 500 and CP, (e) pre-treatment with silymarin and CP (H and E, ×10 (a) Renal tubules are normal. (b) Tubules show severe (+++) necrosis. (c) Tubules show necrosis and slight degenerative changes (++). (d) Tubules show necrosis and slight degenerative changes (+). (e) Tubules show slight necrosis and no degenerative changes (+)
Discussion
In the present study, rats treated with CP showed a decrease in body weight. This weight-loss was attenuated, but not completely prevented by pre-treatment of HSME.[16] Suggesting that CP-induced weight-loss might be due to gastrointestinal toxicity and reduced ingestion of food. The impairment of kidney function by CP is recognized as the main side-effect and the most important dose limiting factor associated with its clinical use. Several investigators[15,17] reported that the alterations induced by CP in the kidney functions were characterized by signs of injury, such as increase of products of lipid peroxidation (LPO) and changes in GSH levels in kidney tissue, creatinine and urea levels in plasma. However, in HSME and silymarin pre-treated groups the body weight of animals in comparison with CP treated group representing prevention of gastrointestinal toxicity or maintenance of normal diet.
The renal antioxidant status, such as SOD, CAT, GPx activities, and reduced GSH concentration are significantly decreased in the CP treated group of animals compared to the control group. The declined antioxidant status partially explains the mechanism of nephrotoxicity induced by CP. The renal accumulation of platinum and covalent binding of renal protein may also play a role in the nephrotoxicity.[5,14] In the present study, increased serum creatinine and urea were observed in CP treated rats may be due to reduction in glomerular filtration rate. The impairment in kidney function was accompanied by an increase in MDA concentrations in kidney tissue. The above findings were well-correlated with the renal histological results. These observations indicated that CP induced nephrotoxicity and the results are in accordance with previous findings. The pre-treatment of HSME provides a significant protection against CP-induced nephrotoxicity, with lowering the level of plasma creatinine and blood urea in CP treated animals.
Decreased concentration of GSH increases the sensitivity of organs to oxidative and chemical injury. The role of GSH, non-protein thiols in the cells, in the formation of conjugates with electrophilic drug metabolites, most often formed by cytochrome P-450-linked monooxygenase, is well-established.[18] Studies with a number of models show that the metabolism of xenobiotics often produced GSH depletion. Reduced renal GSH can markedly increase the toxicity of CP. The depletion of GSH also seems to be a prime factor that permits lipid peroxidation in the CP-treated group. Moreover, the protection of GSH is also by forming the substrate for the GPx activity that can react directly with various aldehydes produced from the peroxidation of membrane lipids. The enhanced GPx activity could partially explain the protection of biomembranes from oxidative attack.
Decreased SOD activity could cause the initiation and propagation of lipid peroxidation in the CP treated group. This may be either due to loss of copper and zinc, essential for the activity of enzyme, or due to ROS induced inactivation of enzyme proteins. The decrease in activities of CAT and GPx could enhance the lipid peroxidation. Thus, the levels of MDA, as a result of lipid peroxidation, were increased in the CP-treated animals. Although, the exact mechanism of CP-induced nephrotoxicity is not well-understood, several investigators have shown that CP nephrotoxicity is associated with LPO in renal tissue. LPO is ascribed to a free radical-mediated chain reaction that damages cell membranes, and inhibition of this process by HSME is mainly attributed to the ability of scavenger free radicals.[19] In the present investigation, pre-treatment with HSME inhibited the increase in LPO induced by CP in renal tissue, indicating antioxidant activity of HSME.[11]
The histopathological evaluation of the kidney preparations in treatment group also revealed a decreased CP-induced tubular necrosis. CP-induced renal damage is associated with increased renal vascular resistance and histopathological damage to proximal tubular cells. On the other hand, an increase in GSH levels in the renal tissue indicates that pretreatment with HSME was due to oxidative stress. The effects of HSME on cellular GSH may be due to antioxidant effects. The treatment with HSME prevented the lipid peroxidation by enhancing the renal CAT, SOD and GPx activities.
Conclusion
In conclusion, it was shown that CP treatment induced renal damage and pretreatment with HSME provided protective effect against this CP-induced nephrotoxicity. However, before concluding a potential usefulness of HSME as adjunct to the CP therapy, further clinical investigation is warranted.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Lynch ED, Gu R, Pierce C, Kil J. Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res. 2005;201:81–9. doi: 10.1016/j.heares.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Schaaf GJ, Maas RF, de Groene EM, Fink-Gremmels J. Management of oxidative stress by heme oxygenase-1 in cisplatin-induced toxicity in renal tubular cells. Free Radic Res. 2002;36:835–43. doi: 10.1080/1071576021000005267. [DOI] [PubMed] [Google Scholar]
- 3.Xiao T, Choudhary S, Zhang W, Ansari NH, Salahudeen A. Possible involvement of oxidative stress in cisplatin-induced apoptosis in LLC-PK1 cells. J Toxicol Environ Health A. 2003;66:469–79. doi: 10.1080/15287390306449. [DOI] [PubMed] [Google Scholar]
- 4.Cummings BS, Schnellmann RG. Cisplatin-induced renal cell apoptosis: Caspase 3-dependent and -independent pathways. J Pharmacol Exp Ther. 2002;302:8–17. doi: 10.1124/jpet.302.1.8. [DOI] [PubMed] [Google Scholar]
- 5.Mohan IK, Khan M, Shobha JC, Naidu MU, Prayag A, Kuppusamy P, et al. Protection against cisplatin-induced nephrotoxicity by Spirulina in rats. Cancer Chemother Pharmacol. 2006;58:802–8. doi: 10.1007/s00280-006-0231-8. [DOI] [PubMed] [Google Scholar]
- 6.Parlakpinar H, Tasdemir S, Polat A, Bay-Karabulut A, Vardi N, Ucar M, et al. Protective role of caffeic acid phenethyl ester (cape) on gentamicin-induced acute renal toxicity in rats. Toxicology. 2005;207:169–77. doi: 10.1016/j.tox.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Nadkarni AK. Indian Materia Medica. 2nd ed. Mumbai: Popular Prakashan; 2007. Asteracantha longifolia; pp. 668–9. [Google Scholar]
- 8.Khare CP. Indian Medicinal Plants: An Illustrated Dictionary. 3rd ed. New York: Springer publication; 2007. Asteracantha longifolia; pp. 317–8. [Google Scholar]
- 9.Ahmed S, Rahman A, Mathur M, Athar M, Sultana S. Anti-tumor promoting activity of Asteracantha longifolia against experimental hepatocarcinogenesis in rats. Food Chem Toxicol. 2001;39:19–28. doi: 10.1016/s0278-6915(00)00103-4. [DOI] [PubMed] [Google Scholar]
- 10.Vijayakumar M, Govindarajan R, Shriwarkar A, Kumar V, Rawat A, Mehrotra S, et al. Free radical scavenging and lipid peroxidation inhibition potential of Hygrophila auriculata. Nat Prod Sci. 2005;11:22–6. [Google Scholar]
- 11.Kshirsagar AD, Ashok P. Hepatoprotective and antioxidant effects of Hygrophila spinosa (K. Schum) Heine Acanthaceae stem extract. Biosci Biotech Res Asia. 2008;5:657–62. [Google Scholar]
- 12.Kshirsagar AD, Ingale KG, Vyawahare NS, Thorve VS. Hygrophila spinosa: A comprehensive review. Pharmacogn Rev. 2010;4:167–71. doi: 10.4103/0973-7847.70912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingale KG, Thakurdesai PA, Vyawahare NS. Effect of Hygrophila spinosa in ethylene glycol induced nephrolithiasis in rats. Indian J Pharmacol. 2012;44:639–42. doi: 10.4103/0253-7613.100402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khandelwal KR. Practical Pharmacognosy-Techniques in Experiments. 8th ed. Pune: Nirali prakashan; 2001. Preliminary phytochemical screening; pp. 149–53. [Google Scholar]
- 15.Naziroglu M, Karaoğlu A, Aksoy AO. Selenium and high dose vitamin E administration protects cisplatin-induced oxidative damage to renal, liver and lens tissues in rats. Toxicology. 2004;195:221–30. doi: 10.1016/j.tox.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Mora Lde O, Antunes LM, Francescato HD, Bianchi Mde L. The effects of oral glutamine on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2003;47:517–22. doi: 10.1016/s1043-6618(03)00040-9. [DOI] [PubMed] [Google Scholar]
- 17.Antunes LM, Darin JD, Bianchi MD. Protective effects of vitamin c against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: A dose-dependent study. Pharmacol Res. 2000;41:405–11. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 18.Rana SV, Allen T, Singh R. Inevitable glutathione, then and now. Indian J Exp Biol. 2002;40:706–16. [PubMed] [Google Scholar]
- 19.Shanmugasundaram P, Venkataraman S. Hepatoprotective and antioxidant effects of Hygrophila auriculata (K. Schum) Heine Acanthaceae root extract. J Ethnopharmacol. 2006;104:124–8. doi: 10.1016/j.jep.2005.08.058. [DOI] [PubMed] [Google Scholar]


