Abstract
Objectives:
The present study was undertaken to evaluate the radioprotective and cytoprotective potential of cordifolioside-A, a primary active constituent of n-butanol fraction of Tinospora Cordifolia (NBTC) against 4 Gy-γ radiation in mice and cyclophosphamide induced genotoxicity.
Materials and Methods:
Presence of cordifolioside-A in NBTC stem ethanolic extract was confirmed by high performance thin layer chromatography (HPTLC) analysis. Radioprotective activity was evaluated at 80 and 120 mg/kg, intraperitoneal (i.p.) dose of NBTC administered 15 days prior to whole body radiation exposure by observing survival rate, change in body weight, hematology, spleen colony forming unit (CFU), and micronucleus (MN) expression. Cytoprotective activity of NBTC was evaluated at 5, 10, and 15 mg/ml concentrations on Allium cepa root meristem growth against cyclophosphamide.
Results:
HPTLC analysis of standard cordifolioside A, and NBTC confirmed the presence of cordifolioside-A in NBTC with the retention factor value of 0.86. Administration of NBTC (120 mg/kg, i.p.) produced significant protection against radiation in terms of increased survival rate, body weight retention, hematological parameters, spleen CFU assay (P < 0.01), and decreased MN expression (P < 0.01). Cytoprotectivity was observed maximally at 10 mg/ml NBTC concentration with significant increase in root growth (P < 0.01), non-toxic mitotic index (MI) (65.9%) and lesser chromosomal aberrations (15.4%). NBTC at 10 mg/ml concentration showed very few C-anaphase compared to aberrations like fragmentation, C-anaphase, multipolarity and sticky chromosome in cyclophosphamide alone.
Conclusion:
The results suggest that enriched NBTC containing cordifolioside-A has a potential in vivo radioprotective effect as well as in vitro cytoprotective activity.
KEY WORDS: Cordifolioside-A, cytoprotective activity, radioprotective activity, Tinospora cordifolia
Introduction
Tinospora cordifolia Miers. (Fam: Menispermaceae) is an important medicinal plant cultivated throughout the Indian sub-continent. Through centuries, it has been extensively used in Ayurveda for the treatment of various ailments.[1] T. cordifolia contains several classes of secondary metabolites such as alkaloids, glycosides, diterpenoids, lactones, steroids, sesquiterpenoids, aliphatics, and phenolic compounds. Among the alkaloids, the roots and stems of the plants are reported to contain berberine, palmatine, tembatarine, magniflorine, choline, tinosporine, isocolumbine, and minor amount of jatrorhizine. The major isolated compounds include the norditerpene furan glycosides such as cordifolioside-A, B, C, D, and E,[2] the ducan type sesquiterpenes tinocordifolin and tinocordifolioside[3] and the furanoids diterpene glycoside palamatoside C and F and amritoside.[4] The clerodane diterpenoides, cordioside tinosponone, and tinocordiside are also present.[5] Other constituents reported include phenolic lignane, octacosanol, heptacosane, beta sitosterol, tinosporidine, cordifolia and siringine.[6]
Literature survey and ethnopharmacological background of T. cordifolia suggested its manifold pharmacological responses such as immunostimulant,[1,7] hepatoprotective,[8] diuretic,[9] anti-inflammatory,[10] radioprotective,[11] antioxidant,[12] hypoglycemic,[13] and antipyretic activity.[14] Among the above described activities, immunostimulating, hepatoprotective, and antioxidant activities may be responsible for protection against radiation and alkylating chemicals. Cordifolioside-A and B isolated from T. cordifolia has been reported to possess immunostimulating activities, which can reduce the side-effects of chemotherapy such as immunosuppression, cytotoxicity, and genotoxicity.[6,15] Though, the researcher assumes that the primary active constituents of T. cordifolia are cordifolioside- A and B,[2,6] until date detail studies has not been performed for phytopharmacological correlation. Hence, our aim was to isolate cordifolioside-A from T. cordifolia stem extract and to evaluate its in vivo radioprotective activity on male Wistar Albino mice against 4 Gy-γ radiation exposure and in vitro cytoprotective activity on Allium cepa root meristem growth.
Materials and Methods
Plant material
T. cordifolia stems were collected from Sanjivani Ayurveda, Bhopal in September 2010. Plant was authenticated by Dr. Ziaul Hasan, Asst. Prof., Department of Botany, Safia College of Science, Bhopal, M.P (voucher specimen no. 184/Bot/Safia/10).
Reagents
Cyclophosphamide was provided by Cadila Health-care Ltd., Ahmedabad; Tocopheryl acetate (Evion) was obtained from Merck (India) Ltd., Goa.
Enrichment and identification of cordifolioside A
The coarse powder of T. cordifolia stem was extracted for 48 h with 50% v/v ethanol in soxhlet at 40-45°C.[16] The crude extracts was subjected to various qualitative tests to detect the presence of common chemical constituents such as alkaloids, glycosides, carbohydrates, flavonoids, phytosterols, saponins, tannins, and phenolic compounds.[17] Enrichment of ethanolic extract for furan glycosides were carried out following the method reported by Gangan et al. The non-polar compounds of dried ethanolic extract were removed by successive extraction with petroleum ether and ethyl acetate and dried. The ethyl acetate insoluble fraction was then extracted with n-butanol, filtered, and dried.[2] The presence of cordifolioside-A in n-butanol fraction of T. cordifolia (NBTC) stem ethanolic extract was characterized by phytochemical screening, thin layer chromatography (TLC) and HPTLC analysis.
TLC analysis
Different mobile phase combinations were tried for better separation of phyto-constituents. The mixture of chloroform:methanol in a ratio 85:15 was found to be optimum for better separation. Detection was done by spraying with 20% SbCl3 in CHCl3 and heating at 105°C for 10 min followed by visualization in ultra violet chamber at 254 nm.
HPTLC analysis
HPTLC was performed on a CAMAG system, which consists of the pre-coated plates (silica gel-F 254, Merck) and high presser sample injector of 100 μl capacity. All the three separated spots on preparative TLC of n-butanol fraction (NBTC-1: retention factor [Rf] 0.52, NBTC-2: Rf 0.56 and NBTC-3: Rf 0.86) were scrapped, dissolved in n-butanol and filtered. The plate was scanned at 254 nm with the help of CAMAG-Linomat 5 software system after development in mobile phase chloroform:methanol (85:15).
Animals
All the experimental procedures and protocols used in this study were reviewed by Institutional Animal Ethics Committee (approval no. IAEC/RCP/OCT-2010/02) as per CPCSEA guidelines. Inbreed Wistar Albino mice weighing between 30 g and 35 g were housed in well-cross ventilated animal house at temperature 25°C ± 2°C, relative humidity 65% ± 5% and light and dark cycles of 12:12 h respectively. During the experiment, animals were fed with standard pelleted diet and housed in polypropylene cages with paddy husk as bedding with free access to water ad libitum.
Acute toxicity studies/lethal dose (LD50)
The acute toxicity study of NBTC was performed on 12 h fasted mice as per OECD guideline 425. Extract was dissolved in 0.5% tween 80 and sterilized by filtration through 0.45 μm membrane filter attached with vacuum pump. Different doses of extract was administered intraperitoneally and observed for mortality up to 72 h.[18]
Radioprotective activity
Animals were divided into four groups of six animals each. Group I (radiation control was treated with vehicle, 0.2 ml/100 g), Group II (with tocopheryl acetate 300 mg/kg), Group III and IV with NBTC 80 and 120 mg/kg respectively. Standard drug tocopheryl acetate was solubilized in corn oil and administered intraperitoneally. Animals of all groups were exposed to 4 Gy-γ radiations by a 60 Co Gammatron teletherapy unit (Theratron 780 C, Canada) at a dose rate of 0.77 Gy/min. Drugs were administered 1 h prior to the radiation exposure and continued upto 15th day. Parameter such as body weight and survival were monitored at regular interval. Endogenous spleen colony forming unit (CFU), micronucleus (MN) and hematological parameters[19] were assessed on the 15th day after sacrifice by cervical dislocation.
Endogenous spleen CFU assay
After sacrifice, spleens were dissected out and fixed in Bouin's solution for 24 h. Macroscopic colonies of CFU visible to naked eyes were scored.
MN assay
Bone marrow cells were flushed out with phosphate buffer saline from femur bones after cutting at epiphyses. The cells were centrifuged at 1000 rpm, resuspended in Modified Eagle's Media, smear prepared on clean glass slides fixed with methanol for 30 min and stained with Giemsa. To determine the ratio of polychromatic and normochromatic erythrocytes 1000 cells were scored from each animal and MN frequency calculated.
Hematological parameters
Hemoglobin (Hb) content was measured, total white blood cells (WBCs) and red blood cells (RBCs) were counted in peripheral blood on 15th post-irradiation days.
Cytoprotective activity
A. cepa bulbs of approximately equal size were divided into seven groups each containing four bulbs. Group I 0.5% tween 80 in distilled water, Group II cyclophosphamide 10 mg/ml treated, Group III NBTC (10 mg/ml), Group IV NBTC (15 mg/ml), Group V NBTC + cyclophosphamide (5 + 10 mg/ml), Group VI NBTC + cyclophosphamide (10 + 10 mg/ml) and Group VII NBTC + cyclophosphamide (15 + 10 mg/ml) treated. A. cepa bulbs of all groups were pre-germinated in tap water for 72 h then transferred to the respective test solutions containing drugs for 48 h. After defined period of treatment A. cepa bulb roots were harvested, root numbers, and length were determined. The root tips were fixed in aceto-alcohol (1:3) to determine mitotic indices and chromosomal aberrations.[20]
Scoring of MI and chromosome aberration (CA)
The mitotic indices of A. cepa root meristem treated cells were determined by scoring approximately 900-1000 cells and observing the cells in interphase, prophase, metaphase, anaphase, and telophase. Anaphase and telophase cells were examined for the following categories of aberrations and scored: Chromosome fragments, bridge, vagrant chromosomes, C-anaphase, multipolar anaphases, and telophases and stick chromosomes.[21]
Statistical analysis
Results were expressed as Mean ± SEM and were analyzed using one way ANOVA followed by Tukey's Kramer multi comparison test using Graph pad prism software (version 5.04).
Results
Phytochemical screening
Ethanolic extract of T. cordifolia (yield 2.44%) showed the presence of alkaloids, glycosides, steroids, tannins, saponins, and flavonoids. The phytochemical screening of n-butanol fraction (yield 0.93%) showed rich presence of glycosides with moderate amount of flavonoids and triterpenoids. HPTLC analysis of standard cordifolioside A, and separated spots of NBTC-1, 2, and 3 confirmed the presence of cordifolioside-A with the Rf value 0.86 in NBTC-3 [Figure 1]. TLC and HPTLC analysis results confirmed the presence of cordifolioside-A in NBTC along with two other unidentified constituents.
Figure 1.
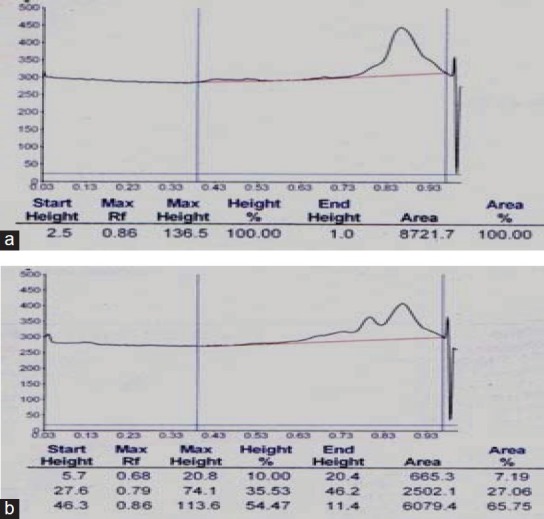
High performance thin layer chromatography densitometric peak of standard cordiofolioside: (a) showing retention factor (Rf) value 0.86 at 254 nm. (b) Peak of n-Butanol fraction of Tinospora cordifolia-3 showing identical peak at Rf value 0.86
LD50 analysis
LD50 was calculated to be 832 mg/kg and 80 and 120 mg/kg dose was selected for experimental study.
Radioprotective activity against 4 Gy-γ radiation exposure
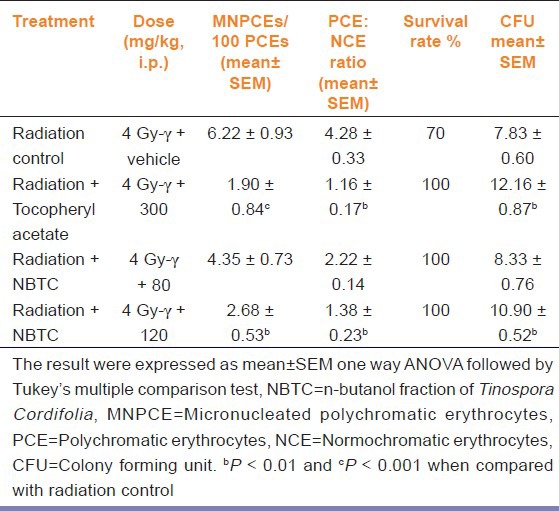
Total RBC, WBC, and Hb concentration was found to be 4.33 ± 0.62 106/mm3, 2.04 ± 0.19 103/mm3, and 7.57 ± 0.82 g/dl in sham radiation exposed group. NBTC 120 mg/kg treated group significantly (P < 0.05-0.01) increased the RBC and Hb concentration with respective value of 8.66 ± 0.85 106/mm3 and 12.17 ± 1.33 g/dl [Figure 2]. Body weight of the negative control and NBTC 80 mg/kg declined constantly (37% and 22% respectively) for 15 days while tocopheryl acetate and NBTC 120 mg/kg dose treatment showed consistent body weight with non-significant decrease (12% and 10% respectively). Sham treated group showed 70% survival rate whereas tocopheryl acetate and NBTC treated groups showed 100% survival. The animals treated with 120 mg/kg NBTC resulted in significant increase (P < 0.01) of CFU count compared to sham control. NBTC at a dose of 120 mg/kg showed radioprotective activity as the micronucleated PCEs/100 PCEs and PCE: NCE ratio was found significantly (P < 0.01) lower compared with radiation control [Table 1].
Figure 2.
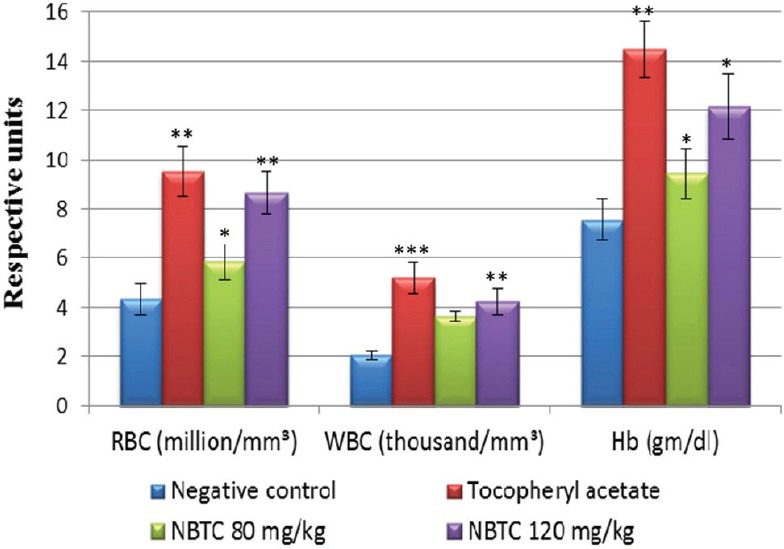
Effects of NBTC on hematological parameters of mice upon 4Gy-γ radiation exposure. The result were expressed as Mean ± SEM, One way ANOVA followed by Tukey's multiple comparison test. NBTC - n-Butanol fraction of Tinospora cordifolia, WBC - White blood cell, RBC - Red blood cell, Hb - Hemoglobin.*P< 0.05, **P< 0.01 and***P< 0.001 when compared with negative control group
Table 1.
Effects of NBTC fraction on frequencies of MNPCEs, spleen CFU and survival rate of mice upon 4 Gy-γ radiation exposure (n=6)
In vitro cytoprotective activity on A. cepa root tip growth
Cyclophosphamide at 10 mg/ml concentration significantly decreased the root length (P < 0.01) and root number (P < 0.001). NBTC at 10 mg/ml had non-significant effect on root length and number but when applied at 15 mg/ml concentration had significant growth retardant effect (P < 0.05). NBTC at 10 mg/ml when applied along with cyclophosphamide showed significant (P < 0.05-0.001) reversal on root length and number [Figure 3].
Figure 3.
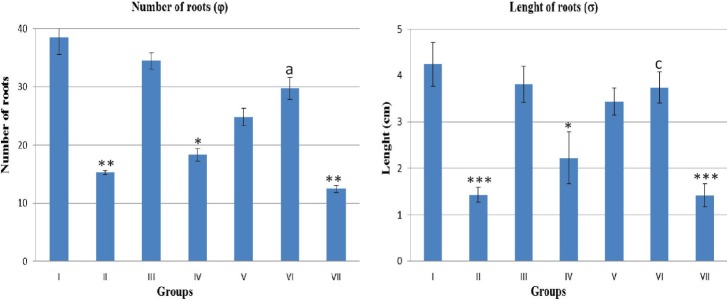
Effect of different drug concentration on Allium cepa root growth number and length. (φ) N= 4 and (σ) N=40. Group I - Normal control (distilled water), Group II - water + cyclophosphamide (10 mg/ml in distilled water), Group III – (n-Butanol fraction of Tinospora cordifolia [NBTC] 10 mg/ml in distilled water), Group IV - NBTC (15 mg/ml in distilled water), Group V- NBTC and cyclophosphamide (5 + 10 mg/ml in distilled water), Group VI - NBTC and cyclophosphamide (10 mg/ml each in distilled water) and Group VII - NBTC and cyclophosphamide (15 + 10 mg/ ml in distilled water).*P< 0.05, **P< 0.01, ***P< 0.001 when compared with normal control. aP < 0.05 and cP < 0.001 when compared with negative control, cyclophosphamide (10 mg/ml)
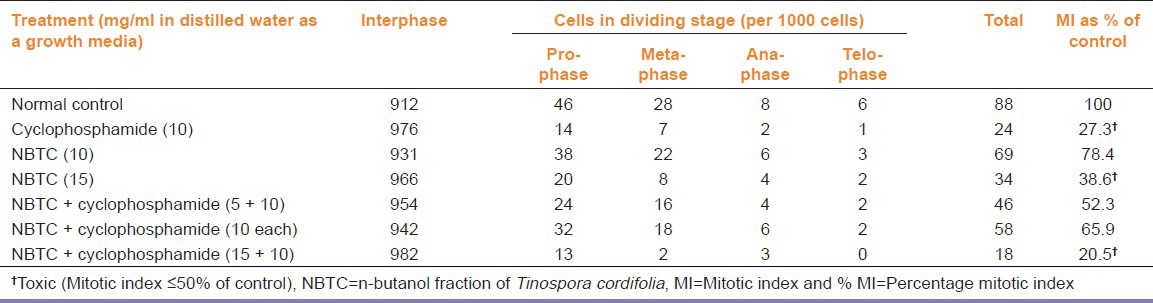
Study of percentage MI of A. cepa root meristems suggests that NBTC (10 mg/ml) and NBTC + cyclophosphamide (5 + 10 and 10 + 10 mg/ml) concentration were non-toxic while cyclophosphamide (10 mg/ml), NBTC (15 mg/ml) and NBTC + cyclophosphamide (15 + 10 mg/ml) were toxic. Cytotoxic effect of cyclophosphamide (10 mg/ml) was evident form the MI 27.3 whereas the same concentration when given in combination with NBTC at 10 mg/ml showed MI 65.9 [Table 2].
Table 2.
Effect of different concentrations of NBTC and cyclophosphamide on mitotic index of A. cepa root meristems
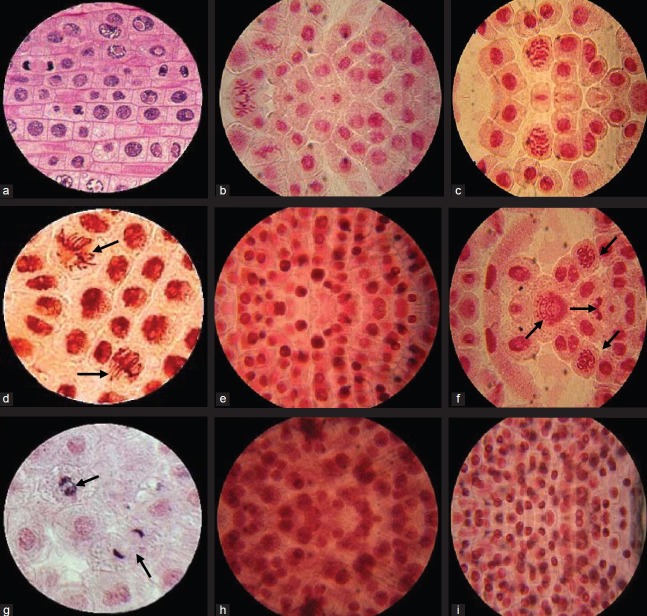
Compared to cyclophosphamide (10 mg/ml) the percent CA in NBTC alone (10 mg/ml) was 12.0 whereas given along with cyclophosphamide (10 mg/ml) it showed only 15.4% CA. Cyclophosphamide at 10 mg/ml showed aberrations like fragmentation, C-anaphase, multipolarity, and sticky chromosome [Figures 4b and c] whereas normal cells in telophase showed mitomic dividing stage [Figure 4a]. NBTC at 10 mg/ml treated A. cepa root meristem showed normal dividing cells in anaphase as well as few abnormal sticky and vagrant chromosome [Figure 4d]. Multipolarity with fragmented and sticky chromosome were present in NBTC 5 mg/ml and cyclophosphamide 10 mg/ml treatment [Figure 4f]. Cyclophosphamide (10 mg/ml) with NBTC 10 mg/ml treatment showed cells in prophase and telophase dividing stage with very few C-anaphase [Figure 4g]. Root treated with NBTC 15 mg/ml alone or in combination with cyclophosphamide 10 mg/ml showed many cells in prophase indicating arresting of growth [Figure 4e, h and i].
Figure 4.
Allium cepa root meristem cells in mitomic dividing stage. (a) Normal cells in telophase mitomic dividing stage. (b and c) Abnormal dividing cells in cyclophosphamide (10 mg/ml) treated group showing multipolarity and sticky chromosome. (d) Normal as well as few abnormal dividing cells in n-Butanol fraction of Tinospora cordifolia (NBTC) (10 mg/ml) treated root showing sticky and vagrant chromosome. (e) NBTC (15 mg/ml) treated showing most of the cells in prophase indicating arresting of growth. (f) NBTC 5 mg/ml and cyclophosphamide 10 mg/ml treated showing multipolarity, vagrant, fragmented and sticky chromosome. (g) NBTC 10 mg/ml and cyclophosphamide 10 mg/ml treated meristem showing normal mitotic dividing stage prophase and telophase. (h and i) NBTC 15 mg/ml and cyclophosphamide 10 mg/ml treated root showing many cells in prophase indicating arresting of growth along with few swelled and deformed cells (×100)
Discussion
Exposures of 4 Gy-γ Radiation cause breaks in DNA and generates free radicals that damage cell membranes, proteins, and organelles in bio-system consequently the highly dividing units such as RBCs and WBCs get majorly affected and there level decline. Whole body irradiation of a moderate dose range (5-10 Gy) leads to decreased concentration of all the cellular elements in the blood due to direct destruction of mature circulating cells, loss of cells from circulation by hemorrhage or leakage through capillary wall and loss of production of cells. Lowering of RBCs cause low Hb concentration along with decrease in the spleen CFUs and formation of MN.[22]
The results of this study showed that NBTC at 120 mg/kg/day given intraperitonealy upto 15th day of radiation exposure effectively protects against gamma radiation. NBTC showed highly significant increase in WBC and RBC count as well as in Hb content signifying its hemopoietic effect. The damage to the hematopoietic system is a major factor resulting in mortality following acute radiation exposure in animals. Radiation control group showed upto 30% mortality within 7 days of exposure whereas NBTC showed no motality.[23]
The basic mechanism of radiation damage is free radical production leading to the formation of peroxides. Tocopheryl acetate is an important radical scavenging antioxidant that interrupts the chain reaction of lipid per-oxidation by reacting with lipid peroxy radicals resulting in highly significant increase in WBC, RBC and Hb content.[12] T. cordifolia is reported to increase percentage body weight gain, Hb content and activity of hexokinase in the liver. T. cordifolia also decreased phospholipids and free fatty acids, inhibits lipid per-oxidation, superoxides, and hydroxyl radicals.[6] NBTC showed the ability to prevent radiation damage, which may be due to free radical scavenging. The protection from irradiation effects may be as a result of several factors, such as the prevention of damage through inhibition of free radical generation or efficient scavenging of free radicals, repair of DNA, membrane, and other damaged target molecules, and the replenishment of severely damaged or dead cells.
Chemicals or other agents which enhance stem cell proliferation can therefore yield an appreciable recovery of the damaged tissue following radiation exposure and thereby contribute to survival.[24] The enhancement of CFU counts in the spleen of NBTC treated irradiated mice indicates the role of NBTC in protecting the stem cells and/or stimulating the proliferation of the surviving cells. The hemoglobin content was also observed to improve in NBTC treated irradiated animals. Liver and spleen sequester the defective RBCs caused due to irradiation damage resulting in the decrease of Hb. T. cordifolia is a blood purifier, possibly acts by stimulating liver and spleen to remove defective and damaged RBCs from peripheral blood circulation.[9] The feedback mechanism however can stimulate hemopoiesis in the bone marrow and therefore higher Hb levels were observed on the 15th day post-treatment of NBTC. NBTC also protects against radiation-induced genotoxicity as observed by MN frequency depicting that 120 mg/kg dose was maximally effective.
The A. cepa root growth study is an efficient test for screening and in situ monitoring for genotoxicity of chemicals revealing their capability of inducing chromosomal aberrations.[25] Anticancer drug, cyclophosphamide acts by intercalating with the DNA of cell retarding growth. Cyclophosphamide produces highly reactive carbonium intermediates, which transfer alkyl groups to cellular macro molecules by forming covalent bonds. The position 7 of guanine residue in DNA is especially susceptible, but other molecular sites are also involved. This results in cross linking/abnormal base pairing and scission of DNA strand. At higher and continuously administered doses cyclophosphamide causes cytotoxicity and is hence used as a media for growth of A. cepa.[24] In this study, cytotoxic effect of cyclophosphamide was evident in the form of shortening and decaying of roots. Anti-tumor drugs that interact with microtubules and tubulin are known to block mitosis and induce cell death by apoptosis.[26]
MI is used as a bio-monitor to assess the mutagenicity of effluents.[27] MI was significantly decreased in cyclophosphamide treatment at 10 mg/ml concentration and the number of cells entering mitotic cell division reduced drastically. A MI below 22% indicates lethal effects on test organisms while that below 50% has sublethal effects. The sublethal concentration of cyclophosphamide and NBTC was found to be 10 and 15 mg/ml respectively, having potent antimitotic property so 10 mg/ml concentration of cyclophosphamide was chosen as cytotoxicity inducer. Prophase accumulation was a common feature in high concentration of both NBTC and cyclophosphamides. The number of abnormal dividing cells at prophase was more than the normal dividing cells in the same phase. Prophase accumulation is attributed to a delay in the breakdown of the nuclear membrane due to ‘carry over’ inhibitory effects of treatments from interphase stage or disturbance/breakdown in spindle apparatus.[28] Molecules like furan glycoside cordifolioside with a similar degree of hydrophilicity and hydrophilicity may show different cytotoxic and cytoprotective properties. In vitro NBTC at 15 mg/ml concentration may be relatively higher in media fixed for 72 h.
In the present study, the percentage MI of NBTC at 10 mg/ml concentration was 78.4% of the control which is non-toxic and 38.6% at 15 mg/ml thereby suggesting sublethal effect. NBTC showed minimum cytotoxicity with MI 65.9% and CA 15.4% reversing the cytotoxicity of cyclophosphamide, which was also confirmed by the appreciable number and length of roots grown. NBTC at 10 mg/ml concentration was potentially less toxic while still inhibiting cell division and protecting from antiproliferative effect of cyclophosphamide. NBTC at 10 mg/ml concentration restorated cell division as evident from very less aberration, mutation and MN expression in cyclophosphamide intoxicated medium. A mutation causing irreversible changes appears in the form of chromosome fragmentations resulting in creation of micronuclei. Such deformation of genetic material together with sticking of chromosomes, inhibits the normal progress of mitotic division even long after removal of the causative factor.[29]
HPTLC showed that NBTC contains cordifolioside-A along with another unidentified phytocompounds. Though this study does not report clearly the mechanism of NBTC but the results suggest that NBTC at 120 mg/kg dose have significant radioprotective effect and cytoprotective efficacy against cyclophoaphamide induced genotoxicity at 10 mg/ml concentration. NBTC posses in vivo radioprotective and in vitro cytoprotective activity, which may be due to presence of cordifolioside-A as a bioactive constituent. NBTC restricts the chromosomal aberration with a nonlethal MI, indicating its cytoprotective capability alone as well as in presence of cyclophosphamide.
From the results of the current investigation, it can be concluded that NBTC stem extract containing cordifolioside A, have radioprotective and cytoprotective activity at 120 mg/kg (i.p.) dose and in vitro at 10 mg/ml concentration. The NBTC contains cordifolioside-A as confirmed by phytochemical, TLC, and HPTLC analysis, which may responsible for the investigated activities along with another unidentified compound. Hence, n-butanol fraction of T. cordifolia stem extract contains cordifolioside-A, which posses radioprotective and cytoprotective activity but the exact mechanism responsible for these activities were yet unknown and needs further study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Aher DV, Wahi AK. Pharmacological study of Tinospora cordifolia as an immunomodulator. Int J Curr Pharm Res. 2010;2:52–4. [Google Scholar]
- 2.Gangan VD, Pradhan P, Sipahimalani AT, Banerji A. Cordifolisides A, B, C: Norditerpene furan glycosides from Tinospora cordifolia. Phytochemistry. 1994;37:781–6. doi: 10.1016/s0031-9422(00)90358-3. [DOI] [PubMed] [Google Scholar]
- 3.Maurya R, Wazir V, Kapil A, Kapil SR. Clerodane diterpenoids from Tinospora cordifolia. Phytochemistry. 1995;38:559–61. [Google Scholar]
- 4.Montagner JP, Lognonne P, Beauduin R, Roult G, Karczewski JF, Stutzmann E, et al. Tinocordifolin, a sesquiterpene from Tinospora cordifolia. Phytochemistry. 1998;49:1343–5. [Google Scholar]
- 5.Maurya R, Manhas LR, Gupta P, Mishra PK, Singh G, Yadav PP. Amritosides A, B, C and D: Clerodane furano diterpene glucosides from Tinospora cordifolia. Phytochemistry. 2004;65:2051–5. doi: 10.1016/j.phytochem.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Kapil A, Sharma S. Immunopotentiating compounds from Tinospora cordifolia. J Ethnopharmacol. 1997;58:89–95. doi: 10.1016/s0378-8741(97)00086-x. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee R, De UK, Ram GC. Evaluation of mammary gland immunity and therapeutic potential of Tinospora cordifolia against bovine subclinical mastitis. Trop Anim Health Prod. 2010;42:645–51. doi: 10.1007/s11250-009-9471-z. [DOI] [PubMed] [Google Scholar]
- 8.Rege N, Sharadini D, Karandikar SM. Hepatoprotective effects of Tinospora cordifolia against carbon tetracholoride induced liver damage. Ind Drugs. 1984;21:1–2. [Google Scholar]
- 9.Singh KP, Gupta AS, Pendse UK, Mahatma O, Bhandari DS, Mahawar MM. Experimental and clinical studies on Tinospora cordifolia. J Res Ind Med. 1975;10:9–14. [Google Scholar]
- 10.Rawal AK, Nath DK, Yadav N, Pande S, Meshram SU, Biswas SK. Rubia Cordifolia, Fagonia cretica linn and Tinospora cordifolia exert anti-inflammatory properties by modulating platelet aggregation and VEGF, COX-2 and VCAM gene expressions in rat hippocampal slices subjected to ischemic reperfusion injury. Int J Appl Res Nat Prod. 2009;2:19–26. [Google Scholar]
- 11.Pahadiya S, Sharma J. Alteration of lethal effects of gamma rays in Swiss albino mice by Tinospora cordifolia. Phytother Res. 2003;17:552–4. doi: 10.1002/ptr.1156. [DOI] [PubMed] [Google Scholar]
- 12.Mathew S, Kuttan G. Antioxidant activity of Tinospora cordifolia and its usefulness in the amelioration of cyclophosphamide induced toxicity. J Exp Clin Cancer Res. 1997;16:407–11. [PubMed] [Google Scholar]
- 13.Rajalakshmi M, Eliza J, Priya CE, Nirmala A, Daisy P. Anti-diabetic properties of Tinospora cordifolia stem extracts on streptozotocin-induced diabetic rats. Afr J Pharm Pharmacol. 2009;3:171–80. [Google Scholar]
- 14.Singla A. Review of biological activities of Tinospora cordifolia. Webmed Central Pharm Sci. 2010;1:1–6. [Google Scholar]
- 15.Maurya R, Wazir V, Kapil A, Kapil RS. Cardifoliosides A and B, two new phenylpropene disaccharides from Tinospora cordifolia possessing immunostimulant activity. Nat Prod Lett. 1996;8:7–10. [Google Scholar]
- 16.Pradhan P, Joseph L, George M, Kaushik N, Rahul C. Pharmacognostic, phyotochemical and quantitative investigation of Saraca asoca leaves. J Pharm Res. 2010;3:776–8. [Google Scholar]
- 17.Diener W, Mischke U, Schlede E, Kayser D. The biometrical evaluation of the OECD modified version of the acute toxic class method (oral) Arch Toxicol. 1995;69:729–34. doi: 10.1007/BF03035438. [DOI] [PubMed] [Google Scholar]
- 18.Goel HC, Prasad J, Singh S, Sagar RK, Agrawala PK, Bala M, et al. Radioprotective potential of an herbal extract of Tinospora cordifolia. J Radiat Res. 2004;45:61–8. doi: 10.1269/jrr.45.61. [DOI] [PubMed] [Google Scholar]
- 19.Quilang JP, Guzman MC, Hitta-Catalan MH, Rubio RO, Jacinto SD, Santiago EC, et al. Effects of polychlorinated biphenyls (pcbs) on root meristem cells of common onion (Allium cepa L.) and on early life stages of Zebrafish (Danio rerio) Philipp J Sci. 2008;137:141–51. [Google Scholar]
- 20.Asita OA, Matebesi LP. Genotoxicity of hormoban and seven other pesticides to onion root tip meristematic cells. Afr J Biotechnol. 2010;9:4225–32. [Google Scholar]
- 21.Potten CS. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol. 1990;58:925–73. doi: 10.1080/09553009014552281. [DOI] [PubMed] [Google Scholar]
- 22.Casarett AP. Radiation Biology. New Jersey: Prentice Hall Englewood Cliffs; 1968. Dose Response Relationships for Model Tissues; pp. 305–324. [Google Scholar]
- 23.Takenaka Y, Miki M, Yasuda H, Mino M. The effect of alpha-tocopherol as an antioxidant on the oxidation of membrane protein thiols induced by free radicals generated in different sites. Arch Biochem Biophys. 1991;285:344–50. doi: 10.1016/0003-9861(91)90370-x. [DOI] [PubMed] [Google Scholar]
- 24.Sehgal R, Roy S, Kumar VL. Evaluation of cytotoxic potential of latex of Calotropis procera and podophyllotoxin in Allium cepa root model. Biocell. 2006;30:9–13. [PubMed] [Google Scholar]
- 25.Fernandes TC, Mazzeo DE, Marin-Morales MA. Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pestic Biochem Physiol. 2007;88:252–9. [Google Scholar]
- 26.Jordan MA. Mechanism of action of antitumor drugs that interact with microtubules and tubulin. Curr Med Chem Anticancer Agents. 2002;2:1–17. doi: 10.2174/1568011023354290. [DOI] [PubMed] [Google Scholar]
- 27.Fiskesjo G. Allium test on river water from Bran and Sexan before and after closure of a chemical factory. Amobiol. 1985;14:99–103. [Google Scholar]
- 28.Panda BB, Sahu UK. Induction of abnormal spindle function and cytokinensis inhibition in mitotic cells of Allium cepa by organophosphorus insecticide fensulfothion. Cytobios. 1985;42:147–55. [Google Scholar]
- 29.Kuraœ M, Pilarski R, Nowakowska J, Zobel A, Brzost K, Antosiewicz J, et al. Effect of alkaloid-Free and alkaloid-rich preparations from uncaria tomentosa bark on mitotic activity and chromosome morphology evaluated by allium test. J Ethnopharmacol. 2009;121:140–7. doi: 10.1016/j.jep.2008.10.023. [DOI] [PubMed] [Google Scholar]